The most effective therapy demands the most complete understanding of the mechanism. Noninvasive imaging has provided key early observations of left ventricular outflow tract (LVOT) narrowing and flow acceleration in hypertrophic cardiomyopathy (HCM) (1,2), resulting from developmental abnormalities (3). On the basis of these observations, the original lift (Venturi) theory for LVOT obstruction by systolic anterior motion (SAM) of the mitral valve in HCM implicated upper septal hypertrophy as the sole culprit, but was therefore unable to explain residual SAM after upper septal reduction or SAM without septal hypertrophy (4–12). Those observations were subsequently explained by a more complete understanding of the interaction of mitral valve structural abnormalities with altered flow, which positions slack leaflet portions into the path of flow diverted by a bulging septum to affect the posterior leaflet surface and generate form-drag forces pushing the leaflet anteriorly; these are compounded by Venturi lift forces later in systole as the LVOT narrows and flow accelerates above the valve (13–23). An important element in understanding the role of mitral valve abnormalities in causing SAM was the observation of Sherrid et al. (24,25) that the distal leaflet (13) is pre-positioned in the outflow tract, such that even low flow rates can move the distal leaflet anteriorly and superiorly to occlude the outflow tract.
Why does this pre-positioning occur (Figure 1)? Certainly, anterior papillary muscle (PM) positioning anteriorly and leaflet elongation may predispose to it; but, because motion of structures is driven by flow-induced forces, this leaflet orientation suggests the influence of as yet unknown flow vectors oriented to push the leaflet anteriorly. Because Doppler color flow mapping can only provide velocity components parallel to the beam, until now, actual flow vectors relative to cardiac structures, which could provide greater pathophysiologic information, could be estimated but not precisely determined (26–28).
FIGURE 1. Comparison of Positioning and Movement of the Distal Mitral Leaflet From End Diastole to Early Systole in Normal Control and HCM Patients.
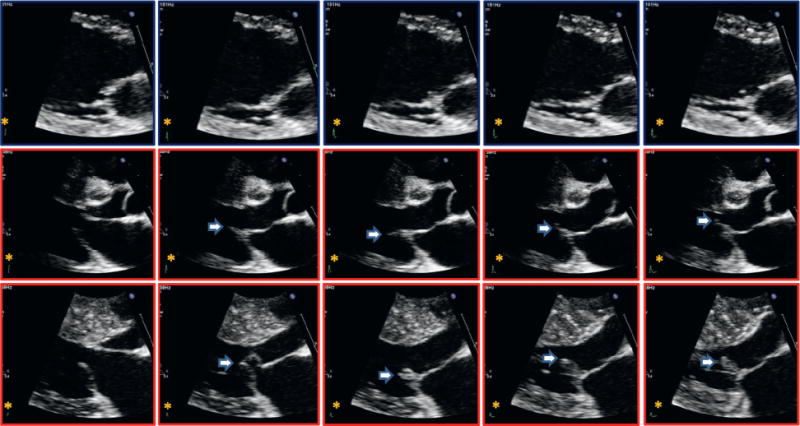
(Top) Normal control patients and (middle and bottom) hypertrophic cardiomyopathy (HCM) patients. Anterior motion of the posterior leaflet as the leaflets move toward closure (late diastole and during isovolumic contraction) before onset of systolic anterior motion (arrows) is prominent in HCM, normally absent.
As in the case of noninvasive measurement of pressure gradients from Doppler velocities, Doppler technology has yielded its richest clinical rewards when combined with fluid mechanics, the conservation principles of classical mechanics applied to fluids within ingeniously-selected control volumes. In this issue of the Journal, Ro et al. (29) have now applied a novel technology that provides vector maps of intracardiac flows from Doppler velocity components. The vectors are derived using the stream function of flow by applying conservation laws to measured Doppler velocity components within volumes of interest containing noncompressible blood.
The investigators applied this technology to answer our original question of leaflet pre-positioning in HCM: the majority of patients with obstruction have swirling flow vortices (26) that affect the posterior leaflet surfaces from below, moving them anteriorly. These vortices are generated by late-diastolic mitral inflow within the confined upper ventricular cavity, and pre-position the distal leaflets into the oncoming systolic wave, creating what can be termed diastolic anterior motion (DAM) as a precursor to SAM. In the remainder of patients, septal geometry redirects even the earliest, low-velocity systolic flows beneath the posterior leaflet, producing drag or pushing forces that drive SAM.
The study confirms that SAM results from anatomically-predisposed leaflets that protrude into these late diastolic/early systolic flows. Once SAM has begun, additional vectors directed posteriorly into the cul-de-sac beneath the posterior leaflet provide insight into its progression, and might conceivably help explain its resolution through a competing “negative or reverse Venturi” (14). Venturi forces act perpendicular to the axis of high-velocity jets. Such forces may act not only on the outflow side of the leaflets in an anterior direction (reinforcing SAM-septal contact), but also on their posterior surface in an opposing direction, initiating detachment of the leaflet from the septum, and thus promoting resolution of SAM.
Clinical implications of these findings include a better understanding of how abnormal valvular structures interact with flows determined by abnormal ventricular geometry, for example, to cause residual SAM with potential for dynamic obstruction in patients following septal reduction. These findings highlight the importance of septal reduction over a sufficient axial length to minimize anteriorly-directed vortex flows and flow redirected to affect the posterior leaflet surface to reduce residual SAM. These results further support the potential of leaflet modification and papillary muscle reorientation to reduce the anatomic substrate for SAM (30–39).
It will, therefore, be of great interest to learn how these flows change in patients with septal reduction, and how those changes correlate with residual SAM, obstruction, and mitral regurgitation (20). An intriguing future direction would combine 3-dimensional assessment of cardiac structure with computational flow modeling to answer the even more basic question of how these structures create abnormal vortices in the first place, and how we might ultimately “sculpt” LV geometry to ensure the most physiologic outflow without obstruction (26).
Thus, this study uses a new technology to answer a longstanding question, indicating how SAM has a late-diastolic impetus, generated by diastolic anterior motion—DAM leading to SAM—which may be modified therapeutically. On a more general note, these observations emphasize that, due to the cyclic nature of cardiac action, phenomena occurring during one cardiac phase can best be understood by also considering the impact of the preceding one.
Acknowledgments
The authors’ work has been supported, in part, by grant 07CVD04 from the Leducq MITRAL Transatlantic Network of Fondation Leducq and National Institutes of Health grants R01 HL72265 and HL109506.
Footnotes
Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology.
References
- 1.Shah PM, Gramiak R, Kramer DH. Ultrasound localization of left ventricular outflow obstruction in hypertrophic obstructive cardiomyopathy. Circulation. 1969;40:3–11. doi: 10.1161/01.cir.40.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Spirito P, Maron BJ. Significance of left ventricular outflow tract cross-sectional area in hypertrophic cardiomyopathy: a two-dimensional echocardiographic assessment. Circulation. 1983;67:1100–8. doi: 10.1161/01.cir.67.5.1100. [DOI] [PubMed] [Google Scholar]
- 3.Olivotto I, Cecchi F, Poggesi C, et al. Developmental origins of hypertrophic cardiomyopathy phenotypes: a unifying hypothesis. Nat Rev Cardiol. 2009;6:317–21. doi: 10.1038/nrcardio.2009.9. [DOI] [PubMed] [Google Scholar]
- 4.Ralph-Edwards A, Woo A, McCrindle BW, et al. Hypertrophic obstructive cardiomyopathy: comparison of outcomes after myectomy or alcohol ablation adjusted by propensity score. J Thorac Cardiovasc Surg. 2005;129:351–8. doi: 10.1016/j.jtcvs.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 5.Delling FN, Sanborn DY, Levine RA, et al. Frequency and mechanism of persistent systolic anterior motion and mitral regurgitation after septal ablation in obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:1691–5. doi: 10.1016/j.amjcard.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Smedira NG, Lytle BW, Lever HM, et al. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127–33. doi: 10.1016/j.athoracsur.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 7.ten Cate FJ, Soliman OI, Michels M, et al. Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution. Circ Heart Fail. 2010;3:362–9. doi: 10.1161/CIRCHEARTFAILURE.109.862359. [DOI] [PubMed] [Google Scholar]
- 8.Sorajja P, Ommen SR, Holmes DR, et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2012;126:2374–80. doi: 10.1161/CIRCULATIONAHA.111.076257. [DOI] [PubMed] [Google Scholar]
- 9.Desai MY, Bhonsale A, Smedira NG, et al. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209–16. doi: 10.1161/CIRCULATIONAHA.112.000849. [DOI] [PubMed] [Google Scholar]
- 10.Jebara VA, Mihaileanu S, Acar C, et al. Left ventricular outflow tract obstruction after mitral valve repair. Results of the sliding leaflet technique. Circulation. 1993;88(Pt 2):II30–4. [PubMed] [Google Scholar]
- 11.Lee KS, Stewart WJ, Lever HM, et al. Mechanism of outflow tract obstruction causing failed mitral valve repair. Anterior displacement of leaflet coaptation. Circulation. 1993;88(Pt 2):II24–9. [PubMed] [Google Scholar]
- 12.Maslow AD, Regan MM, Haering JM, et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol. 1999;34:2096–104. doi: 10.1016/s0735-1097(99)00464-7. [DOI] [PubMed] [Google Scholar]
- 13.Shah PM, Taylor RD, Wong M. Abnormal mitral valve coaptation in hypertrophic obstructive cardiomyopathy: proposed role in systolic anterior motion of mitral valve. Am J Cardiol. 1981;48:258–62. doi: 10.1016/0002-9149(81)90605-6. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Levine RA, King ME, et al. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am Heart J. 1987;113:633–44. doi: 10.1016/0002-8703(87)90701-0. [DOI] [PubMed] [Google Scholar]
- 15.Klues HG, Roberts WC, Maron BJ. Morphological determinants of echocardiographic patterns of mitral valve systolic anterior motion in obstructive hypertrophic cardiomyopathy. Circulation. 1993;87:1570–9. doi: 10.1161/01.cir.87.5.1570. [DOI] [PubMed] [Google Scholar]
- 16.Yoganathan AP, Lemmon JD, Jr, Kim YH, et al. A three-dimensional computational investigation of intraventricular fluid dynamics: examination into the initiation of systolic anterior motion of the mitral valve leaflets. J Biomech Eng. 1995;117:94–102. doi: 10.1115/1.2792276. [DOI] [PubMed] [Google Scholar]
- 17.Levine RA, Vlahakes GJ, Lefebvre X, et al. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation. 1995;91:1189–95. doi: 10.1161/01.cir.91.4.1189. [DOI] [PubMed] [Google Scholar]
- 18.Nakatani S, Schwammenthal E, Lever HM, et al. New insights into the reduction of mitral valve systolic anterior motion after ventricular septal myectomy in hypertrophic obstructive cardiomyopathy. Am Heart J. 1996;131:294–300. doi: 10.1016/s0002-8703(96)90357-9. [DOI] [PubMed] [Google Scholar]
- 19.He S, Hopmeyer J, Lefebvre XP, et al. Importance of leaflet elongation in causing systolic anterior motion of the mitral valve. J Heart Valve Dis. 1997;6:149–59. [PubMed] [Google Scholar]
- 20.Schwammenthal E, Nakatani S, He S, et al. Mechanism of mitral regurgitation in hypertrophic cardiomyopathy: mismatch of posterior to anterior leaflet length and mobility. Circulation. 1998;98:856–65. doi: 10.1161/01.cir.98.9.856. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Handschumacher MD, Levine RA, et al. In vivo measurement of mitral leaflet surface area and subvalvular geometry in patients with asymmetrical septal hypertrophy: insights into the mechanism of outflow tract obstruction. Circulation. 2010;122:1298–307. doi: 10.1161/CIRCULATIONAHA.109.935551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagege AA, Bruneval P, Levine RA, et al. The mitral valve in hypertrophic cardiomyopathy: old versus new concepts. J Cardiovasc Transl Res. 2011;4:757–66. doi: 10.1007/s12265-011-9319-6. [DOI] [PubMed] [Google Scholar]
- 23.Maron MS, Olivotto I, Harrigan C, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124:40–7. doi: 10.1161/CIRCULATIONAHA.110.985812. [DOI] [PubMed] [Google Scholar]
- 24.Sherrid MV, Gunsburg DZ, Moldenhauer S, et al. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2000;36:1344–54. doi: 10.1016/s0735-1097(00)00830-5. [DOI] [PubMed] [Google Scholar]
- 25.Sherrid MV, Chu CK, Delia E, et al. An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993;22:816–25. doi: 10.1016/0735-1097(93)90196-8. [DOI] [PubMed] [Google Scholar]
- 26.Reul H, Talukder N, Müller EW. Fluid mechanics of the natural mitral valve. J Biomech. 1981;14:361–72. doi: 10.1016/0021-9290(81)90046-4. [DOI] [PubMed] [Google Scholar]
- 27.Bolger AF, Heiberg E, Karlsson M, et al. Transit of blood flow through the human left ventricle mapped by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9:741–7. doi: 10.1080/10976640701544530. [DOI] [PubMed] [Google Scholar]
- 28.Sengupta PP, Pedrizetti G, Narula J. Multiplanar visualization of blood flow using echocardiographic particle imaging velocimetry. J Am Coll Cardiol Img. 2012;5:566–9. doi: 10.1016/j.jcmg.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Ro R, Halpern D, Sahn DJ, et al. Vector flow mapping in obstructive hypertrophic cardiomyopathy to assess the relationship of early systolic left ventricular flow and the mitral valve. J Am Coll Cardiol. 2014;64:I984–95. doi: 10.1016/j.jacc.2014.04.090. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh CL, Maron BJ, Cannon RO, 3rd, et al. Initial results of combined anterior mitral leaflet plication and ventricular septal myotomy-myectomy for relief of left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Circulation. 1992;86:II60–7. [PubMed] [Google Scholar]
- 31.Schwammenthal E, Levine RA. Dynamic subaortic obstruction: a disease of the mitral valve suitable for surgical repair? J Am Coll Cardiol. 1996;28:203–6. doi: 10.1016/0735-1097(96)00213-6. [DOI] [PubMed] [Google Scholar]
- 32.van der Lee C, Kofflard MJ, van Herwerden LA, et al. Sustained improvement after combined anterior mitral leaflet extension and myectomy in hypertrophic obstructive cardiomyopathy. Circulation. 2003;108:2088–92. doi: 10.1161/01.CIR.0000092912.57140.14. [DOI] [PubMed] [Google Scholar]
- 33.Sherrid MV, Chaudhry FA, Swistel DG. Obstructive hypertrophic cardiomyopathy: echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann Thorac Surg. 2003;75:620–32. doi: 10.1016/s0003-4975(02)04546-0. [DOI] [PubMed] [Google Scholar]
- 34.Dearani JA, Ommen SR, Gersh BJ, et al. Surgery insight: septal myectomy for obstructive hypertrophic cardiomyopathy—the Mayo clinic experience. Nat Clin Pract Cardiovasc Med. 2007;4:503–12. doi: 10.1038/ncpcardio0965. [DOI] [PubMed] [Google Scholar]
- 35.Swistel DG, DeRose JJ, Jr, Sherrid MV. Management of patients with complex hypertrophic cardiomyopathy: resection/plication/release. Operative Techniques in Cardiovascular and Thoracic Surgery. 2004;9:261–7. [Google Scholar]
- 36.Kaple RK, Murphy RT, DiPaola LM, et al. Mitral valve abnormalities in hypertrophic cardiomyopathy: echocardiographic features and surgical outcomes. Ann Thorac Surg. 2008;85:1527–35. doi: 10.1016/j.athoracsur.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 37.Bryant R, 3rd, Smedira NG. Papillary muscle realignment for symptomatic left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg. 2008;135:223–4. doi: 10.1016/j.jtcvs.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 38.Balaram SK, Tyrie L, Sherrid MV, et al. Resection-plication-release for hypertrophic cardiomyopathy: clinical and echocardiographic follow-up. Ann Thorac Surg. 2008;86:1539–44. doi: 10.1016/j.athoracsur.2008.07.048. discussion 1544–5. [DOI] [PubMed] [Google Scholar]
- 39.Seeburger J, Passage J, Borger MA, et al. A new concept for correction of systolic anterior motion and mitral valve regurgitation in patients with hypertrophic obstructive cardiomyopathy. J Thorac Cardiovasc Surg. 2010;140:481–3. doi: 10.1016/j.jtcvs.2010.01.010. [DOI] [PubMed] [Google Scholar]


