Abstract
OBJECTIVES
This study was designed to assess the role of left atrial (LA) shape in predicting embolic cerebrovascular events (ECE) in patients with mitral stenosis (MS).
BACKGROUND
Patients with rheumatic MS are at increased risk for ECE. LA remodeling in response to MS involves not only chamber dilation but also changes in the shape. We hypothesized that a more spherical LA shape may be associated with increased embolic events due to predisposition to thrombus formation or to atrial arrhythmias compared with an elliptical-shaped LA of comparable volume.
METHODS
A total of 212 patients with MS and 20 control subjects were enrolled. LA volume, LA emptying fraction, and cross-sectional area were measured by 3-dimensional (3D) transthoracic echocardiography. LA shape was expressed as the ratio of measured LA end-systolic volume to hypothetical sphere volume ([4/3π r3] where r was obtained from 3D cross-sectional area). The lower the LA shape index, the more spherical the shape.
RESULTS
A total of 41 patients presented with ECE at the time of enrollment or during follow-up. On multivariate analysis, LA 3D emptying fraction (adjusted odds ratio [OR]: 0.96; 95% confidence interval [CI]: 0.92 to 0.99; p = 0.028) and LA shape index (OR: 0.73; 95% CI: 0.61 to 0.87; p < 0.001) emerged as important factors associated with ECE, after adjustment for age and anticoagulation therapy. In patients in sinus rhythm, LA shape index remained associated with ECE (OR: 0.79; 95% CI: 0.67 to 0.94; p = 0.007), independent of age and LA function. An in vitro phantom atrial model demonstrated more stagnant flow profiles in spherical compared with ellipsoidal chamber.
CONCLUSIONS
In rheumatic MS patients, differential LA remodeling affects ECE risk. A more spherical LA shape was independently associated with an increased risk for ECE, adding incremental value in predicting events beyond that provided by age and LA function.
Keywords: 3D echocardiography, embolic events, left atrial function, left atrial shape, mitral stenosis
Patients with rheumatic mitral stenosis (MS) are at increased risk for embolic events. Systemic embolization occurs in 10% to 20% of MS patients, with 75% of cases manifesting as cerebral embolism (1–3). Various factors are related to an increased risk for embolization, including age, atrial fibrillation (AF), and left atrial enlargement (4–8). Embolic events do not appear to be related to the severity of MS, and they can be the first clinical manifestations of the disease (1,2). Additionally, systemic embolism is also known to occur in patients in sinus rhythm (5–9).
Rheumatic MS causes chronic pressure overload on the left atrium (LA), which leads to a range of adaptive processes, including LA structural remodeling (10). LA remodeling involves changes not only in atrial size and function but also in the shape of the chamber. Although LA enlargement by itself contributes to an increased risk for thrombus formation (4,7,8,11), the influence of the LA anatomic shape on blood flow pattern and consequently risk for embolic events has not been well defined.
Three-dimensional (3D) echocardiography allows for the assessment of global LA shape (12) and standardized direct volume and area measurements of the LA without geometric assumptions (13), with superior accuracy compared with conventional echocardiography, especially for an enlarged LA (14). The assessment of LA shape by 3D echocardiography may provide insights into the mechanism that determines blood stasis, which predisposes to embolic events in the setting of MS.
We hypothesized that differential LA remodeling in response to chronic pressure overload contributes to the risk for embolic events. Specifically, a spherical-shaped LA is predisposed to thrombus due to greater stagnant blood flow or to atrial arrhythmias compared with an elliptical-shaped LA of comparable volume. The aim of this study was to assess the role of LA anatomic shape in predicting embolic cerebrovascular events (ECE) in patients with rheumatic MS.
METHODS
Study population
The study prospectively enrolled 212 consecutive patients with mild to severe rheumatic MS referred to a tertiary care center (Hospital das Clinicas, Federal University of Minas Gerais, Belo Horizonte, Brazil) from 2009 to 2012. Patients with moderate or greater mitral regurgitation; significant aortic valve disease; and/or associated systemic diseases that are predictors of ischemic cerebrovascular events, including hypertension and diabetes, were excluded. Transesophageal echocardiography was performed in all patients with AF or with previous embolic events to exclude LA and LA appendage thrombus (n = 73). The presence of LA or LA appendage thrombus was also an exclusion criteria.
Written informed consent was obtained from all patients, and the study was approved by the institutional clinical research and ethics committee.
Outcome assessment
The outcome of this study was defined as the first ECE, including stroke or transient ischemic attack, suspected to be of thromboembolic origin from cardiac source before enrollment into the study or during the follow-up. Transient ischemic attack was defined as an acute neurological deficit of presumed vascular origin lasting <24 h. Stroke was defined as an acute neurological deficit of presumed vascular origin lasting >24 h, or the presence of brain infarction on neuroimaging (15).
Patients were clinically managed on the basis of the American College of Cardiology/American Heart Association 2008 guidelines (16). According to these guidelines, anticoagulation was indicated in patients with MS and AF, or with a prior embolic event.
Twenty healthy subjects with similar sex and age distribution as the cases, and normal standard echocardiograms with good-quality images, were selected as controls.
Echocardiographic evaluation
Transthoracic 2-dimensional (2D) echocardiography was performed according to the recommendations of the American Society of Echocardiography (17). All measurements were performed by a single investigator, blinded to clinical data.
Left atrial volume (LAV) by 2D echocardiography was determined using the modified Simpson rule at end-systole (17). LA linear dimensions were measured in 3 planes: anteroposterior, lateral, and superoinferior at end-systole. A 2D sphericity index was calculated as the ratio between the superoinferior and mediolateral diameters.
Assessment of LAVs by 3D echocardiography
Real-time 3D echocardiography was collected in full-volume mode from 4 heartbeats of breath-holding in expiration.
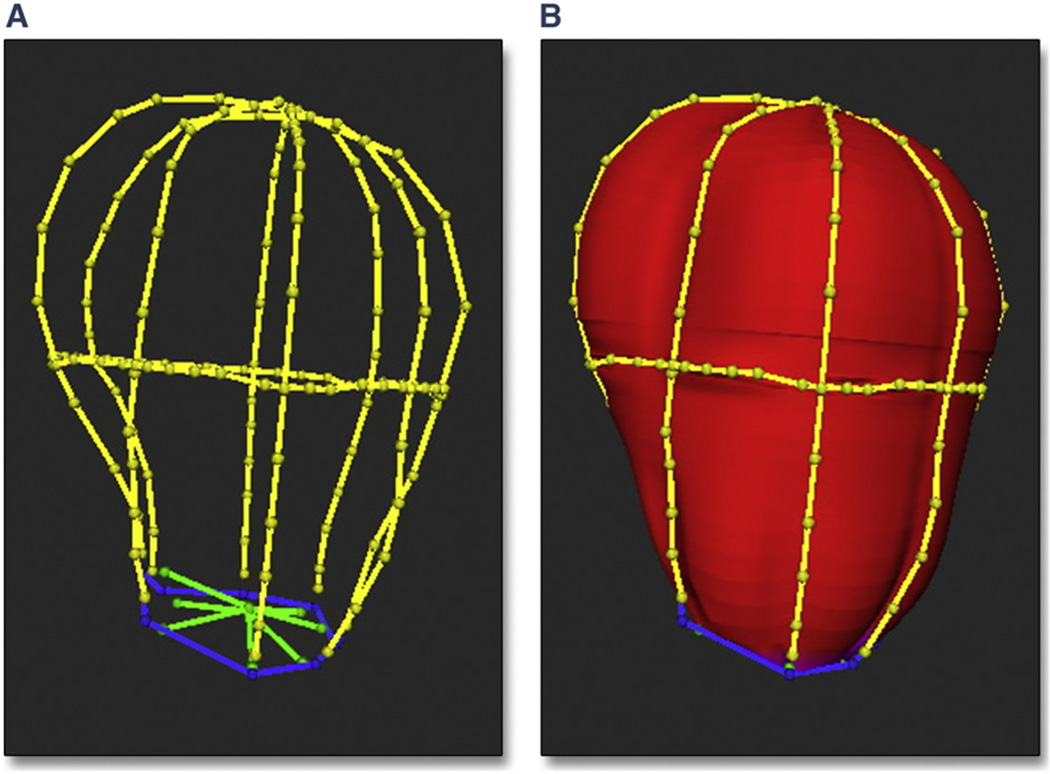
LAV was measured using customized software (Omni4D, Inc., Beverly Hills, California) (18). LA endocardial borders of the LA on 3D images were traced on 4 equiangular image planes (0°, 45°, 90°, and 135°) intersecting a reference axis from the LA center to the mitral annular center (approximating the direction of blood flow in the LA) (Fig. 1A). A 3D endocardial surface of the LA was formed using an automated algorithm fitting polygonal mesh to traced borders (Figs. 1B and 2). This 3D reconstruction of the left atrium allows it to be visualized from a number of different views (Online Video 1).
Figure 1. Endocardial Borders Conforming to Delineated Borders.
In this example, the endocardial borders of the left atrium (LA) (A) and the polygonal mesh surface conform to the delineated borders (B). Borders were traced on 4 equiangular planes (0°, 45°, 90°, and 135°) passing through the long axis of the LA and 1 cross-sectional plane perpendicular to the axis at the mid-atrium. Red indicates left atrial endocardial surface; blue indicates mitral valve annulus; yellow indicates left atrial borders; and green indicates mitral valve leaflets.
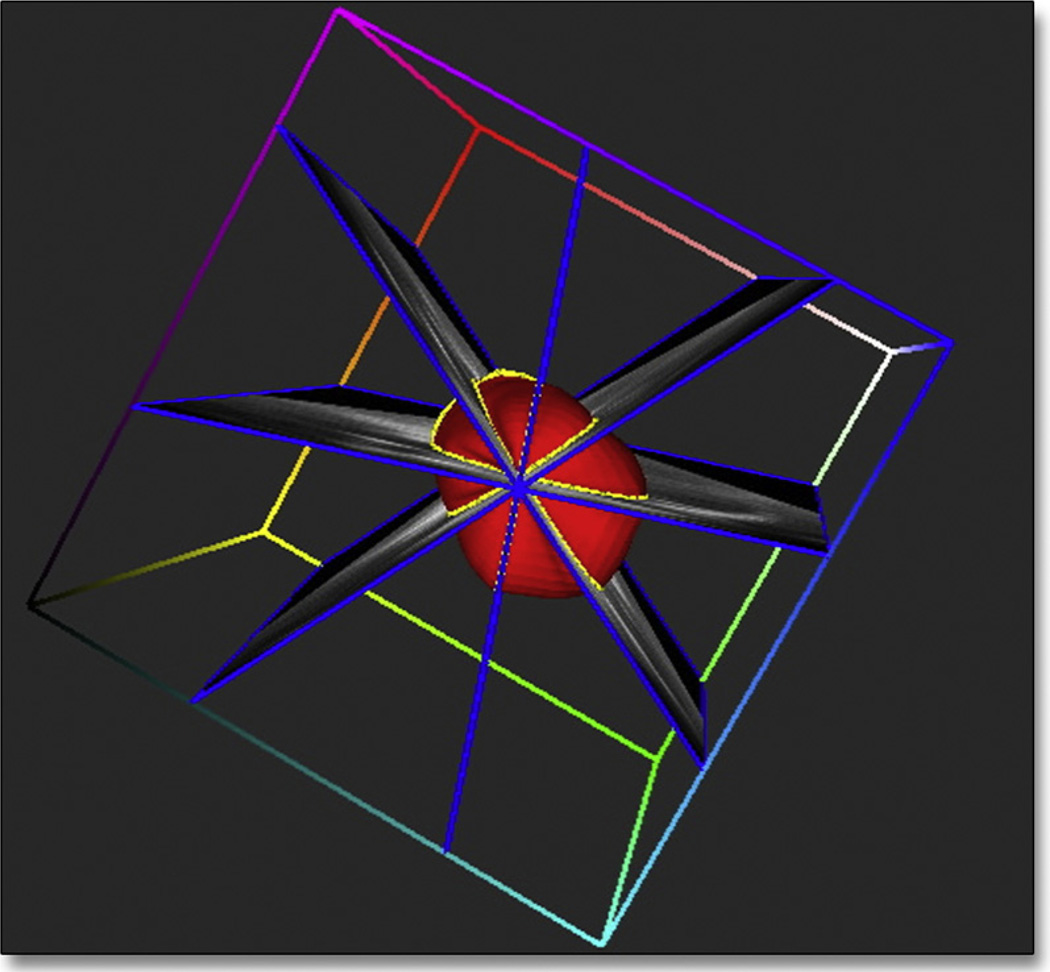
Figure 2. 3D Reconstruction of the LA.
Example of image planes intersecting the reconstructed left atrial (LA) surface, enabling its volume and shape indexes to be computed. Online Video 1 shows a 3D structure of the left atrium highlighting its shape. 3D = 3-dimensional.
The maximal LAV was measured at end-systole, just before mitral valve opening and the minimum volume at end diastole, before mitral valve closure. LAVs were indexed to body surface area. LA emptying fraction (LAEF) to assess LA function was expressed as: LAEF (%) = 100 · [(LAVmax − LAVmin)/LAVmax] (19–21).
Measures of LA cross-sectional area and shape by 3D echocardiography
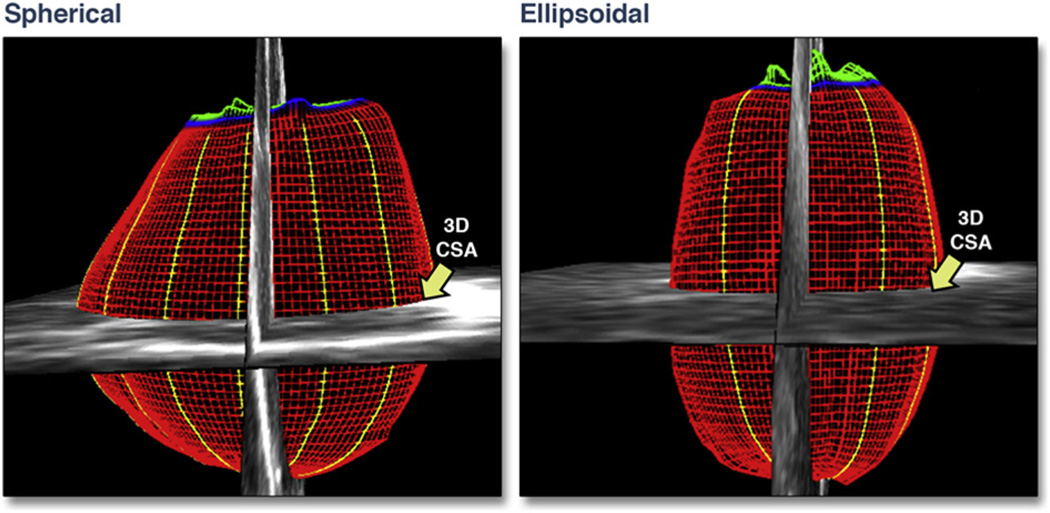
LA cross-sectional area (CSA) at the mid-atrial level was obtained by adjusting a cross-sectional image plane perpendicular to the reference axis to the midpoint using 3D guidance, tracing the endocardial border and computing the 3D area (Fig. 3). LA CSA was divided by body surface area to normalize for body size.
Figure 3. LA 3D CSA Measured in 2 Different LA Anatomic Shapes.
The 3D cross-sectional area (CSA) is larger in the spherical shape compared with the ellipsoidal shape. Red indicates left atrial endocardial surface; blue indicates mitral valve annulus; yellow indicates left atrial borders; green indicates mitral valve leaflets. Abbreviations as in Figures 1 and 2.
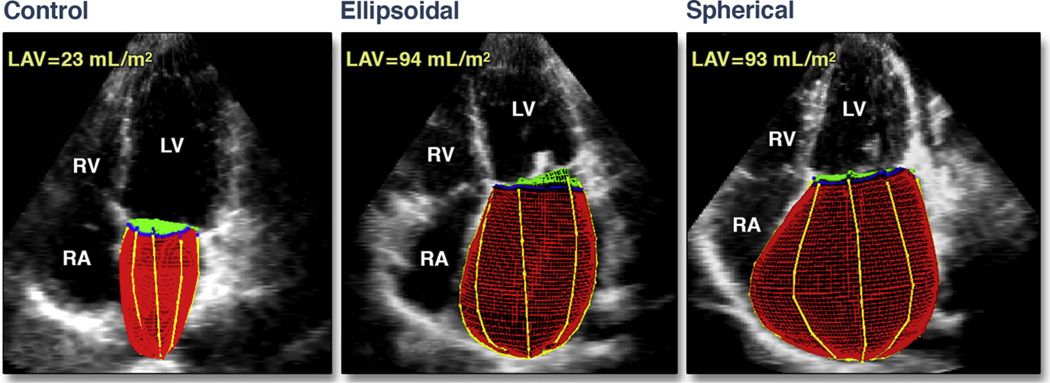
The shape of the LA (LA shape index) was expressed as the ratio of the LA end-systolic volume to a calculated atrial volume from the mid-atrial CSA (assuming that the atrium is completely spherical). This hypothetical volume was derived from the formula for the volume of a sphere (volume = 4/3π r3), where r was obtained from the mid-atrial CSA. Compared with the normally elongated ellipsoidal LA, a more spherically-shaped LA corresponded to a lower LA shape ratio index (Figs. 3 and 4).
Figure 4. Normal LA, Ellipsoidal, and Spherical Shapes.
In these examples of normal 3D LA (left), ellipsoidal (middle), and spherical (right) shapes, the left atrial volumes (LAV) in both the ellipsoidal shape and the spherical shape are similar. LV = left ventricle; RA = right atrium; RV = right ventricle; other abbreviations as in Figures 1 and 2.
Phantom model
To explore whether differences in chamber shape results in variations in flow velocities that might influence thrombus formation, flow-velocity profiles were measured across the mid-plane of a spherical versus an ellipsoidal polyethylene model with constant longitudinal flow rate and CSA of 7 cm2. Flow rates of 1.5, 2.5, 3.5, and 4.5 l/min were studied.
Statistical analysis
Data are summarized as mean ± SD or as n (%). Differences in clinical and echocardiographic variables between the patients with and without ECE were evaluated using unpaired Student t tests or chi-square tests as appropriate. Logistic regression analysis was performed to determine characteristics that were associated with ECE. Clinical and echocardiographic variables that were found to be significantly associated with ECE on univariate analysis were included in the multivariate logistic regression analysis. To satisfy the assumption of the independence of events, only the first event was considered as the endpoint, and recurrent embolic events were not included in the analysis.
The potential predictive variables of ECE included in the multivariate analysis were age, previous valvuloplasty, AF, anticoagulant therapy, blood pressure, transmitral pressure gradient, and left ventricular (LV) ejection fraction. The variables of 3D LA assessment were checked for collinearity, and obviously interdependent covariates were not used simultaneously in any of the analyses. We constructed the first multivariate model including the overall patient population and then a second model with a subset of patients in sinus rhythm. A shrinkage factor was estimated from the bootstrap procedure, and we shrunk the regression coefficients to correct for optimism of the models (22). The discrimination and calibration of the multivariate models were measured to assess their performance in ECE prediction (23,24).
Reproducibility of 3D LAVs (emptying fraction) and CSA was assessed by the intraclass correlation coefficients for repeated measures in a random sample of 15 patients. Statistical analysis was performed using SPSS version 18.0 (IBM SPSS Statistics, IBM Corporation, Armonk, New York) and R for Statistical Computing version 2.15.1 (R Foundation, Vienna, Austria).
RESULTS
Clinical and echocardiographic characteristics
The mean age was 44.1 ± 11.7 years, with 84% women. A total of 167 (79%) were in New York Heart Association functional class I or II, and 45 patients (21%) were in class III or IV. On entry into the study, 46 patients (22%) were in AF, and 72 patients (34%) had a history of mitral valvuloplasty (either percutaneous or surgical).
The baseline clinical characteristics of the study patients stratified by the presence of ECE are summarized in Table 1. Patients who had experienced an embolic event or those with paroxysmal or persistent AF were on anticoagulant therapy.
Table 1.
Baseline Clinical and Echocardiographic Features of the Study Population According to the Presence of ECE
| Patients Without Events (n = 171) |
Patients With Events (n = 41) |
OR (95% CI) |
p Value | |
|---|---|---|---|---|
| Age, yrs | 42.8 ± 11.4 | 49.2 ± 11.6 | 1.05 (1.02–1.14) | 0.001 |
| History | ||||
| Valvuloplasty | 52 (30) | 20 (49) | 2.27 (1.13–4.57) | 0.022 |
| Anticoagulation therapy* | 37 (22) | 25 (61) | 0.164 (0.08–0.34) | <0.001 |
| Atrial fibrillation† | 32 (19) | 14 (34) | 2.25 (1.06–4.77) | 0.034 |
| BP, mm Hg | ||||
| Systolic | 117.4 ± 14.6 | 121.6 ± 17.8 | 1.02 (0.99–1.04) | 0.121 |
| Diastolic | 73.9 ± 11.0 | 79.6 ± 11.4 | 1.05 (1.02–1.09) | 0.004 |
| Cardiovascular measurements | ||||
| LVEF, % | 63.9 ± 7.4 | 61.1 ± 9.1 | 0.96 (0.92–0.99) | 0.044 |
| Mitral valve area, cm2‡ | 1.19 ± 0.4 | 1.24 ± 0.3 | 1.35 (0.59–3.01) | 0.473 |
| Mean gradient, mm Hg | 9.9 ± 5.2 | 8.1 ± 3.7 | 0.91 (0.84–0.99) | 0.037 |
| LA diameter, mm | 50.2 ± 6.5 | 52.1 ± 8.6 | 1.04 (0.99–1.09) | 0.126 |
| 2D LA sphericity index | 1.29 ± 0.17 | 1.24 ± 0.24 | 0.23 (0.02–3.42) | 0.282 |
| 2D LA volume, ml/m2 | 58.9 ± 22.5 | 69.1 ± 45.5 | 1.01 (0.99–1.02) | 0.070 |
| 3D LA volume, ml/m2 | 53.3 ± 20.1 | 64.8 ± 34.4 | 1.02 (1.01–1.03) | 0.014 |
| 3D LA CSA, cm2/m2 | 12.7 ± 3.2 | 15.5 ± 5.5 | 1.17 (1.07–1.28) | <0.001 |
| 3D LAEF, % | 30.9 ± 15.0 | 23.5 ± 15.3 | 0.97 (0.94–0.99) | 0.029 |
| LA 3D shape index§ | 1.59 ± 0.4 | 1.28 ± 0.3 | 0.78 (0.69–0.88) | <0.001 |
Values are mean ± SD or n (%).
Anticoagulation therapy at the time of enrollment into the study.
Atrial fibrillation at the time of enrollment into the study.
Mitral valve area was assessed by planimetry.
OR per 0.1-U increase.
2D = 2-dimensional; 3D = 3-dimensional; BP = blood pressure; CI = confidence interval; CSA = cross-sectional area; ECE = embolic cerebrovascular events; LA = left atrial; LAEF = left atrial emptying fraction; LVEF = left ventricular ejection fraction; OR = odds ratio.
The echocardiographic features of the patients according the presence of ECE are also summarized in Table 1. The mean mitral valve area was 1.2 cm2 in the overall population, including a wide spectrum of MS severity (range 0.42 to 2.78 cm2; interquartile range: 0.89 to 1.42 cm2). There was no significant difference in the degree of MS severity between the patients with ECE and those without.
Patients with AF had larger LAVs and LA CSA and lower LAEF compared with patients in sinus rhythm. However, the LA shape index was similar between patients with and without persistent AF at baseline (1.54 ± 0.4 vs. 1.48 ± 0.5; p = 0.45).
ECE in the overall study population
ECE occurred in 41 patients (19%); 33 of these patients were diagnosed with an ECE before enrollment into the study, and the remaining 8 patients were diagnosed during the course of follow-up. At 2-year follow-up, 2 patients had died, 34 patients had undergone percutaneous mitral valvuloplasty, and 17 patients had undergone mitral valve replacement. Additionally, during the follow-up, 5 patients showed onset of AF, of whom 3 had ECE.
Several clinical and echocardiographic variables were tested for a possible association with ECE (Table 1). In multivariate logistic regression analysis including the potential predictors of ECE, the independent factors associated with ECE were age, 3D LAEF, LA shape index, and having received anticoagulation therapy (Table 2).
Table 2.
Multivariate Logistic Regression Model for Predicting ECE in the Overall MS Population*
| Covariate† | OR | 95% CI | p Value |
|---|---|---|---|
| Age, yrs | 1.07 | 1.02–1.12 | 0.006 |
| Anticoagulation therapy | 0.09 | 0.03–0.31 | <0.001 |
| 3D LAEF, % | 0.96 | 0.92–0.99 | 0.028 |
| 3D LA shape index‡ | 0.73 | 0.61–0.87 | <0.001 |
Shrinkage factor of 0.8561.
The c statistic for predicting ECE was 0.87 (95% CI: 0.81 to 0.94).
OR per 0.1-U increase.
Abbreviations as in Table 1.
As absolute LAV and CSA were strongly correlated, these variables were not included in the same model. When indexed 3D LAV was included, this variable was removed from the model (odds ratio [OR]: 1.011; 95% confidence interval [CI]: 0.99 to 1.03; p = 0.197) for not being statistically significant. Therefore, although LAV index was a predictor of ECE on univariate analysis, the 3D LAEF, which expresses LA function, was superior in ECE prediction.
LA shape index calculated by parameters derived from 2D echocardiography measurements, including diameter and sphericity index, were not predictors of ECE.
ECE in patients with sinus rhythm
A subanalysis excluding subjects with AF was performed. In the subset of patients in sinus rhythm, ECE occurred in 19 patients before enrollment into the study and in 8 patients during the course of the follow-up. The variables that were selected for the multivariate model were age, previous valvuloplasty, blood pressure, transmitral pressure gradient, LV ejection fraction, LAV, LA CSA, LAEF, and LA shape index. LA shape index was a powerful predictor of ECE (OR: 0.79; 95% CI: 0.67 to 0.94; p = 0.007), adding incremental value to age (OR: 1.05; 95% CI: 1.01 to 1.10; p = 0.043) and LAEF (OR: 0.96; 95% CI: 0.93 to 0.99; p = 0.031), whereas LAV did not show a significant association with ECE (Table 3). The c statistic for predicting ECE in the final model was 0.85 (95% CI: 0.76 to 0.94).
Table 3.
Predictors of ECE in Patients With Sinus Rhythm*
| Covariate | OR | 95% CI | p Value |
|---|---|---|---|
| Age, yrs | 1.05 | 1.01–1.10 | 0.043 |
| 3D LAEF, % | 0.96 | 0.93–0.99 | 0.031 |
| 3D LA shape index† | 0.79 | 0.67–0.94 | 0.007 |
To assess the value of LA shape in predicting ECE, we excluded patients who had events before recruitment into the study. This subset of patients was in sinus rhythm, without the confounding effects of arrhythmia or anticoagulant therapy on the outcome. Among all variables tested, LA shape index was the only predictor of events (adjusted OR: 0.70; 95% CI: 0.53 to 0.92; p = 0.012).
LA measurements in patients with sinus rhythm stratified by the presence of ECE and controls
The mean age of the control group was 40.4 ± 10.4 years, and 15 were women (75%), similar to the case group. The reference values of 3D LA measurements are shown in Table 4.
Table 4.
Parameters of LA Assessment According to the Presence of ECE in MS Patients in Sinus Rhythm and in Controls
| Patients Without Events (n = 139) |
Patients With Events (n = 27) |
p Value | Reference Controls* (n = 20) |
|
|---|---|---|---|---|
| LA anteroposterior diameter, mm | 48.7 ± 5.7 | 49.4 ± 6.8 | 0.773 | 32.9 ± 4.6 |
| 3D LA volume, ml/m2 | 49.6 ± 13.2 | 54.2 ± 20.8 | 0.294 | 17.1 ± 4.5 |
| 3D LA CSA, cm2/m2 | 12.3 ± 2.5 | 14.2 ± 4.5 | 0.002 | 6.9 ± 2.1 |
| 3D LAEF, % | 32.6 ± 15.2 | 24.9 ± 17.2 | 0.031 | 58.6 ± 6.2 |
| 3D LA shape index | 1.59 ± 0.41 | 1.28 ± 0.36 | <0.001 | 1.73 ± 0.37 |
Echocardiographic parameters to assess LA were compared between patients with and without ECE (Table 4). LAEF and LA shape index were lower in patients with ECE compared with those in patients without ECE, whereas LAV was similar between the 2 groups. There was also an increase in the LA CSA in patients with ECE compared with that in those without ECE.
Reproducibility
For the LA CSA, the intraclass correlation coefficients were 0.96 for interobserver variability and 0.98 for intraobserver variability. For LAEF, the intraclass correlation coefficients were 0.88 for interobserver variability and 0.93 for intraobserver variability.
Validation in the phantom model
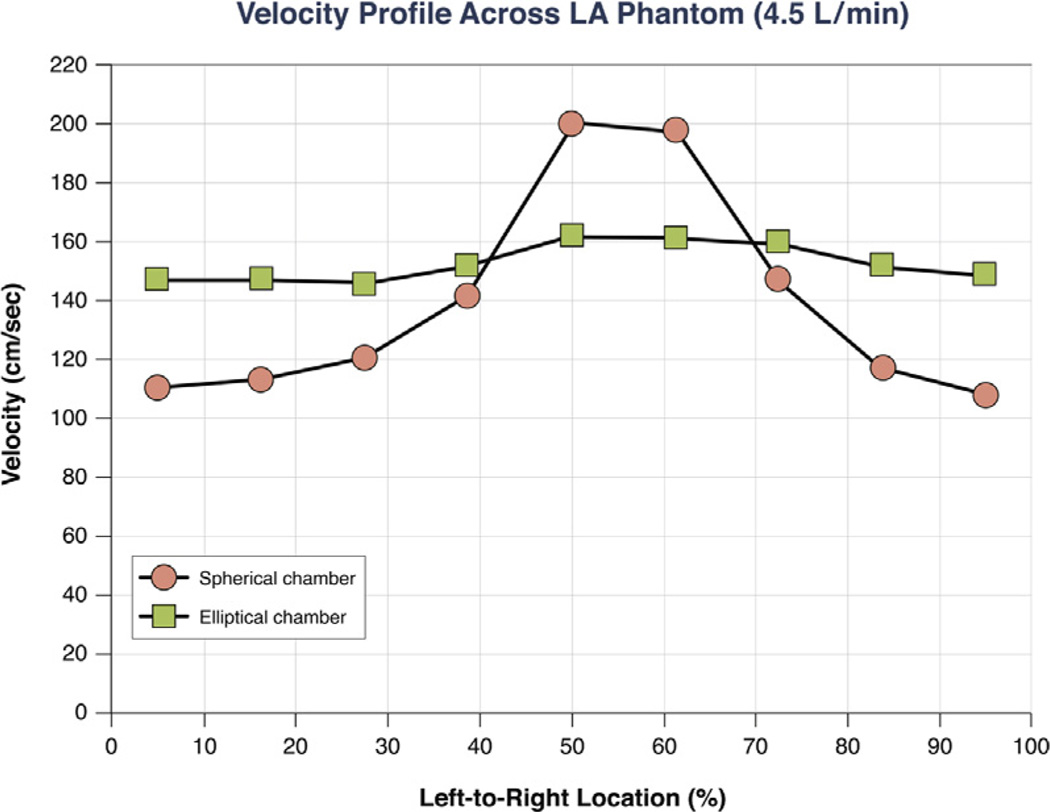
Atrial inflow velocities measured within the ellipsoidal-shaped model remained relatively constant at the mid-level of the model, whereas velocity magnitudes in the spherical-shaped model were maximal in the center of the model but were significantly lower in the peripheral regions. The velocity waveforms for both chambers are shown in Figure 5. In the spherical-shaped model, the mean velocity in the sides was 121 ± 15 cm/s, whereas in the ellipsoidal one, it was 148 ± 7 cm/s (p = 0.002).
Figure 5. In Vitro Velocity–Flow Profiles of a Phantom Model.
In these in vitro velocity–flow profiles of a spherical and an ellipsoidal phantom model (constant inflow rate of 4.5 l/min), velocities were measured at 9 equidistant points across the mid-chamber for each model, from left to right.
DISCUSSION
The present study examined the influence of LA shape on ECE in patients with rheumatic MS. Besides age, 3D LA emptying fraction and LA shape index were found to be important factors associated with ECE. In patients in sinus rhythm, LA shape index was a powerful predictor of ECE. These findings demonstrate the role of pathological LA remodeling pattern in predicting ECE.
LA enlargement and embolic events
In patients with MS, several studies have demonstrated that LA enlargement is a marker of increased thromboembolic risk (4,6,7,9,11,25,26). However, the great majority of studies considered LA size or volume as an indicator of risk. This study aimed to look specifically at LA shape and not just LAVs and LA function in predicting stroke. As LA dilation may not occur in a uniform fashion, LA shape might be a better measure of the pattern of LA remodeling and a better predictor of embolic risk than absolute LAV.
The results of this study show that the atrial shape adds incremental value in predicting embolic events. Additionally, in agreement with a recently published study (27), we found that LA reservoir function was also associated with an increased risk for ECE. Although the LA has 3 major roles that affect LV filling, its reservoir function represents the most important component of the LA function in MS. LA contractile force cannot overcome the mechanical obstruction across the valve and does not significantly contribute to LV filling (28). Therefore, in our study we assessed LA function by measuring LAEF. Our findings are in line with those from previous studies, and add novel evidence indicating that LA shape is a strong and independent predictor of ECE after controlling for potential confounders.
We developed an index to assess LA shape on the basis of 3D reconstruction that takes into account 2 important determinants of blood flow stagnation with potential for thrombus formation: the short-axis CSA that is orthogonal to direction of blood flow, and volume of the LA. For a given volume, the CSA is inversely proportional to the velocity of the blood flow. The index assesses the relationship between the measured volume and the calculated volume derived from a virtual sphere. Therefore, the ratio between these 2 volumes can express the degree of difference in shape of the chamber. Although increases both in volume and in CSA are observed in MS, a predominant increase of the CSA relative to volume is expected in a more spherical LA shape. Hence, the ratio between these parameters seems to be more accurate and better predicts the ECE risk.
Insights into the mechanism of ECE in mitral stenosis: role of LA shape
Pressure overload on the atrium induces not only LA dilation but also myocardial hypertrophy and interstitial fibrosis (29), which may further influence the remodeling pattern (30). The atrial remodeling can be different in MS compared with mitral regurgitation (31). Cho et al. (32) demonstrated that after mitral valve surgery, patients with mitral regurgitation showed a larger LAV reduction compared with those with MS.
Patients with MS are a heterogeneous group with respect to LA shape. Patients in whom the LA remodeling leads to morphological changes in the atrium, from an ellipsoidal into a more spherical shape, appear to be at increased risk for embolic events. Additionally, spherical remodeling of the LA can lead to a further increase in the atrial wall tension, which may predispose to onset of AF. Thus, spherical LA morphology is probably less effective for atrial contraction and is a marker for future AF episodes, representing a higher-risk condition for ECE.
Clinical implications
The identification of patients who are at higher risk for ECE could lead to changes in the management of patients with MS. Presently, there are no data to support the concept that oral anticoagulation is beneficial in patients with MS and in sinus rhythm (16). Assessment of LA shape may therefore be helpful in guiding the decision of anticoagulation in patients with MS who are in sinus rhythm.
Study limitations
Patients with ECE before enrollment into the study were included, which may potentially affect the prediction of the outcome. Prospective studies are needed for the demonstration of the cause–effect relationship between the LA shape and embolic events.
AF was diagnosed on the basis of symptoms, electrocardiography, and periodic Holter examinations. Paroxysmal AF may still have been present at the time of the ECE but not diagnosed on the later electrocardiographic tracings obtained during the medical visit.
Although patients with MS and ECE have a high risk for cardiogenic embolism, the precise mechanism is difficult to determine. Nonetheless, we attempted to exclude patients with other potential causes for the ECE, and therefore, a cardiac source of embolism was presumed to be the most likely in our patient population.
Doppler imaging measures only a single component of blood velocity along the transducer scan line and, therefore, does not provide quantitative information with respect to vortex formation. The data presented here are not definitive evidence of thrombus formation or stasis, and further research is warranted to establish the role of shape on regional flow.
CONCLUSIONS
In patients with a wide spectrum of rheumatic MS severity, differential LA remodeling affects ECE risk. Low 3D LAEF and a spherical LA shape were independently associated with an increased risk for embolic events. LA shape index was associated with ECE in the setting of MS with LA dilation and sinus rhythm. The 3D assessment of LA shape may be helpful in identifying MS patients at high risk for embolic events.
Supplementary Material
Acknowledgments
This study was partly supported by grants from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasília, Brazil) and by the National Institutes of Health/National Heart, Lung, and Blood Institute grant no. R01 HL092101 (Dr. Hung).
ABBREVIATIONS AND ACRONYMS
- 2D
2-dimensional
- 3D
3-dimensional
- AF
atrial fibrillation
- CSA
cross-sectional area
- ECE
embolic cerebrovascular event(s)
- LA
left atrium/atrial
- LAEF
left atrial emptying fraction
- LV
left ventricular
- MS
mitral stenosis
APPENDIX
For a supplemental video and legend, please see the online version of this article.
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Coulshed N, Epstein EJ, McKendrick CS, Galloway RW, Walker E. Systemic embolism in mitral valve disease. Br Heart J. 1970;32:26–34. doi: 10.1136/hrt.32.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood P. An appreciation of mitral stenosis. I. Clinical features. Br Med J. 1954;1:1051–1063. doi: 10.1136/bmj.1.4870.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selzer A, Cohn KE. Natural history of mitral stenosis: a review. Circulation. 1972;45:878–890. doi: 10.1161/01.cir.45.4.878. [DOI] [PubMed] [Google Scholar]
- 4.Conradie C, Schall R, Marx JD. Left atrial size—a risk factor for left atrial thrombi in mitral stenosis. Clin Cardiol. 1995;18:518–520. doi: 10.1002/clc.4960180907. [DOI] [PubMed] [Google Scholar]
- 5.Acarturk E, Usal A, Demir M, Akgul F, Ozeren A. Thromboembolism risk in patients with mitral stenosis. Jpn Heart J. 1997;38:669–675. doi: 10.1536/ihj.38.669. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CW, Lo SK, Ko YS, Cheng NJ, Lin PJ, Chang CH. Predictors of systemic embolism in patients with mitral stenosis. A prospective study. Ann Intern Med. 1998;128:885–889. doi: 10.7326/0003-4819-128-11-199806010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Keenan NG, Cueff C, Cimadevilla C, et al. Usefulness of left atrial volume versus diameter to assess thromboembolic risk in mitral stenosis. Am J Cardiol. 2010;106:1152–1156. doi: 10.1016/j.amjcard.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Goswami KC, Yadav R, Rao MB, Bahl VK, Talwar KK, Manchanda SC. Clinical and echocardiographic predictors of left atrial clot and spontaneous echo contrast in patients with severe rheumatic mitral stenosis: a prospective study in 200 patients by transesophageal echocardiography. Int J Cardiol. 2000;73:273–279. doi: 10.1016/s0167-5273(00)00235-7. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein NE, Demopoulos LA, Tunick PA, Rosenzweig BP, Kronzon I. Correlates of spontaneous echo contrast in patients with mitral stenosis and normal sinus rhythm. Am Heart J. 1994;128:287–292. doi: 10.1016/0002-8703(94)90481-2. [DOI] [PubMed] [Google Scholar]
- 10.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal AK, Venugopalan P. Left atrial spontaneous echo contrast in patients with rheumatic mitral valve stenosis in sinus rhythm: relationship to mitral valve and left atrial measurements. Int J Cardiol. 2001;77:63–68. doi: 10.1016/s0167-5273(00)00415-0. [DOI] [PubMed] [Google Scholar]
- 12.Kleijn SA, Aly MF, Terwee CB, van Rossum AC, Kamp O. Comparison between direct volumetric and speckle tracking methodologies for left ventricular and left atrial chamber quantification by three-dimensional echocardiography. Am J Cardiol. 2011;108:1038–1044. doi: 10.1016/j.amjcard.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Hung J, Lang R, Flachskampf F, et al. 3D echocardiography: a review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213–233. doi: 10.1016/j.echo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Anwar AM, Soliman OI, Geleijnse ML, Nemes A, Vletter WB, ten Cate FJ. Assessment of left atrial volume and function by real-time three-dimensional echocardiography. Int J Cardiol. 2008;123:155–161. doi: 10.1016/j.ijcard.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) J Am Coll Cardiol. 2008;52:e1–e142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Handschumacher MD, Lethor JP, Siu SC, et al. A new integrated system for three-dimensional echocardiographic reconstruction: development and validation for ventricular volume with application in human subjects. J Am Coll Cardiol. 1993;21:743–753. doi: 10.1016/0735-1097(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 19.Miyasaka Y, Tsujimoto S, Maeba H, et al. Left atrial volume by real-time three-dimensional echocardiography: validation by 64-slice multidetector computed tomography. J Am Soc Echocardiogr. 2011;24:680–686. doi: 10.1016/j.echo.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Caldas MC, Meira ZA, Barbosa MM. Evaluation of 107 patients with sickle cell anemia through tissue Doppler and myocardial performance index. J Am Soc Echocardiogr. 2008;21:1163–1167. doi: 10.1016/j.echo.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Dham N, Ensing G, Minniti C, Campbell A, Arteta M, Rana S, et al. Prospective echocardiography assessment of pulmonary hypertension and its potential etiologies in children with sickle cell disease. Am J Cardiol. 2009;104:713–720. doi: 10.1016/j.amjcard.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steyerberg EW. Clinical Prediction Models: A Practical Approach To Development, Validation, and Updating. New York, NY: Springer; 2009. [Google Scholar]
- 23.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 25.Li YH, Hwang JJ, Ko YL, et al. Left atrial spontaneous echo contrast in patients with rheumatic mitral valve disease in sinus rhythm. Implication of an altered left atrial appendage function in its formation. Chest. 1995;108:99–103. doi: 10.1378/chest.108.1.99. [DOI] [PubMed] [Google Scholar]
- 26.Kaymaz C, Ozdemir N, Erentug V, Sismanoglu M, Yakut C, Ozkan M. Location, size, and morphologic characteristics of left atrial thrombi as assessed by transesophageal echocardiography in relation to systemic embolism in patients with rheumatic mitral valve disease. Am J Cardiol. 2003;91:765–769. doi: 10.1016/s0002-9149(02)03428-8. [DOI] [PubMed] [Google Scholar]
- 27.Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. J Am Coll Cardiol Img. 2013;6:313–323. doi: 10.1016/j.jcmg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Khan A, Moe GW, Nili N, et al. The cardiac atria are chambers of active remodeling and dynamic collagen turnover during evolving heart failure. J Am Coll Cardiol. 2004;43:68–76. doi: 10.1016/j.jacc.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Kim KH, Kim YJ, Shin DH, et al. Left atrial remodelling in patients with successful percutaneous mitral valvuloplasty: determinants and impact on long-term clinical outcome. Heart. 2010;96:1050–1055. doi: 10.1136/hrt.2009.187088. [DOI] [PubMed] [Google Scholar]
- 31.Qian Y, Meng J, Tang H, et al. Different structural remodelling in atrial fibrillation with different types of mitral valvular diseases. Europace. 2010;12:371–377. doi: 10.1093/europace/eup438. [DOI] [PubMed] [Google Scholar]
- 32.Cho DK, Ha JW, Chang BC, et al. Factors determining early left atrial reverse remodeling after mitral valve surgery. Am J Cardiol. 2008;101:374–377. doi: 10.1016/j.amjcard.2007.09.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.