Abstract
Great progress has been made in understanding the HCV genome and its molecular virology. This understanding has culminated in the development of direct acting antiviral (DAA) agents targeting HCV viral proteins. Telaprevir (TVR) and boceprevir (BOC) were the first DAAs introduced for treatment of genotype1 HCV in 2011; when used in combination with pegylated interferon (pegIFN) and ribavirin (RBV), these protease inhibitors improved efficacy in patients with chronic HCV infection compared to the traditional dual therapy. However, this combination was associated with adverse events that often led to early termination of therapy. In late 2013, the FDA approved a second wave of DAAs, sofosbuvir (SOF) and simeprevir (SMV). The use of SOF with SMV opened the door for IFN-free combination regimens. This combination was highly efficacious and well tolerated in patients with HCV genotype 1. Sofosbuvir and ledipasvir (LDV) fixed dose oral combination (FDC) therapy were recently approved, elevating SVR rates to over 95%. We are anticipating the approval of additional IFN-free regimens with comparable efficacy and tolerability but with the addition of pangenotypic coverage, fewer drug-drug interactions and a high barrier to resistance. This review will summarize current management for chronic HCV infection.
Keywords: Direct acting antivirals (DAA), Protease Inhibitors, NS5A inhibitors, NS5B Polymerase Inhibitors, HCV/HIV co-infection
Introduction
HCV is a single stranded positive RNA virus first discovered in 1989[1]. Prior to discovery of the viral agent, HCV was mainly transmitted via blood products[2]. Since then, injection drug use has emerged as the major mode of transmission. Other modes of transmission include vertical transmission from mother to infant and contaminated drug paraphernalia shared by non-injecting drug users (via nasal and rectal routes). While Heterosexual transmission rates are rare, MSM (men who have sex with men) are at risk for HCV transmission and the risk is compounded if they have HIV co-infection (0.07% vs. 5.6% prevalence per year)[3, 4]. It is estimated that about 130–170 million people or 3% of the world population is chronically infected with HCV[5]. There is an increasing burden of HCV/HIV co-infection due to overlapping modes of transmission[6]. The worldwide estimated prevalence of HCV/HIV co-infection is 5–7 million people. Of the 1.2 million HIV infected individuals in the US, approximately 25% of them are co-infected with HCV[7, 8]. Chronic HCV infection is the leading cause of liver related death and the most prominent indication for liver transplant in the United States. The estimated mortality related to HCV infection was 16,627 deaths in 2010 and this is expected to double by 2030[9]. It takes approximately 20 to 30 years for individuals with HCV monoinfection to develop cirrhosis. This process is accelerated in patients with HIV co-infection[10]. In the era of highly active anti-retroviral therapy (HAART), chronic HCV infection surpassed HIV infection as the leading cause of viral associated mortality and morbidity. HCV treatment in this subgroup has been challenging in the era of pegIFN and RBV due to increased frequency of adverse events[11–13]. The primary goal for HCV therapy is to achieve a sustained virologic response (SVR), which is defined as undetectable HCV RNA 12 weeks after completion of therapy. HCV eradication is associated with reduction of HCV related complications, including progression to cirrhosis, hepatic decompensation, hepatocellular carcinoma (HCC) and death[14]. When making clinical decisions regarding when or who to treat, preference should be given to those patients with the greatest risk for HCV related morbidity and mortality. Currently available treatments can be divided into two genres, indirect and direct acting antiviral regimens.
HCV lifecycle
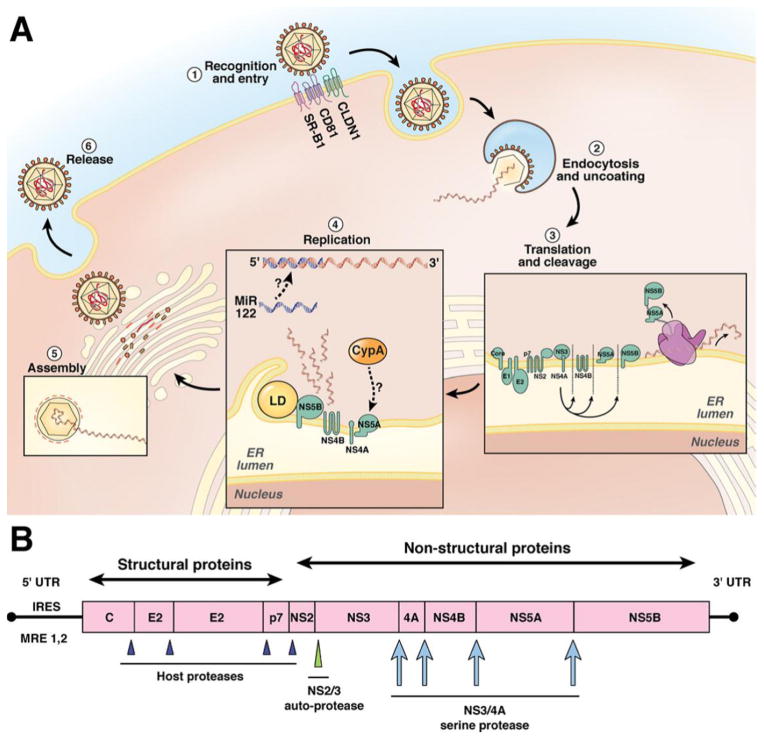
HCV circulates as a lipoviral particle until it enters the hepatocytes via the binding of its envelope proteins to CD81, SR-B1, claudin 1 and occludin co-receptors[15]. (Figure 1) Once the virus is internalized into endosomal vesicles, the acidic pH environment results in fusion of viral and endosomal membranes. The viral RNA is then released into the cytoplasm, whereupon it undergoes translation, resulting in a single viral polyprotein. This polyprotein is subsequently cleaved by viral and host proteases into ten viral proteins, three of which are structural (Core, E1 and E2), while the remainder are non-structural (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B)[16, 17]. NS3-4A protease is required for cleavage of the downstream viral peptides. It also has the ability to cleave and inactivate cellular proteins that are required for antiviral activity. Viral RNA replication takes place on an altered ER membrane, where a positive strand RNA is copied by the NS5B RNA-dependent RNA polymerase (RdRp) into a negative strand RNA intermediate, which in turn serves as a template for the new viral genomic RNA. This replication also requires host factors such as proteins involved in lipid metabolism, as well as micro RNA-122. The NS5B RdRp lacks proofreading capability thus mutations in the HCV genome develop at a rate of 10−4 per nucleotide[18]. The viral particles mature in the Golgi apparatus and NS5A, a non-enzymatic protein, aids in both viral replication and assembly. Once packaged, the mature virions are released into the circulation. The DAAs target the non-structural proteins and inhibit their functions[19].
Figure 1.
HCV viral lifecycle, HCV polypeptide structure, and cleavage sites. (A) The HCV viral lifecycle. The virus circulates as a highly lipidated lipoviral particle (LVP). The LVP requires several cells surface receptors for entry (step 1) into the hepatocyte, including scavenger receptor class B1 (SR-B1), CD-81, claudin (CLDN1), and occludin (not pictured). Once internalized, the viral genome is uncoated, revealing the naked viral RNA and viral nucleocapsid. The viral RNA is translated by host ribosomes into the viral polypeptide (step 3), which is then cleaved by a combination of host and viral proteases into the 10 viral proteins. Replication occurs at an endoplasmic reticulum membrane derived replication complex (the membranous web), which includes the lipid droplet (LD) and nonstructural viral proteins NS4A NS5B (step 4). Viral replication is also dependent on the participation of key host factors, which include miR-122 and cyclophilin A (CypA). The newly synthesized viral RNA is assembled into new LVP by the Golgi apparatus and subsequently released by the cell (steps 5 and 6). (B) HCV viral genome. The viral genome is a positive-sense, single-stranded RNA genome. The 5′ untranslated region (UTR) contains 2 important domains. The internal ribosome entry site (IRES) directs translation in a cap-independent manner. The 5′ UTR also contains 2 recognition sites by miR-122 that are critical for viral replication. After translation, a single viral polypeptide is generated. The structural proteins are cleaved by host proteases. The NS2/3 autoprotease cleaves the NS2 NS3 junction. The NS3/4A protease initially serves as an autoprotease and separates NS3 NS4A, but then subsequently cleaves the remaining nonstructural proteins.
Treatment of HCV using indirect acting antivirals
Historically, pegIFN and RBV have been the mainstay of therapy for more than 25 years. It is important to acknowledge that these are still part of the standard of care for all genotypes for some of the developing countries. We will synopsize both the mechanism of actions and the treatment recommendations for genotypes 1–6. This is covered extensively in the AASLD/IDSA and EASL Clinical Practice Guidelines 2014[20].
PegInterferon-alfa (pegIFN)
Interferons are host proteins released in response to viral infections. These aid in elimination of infected cells and have both antiviral activity, and immunomodulatory effects[21]. There are two types of pegylated interferon, 2a and 2b. PegIFN 2a is administered subcutaneously at a fixed dose of 180μg/week while pegIFN 2b is weight-based dosing, at 1.5μg/kg/week.
Ribavirin (RBV)
It is a guanosine analogue. Its mechanism of action is not precisely understood but it has been proposed that it induces viral mutagenesis, inhibits viral replication, and possesses immunomodulatory effects. It is administered orally twice daily and it is weight-based dosing (<75kg 1000mg/day; >75kg 1200mg/day). It is used in combination with PegIFN for all genotypes for variable durations.
Treatment of Genotype 1 using IFN-based regimens
Treatment with pegIFN/RBV for 48 weeks yields SVR rates of approximately 50%. A recent review by Jia et al, which included 46 independent studies, demonstrated that the favorable IL28B single nucleotide polymorphisms (CC at rs12979860 and TT at rs8099917) were associated with high rates of SVR[22]. Other predictors of good clinical response included lower baseline viral load and mild degree of fibrosis (Metavir F0-F2). Patients who are being treated with pegIFN/RBV should be monitored routinely for adverse events and have regular assessments for virological response such that patients who failed to achieve a significant viral load reduction earlier in the course should discontinue therapy early. The most common reason for treatment discontinuations are due to side effects such as influenza-like illness, psychiatric symptoms and hematological abnormalities.
Treatment of genotype 2–6 using IFN-based regimens
Treatment duration for patients infected with HCV genotype 2 (GT2) varies from 16 to 24 weeks in a treatment response-guided manner. For patients with low baseline viral load, minimal fibrosis and rapid viral response (defined as undetectable viral load at 4 weeks of treatment) could be treated for 16 weeks. Patients with advanced fibrosis, cirrhosis, or those with medical comorbidities such as insulin resistance, metabolic syndrome and non viral steatosis should be treated for 24 weeks irrespective of RVR[23]. Patients with GT2 who do not achieve RVR could have their treatment duration extended to 48 weeks provided they have undetectable RNA at 24 weeks. Most studies demonstrated that patients with GT2 treated with this combination achieved highest SVR rates of about 76–82%. Patients with GT3 treated with pegIFN/RBV for 24–48 weeks have much lower response rate (SVR 70%) compared to GT2, especially in patients with cirrhosis (42%- 48%)[24]. Data on the use of pegIFN/RBV for other genotypes is scant. Patients with GT4 were under represented in the pivotal trials, although in the subsequent studies the SVR were reported to be between 43–70% and lower in the European descent.
Direct acting antivirals (DAAs)
There are 3 classes of DAAs thus far which could be used in different combination for the treatment of HCV. Currently, we have two FDA approved all-oral regimens for treatment of GT1 and these included sofosbuvir and simeprevir (SOF/SMV), and sofosbuvir and ledipasvir fixed dose combination (SOF/LDV FDC). The EASL and AASLD/IDSA guidelines (2014) recommend that all treatment-naïve patients with compensated chronic liver disease related to HCV, who are willing to be treated and have no contraindications to therapy should be considered for treatment. They also state that treatment priority should be given to patients with advanced fibrosis or cirrhosis (Metavir score F3 to F4), clinically significant extra-hepatic manifestations such as symptomatic cryoglobulinemia or HCV immune complex nephropathy, liver transplant recipients and patients with high risk of HCV transmission. In patients with minimal or no fibrosis, treatment may be deferred pending the development and availability of new therapies[25]. This decision should take into consideration the patient’s preference and risk of progression, the presence of co-morbidities and the patient’s age. In situations where treatment is deferred it is imperative to monitor these patients regularly for disease progression. The different classes of DAAs and brief descriptions of the mechanism of actions are summarized below.
NS3/4A Protease Inhibitors (PIs)
NS3/NS4A protease inhibitors block the HCV NS3/NS4A protease enzymatic cleavage of HCV C-terminal polyprotein into discrete nonstructural proteins. Telaprevir (TVR) and boceprevir (BOC) were the first PIs to be approved by the FDA in 2011, each to be used in conjunction with pegIFN and RBV[26]. The second wave consisted of simeprevir (SMV) licensed in November 2013 to be used in combination with pegIFN/RBV for GT1 and just recently approved to be used in combination with SOF based on the COSMOS Study[27]. PI regimens, especially SMV, combined with pegIFN/RBV improved SVR rates from 45% to 80%. SVR rates were influenced by presence of pre-existing resistance mutation in Q80K polymorphism which has a worldwide prevalence of approximately 25%[28]. Patients who are infected with HCV GT1a and harbor the Q80K polymorphisms experience poor response to SMV/pegIFN/RBV regimen, whereas the combination of SOF/SMV eliminates the Q80K mutation influence[29]. The SVR rates of SOF/SMV are similar between GT1a and GT1b in HIV co-infected individuals irrespective of their Q80K mutation status[30]. The ritonavir-boosted PI pariteprevir (ABT-450) is currently in phase III studies and is expected to be approved by the end of 2014[31, 32]. Other PIs expected to be licensed include grazoprevir (MK-5172) and ABT-493[33]. Grazoprevir was evaluated in C-WORTHY phase II trial in combination with elbasvir (MK-8742, an NS5A inhibitor) with or without RBV in patients infected with HCV GT1. The study population consisted of two cohorts, treatment naïve cirrhotics (n=123) and previous null responders with or without cirrhosis (n=130). Each cohort had four arms that were randomly assigned to receive 12 or 18 weeks of grazoprevir, elbasvir with or without RBV. In cohort 1, the SVR rates were 90% (95% CI 74–98) in the 12 weeks ribavirin containing arm and 97% (95% CI 82–100) in the ribavirin free arm. In cohort 2, the SVR rates were 91% (CI 76–98) in the 12 week ribavirin arm and 100% (95% CI 89–100) in the 18 week ribavirin containing arm[29]. This combination was generally well tolerated. The addition of ribavirin had no added benefit and was associated with more side effects. ABT-493 is currently in phase II studies and has been evaluated in combination with ABT-530 (NS5A inhibitor). Unlike other PIs, ABT-493 has high potency against GT3a and the most clinically important resistance-associated variants (R155 or D168 variants in GT1) that are selected by other PIs[34]. Most of the newer PIs are once daily regimens and appear to have less toxicity compared to the first wave PIs[35]. They also provide broader genotype coverage (e.g., pariteprevir has GT1-4 and GT6 coverage)[36].
NS5A Inhibitors
NS5A is a nonstructural protein that is critical for both viral replication and assembly. It exists in phosphorylated and hyperphosphorylated forms which are implicated in different lifecycle functions. The precise mechanism of action for NS5A inhibitors is unknown[37]. Suggested mechanisms include inhibition of hyperphosphorylation, which is required for viral production and it alters the subcellular localization of NS5A resulting in faulty viral assembly. It has three cytoplasmic domains; domain 1 lacks the amphipathic helix and spanning residues and has a potential role in RNA binding; the functions of the other 2 domains remain unknown. It is believed that there is a direct interaction between NS5A and NS5B RdRp such that the in vitro interaction catalyzes RdRp-dependent synthesis of negative RNA strands. The interaction of domain I with the viral 3‘UTR is responsible for the NS5B RdRp medicated viral replication. Domain II interacts with cyclophilin A, a host cell protein that plays a key role in viral packaging and assembly. Ledipasvir is the first FDA approved NS5A inhibitor[38, 39]. Several other NS5A inhibitors, such as Daclatasvir (DCV), ABT-267, elbasvir (MK-8742), MK-8408 and GS-5816, are expected to be approved soon[40, 41]. DCV is the most advanced NS5A inhibitor in clinical trials and has shown activity against all genotypes. It has been evaluated in HCV monoinfected and HCV/HIV co-infected individuals. Current trials are evaluating several combination therapies with DCV, including DCV and pegIFN/RBV; DCV, asunaprevir (ASV) and pegIFN/RBV; DCV and ASV; DCV and SOF with or without RBV. A combination of DCV plus SOF has demonstrated high SVR rates in both previous null responders (100% in the 24 week arm without RBV) and treatment naïve non cirrhotic (100% in the 12 week arm without RBV) in GT1 HCV infected individuals[42]. GS- 5816, a pangenotypic NS5A inhibitor with picomolar potency against GT1-6, has been evaluated in combination with SOF for treatment of all the HCV genotypes with remarkable SVR’s amongst GT1 and GT3 patients. In the ELECTRON-2 study, GT3 HCV infected treatment naive group without cirrhosis demonstrated SVR rates of 100% when GS-5816 (100mg) was used in combination with SOF for 12 weeks and this was irrespective of RBV use. Among the treatment experienced GT3 patients, the SVR rates were 96% and only one patient experienced virologic relapse[43].
NS5B Polymerase Inhibitors
There are two main classes of NS5B polymerase inhibitors, namely nucleos(t)ide inhibitors and non-nucleos(t)ide inhibitors. The nucleos(t)ide inhibitors bind to the polymerase’s active site, while the non-nucleosides bind to allosteric sites of the enzymes causing conformational changes that in turn inhibit the polymerase activity. Sofosbuvir is a nucleotide polymerase inhibitor. It is well tolerated without overt mitochondrial toxicity. It also has pangenotypic coverage given the remarkable conservation of the active site across genotypes and a high barrier to resistance as mutations in the active site of the NS5B polymerase result in profound abrogation of viral fitness. Excellent SVR rates have been observed in most studies using SOF and in vivo resistance mutations are rare and without significant clinical consequences[44]. Dasabuvir is a non-nucleoside NS5B polymerase inhibitor that is expected to be approved by the end of 2014 to be used in combination with ABT-450/r, ombitasvir with or without RBV. It has been evaluated in PEARL 1-4 and SAPPHIRE 1-2 studies and the studies have demonstrated excellent SVR results amongst GT1 HCV infected individuals. The SVR rate is highest amongst GT1b when compared to GT1a (99% vs. 90%); however, with the addition of ribavirin in the GT1a subgroup of non-cirrhotic patients the SVR rate improved to 97%[45, 46].
Current recommendations for the use of DAA
Genotype 1
Recently approved SOF (400 mg)/LDV (90 mg) FDC for 12 weeks is now the first line therapy for GT1 infected individuals. It has the highest SVR rates 94%-99% amongst all the other available therapies. This combination therapy is well tolerated and can be used in patients with advanced fibrosis/cirrhosis. The treatment duration may be shortened to 8 weeks in treatment naïve, non-cirrhotic patients with HCV RNA less than 6 million IU/ml. Patients who are previous non-responders and have advanced fibrosis/cirrhosis should be treated for 24 weeks[47]. SOF (400mg) and SMV (150 mg) for 12–24 weeks is the alternative treatment. The COSMOS study showed excellent SVR rates (93%) across all GT1 patients irrespective of HCV subtype and Q80K polymorphism[48]. This combination is not recommended for patients who were previously treated with HCV protease inhibitors. The combination of SOF, weight based RBV and pegIFN for 12 weeks is a reasonable option for patients who are pegIFN eligible. This combination was evaluated in the NEUTRINO trial and demonstrated SVR rates of >90% in non-cirrhotic patients and 80% in patients with cirrhosis[49]. Other all oral IFN free treatment options that are currently being evaluated include a combination of ritonavir-boosted pariteprevir (ABT-450, a protease inhibitor), ombitasvir (ABT-267, an NS5A inhibitor), dasabuvir (ABT- 333, a nonnucleoside polymerase inhibitor) with or without RBV for 12–24 weeks, SOF and DCV for 12 weeks, and DCV, ASV and BMS-791325 (a non-nucleoside polymerase inhibitor) for 12–24 weeks[45, 46, 50]. Preliminary results from the TURQUOISE II study, which evaluated the combination of pariteprevir, ombitasvir, dasabuvir and ribavirin in both the treatment naive and experienced patients with cirrhosis, demonstrated SVR rates of 92% (n=208) in the 12 week arm and 96% (n=172) in the 24 week arm[51]. DCV in combination with ASV and BMS-791325 for 12 or 24 weeks yield a SVR in excess of 92% in treatment-naive patients (n=66) with HCV genotype 1 infection[50].
Genotype 2
Patients infected with GT2 should be treated with SOF (400 mg) and weight based RBV for 12 weeks. Treatment duration should be extended to 16 weeks in patients with advanced fibrosis/cirrhosis or previous non-responders. This combination has been evaluated in both treatment naïve and treatment experienced individuals with or without cirrhosis. The three major landmark trials that assessed the treatment responses for SOF/RBV in treatment naïve patients with or without cirrhosis were FISSION, POSITRON and VALENCE, these demonstrated SVR rates >90% across all 3 studies with the highest SVR among non cirrhotics (98%). VALENCE AND FUSION trials assessed the treatment response for SOF/RBV in treatment experienced individuals with or without cirrhosis. The SVR rates were lowest (88%) amongst treatment experienced cirrhotics treated with SOF/RBV for 12 weeks in the VALENCE study. In the FUSION study, where SOF/RBV was given for either 12 or 16 weeks, the SVR rate among the cirrhotics was 60% in the 12 week arm and 78% in the 16 week arm[52–54].
Genotype 3
Treatment naive patients infected with GT3 should be treated with SOF (400 mg) and weight based RBV for 24 weeks. The SVR rate associated with this combination have been reported to be as high as 95% in treatment naïve individual without cirrhosis and 92% in those with cirrhosis (VALENCE study)[44]. The SVR rates were lower in treatment experienced group especially those with advanced fibrosis and or cirrhosis (87% vs.62%). The alternative treatment option for IFN eligible individuals would be SOF, weight based RBV and pegIFN for 12 weeks. This yielded similar SVR rates (92%) to the SOF/RBV combination in treatment naive patients with early fibrosis. Patients with advanced fibrosis/cirrhosis had lower SVR rate (83%) with this combination. A few studies (VALENCE and FISSION) have suggested that a longer duration of therapy with SOF/RBV for patients with cirrhosis may yield better results. The combination of SOF and DCV has been evaluated amongst patients infected with HCV GT3. This study included 18 treatment naïve non-cirrhotic HCV GT3 patients treated for 12 weeks, SVR rates was 89%. Of the two patients that failed to achieve SVR, one demonstrated a preexisting NS5A-A30K polymorphism which is associated with DCV resistance[41]. DCV is expected to be approved by the end of 2014. SOF/LDV FDC in combination with RBV has recently been shown to produce highest SVR (100%) among treatment naïve HCV GT3 patients[55].
Genotype 4
Data for treatment of patients with GT4 is scant, however, for those patients who cannot wait to be treated, the recommendation is the use of SOF (400 mg), weight based RBV and pegIFN for 24 weeks. The NEUTRINO trial, which included 28 patients infected with GT4, showed an SVR rate of 96%[56]. Alternatively, for patients who are ineligible for pegIFN based therapy, one could consider the combination of SOF and weight based RBV for 24 weeks. The SVR rate are 100% in treatment naive individuals and 93% in treatment experienced[57]. A promising alternative expected to be approved soon is the combination of pariteprevir/ritonavir, ombitasvir, dasabuvir with or without ribavirin. This combination was evaluated in the PEARL-1 trial that consisted of 3 arms of both treatment naïve and treatment experienced individuals[58]. The treatment-experienced arm was assigned to receive pariteprevir/ritonavir, ombitasvir, dasabuvir and ribavirin for 12 weeks and the treatment naïve groups were assigned to either ribavirin containing and ribavirin free arms. The SVR rates were 100% in both the treatment experienced and treatment naïve ribavirin containing arms. In the treatment naïve ribavirin free group SVR rate was 91%, one patient experienced rebound and two patients had virologic relapse. None of the patients in this cohort had cirrhosis. The commonest adverse events were fatigue, headache and insomnia and four patients had RBV dose reduction for anemia[58]. The SYNERGY trial evaluated the efficacy of the approved SOF/LDV FDC for 12 weeks in 21 patients infected with GT4. Majority of the patients were black, 10% had advanced fibrosis and 33% had compensated cirrhosis. The SVR rates were 95% and this combination was well tolerated, even in patients with cirrhosis[59].
Genotype 5 and 6
There is limited data to guide treatment in patients with these two genotypes. For those patients who cannot afford to wait to be treated, SOF (400 mg), weight based RBV and pegIFN for 12 weeks is the most optimal therapy based on the NEUTRINO trial[56]. The alternative therapy remains pegIFN and RBV for 48 weeks. We are looking forward to clinical trials that will include countries where genotypes 5 and 6 are prevalent to pave the way for IFN-free regimens in these patients. A two center open-label study that evaluated efficacy of SOF/LDV FDC for 12 weeks in 25 patients infected with HCV GT6 has demonstrated promising results. The SVR rates reported were 96% (24/25) and the one patient who relapsed had discontinued therapy at 8 weeks due to intravenous drug use. Majority of these patients were treatment naïve (92%) and only 8% had cirrhosis[60]. The treatment summary for all genotypes can be found in Table 1.
Table 1.
Treatment summary for all genotypes
| Recommended | Genotype 1 | |||
|---|---|---|---|---|
| Treatment | Duration | SVR | Study | |
| SOF/LDV FDC | 8–24 WEEKS | 94–99% | ION I, II, III[38, 39, 47] | |
| SOF/SIM | 12–24 WEEKS | 92% | COSMOS[27] | |
| Alternative Therapy IFN eligible | SMV +RBV/PegIFN | 24WEEKS | 93% | QUEST[61] |
| SOF + RBV/PegIFN | 12 WEEKS | 89% (80% in cirrhotics) | NEUTRINO[49, 56] | |
| Genotype 2 | ||||
| Recommended | SOF +RBV | 12–16 WEEKS | 94% | FISSION, POSITRON & VALENCE[44, 52] |
| Genotype 3 | ||||
| Recommended Rx naive) | SOF +RBV | 24 WEEKS | 93% | VALENCE[44] |
| Alternative | SOF +RBV +PegIFN | 12 WEKS | 97% | PROTON & ELECTRON[53, 54] |
| Genotype 4 | ||||
| Recommended | SOF + RBV + PegIFN | 12 WEEKS | NEUTRINO[56] | |
| SOF +RBV | 24 WEEKS | |||
| SOF/LDV FDC | 12 WEEKS | 95% | SYNERGY[59] | |
| Paritepravir/ritonavir, ombitasvir, dasabuvir ± RBV | 12 WEEKS | 91%-100% | PEARL-1[58] | |
| Genotype 5/6 | ||||
| Recommended | SOF +RBV + PegIFN | 12 WEEKS | NEUTRINO[56] | |
| (GT6) | SOF/LDV FDC | 12 WEEKS | 96% | AASLD[60] |
Abbreviations: SOF=Sofosbuvir (400mg/daily); SMV= Simeprevir (150mg/daily); PegIFN= Pegylated interferon (180μg/weekly sc); RBV= Ribavirin (weight based 1000mg <75kg and 1200 >75kg; LDV= Ledipasvir (90mg);
Treatment options for specific groups
Cirrhosis
All patients with compensated cirrhosis should be treated. PegIFN/RBV should be avoided given the risk of hepatic decompensation. Most oral DAAs are well tolerated with the exception of SMV. Simeprevir is primarily metabolized by the liver and patients with moderate to severe hepatic impairment (Child-Pugh class B and C) could experience accumulation of drug levels; thus SMV should be avoided beyond Child-Pugh class A. Treatment naïve patients with compensated cirrhosis including those with HCC should receive the same treatment as recommended for patients without cirrhosis. Patients with decompensated cirrhosis should be referred for liver transplant and if liver transplant is not an option, SOF/RBV for 48 weeks is the recommended therapy (AASLD/IDSA/IAS-USA) for all genotypes. The benefit of treating these patients is a significant reduction in incidence for decompensation and the lower rates of HCV recurrence post transplant. These patients require close monitoring due to increased frequency of hematological side effects. SOF/LDV FDC for 12 weeks is well tolerated and efficacious (SVR 65% ELECTRON Study).
HCV/HIV co- infection
Patients with HCV/HIV co-infection are at risk for accelerated liver disease progression and thus it is imperative to treat the HCV infection. Successful HCV eradication in this subgroup is associated with a reduction in all cause and liver related mortality[62]. The previous standard therapy of pegIFN and RBV was associated with disappointingly low SVR rates (<30%) for GT1[63]. The first generation PI (TVR and BOC-based regimens) improved the treatment efficacy such that the SVR rates were on par with the HIV negative patients. However, treatment toxicities and drug-drug interactions posed challenges for their use in the co-infected population. Sofosbuvir metabolism is independent of cytochrome P450 thus it has less drug-drug interaction with the contemporary HAART regimens. The PHOTON-1 and PHOTON-2 studies evaluated the use of SOF with RBV in both treatment naïve and treatment experienced HCV/HIV co-infected individuals. This combination produced SVR rates exceeding 80% across GT1 to GT3, including patients with advanced fibrosis[64]. The recently approved SOF/LDV FDC, with remarkably high SVR rates amongst the HCV monoinfected individuals, is currently being evaluated in the phase III ION-4 study of HCV/HIV co-infected population. A recent phase II study of 50 HCV/HIV co-infected patients on a wide range of ART regimens evaluating use of SOF/LDV FDC has revealed 100% SVR8 and SVR12 rates[65]. High rates of response may also be accomplished with other DAA combinations. A study assessing the use of pariteprevir, ombitasvir, dasabuvir, and RBV in 63 HCV/HIV co-infected patients showed promising SVR rate [66]. These subjects were on raltegravir or boosted atazanavir-based ART regimens. There was no drug-drug interaction observed except for a rise in total bilirubin in patients receiving atazanavir. Overall, all oral pegIFN-free regimens with comparable SVR, good tolerability and safety profile appear to be achievable in HCV/HIV co-infected patients.
Renal failure and Patients on Hemodialysis
The recommendation prior availability of all oral DAAs has been to treat patients with renal failure or patients on hemodialysis with pegIFN/RBV prior to planned renal transplant because of deleterious effects of pegIFN on graft survival[67]. Treatment in this population is challenging as one needs to modify the medication dosages according to the creatinine clearance (CrCl), leading to suboptimal therapy[68, 69]. The major elimination pathway for SOF is via renal excretion, however, studies have shown that no dosage modification is required in patients with mild (GFR 60–89 ml/min/1.73m2) to moderate (GFR 30–59 ml/min/1.73m2) renal impairment. SOF is contraindicated in patients with severe renal impairment (GFR<30 ml/min/1.73m2) or those that require hemodialysis due to the concern of retained SOF metabolites. Studies of SOF dosing in severe renal impairment are ongoing. The safety and efficacy of SMV and LDV has not been studied in patients with severe renal impairment including patients on dialysis. SMV is highly protein bound and dialysis is unlikely to result in significant removal of its metabolites. It has good oral bioavailability and does not require dose adjustment in mild to moderate kidney disease.
Post liver transplant HCV recurrence
HCV remains the leading indication for liver transplant in the United States. The outcomes are poor for patients with active viremia undergoing transplant. Approximately 20–30% of these patients will develop cirrhosis within 5 years if not treated. In the era of pegIFN and RBV, it was advisable to treat these patients prior liver transplantation to avoid the risk of interferon induced plasma cell hepatitis in the allograft. The new FDA approved DAAs (SOF/LDV and SOF/RBV) are well-tolerated post transplant. With the exception of PIs, which are metabolized in the liver via cytochrome p450 enzyme, SOF and LDV require no dose adjustment when used with calcineurin inhibitors (CNI)[70]. In a prospective multicenter study of 40 previously treated and treatment naive liver transplant patients infected with GT1, 3 and 4, where a combination of SOF and RBV was administered for 24 weeks, 70% of patients achieved SVR. In this study, 40% of the patients had cirrhosis and 83% were treatment experience. Overall this combination was well tolerated with no CNI toxicities or drug interactions[71]. Other recommended treatment options for treating patients with HCV GT1 recurrence post transplant include SOF/SMV with or without RBV for 12–24 weeks or SOF/LDV FDC with RBV for 12 weeks. In a recent prospective study, SOF/LDV FDC with RBV was evaluated in 223 post-liver transplant patients infected with G1 and GT4. The median post-transplant period was 4.4 years. This study consisted of both treatment naïve (17%) and treatment experienced individuals (83%). Approximately 50% of patients had mild to moderate fibrosis (Metavir F0-F3). The study included patients with both compensated and decompensated cirrhosis (Child- Pugh class A to C). The SVR4 in the non-cirrhotic patients were 96% and 94% in the 12 week and 24 week arms respectively. The SVR 4 in patients with cirrhosis was 92% and 82% in the 12 week and 24 week arm respectively. Overall this combination was well tolerated; five deaths were reported in the cirrhotic cohort. The fatalities were not drug related, few were due to complications of cirrhosis, one aortic dissection and other had progressive multifocal luecoencephalitis[72]. More treatment options for GT1 infection will soon include a combination of ritonavir boosted pariteprevir, ombitasvir, dasabuvir and ribavirin. This combination was evaluated in the CORAL-1 study that consisted of 34 liver transplant patients with minimal or no fibrosis. The majority of patients tolerated therapy (33/34 patients) for 24 weeks with a low rate of adverse events. Only one patient discontinued therapy at 18 weeks due to severe rash, memory impairment and anxiety all thought to be drug related. The SVR12 in this cohort was 97%. Ribavirin associated anemia was the most common adverse event, with 9 patients requiring ribavirin dosage adjustment and 5 patients requiring erythropoietin administration. There were no blood transfusion required[73].
Conclusion
Chronic HCV infection has been a major burden in most health care systems for decades. The past three years have been witness to major breakthrough in new drug development against HCV. The once difficult to treat GT1, which predominates in the US and worldwide, can now be cured in 12 weeks or less with all oral interferon-free direct acting antivirals. These new regimens are not only effective in the treatment naïve population, but also effective in the difficult-to-treat populations, such as cirrhotics, patients with HBV and HIV co-infection, and liver transplant recipients. Challenges remain ahead for the prevention, identification and early diagnosis of chronic HCV infection, as well as the delivery of medications to those who need to be treated. The residual damage incurred over the past few decades may still linger and lead to complications even as we successfully treat most chronic infections.
Acknowledgments
Raymond T. Chung reports grant support from Gilead, Abbvie, Mass Biologics, BMS (clinical trials).
Footnotes
Conflict of Interest
Neliswa A. Gogela, Ming V. Lin, and Jessica L. Wisocky declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Chevaliez S, Pawlotsky JM. HCV Genome and Life Cycle. In: Tan SL, editor. Hepatitis C Viruses: Genomes and Molecular Biology. Norfolk (UK): 2006. [Google Scholar]
- 2.Choo QL, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244(4902):359–62. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 3.Urbanus AT, et al. Trends in hepatitis C virus infections among MSM attending a sexually transmitted infection clinic; 1995–2010. AIDS. 2014;28(5):781–90. doi: 10.1097/QAD.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 4.Zielinski A. Sexual behaviour and the risk of HCV infection. Przegl Epidemiol. 2014;68(1):1–3. 99–100. [PubMed] [Google Scholar]
- 5.Smith BD, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 6.Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11(6):362–71. doi: 10.1038/nrgastro.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong GL, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 8.Data Collection on Adverse Events of Anti, H.I.V.d.S.G., et al., . Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24(10):1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 9.Davis GL, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513–21. 521 e1–6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 10.Lee MH, et al. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol. 2014;20(28):9270–80. doi: 10.3748/wjg.v20.i28.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konerman MA, et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014;59(3):767–75. doi: 10.1002/hep.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356(14):1445–54. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thein HH, et al. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 14.Marcellin P. Hepatitis C: the clinical spectrum of the disease. J Hepatol. 1999;31(Suppl 1):9–16. doi: 10.1016/s0168-8278(99)80368-7. [DOI] [PubMed] [Google Scholar]
- 15.Dorner M, et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature. 2013;501(7466):237–41. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer EA, Chung RT. Anti-hepatitis C virus drugs in development. Gastroenterology. 2012;142(6):1340–1350 e1. doi: 10.1053/j.gastro.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19(7):837–49. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.deLemos AS, Chung RT. Hepatitis C treatment: an incipient therapeutic revolution. Trends Mol Med. 2014;20(6):315–21. doi: 10.1016/j.molmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Feeney ER, Chung RT. Antiviral treatment of hepatitis C. BMJ. 2014;348:g3308. doi: 10.1136/bmj.g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liver EAfSo. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 22.Jia Z, et al. Test of IL28B polymorphisms in chronic hepatitis C patients treated with PegIFN and ribavirin depends on HCV genotypes: results from a meta-analysis. PLoS One. 2012;7(9):e45698. doi: 10.1371/journal.pone.0045698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368(20):1907–17. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoeb D, et al. Extended duration therapy with pegylated interferon and ribavirin for patients with genotype 3 hepatitis C and advanced fibrosis: Final results from the STEPS trial. J Hepatol. 2014;60(4):699–705. doi: 10.1016/j.jhep.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 25.American Association for the Study of Liver disease, I.D.S.o.A.a.I.A.S.-U. American Association for the Study of Liver disease, Infectious Diseases Society of America and International Antiviral Society-USA: Recommendations for Testing, Managing, and Treating Hepatits C. 2014. [cited 2014 October 29] [Google Scholar]
- 26.Poordad F, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Lawitz E, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014 doi: 10.1016/S0140-6736(14)61036-9. COSMOS: This study evaluated a combination of sofosbuvir and simeprevir for treatment of HCV GT1 infected individuals. Cohort 1 consisted of treatment naive patients with F3-F4 fibrosis and cohort 2 consisted of treatment experienced (previous null responders to pegIFN and RBV while previous PI use was a contraindication). SVR rates were >90% in both cohorts and this treatment combination was efficacious and well tolerated. [DOI] [PubMed] [Google Scholar]
- 28.Palanisamy N, et al. Implications of baseline polymorphisms for potential resistance to NS3 protease inhibitors in Hepatitis C virus genotypes 1a, 2b and 3a. Antiviral Res. 2013;99(1):12–7. doi: 10.1016/j.antiviral.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Lawitz EGE, Pearlman B, Tam E, Ghesquierre W, Guyader D, Alric L, Bronowicki J, Lester L, Sievert W, Ghalib R, Balart L, Sund F, Lagging M, Dukto F, Shaughnessy M, Hwang P, Howe AYM, Wahl J, Robertson M, Barr E, Harber B. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK -5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype1infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open label phase 2 trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed] [Google Scholar]
- 30.Macias J, et al. Latest pharmacotherapy options for treating hepatitis C in HIV-infected patients. Expert Opin Pharmacother. 2014;15(13):1837–48. doi: 10.1517/14656566.2014.934810. [DOI] [PubMed] [Google Scholar]
- 31•.Zeuzem S, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1604–14. doi: 10.1056/NEJMoa1401561. SAPPHIRE II: Retreatment with ABT-450, ombitasvir, dasabuvir and ribavirin produced high SVR rates (95.2%) in previous null responders. [DOI] [PubMed] [Google Scholar]
- 32•.Poordad F, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973–82. doi: 10.1056/NEJMoa1402869. TURQOISSE II: A combination of ABT-450, ombitasvir, dasabuvir and ribavirin produced high SVR rates even in patients with cirrhosis. None of them had decompensation, only side effects reported were fatigue and nausea. [DOI] [PubMed] [Google Scholar]
- 33.Yau AH, Yoshida EM. Hepatitis C drugs: The end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: A concise review. Can J Gastroenterol Hepatol. 2014;28(8):445–51. doi: 10.1155/2014/549624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawitz EORWD, Freilich BL, Box TD, Overcash S, Liu W, Campbell AL, Lin C, Asatryan A, Kort J. Potent antiviral activity of ABT-493 and ABT-530 with 3-day monotherapy in patients with or without cirrhosis in phase 2 and 3 studies. 2014 AASLD 2014 Abstract. [Google Scholar]
- 35.Schinazi R, et al. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34(Suppl 1):69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muir AJ. The rapid evolution of treatment strategies for hepatitis C. Am J Gastroenterol. 2014;109(5):628–35. doi: 10.1038/ajg.2014.66. quiz 636. [DOI] [PubMed] [Google Scholar]
- 37.Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59(2):375–82. doi: 10.1016/j.jhep.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 38••.Afdhal N, et al. dLedipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–93. doi: 10.1056/NEJMoa1316366. ION II: This study evaluated a fixed dose combination of sofosbuvir and ledipasvir with or without ribavirin in treatment experienced patients infected with GT1. A total of 440 patients were recruited, 20% had cirrhosis and 79% were infected with GT1a. SVR rates varied from 94%–99%, and 99% was observed in the 2 groups that received treatment for 24 weeks. [DOI] [PubMed] [Google Scholar]
- 39••.Afdhal N, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98. doi: 10.1056/NEJMoa1402454. ION I: This study evaluated a fixed dose combination of sofosbuvir and ledipasvir with or without ribavirin in treatment naive patients infected with GT1. Total number of patients recruited was 865 of which 12% had cirrhosis, 12% black and 67% GT1a. Patients were randomly assigned in a 1:1:1:1 ratio and were stratified according to HCV genotype (1a or 1b) and cirrhosis to receive treatment for either 12 or 24 weeks. Except for 1 patient who experienced virological breakthrough from non compliance this combination had SVR rates of 97%–99% across all four arms. The few patients that relapsed were older males with cirrhosis. [DOI] [PubMed] [Google Scholar]
- 40.Kim DY, Ahn SH, Han KH. Emerging Therapies for Hepatitis C. Gut Liver. 2014;8(5):471–479. doi: 10.5009/gnl14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Sulkowski MS, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–21. doi: 10.1056/NEJMoa1306218. A combination of daclatasvir and sofosbuvir was evaluated amongst patients infected with HCV GT1-3. Majority of the patients in this study were infected with GT1 (both treatment naive and previous PI failures) and only 18 had GT3. This combination proved highly efficacious and was well tolerated. [DOI] [PubMed] [Google Scholar]
- 42.Stedman CA. Current prospects for interferon-free treatment of hepatitis C in 2012. J Gastroenterol Hepatol. 2013;28(1):38–45. doi: 10.1111/jgh.12028. [DOI] [PubMed] [Google Scholar]
- 43.Gane EHRH, McNally J, Svarovskaia E, Brainard DM, Symonds WT, McHutchison JG, Stedman C. Once- Daily sofosbuvir with GS-5816 for 8 weeks with or without ribavirin in patients with HCV genotype 3 without cirrhosis results in high rates of SVR: The ELECTRON-2 Study. 2014 AASLD 2014 Abstract. [Google Scholar]
- 44.Zeuzem S, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 45•.Feld JJ, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–603. doi: 10.1056/NEJMoa1315722. SAPPHIRE I: In this study a total of 631 previously untreated patients infected with HCV genotype 1 were randomized to receive an active regimen that consisted of ABT-450/r, ombitasvir, dasabuvir and ribavirin for group A and matching placebos for group B. SVR rates were 96.2% in group A and when genotype 1 was subtype GT1b had highest SVR rates of 98%. [DOI] [PubMed] [Google Scholar]
- 46•.Ferenci P, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983–92. doi: 10.1056/NEJMoa1402338. PEARL III and IV: A combination of ABT-450/r, ombitasvir, dasabuvir with or without ribavirin produced highest SVR rates (99.5%) among previously untreated patients infected with GT1b compared to GT1a (97%). Among patients infected with GT1a the rate of virological failure was higher ribavirin free arm (7.8% vs.2.0%) [DOI] [PubMed] [Google Scholar]
- 47••.Kowdley KV, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–88. doi: 10.1056/NEJMoa1402355. ION III: This study evaluated a fixed dose combination of sofosbuvir and ledipasvir with or without ribavirin in treatment naive patients without cirrhosis infected with GT1. Patients were randomly assigned to receive treatment for either 8 or 12 weeks. SVR rate in the 12 week arm was one percent higher than in the 8 week arm (95% vs. 94%), and ribavirin had no added benefits. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson IM, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–77. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 49.Koff RS. Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39(5):478–87. doi: 10.1111/apt.12601. [DOI] [PubMed] [Google Scholar]
- 50.Everson GT, et al. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146(2):420–9. doi: 10.1053/j.gastro.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 51.Lok A. New All-Oral HCV Therapies for Genotype 1: A Final Good-bye to Interferon. Clinical Liver Disease. 2014;3(6):137–140. doi: 10.1002/cld.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asselah T. Sofosbuvir-based interferon-free therapy for patients with HCV infection. J Hepatol. 2013;59(6):1342–5. doi: 10.1016/j.jhep.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 53.Lawitz E, et al. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2014 doi: 10.1002/hep.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heidrich B. P1109 Overtreatment of patients with chronic Hepatitis C (CHC) genotype 2/3 with low baseline viral load and RVR: Experiences in a real world. Journal of Hepatology. 2014;60(1) [Google Scholar]
- 55.Gane EHRH, An D, Pang PS, Symonds WT, McHutchison JG, Stedman C. Ledipasvir/Sofosbuvir Fixed-Dose Combination Is Safe and Effective in Difficult-to-Treat Populations Including GT 3 Patients, Decompensated GT 1 Patients, and GT 1 Patients With Prior Sofosbuvir Experience. EASL - The International Liver Congress; 2014; 2014. [Google Scholar]
- 56.Lawitz E, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;369(7):678–9. doi: 10.1056/NEJMc1307641. [DOI] [PubMed] [Google Scholar]
- 57.Degasperi E, Aghemo A. Sofosbuvir for the treatment of chronic hepatitis C: between current evidence and future perspectives. Hepat Med. 2014;6:25–33. doi: 10.2147/HMER.S44375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pol SRKR, Baykal T, Hezode C, Hassanein T, Marcellin P, Berenguer M, Fleischer-Stepniewska KM, Hall C, Collins C, Vilchez RA. Interferon-Free Regimens of Ombitasvir and ABT-450/r with or without Ribavirin in Patients with HCV Genotype 4 Infection: PEARL-I Study Results. American Association for the Study of Liver Diseases (AASLD) Liver Meeting; 2014. [Google Scholar]
- 59.Kapoor RKA, Sidharthan S, Sims Z, Petersen TL, Osinusi A, Nelson AK, Silk R, Kotb C, Sugarman K, Lam BP, Pang PS, Subramanian M, McHutchinson JG, Masur H, Kottilil S, Rustgi VD. Treatment of hepatitis C genotype 4 with ledipasvir and sofosbuvir for 12 weeks: results of the SYNERGY trial. American Association for the Study of Liver Diseases (AASLD) Liver Meeting; 2014. [Google Scholar]
- 60.Gane EHRH, An D, An D, Svaroskaia ES, Pang PS, Symonds WT, McHutchison JG, Stedman C. High efficacy of LDV/SOF regimens for 12 weeks for patients with HCV GT3 or GT6 infection. American Association for the Study of Liver Diseases (AASLD) Liver Meeting; 2014. [Google Scholar]
- 61.Manns M, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384(9941):414–26. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 62.van der Meer AJ, Hansen BE, Janssen HL. Sustained virological response to treatment in patients with chronic hepatitis C--reply. JAMA. 2013;309(14):1457. doi: 10.1001/jama.2013.2733. [DOI] [PubMed] [Google Scholar]
- 63.Shire NJ, Welge JA, Sherman KE. Response rates to pegylated interferon and ribavirin in HCV/HIV coinfection: a research synthesis. J Viral Hepat. 2007;14(4):239–48. doi: 10.1111/j.1365-2893.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 64.Sulkowski MS, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159(2):86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 65••.Osinusi ATK, Nelson A, Kohli A, Gross C, Polis MA, Pang PS, Symonds WT, Talwani R, Sajadi MM, Hogan J, Benator D, Subramanian M, Mchutchison J, Masur H, Kottilil S for the NIAID ERADICATE Study Team, . Use of sofosbuvir/ledipasvir fixed dose combination for treatment of HCV genotype-1 in patients coinfected with HIV. Journal of Hepatology. 2014;60(1):S7. ERADICATE: This study evaluated use of sofosbuvir and ledipasvir in patients with HIV and HCV GT1 coinfection. A total of 50 patients (80% with GT1a) majority on ART and few (20%) not on ART but with reserved CD4 of >500 cells/mm3. All patients on ART were on tenofovir/emtricitabine, 41% were on efavirenz, 27% raltegravir and 21% on rilpivirine. 100% SVR12 rates were observed in the untreated group and 100% SVR4 in the ART group. No adverse events reported thus far. [Google Scholar]
- 66••.Sulkowski MEJ, Wyles D, Trinh R, Lalezari J, Slim J, Gathe J, Ruane PJ, Wang C, Elion R, Bredeek F, Brennan R, Blick G, Khatri A, Gibbons K, Hu YB, Fredrick L, Pilot-Matias T, Da Silva-Tillmann McGovern BB, Campbell AL, Podsadecki T. TURQUOISE-I: safety and efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in patients co-infected with hepatitis C and HIV-1. International AIDS conference; 2014; TURQUOISE-1: This study has evaluated the safety and efficacy of ABT-450/r/ombitasvir, dasabuvir and ribavirin in HIV/HCV-1 co-infected individuals. A total number 63 individuals, majority males (92.1%), white race (76.2), GT1a (88.9%), 19% had cirrhosis and a few (33.3%) were previously treated. SVR 4 for the 12 week arm was 96.8% and EOTR for the 24 week arms was 96.7%. No adverse events reported thus far. ART regimen used consisted of atazanavir or raltegravir. [Google Scholar]
- 67.Baid-Agrawal S, et al. Hepatitis C Virus Infection and Kidney Transplantation in 2014: What’s New? Am J Transplant. 2014;14(10):2206–20. doi: 10.1111/ajt.12835. [DOI] [PubMed] [Google Scholar]
- 68.Fabrizi F, Messa P, Martin P. Recent Advances on Hepatitis C Virus in Dialysis Population. Kidney Blood Press Res. 2014;39(4):260–271. doi: 10.1159/000355803. [DOI] [PubMed] [Google Scholar]
- 69.Liu CH, et al. Pegylated interferon-alpha2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med. 2013;159(11):729–38. doi: 10.7326/0003-4819-159-11-201312030-00005. [DOI] [PubMed] [Google Scholar]
- 70.Gane EJ, Agarwal K. Directly acting antivirals (DAAs) for the treatment of chronic hepatitis C virus infection in liver transplant patients: “a flood of opportunity”. Am J Transplant. 2014;14(5):994–1002. doi: 10.1111/ajt.12714. [DOI] [PubMed] [Google Scholar]
- 71.Samuel DCM, Gane E, et al. Sofosbuvir and ribavirin for the treatment of recurrent hepatitis C infection after liver transplantation: results of a prospective, multicenter study. 49thEuropean Association for the Study of the Liver International Liver Congress (EASL 2014); London. 2014. [Google Scholar]
- 72.Reddy KRE, GT, Flamm SL, Denning JM, Arteburn S, Brandt-Sarif T, Pang PS, McHutchinson JG, Curry MP, Charlton M. Ledipasvir/ Sofosbuvir with Ribavirin for the treatment of HCV in patients with post transplant recurrence: Preliminary results of a prospective study. AASLD. 2014;2014 Abstract. [Google Scholar]
- 73.Kwo PY, et al. An Interferon-free Antiviral Regimen for HCV after Liver Transplantation. N Engl J Med. 2014 doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]