Abstract
Establishment of cell polarity is important for epithelial lumen formation, and the molecular mechanisms directing this process are only partially understood. In this issue of Developmental Cell, Bryant et al. (2014) show that disassembly, membrane translocation, and reassembly of podocalyxin complexes controls epithelial cell polarization and lumen formation in 3D matrices.
Tubes are cellular structures, present within many tissues, whose lumens are lined by either epithelial or endothelial cells (ECs). Considerable work has identified key molecules and signaling events within these two cell types that are required for lumen and tube formation by controlling cell and cytoskeletal polarization, as well as membrane trafficking events involved in these processes (Bryant et al., 2010; Bryant and Mostov, 2008; Datta et al., 2011; Davis et al., 2011; Sacharidou et al., 2012). When generating lumen structures in a 3D environment, cells endocytose membrane vesicles from peripheral membranes (i.e., basal surface), which then traffic via membrane transcytosis to create and expand an apical membrane surface. Despite our advancing knowledge of lumen formation, considerably more information is necessary to understand this fundamental cellular process, which plays critical functions in tissue development, differentiation, homeostasis, regeneration, and repair.
Now in Developmental Cell, Mostov and colleagues (Bryant et al., 2014) provide new insights into the molecular control of apical membrane biogenesis during epithelial morphogenesis. They demonstrate that podocalyxin, an apically expressed sialoprotein in epithelial and ECs (Dekan et al., 1990), is an important regulator of epithelial cell polarization and lumen formation (Bryant et al., 2014). In response to a specific set of signals and membrane trafficking events, podocalyxin and associated proteins switch from a basal to an apical membrane position, thereby controlling lumen formation (Figure 1). The authors first demonstrate that small, two- to three-cell clusters of MDCK cells in 3D Matrigel localize podocalyxin to a basal region at the cell-extracellular matrix (ECM) interface. At this location, podocalyxin forms a complex with the PDZ scaffold protein, NHERF1, and the actin-binding protein, ezrin. Podocalyxin is then removed from basal membrane sites and transported within Rab11a-containing vesicles (containing a different NHERF, NHERF2) to an apical membrane position, initiating formation of a single lumen compartment at the center of a group of polarized epithelial cells (i.e., polarity inversion) (Figure 1).
Figure 1. Podocalyxin-Dependent Polarity Inversion Controls Epithelial Lumen Formation.
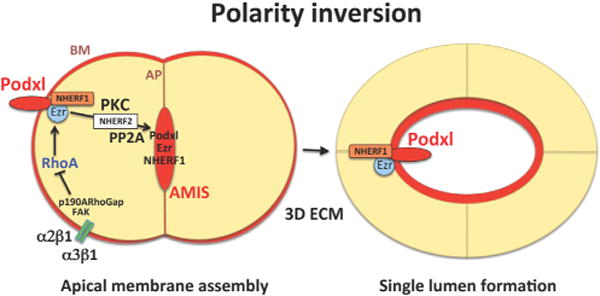
Podocalyxin (Podxl) and its interacting partners, NHERF1, NHERF2, and Ezrin (Ezr), control lumen formation via polarity inversion in 3D matrices. Integrin signaling through a2b1 and a3b1 leads to FAK and p190A RhoGap activation and RhoA inactivation. This signal, in conjunction with PKC activation, leads to disassembly of Podxl/NHERF1/Ezr complexes at the basal membrane (BM). Membrane translocation events lead to nascent assembly of pre-apical membrane surfaces called apical membrane initiating sites (AMIS), which then mature into apical membranes (AP) during lumen formation between multiple epithelial cells. Podxl associates with NHERF2 during vesicular trafficking, while Podxl/NHERF1/Ezr complexes reform during apical membrane assembly in a manner dependent on the protein phosphatase, PP2A.
Bryant et al. demonstrate that these processes require integrin-ECM signaling and protein kinase C (PKC)-dependent phosphorylation. These events result in disassembly of podocalyxin/NHERF1/Ezrin complexes within the basal membrane, and this is necessary for them to reassemble at the developing apical membrane. The authors further show that disruption of these pathways leads to retention of the complexes at the basal surface, thereby blocking lumen formation. As vesicles traffic toward the apical surface, they reacquire both NHERF1 and Ezrin, which colocalize with podocalyxin again at the epithelial apical membrane (Figure 1), and the authors further show that podocalyxin is required for NHERF1 and ezrin to target apically. Thus, podocalyxin-containing complexes are necessary for development of polarized single-lumen structures in MDCK cysts.
Bryant et al. next identified the molecular events that govern podocalyxin complex disassembly and subsequent membrane translocation events, demonstrating that RhoA activity must be suppressed during the lumen formation process. They found that integrin-dependent (i.e., α2b1 and α3β1) activation of focal adhesion kinase (FAK) and FAK-dependent phosphorylation of p190A RhoGAP led to inactivation of RhoA/Rho kinase and that this is a necessary step for podocalyxin translocation. In addition, PKCβII and PKCα (to a lesser extent) were found to cause phosphorylation-dependent dissociation of podocalyxin/NHERF1/ezrin complexes. Blockade of PKCα/β with chemical inhibitors interferes with single-lumen formation between groups of epithelial cells due to retention of these complexes at the basal membrane. Consistently, small hairpin RNA suppression of protein phosphatase 2A (PP2A), which dephosphorylates NHERF1, interrupts lumen formation by interfering with the ability of podocalyxin/NHERF1/ezrin complexes to reassemble during transcytosis.
When these events are disrupted by blockade of integrin signaling, PKC activity, or inducing RhoA activation, epithelial cell clusters were found to enter a state of front-rear polarity, wherein they fail to remove podocalyxin from the basal membrane while simultaneously expressing it on the cell surface in an asymmetrical manner. These clusters lack distinct central lumens and actively migrate together in the direction of polarized podocalyxin expression, which resembles the process of collective cell motility. Similar findings have been observed during EC tubulogenesis, in which signaling cascades and proteins such as Rasip1, Arhgap29, and cerebral cavernous malformation (CCM)-1/2 suppress RhoA during lumen formation (Davis et al., 2011; Sacharidou et al., 2012; Xu et al., 2011).
Overall, these detailed studies provide important new insights into how apical-basal polarization is established during morphogenic events in 3D matrices. One of the many strengths of this study is its temporal analysis of signaling and protein trafficking (Bryant et al., 2014), an essential approach in the molecular analysis of a complex morphogenic process that occurs over days. Many of the basic principles and signaling interactions identified in this work will be relevant to other morphogenic processes, including lumen formation of different epithelial cell types, as well as the developing endothelium (Bryant et al., 2014; Davis et al., 2011). Interestingly, in mouse knockout studies with podocalyxin, the principle defect observed was in the kidney, where a failure to produce urine (anuria) suggested disruption of epithelial tube networks and/or glomerular ultrafiltration due to loss of specialized podocyte foot processes (Doyonnas et al., 2001). By contrast, endothelial tubulogenesis and lumen formation were normal in these animals (Doyonnas et al., 2001), despite the fact that podocalyxin is apically expressed in ECs (Dekan et al., 1990).
Although it is clear that epithelial and endothelial tubulogenic mechanisms have similar features, these cell types are functionally different. The mechanisms controlling apical-basal polarization in these cells, while similar, also have distinct features (Datta et al., 2011; Davis et al., 2011; Sacharidou et al., 2012). In contrast to epithelial polarization, EC polarization depends on unique signals derived from blood flow and shear stress on the EC apical surface, the vascular basement membrane composed of different laminin isoforms (compared to epithelial basement membranes), and the close association of mural cells along the basal surface (Davis et al., 2011; Sacharidou et al., 2012). Together, these studies demonstrate common themes in both epithelial and endothelial systems as to how molecular switches control cell polarization. Bryant et al. (2014) provide a key lesson for future studies using different cell types and model systems by demonstrating that detailed molecular studies are necessary to elucidate the underlying basis for complex morphogenic processes, including lumen and tube formation.
References
- Bryant DM, Mostov KE. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Roignot J, Datta A, Overeem AW, Kim M, Yu W, Peng X, Eastburn DJ, Ewald AJ, Werb Z, Mostov KE. Dev Cell. 2014;2014 doi: 10.1016/j.devcel.2014.08.027. in press Published online October 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE. Curr Biol. 2011;21:R126–R136. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Stratman AN, Sacharidou A, Koh W. Int Rev Cell Mol Biol. 2011;288:101–165. doi: 10.1016/B978-0-12-386041-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekan G, Miettinen A, Schnabel E, Farquhar MG. Am J Pathol. 1990;137:913–927. [PMC free article] [PubMed] [Google Scholar]
- Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM. J Exp Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharidou A, Stratman AN, Davis GE. Cells Tissues Organs (Print) 2012;195:122–143. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O. Dev Cell. 2011;20:526–539. doi: 10.1016/j.devcel.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


