Abstract
Background and aims
Patients with non-alcoholic fatty liver disease (NAFLD) are thought to be at increased risk of cardiovascular morbidity and mortality. However, the relationships NAFLD to subclinical myocardial injury or structural heart disease are unknown
Methods
We conducted a cross-sectional analysis of 8668 participants from the Atherosclerosis Risk in the Communities (ARIC) Study without clinical evidence of cardiovascular disease. We used levels of liver enzymes (Alanine aminotrasferase [ALT], Aspartate aminotranferase [AST] and Gamma-glutamyl transpeptidase [GGT]), in the context of no history of elevated alcohol consumption as non-invasive surrogates of NAFLD. We used highly sensitive cardiac troponin T (hs-cTnT) and n-terminal pro-Brain natriuretic peptide (NT-proBNP) as biomarkers of myocardial damage and function.
Results
In this population-based study (mean age 63 years, 60% women, 78% white), higher levels of ALT, AST and GGT, even within the normal range, were significantly and independently associated with detectable (hs-cTnT>3 ng/L) and elevated (hs-cTnT≥14 ng/L) concentrations of hs-TnT. The adjusted odds ratios (95% confidence interval) for elevated liver enzymes (vs normal levels) with elevated hs-cTnT were: 1.65 (1.28–2.14) for ALT, 1.90 (1.36–2.68) for AST, and 1.55 (1.13–2.12) for GGT. Furthermore, there was evidence for inverse associations of ALT and AST with NT-proBNP.
Conclusion
Our results suggest that elevated liver enzymes in the absence of elevated alcohol consumption, may be associated with subclinical myocardial injury. The inverse association between NT-proBNP and ALT and AST support the recently described metabolic role of natriuretic peptides.
Keywords: NAFLD, subclinical cardiac damage, natriuretic peptides, troponin T
Hepatic steatosis, or fatty liver, is characterized by the excessive accumulation of triglycerides in the liver. This, in the absence of excessive alcohol consumption, is termed nonalcoholic fatty liver disease (NAFLD), the most common liver condition in western countries. Strictly speaking, liver biopsy is the gold standard method to diagnose NAFLD, however given its limitations it is not routinely performed in clinical practice [1]. The majority of studies therefore define NAFLD using surrogate markers of the disease such as levels of liver enzymes together with clinical information such as insulin resistance and low levels of alcohol consumption [2].
Besides obesity, NAFLD is associated with type 2 diabetes, dyslipidemia, and hypertension [3, 4]. While NAFLD is known to lead to liver related complications [5–9], the role of NAFLD in the development of cardiovascular disease is controversial [10–13]. In addition to shared risk factors, the presence of ectopic fat in the liver is thought to be an important contributor to systemic inflammatory changes [14, 15]. Previous epidemiologic studies of the association between NAFLD and cardiovascular disease have mostly focused on atherosclerotic disease and have been limited by the use of small, highly selected samples (e.g. patients with liver biopsy), or the study of patients with already clinically evident atherosclerotic cardiovascular disease [16].
To our knowledge little evidence exists examining the association between NAFLD and myocardial damage. Cardiac troponin T and N-terminal pro-brain natriuretic peptide (NT-proBNP) are biomarkers with established value for the identification of subclinical myocardial damage and structural heart disease, respectively [17]. Cardiac troponin T is widely used in the acute care setting to diagnose myocardial infarction. However, recent studies have shown that minute levels of circulating cardiac troponin T measured using novel (pre-commercial) highly sensitivity assays may reflect chronic subclinical myocardial injury [18] and have recently been shown to improve prediction of cardiovascular morbidity and mortality in subjects with stable coronary artery disease, and in persons without clinically evident cardiovascular disease [19–21].
NT-proBNP, an inert fraction of the prohormone for B-type natriuretic peptide (BNP), is secreted by ventricular myocytes in response to increased wall stress and ventricular filling pressure [22–24]. NT-proBNP is a robust biomarker of subclinical left ventricular dysfunction and heart failure, and is associated with cardiovascular and all cause mortality [22, 25–30].
The objective of the study was to examine the association between NAFLD and subclinical myocardial injury and structural heart disease. We hypothesized that NAFLD, as assessed by liver enzymes levels among people with low alcohol consumption, would be associated with subclinical myocardial damage as indicated by elevated hs-cTnT, and with subclinical structural heart disease, as indicated by elevated NT-proBNP.
Patients and Methods
Study Population
The ARIC Study is an ongoing cohort of 15,792 middle-aged adults recruited from four U.S. communities: Forsyth Country, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland [31]. The first examination of participants took place from 1987 to 1989, with three follow-up visits, occurring approximately three years apart, and a fifth from 2011 to 2013. The fourth visit (1996–1998) was attended by 11,656 participants and is the baseline for the present study because both liver enzymes and cardiac biomarkers data are available at this visit. We excluded participants with race/ethnicity other than black or white, persons with a history of coronary heart disease (defined as history of a physician diagnosed myocardial infarction, evidence of a prior myocardial infarction by electrocardiogram, or self-reported prior coronary reperfusion procedure), heart failure (defined as self-reported treatment for heart failure, hospitalization for heart failure, the Gothenburg stage 3, dyspnea due to cardiac causes and under treatment with digitalis or loop diuretics), elevated alcohol consumption (>20 grams/day), individuals with any liver enzyme >4 SD (n=127), and persons with missing data. The final sample size was 8,668 adults.
All participants signed written informed consent and the institutional review boards at each clinical site approved the study.
Measurements of high sensitive cardiac troponin T (hs-cTnT), N-terminal pro–B-type natriuretic peptide (NT-proBNP) and liver enzymes
hs-cTnT concentrations were measured with a novel pre-commercial highly sensitive assay, Elecsys Troponin T (Roche Diagnostics), on an automated Cobas e411 analyzer. The between-assay coefficients of variation were 2.6% and 6.9% for control materials with mean troponin T concentrations of 2.378 μg/L and 0.029 μg/L, respectively [32].
NT-proBNP was measured by using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics) with coefficients of variation ranging from 3.5–4.7%.
Alanine aminotransferase (ALT), aspartate aminotransferace (AST), and gamma-glutamyl transferase (GGT) were measured using an Olympus AU400e automated chemistry analyzer according to the manufacturer’s protocol. Intra-assay coefficients of variation were 11.1% for ALT, 8.5% for AST, and 9.3% for GGT.
Other Measurements
Smoking history, alcohol consumption, and family history of diabetes were assessed during interviews with the participants [31].
Using standardized methods, height, weight, waist circumference, and blood pressure were measured. Fasting blood samples were obtained, and the following assays were performed using standard methods: serum glucose, insulin, cholesterol levels (total, LDL- and HDL-cholesterol), triglycerides, creatinine and C reactive protein [33].
Statistical Analyses
We modeled liver enzymes using quartiles and dichotomously (normal or elevated). We defined “elevated” as levels >95th percentile based on the gender-specific distributions of each liver enzyme in a healthy subgroup of the ARIC population (normal weight persons without diabetes). Thus, elevated ALT was defined as >24 U/L among men and >21 U/L among women; elevated AST was defined as >32 U/L for men and >28 U/L for women; and elevated GGT was defined as >56 U/L for men and >50 U/L for women.
We modeled hs-cTnT in 3 different ways. 1) Two categories based on commonly used cut-offs: elevated (≥14 ng/L) versus normal (<14 ng/dL); 2) Two categories based on the limit of measurement using this hs-cTnT assay: measurable (≥3 ng/L) versus non-measurable (i.e. normal, <3 ng/L); and 3) As a continuous variable, participants with non-measurable hs-cTnT were assigned the value 1.5 ng/L.
We also modeled NT-proBNP in 3 different ways: 1) Two categories based on previously used cut-off: elevated (>400 pg/mL) versus normal (<400 pg/mL); 2) Two categories based on detectability of the assay: detectable (≥5 pg/mL) versus non-detectable (<5 ng/mL); 3) As a continuous variable, participants with non-detectable NT-proBNP were assigned the value 2.5 pg/mL.
We used logistic regression to model the associations of liver enzymes with elevated hs-cTnT or NT-proBNP with multivariable adjustment for potential confounding factors. We tested for interactions by race, sex, obesity, statin use, and diabetes in Model 3. We also implemented linear regression models with each liver enzyme modeled as restricted cubic splines with 4 knots (percentiles 5, 35, 65 and 95) to characterize the shape of the associations of the liver enzymes with hs-cTnT and NT-proBNP. All spline models were truncated at the 1st and 99th percentile of the distributions of the liver enzymes.
Results
Overall, the mean age of the participants included in the analyses was 62.6 (SD 5.6), 60% were female and 78% were white.
Compared to participants in the lowest quartile of ALT, those in the upper quartiles were more likely to be men and white, had higher body mass index, were more likely to have insulin resistance and diabetes, had lower HDL and higher triglycerides levels, lower levels of c-reactive protein and were less likely to be current smokers (Table 1). Similarly, those in the upper quartiles of AST were also more likely to be men, white and more likely to have insulin resistance. Compared to those in the lowest quartile of GGT, those in the upper quartiles were more likely to be male, black, had a higher body mass index, higher c-reactive protein, had higher LDL and triglycerides levels, and lower HDL, they were more likely to have hypertension, insulin resistance and diabetes.
Table 1.
Characteristics of the ARIC Study participants without a history of cardiovascular disease by quartiles of ALT, AST and GGT*, 1996–98 (N=8668)
| Q1 | Q2 | Q3 | Q4 | P for trend | |
|---|---|---|---|---|---|
| Mean Age (years) | |||||
| ALT | 62.9 | 62.8 | 62.8 | 61.8 | <0.001 |
| AST | 61.9 | 62.7 | 62.8 | 62.5 | 0.16 |
| GGT | 62.4 | 63.1 | 62.6 | 62.2 | 0.002 |
| Women (%) | |||||
| ALT | 76.7 | 69.8 | 58.1 | 46.2 | <0.001 |
| AST | 72.3 | 64.5 | 58.8 | 54.5 | <0.001 |
| GGT | 80.6 | 63.9 | 52.0 | 52.9 | <0.001 |
| Black (%) | |||||
| ALT | 29.4 | 23.5 | 20.2 | 17.9 | <0.001 |
| AST | 28.7 | 22.3 | 21.3 | 20.0 | <0.001 |
| GGT | 12.6 | 19.3 | 26.2 | 31.5 | <0.001 |
| Education, Less than High School (%) | |||||
| ALT | 20.4 | 19.8 | 16.6 | 16.0 | <0.001 |
| AST | 22.1 | 18.2 | 17.2 | 16.1 | <0.001 |
| GGT | 13.8 | 17.4 | 20.2 | 20.7 | <0.001 |
| Hypertension (%) | |||||
| ALT | 30.4 | 28.6 | 30.7 | 30.9 | 0.34 |
| AST | 30.5 | 29.4 | 30.9 | 30.1 | 0.82 |
| GGT | 21.4 | 27.7 | 32.5 | 38.4 | <0.001 |
| Current Smoker (%) | |||||
| ALT | 19.7 | 14.2 | 11.5 | 9.2 | <0.001 |
| AST | 22.5 | 15.3 | 9.8 | 8.9 | <0.001 |
| GGT | 14.0 | 14.1 | 12.7 | 13.2 | 0.001 |
| Obese (%) | |||||
| ALT | 29.2 | 32.8 | 35.6 | 41.8 | <0.001 |
| AST | 39.3 | 34.4 | 31.2 | 36.0 | <0.001 |
| GGT | 20.2 | 31.9 | 40.5 | 46.3 | <0.001 |
| Diabetes† (%) | |||||
| ALT | 9.5 | 9.8 | 13.8 | 17.8 | <0.001 |
| AST | 17.5 | 11.5 | 10.5 | 13.0 | <0.001 |
| GGT | 4.4 | 8.5 | 15.0 | 22.9 | <0.001 |
| Mean body mass index (kg/m2) | |||||
| ALT | 28.1 | 28.4 | 28.9 | 29.8 | <0.001 |
| AST | 29.6 | 28.7 | 28.4 | 28.8 | 0.34 |
| GGT | 26.7 | 28.5 | 29.6 | 30.3 | <0.001 |
| Mean eGFR (ml/min/1.73 m2) | |||||
| ALT | 81.8 | 82.5 | 82.4 | 83.2 | 0.01 |
| AST | 85.0 | 82.6 | 81.8 | 81.4 | 0.12 |
| GGT | 81.0 | 81.5 | 83.3 | 84.2 | <0.001 |
| Median hs-CRP (mg/L) | |||||
| ALT | 2.9 | 2.4 | 2.2 | 2.2 | <0.001 |
| AST | 3.4 | 2.5 | 2.1 | 2.1 | 0.001 |
| GGT | 1.9 | 2.1 | 2.5 | 3.1 | <0.001 |
| Mean LDL (mg/dL) | |||||
| ALT | 122.8 | 123.8 | 124.9 | 123.5 | 0.62 |
| AST | 123.4 | 125.2 | 122.9 | 123.1 | 0.05 |
| GGT | 118.6 | 124.2 | 125.0 | 126.6 | <0.001 |
| Mean HDL (mg/dL) | |||||
| ALT | 53.7 | 52.8 | 49.9 | 46.5 | <0.001 |
| AST | 50.7 | 51.6 | 50.8 | 49.5 | <0.001 |
| GGT | 57.3 | 50.6 | 47.5 | 47.5 | <0.001 |
| Median Triglycerides (mg/dL) | |||||
| ALT | 109 | 118 | 122.5 | 130 | <0.001 |
| AST | 117 | 121 | 120 | 121 | 0.40 |
| GGT | 106 | 117 | 124 | 135 | <0.001 |
| Insulin Resistance § (%) | |||||
| ALT | 18.1 | 20.4 | 23.8 | 38.7 | <0.001 |
| AST | 19.2 | 25.9 | 22.1 | 32.7 | <0.001 |
| GGT | 9.7 | 21.1 | 29.4 | 39.9 | <0.001 |
Quartiles ALT U/L, median [range]: Q1: 8 [1–9], Q2:11 [10–12], Q3:14 [13–16], Q4: 21 [17–322];
Quartiles AST U/L, median [range]: Q1:13 [5–14], Q2:16 [15–17], Q3:19 [18–20], Q4: 25 [21–58];
Quartiles GGT U/L, median [range]: Q1:12 [2–14], Q2:17 [15–20], Q3:24 [21–29], Q4: 40 [30–149]
Includes diagnosed and undiagnosed diabetes defined as fasting blood glucose ≥126 mg/dL.
Fasting HOMA-IR >3.5
The characteristics associated with elevated hs-cTnT and NT-proBNP are shown in supplemental tables 2 and 3. The following factors were significantly associated with elevated hs-cTnT: older age, gender men, lower education, obesity, diabetes, and hypertension. The factors associated with elevated NT-proBNP included: older age, gender female, current smoking, higher c-reactive protein and hypertension.
Association of liver enzymes with hs-cTnT
Higher quartiles of ALT, AST and GGT quartiles were significantly and consistently associated with detectable and elevated hs-cTnT (Figure 1 and Supplemental Table 1). Even after adjusting for traditional cardiovascular risk factors, those in the upper quartile of each of the liver enzymes were significantly associated with the presence of elevated hs-cTnT (Table 2). Further adjusting for NT-proBNP did not change the results significantly (Supplemental Table 5). We observed similar results in analyses modeling liver enzymes dichotomously (elevated vs not) (Supplemental Table 4). The continuous relationships between each of the liver enzymes and hs-cTnT is shown in Figure 2. Consistent with the analyses using quartiles, there was some evidence of threshold effect at: 12 U/L for ALT, 18 for AST, and 20 for GGT. In addition, to take into account the correlation between liver enzymes, we examined the association between elevated hs-TnT and the combined elevation of ALT and AST. The results were very similar. After adjusting for traditional cardiovascular risk factors, those in the upper quartile of ALT and AST had significantly increased odds of elevated hs-TnT, OR=1.61 (95%CI 1.12–2.30), fairly similar to the estimates observe in table 2, OR=1.46 (95% CI 1.12–1.91) for ALT and OR=1.72 (95% CI 1.30–2.29) for AST. No significant interactions were observed by sex, race, obesity, statin use, or diabetes status (all p-values >0.05) (Supplemental Tables 6–10).
Figure 1. Age-, sex- and race adjusted proportion (95% confidence interval) of participants with elevated hs-cTnT and NT-proBNP by quartiles* of AST, ALT, or GGT.
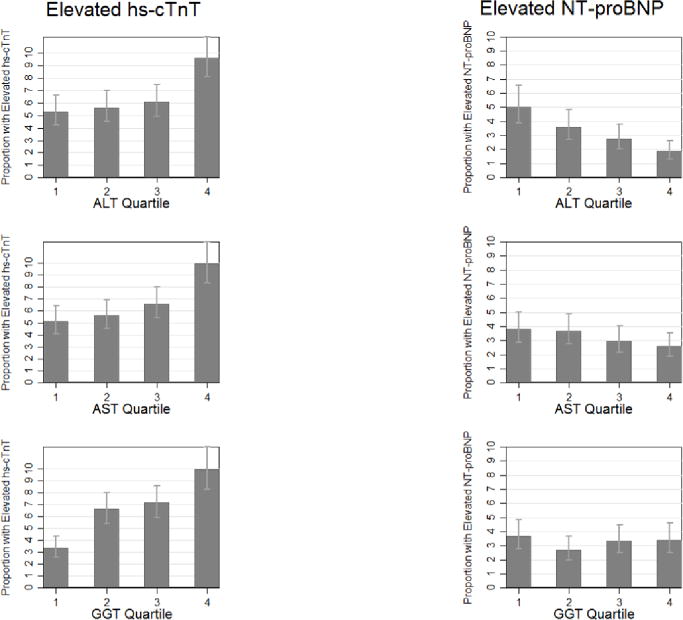
*Quartiles ALT U/L, median [range]: Q1: 8 [1–9], Q2:11 [10–12], Q3:14 [13–16], Q4:21 [17–322];
Quartiles AST U/L, median [range]: Q1:13 [5–14], Q2:16 [15–17], Q3:19 [18–20], Q4:25 [21–58];
Quartiles GGT U/L, median [range]: Q1:12 [2–14], Q2:17 [15–20], Q3:24 [21–29], Q4:40 [30–149]
Table 2.
Adjusted Odds Ratios (95% Confidence Intervals) of elevated hs-troponin T and elevated NT-proBNP by quartiles of liver enzymes
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|
|
Elevated hs-troponin T (>14 ng/L)
| |||
| ALT | |||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.01 (0.77–1.34) | 1.01 (0.76–1.33) | 1.02 (0.76–1.36) |
| Q3 | 0.96 (0.73–1.27) | 0.96 (0.73–1.27) | 0.95 (0.71–1.26) |
| Q4 | 1.54 (1.20–1.99) | 1.54 (1.19–1.99) | 1.46 (1.12–1.91) |
| AST | |||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 0.76 (0.57–1.01) | 0.75 (0.56–1.01) | 0.92 (0.68–1.25) |
| Q3 | 0.94 (0.70–1.24) | 0.93 (0.70–1.24) | 1.19 (0.87–1.61) |
| Q4 | 1.37 (1.05–1.78) | 1.36 (1.05–1.78) | 1.72 (1.30–2.29) |
| GGT | |||
| Q1 | 1 (Reference) | 1 (Reference | 1 (Reference) |
| Q2 | 1.44 (1.06–1.94) | 1.44 (1.07–1.96) | 1.33 (0.97–1.82) |
| Q3 | 1.36 (1.00–1.84) | 1.37 (1.01–1.86) | 1.14 (0.83–1.58) |
| Q4 | 1.84 (1.38–2.48) | 1.86 (1.39–2.50) | 1.44 (1.05–1.97) |
|
| |||
|
Elevated NT-proBNP (>400 pg/mL)
| |||
| ALT | |||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 0.71 (0.53–0.97) | 0.74 (0.54–1.01) | 0.81 (0.59–1.12) |
| Q3 | 0.53 (0.38–0.74) | 0.57 (0.40–0.79) | 0.64 (0.45–0.92) |
| Q4 | 0.40 (0.28–0.58) | 0.44 (0.30–0.63) | 0.49 (0.33–0.72) |
| AST | |||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 0.82 (0.58–1.14) | 0.86 (0.61–1.20) | 0.94 (0.66–1.35) |
| Q3 | 0.79 (0.56–1.12) | 0.87 (0.61–1.23) | 0.92 (0.63–1.33) |
| Q4 | 0.58 (0.40–0.83) | 0.64 (0.45–0.93) | 0.67 (0.46–0.99) |
| GGT | |||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 0.70 (0.49–0.99) | 0.68 (0.48–0.97) | 0.73 (0.51–1.06) |
| Q3 | 1.03 (0.73–1.45) | 1.04 (0.74–1.46) | 1.15 (0.80–1.65) |
| Q4 | 0.95 (0.68–1.34) | 0.96 (0.69–1.37) | 0.98 (0.67–1.43) |
Model 1: age (years), race-center, sex, education (less than high school, high school, college or above)
Model 2: variables in Model 1 + smoking (current, former, or never smoker), alcohol consumption (current, former, or never drinker)
Model 3: variables in Model 2+ body mass index (kg/m2), systolic blood pressure (mm/Hg), diastolic blood pressure (mm/Hg), fasting glucose (mg/dL), LDL (mg/dL) and HDL-cholesterol (mg/dL), hypertension medication use, C-reactive protein (mg/L) and eGFR (mL/min/1.73m2)
Figure 2. Association between levels of liver enzymes and hs-troponin T and NT-proBNP levels.
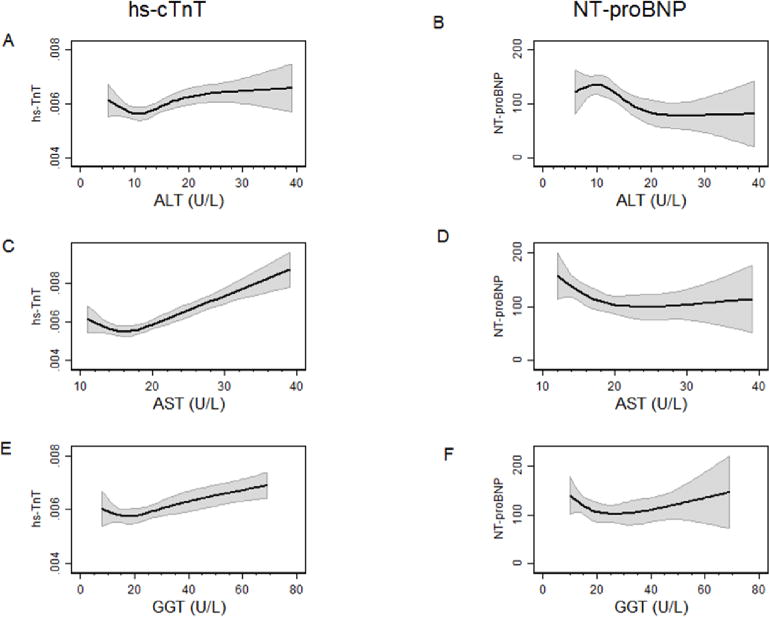
A: Association between ALT and hs-cTnT, B: Association between ALT and NT-proBNP,
C: Association between AST and hs-cTnT, D: Association between AST and NT-proBNP,
E: Association between GGT and hs-cTnT, D: Association between GGT and NT-proBNP,
All linear regression models, with cubic splines with 4 knots and adjusted for: age (years), sex, race, and body mass index (kg/m2).
Association of liver enzymes with NT-proBNP
Persons in the upper quartiles of ALT and AST had a lower prevalence of elevated NT-proBNP compared to those persons in the lower quartiles of these liver enzymes (Figure 1, Supplemental Table 1). Further adjusting for hs-cTnT did not change the results significantly (Supplemental Table 5). The prevalence of elevated NT-proBNP did not show a clear pattern of increase or decrease across quartiles of GGT. After adjustment for cardiovascular risk factors, those in the upper quartiles of ALT and AST were significantly and inversely associated with elevated NT-proBNP (Model 3, Table 2). When we examined the association between elevated NT-proBNP and the combined elevation in ALT and AST the results were very similar. After adjusting for traditional cardiovascular risk factors, those in the upper quartile of ALT and AST had significantly decreased odds of elevated NT-proBNP, OR=0.48 (95%CI 0.29–0.78), fairly similar to the estimates observed in table 2, OR=0.49 (95% CI 0.33–0.72) for ALT and OR=0.67 (95% CI 0.46–0.99) for AST.
When modeled in quartiles, GGT was not strongly associated with elevated NT-proBNP. In contrast, in analyses modeling the associations of elevated liver enzymes with elevated NT-proBNP, elevated GGT was positively associated with elevated NT-proBNP whereas elevated ALT and AST showed inverse but not statistically significant associations with elevated NT-proBNP (Table 3). The paradoxical results for GGT become clear when examining Figure 2: the association of GGT with NT-proBNP is U-shaped, with significant associations at both high and low levels of GGT. The observed shape of the associations for ALT and AST with NT-proBNP appear more consistently inverse. In models of ALT and AST with NT-proBNP, no significant interactions were observed by sex, race and presence of obesity (all p-values-for-interaction >0.05). However, there was some evidence that the association between GGT and elevated NT-proBNP was modified by diabetes status (p-for-interaction = 0.04). (Supplemental Table 9)
Discussions
We found that liver enzymes were consistently and strongly associated with minute elevations in circulating concentrations of cardiac troponin T. The observed association was independent of traditional cardiovascular risk factors, suggesting that NAFLD may contribute to cardiovascular disease via non-atherosclerotic pathways that are not yet entirely clear. In contrast to the results for hs-cTnT, the associations of liver enzymes and NT-proBNP were largely inconsistent: ALT and AST predominantly displayed an inverse association, whereas GGT had a more positive association.
The association between NAFLD and cardiovascular disease is an area of interest and controversy. Longitudinal studies assessing the association between liver enzymes as surrogates of NAFLD with cardiovascular mortality have been inconsistent. Ruhl et al. using data from a nationally representative sample of the U.S. reported no increased risk of cardiovascular death among persons with increased ALT or GGT after adjusting for potential confounders [34]. In contrast, Ruttman et al. using a large prospective cohort study of more than 160,000 Austrians, found an independent and dose-response relationship between GGT with cardiovascular mortality, with statistically significant hazard ratios (95% confidence interval) of 1.17 (1.02–1.33), 1.28 (1.08–1.53), 1.39 (1.09–1.78), and 1.64 (1.35–2.00) for GGT between 14–27, 28–41, 42–55 and >55 U/L, respectively, compared to persons with GGT levels <14 U/L [35]. Fraser et al., using a sample of ~3000 British women, found that ALT levels were not associated with cardiovascular events regardless the level of adjustment, however, GGT levels were associated with cardiovascular events in age adjusted models but after adjusting for confounders the associations became non significant [36]. The same group conducted a pooled meta-analysis of data from 10 different studies and found no significant, independent association exists between ALT and incident coronary heart disease [36]. However, in the pooled results they did observe a significant association between GGT (1 U/L natural log increase) and incident coronary heart disease (HR=1.20, 95% CI 1.02, 1.40). Ford et al., pooled three European cohorts and found no significant association between ALT quartiles and risk of cardiovascular morbidity or mortality [37].
Few studies have assessed the association between NAFLD and subclinical cardiovascular disease. The vast majority have relied on imaging techniques to characterize subclinical atherosclerotic outcomes. Sookian et al. pooled data from 7 studies (5 small hospital–based samples and 2 population based studies) and demonstrated that patients with NAFLD, had higher odds of carotid plaques, as measured by carotid intima media thickness; additionally they found a significant positive association between levels of liver enzymes (ALT and GGT) and carotid IMT [38]. Jung et al. found that ALT was positively associated with coronary calcium score detected using multi-detected row computed tomography [39]. Santos et al. also found an independent association between ultrasound-defined NAFLD and presence of coronary artery calcification [40].
Cardiac troponin T is an established biomarker representing myocardial injury and it has been shown to strongly predict the development of heart failure, cardiovascular events and death. In the same population of the current study, compared to those with undetectable levels, those with elevated levels of hs-cTnT had a hazard ratio of 2.29 (95% CI 1.81–2.89) for coronary heart disease, 2.96 (95% CI 3.21–4.88) for total mortality and 5.95 (95% CI 4.47–7.92) for heart failure [19]. In the current study we demonstrated a robust and independent association between liver enzymes (presumed NAFLD) and hs-cTnT, among people without a history of atherosclerotic cardiovascular disease. The mechanisms by which presumed NAFLD may be directly associated to subclinical non-atherosclerotic myocardial injury may involve coexisting ectopic lipid accumulation in the liver and myocardium leading to cardiac lipotoxicity, subsequent myocardial injury and eventually dilated cardiomyopathy [41, 42]. In addition the accumulation of ectopic fat in the liver has been shown to be accompanied by oxidative stress [15] and hypoxia-ischemia [43], factors that are also involved in myocardial damage; and thus may constitute additional pathways linking both.
We also examined the associations between liver enzymes and NT-proBNP as a marker of subclinical structural heart disease (e.g. left ventricular dysfunction). We found that, contrary to our hypothesis, levels of ALT and AST were inversely correlated with NT-proBNP levels, possibly suggesting less structural heart disease among people with NAFLD. However, one alternative, novel, and potentially more plausible mechanism for these inverse associations involves the recently described direct metabolic effects of BNP which include: increases in the mitochondrial biogenesis, adipose tissue lipolysis, and the “browning” of white adipocytes (inducing energy expenditure)[44–46]. In fact, some epidemiological analyses have shown an inverse association between obesity, diabetes, and BNP [47–49]. The somewhat different association between GGT and NT-proBNP, with a “U” shape with both low and high levels of GGT associated with higher NT-proBNP, is at least in part consistent to the original hypothesis. A potential explanation for the disagreement is that GGT is present in cell membranes of many tissues, including the kidney, liver, pancreas, heart and brain. As mentioned before, a number of studies have demonstrated an independent association between GGT and cardiovascular disease but no association of ALT or AST and cardiovascular disease. In contrast, the mechanism for the observed association between low GGT and higher NT-proBNP is consistent to the putative metabolic effects of NT-proBNP. We also found a significant interaction between diabetes and GGT. We believe that the interaction stems from the potentially different meaning of elevated GGT given the diabetes status: higher GGT in the context of diabetes is highly likely a good reflection of liver (NAFLD) related condition, and thus the results are consistent with those observed for more specific liver-NAFLD related enzymes (ALT and AST). In contrast, elevated GGT levels among adults without diabetes and low alcohol drinking is highly suggestive of an extra-hepatic, potentially cardiac, source of GGT. Our results confirm an inverse and independent association between NAFLD and NT-proBNP observed in one smaller study (N=713) [50]. Given the above mentioned limitations for the interpretation of NT-proBNP, future studies using direct measures of ventricular function are needed to provide a more definitive answer to the question whether NAFLD is associated to structural heart disease.
The cross-sectional design limits our ability to establish the temporality of the observed associations. We relied on cardiac biomarkers in the absence of clinically evident cardiovascular disease to assess subclinical myocardial disease, however the prognostic and diagnostic value of hs-cTnT and NT-proBNP are well established [51]. We used single measurements of liver enzymes as surrogate markers of NAFLD. It has been shown that for all liver enzymes, but in particular ALT and AST, there is significant intra-individual short term variation [52]. Furthermore, depending on the cut-off value, the sensitivity for the detection of NAFLD by liver enzymes ranges between 74% to 99% and the specificity ranges between 8% and 45%[3]. In addition, to minimize measurement error, we conducted additional analyses combining the elevation of ALT and AST, and found that the results were very similar. We had information on alcohol consumption, but we lacked information on other potential causes of liver disease (e.g. viral hepatitis, hemochromatosis, autoimmune disease) and therefore we cannot definitely identify the underlying potential cause of liver disease in this population. Nonetheless, based on a prior study in a small subset of the ARIC participants, the prevalence of these conditions in this cohort is known to be low (<1%)[53], and in this study, those with elevated liver enzymes had significantly higher levels of metabolic abnormalities and thus provide construct validity to our surrogate measure of NAFLD. Future studies are needed In ARIC and other similar cohorts to be better positioned to distinguish underlying cause or causes of liver disease. Although we had rigorous measurement of cardiovascular risk factors and we adjusted for a number of potential confounders, the possibility for residual confounding remains.
Our study also has a number of strengths that deserve mention. To our knowledge, this study represents the first attempt to characterize the association between liver enzymes and biomarkers of myocardial damage. This study benefitted from the rigorous and standardized data collection procedures in the ARIC Study, which among other things allowed us to use comprehensive information to exclude persons clinical CVD including silent MI based on ECG and for adjustment for potential confounders. In addition, ARIC constitutes one of the largest biracial community-based sample in the U.S.
In summary, in a large community based sample of middle age and older adults without history of CVD and with low alcohol consumption, each of the liver enzymes studied (ALT, AST, GGT), in the context of low alcohol consumption, were strongly associated with minute elevations in cardiac troponin T, a result of myocardial injury. The observed inverse association between liver enzymes and NT-proBNP may stem from recently described metabolic effects of natriuretic peptides, and suggest that similar to patients with diabetes and obesity, adults with presumed NAFLD have lower levels of NT-proBNP. However, the potential mechanisms for the observed metabolic effects of NT-proBNP in the liver are intriguing and deserve further study.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
FINANCIAL SUPPORT
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts: (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN26820110001 0C, HHSN268201100011C, and HHSN268201100012C).
LIST OF ABBREVIATIONS
- NAFLD
nonalcoholic fatty liver disease
- ARIC
Atherosclerosis Risk in the Communities Study
- ALT
Alanine aminotrasferase
- AST
Aspartate aminotranferase
- GGT
Gamma-glutamyl transpeptidase
- hs-cTnT
highly sensitive cardiac troponin T
- NT-proBNP
and n-terminal pro-Brain natriuretic peptide
- LDL
Low density lipoprotein
- HDL
High density lipoprotein
- hs-CRP
Highly sensitive C-reactive protein
- eGFR
Estimated glomerular filtration rate
- CVD
Cardiovascular disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Contributions: ML conceived the study question, analyzed and interpreted the data, and drafted the manuscript and is the guarantor of this work; JR, JMC, JC, AS, CN, RH, CB, ES interpreted the data and critically revised the manuscript for intellectual content; ES obtained funding and supervised the study.
CONFLICT OF INTEREST
Roche Diagnostics provided reagents and loan of an instrument to conduct the hs-cTnT and NT-proBNP assays. Roche Diagnostics had no role in design, analysis or presentation preparation. Drs. Hoogeveen and Ballantyne: Grant support from Roche Diagnostics (and the National Institutes of Health). The other authors declare no conflict of interests to disclose.
References
- 1.Ratziu V, Cadranel JF, Serfaty L, Denis J, Renou C, Delassalle P, et al. A survey of patterns of practice and perception of NAFLD in a large sample of practicing gastroenterologists in France. J Hepatol. 2012;57:376–383. doi: 10.1016/j.jhep.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 3.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 8.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 11.Ghouri N, Preiss D, Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology. 2010;52:1156–1161. doi: 10.1002/hep.23789. [DOI] [PubMed] [Google Scholar]
- 12.Loria P, Lonardo A, Bellentani S, Day CP, Marchesini G, Carulli N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: an open question. Nutr Metab Cardiovasc Dis. 2007;17:684–698. doi: 10.1016/j.numecd.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Loria P, Lonardo A, Targher G. Is liver fat detrimental to vessels?: intersections in the pathogenesis of NAFLD and atherosclerosis. Clin Sci(Lond) 2008;115:1–12. doi: 10.1042/CS20070311. [DOI] [PubMed] [Google Scholar]
- 14.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 15.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–379. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 16.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 17.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 18.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenning BA, Raymond I, Hildebrandt PR, Nilsson JC, Baumann M, Pedersen F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart. 2004;90:297–303. doi: 10.1136/hrt.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail. 2004;6:257–260. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Nishikimi T, Yoshihara F, Morimoto A, Ishikawa K, Ishimitsu T, Saito Y, et al. Relationship between left ventricular geometry and natriuretic peptide levels in essential hypertension. Hypertension. 1996;28:22–30. doi: 10.1161/01.hyp.28.1.22. [DOI] [PubMed] [Google Scholar]
- 25.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–176. doi: 10.1001/jama.297.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350:1349–1353. doi: 10.1016/S0140-6736(97)06031-5. [DOI] [PubMed] [Google Scholar]
- 27.de Lemos JA, Hildebrandt P. Amino-terminal pro-B-type natriuretic peptides: testing in general populations. Am J Cardiol. 2008;101:16–20. doi: 10.1016/j.amjcard.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 29.McDonagh TA, Holmer S, Raymond I, Luchner A, Hildebrant P, Dargie HJ. NT-proBNP and the diagnosis of heart failure: a pooled analysis of three European epidemiological studies. Eur J Heart Fail. 2004;6:269–273. doi: 10.1016/j.ejheart.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 30.McKie PM, Rodeheffer RJ, Cataliotti A, Martin FL, Urban LH, Mahoney DW, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47:874–880. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 32.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem. 2011;57:891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ARIC Study protocol. Manual 7: Blood Collection and Processing. [Google Scholar]
- 34.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–485. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 35.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130–2137. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 36.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729–2735. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- 37.Ford I, Mooijaart SP, Lloyd S, Murray HM, Westendorp RG, de Craen AJ, et al. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. International journal of epidemiology. 2011;40:1530–1538. doi: 10.1093/ije/dyr172. [DOI] [PubMed] [Google Scholar]
- 38.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49:600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Jung DH, Lee YJ, Ahn HY, Shim JY, Lee HR. Relationship of hepatic steatosis and alanine aminotransferase with coronary calcification. Clin Chem Lab Med. 2010;48:1829–1834. doi: 10.1515/CCLM.2010.349. [DOI] [PubMed] [Google Scholar]
- 40.Santos RD, Nasir K, Conceicao RD, Sarwar A, Carvalho JA, Blumenthal RS. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis. 2007;194:517–519. doi: 10.1016/j.atherosclerosis.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 41.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 42.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, et al. Cardiac steatosis in diabetes mellitus: a 1 H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 43.Musso G, Olivetti C, Cassader M, Gambino R. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis. 2012;32:49–64. doi: 10.1055/s-0032-1306426. [DOI] [PubMed] [Google Scholar]
- 44.Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittle AJ, Vidal-Puig A. NPs – heart hormones that regulate brown fat? J Clin Invest. 2012;122:804–807. doi: 10.1172/JCI62595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 48.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 49.Lazo M, Young JH, Brancati FL, Coresh J, Whelton S, Ndumele CE, et al. N-terminal pro-Brain Natriuretic Peptide and Risk of Diabetes. Diabetes. 2013;62:3189–93. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muscari A, Berzigotti A, Bianchi G, Giannoni C, Ligabue A, Magalotti D, et al. Non-cardiac determinants of NT-proBNP levels in the elderly: relevance of haematocrit and hepatic steatosis. Eur J Heart Fail. 2006;8:468–476. doi: 10.1016/j.ejheart.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 51.Writing Committee M. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 52.Lazo M, Selvin E, Clark JM. Brief communication: clinical implications of short-term variability in liver function test results. Ann Intern Med. 2008;148:348–352. doi: 10.7326/0003-4819-148-5-200803040-00005. [DOI] [PubMed] [Google Scholar]
- 53.Schneider AL, Lazo M, Ndumele CE, Pankow JS, Coresh J, Clark JM, et al. Liver enzymes, race, gender and diabetes risk: the Atherosclerosis Risk in Communities (ARIC) Study. Diabet Med. 2013;30(8):926–33. doi: 10.1111/dme.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


