Abstract
Introduction
We have recently identified a spectrum of fibrinolysis in response to injury, in which there is increased mortality in patients who have either excessive fibrinolysis (hyperfibrinolysis [HF]) or impaired fibrinolysis (shutdown). The regulation of the fibrinolytic system after trauma remains poorly understood. Our group’s previous proteomic and metabolomic work identified elevated red blood cell (RBC) degradation products in trauma patients manifesting HF. We therefore hypothesized that hemolysis was contributory to the pathogenesis of HF. Given the central role of platelets in the cell-based model of coagulation, we further investigated the potential role of platelet lysis in mediation of the fibrinolytic system.
Methods
Red blood cells from healthy donors were frozen in liquid nitrogen and vortexed to create mechanical membrane disruption. Platelets were prepared in a similar fashion. Assays were performed with citrated whole blood mixed ex vivo with either RBC or platelet lysates. Tissue plasminogen activator (tPA) was then added to promote fibrinolysis, mimicking the tPA release from ischemic endothelium during hemorrhagic shock. The degree of fibrinolysis was evaluated with thromboelastography. To identify the mediators of the fibrinolysis system present in RBC and platelet lysates, these lysates were passed over immobilized tPA and plasminogen affinity columns to capture protein-binding partners from RBC or platelet lysates.
Results
The addition of 75 ng/mL of tPA to whole blood increased fibrinolysis from median 30-min lysis of 1.4% (interquartile range [IQR], 0.9%–2.0%) to 8.9% (IQR, 6.5%–11.5%). Red blood cell lysate with tPA increased fibrinolysis to 20.1% (IQR, 12.5%–33.7%), which was nearly three times as much lysis as tPA alone (P < 0.001). Conversely, the addition of platelet lysate decreased tPA-mediated fibrinolysis to 0.35% (IQR, 0.2%–0.8%; P < 0.001). Affinity chromatography coupled with tandem mass spectrometry identified a number of proteins not previously associated with regulation of fibrinolysis and trauma.
Conclusion
Red blood cell lysate is a potent enhancer of fibrinolysis, whereas platelet lysate inhibits fibrinolysis. Intracellular proteins from circulating blood cells contain proteins that interact with the two key proteins of tPA-mediated fibrinolysis. Understanding the effect of tissue injury and shock on the lysis of circulating cells may provide insight to comprehending the spectrum of fibrinolysis in response to trauma.
Keywords: Fibrinolysis shutdown, hyperfibrinolysis, trauma induced coagulopathy, hemolysis, platelets, red blood cells
INTRODUCTION
Coagulopathy in response to trauma has been described in the literature since the 18th century (1). Alteration of coagulation in response to tissue injury and shock involves unique endothelial and circulating blood component pathways (2). Principal component analysis of severely injured trauma patients indicates that abnormalities in clotting factors and fibrinolysis are mechanistically distinct (3). Derangements of fibrinolysis are of particular clinical interest, as they are strongly associated with poor outcomes in trauma. Hyperfibrinolysis (excessive fibrinolysis) is an independent marker of early mortality related to uncontrolled bleeding (4, 5) and associated with mortality rates exceeding 70% (6). Conversely, the shutdown of fibrinolysis (impaired fibrinolysis) is associated with late death from organ failure (7).
In our recent analysis, we recognized that trauma patients can present with a spectrum of fibrinolytic activity in which the extremes (shutdown and hyperfibrinolysis) appear to be pathologic, but between which there is a protective level of physiologic fibrinolysis (8). The hyperfibrinolytic phenotype is associated with early death from exsanguination, whereas the shutdown phenotype is associated with later death from organ failure. However, it remains unclear why patients with a similar degree of shock and injury severity could have substantially different levels of fibrinolysis.
Metabolomic analyses of patients with the hyperfibrinolytic response to trauma identified increased RBC degradation products in circulating plasma (Peltz et al., unpublished data, 2014). There is also evidence that platelets undergo lysis after trauma, supported by data that platelet microparticles increase shortly after injury (9). These data stimulated interest in the potential role of intracellular proteins released from damaged red blood cells (RBCs) and platelets in the pathologic derangements of fibrinolysis observed in trauma. We therefore hypothesized that hemolysis was contributory to hyperfibrinolysis, and platelet lysis would shut down fibrinolysis.
METHODS
Subjects
Blood samples were obtained from healthy volunteers with no known coagulation abnormalities or use of anticoagulants. There were six subjects per each experiment. Median age was 32 years with a range from 26 to 67 years, consisting of four males and two females. Females were not on oral contraceptive medications and were not pregnant. Volunteers were consented, and blood was obtained under an institutional review board–approved protocol (COMIRB no. 14-0366).
Hemolyzed RBC lysate
A unit of leuokreduced AB RBCs was obtained from the Bonfils Blood Center. AB blood ensured that residual plasma would not have preformed antibodies interacting with nonmatched volunteer blood. Cells were flash frozen in liquid nitrogen and thawed with intermittent vortexing. Based on preliminary experiments, three freeze-thaw cycles maximized fibrinolysis enhancement with no residual intact RBCs by manual counting. Hemolyzed RBC lysate (HL) was stored at −80°C prior to use.
Platelet lysate
Platelets were obtained from discarded deidentified platelet-rich plasma. Platelet count averaged 1.7 × 106 cells per milliliter. Six milliliters of platelet-rich plasma was spun at 1,000g for 10 min to remove plasma and washed by reconstituting with 1 mL of normal saline, which was decanted after the next spin. The spin-wash cycle was repeated three times to remove plasma. The final platelet pellet was reconstituted in 0.5 mL of normal saline. This was lysed using the same freeze-thaw process as used for RBCs. To compensate for the variability in platelet count between donors and the difficulty of processing small volumes, a homogenous large-volume pooled platelet lysate (PL) was created for use in our assays. Platelets from 20 deidentified donors went through three spin-wash cycles to remove plasma as previously described. After the final spin, the 0.5-mL aliquots from the 20 different donors were combined. The pooled, washed platelets underwent the triple freeze-thaw process and were aliquoted for later use and stored at −80°C.
Tissue plasminogen activator
Human single-chain tissue plasminogen activator (tPA) from Molecular Innovations (Novi, Mich) was diluted in 5% bovine serum albumin in phosphate-buffered saline (PBS) to a final concentration of 10 ng/µL. Individual, single-use aliquots of tPA were stored at −80°C and thawed immediately before each assay. A concentration of 75 ng/mL of tPA was previously found to be an inflection point for which a citrated native thromboelastography (TEG) had statistically increased fibrinolytic activity compared with a non–tPA-challenged whole-blood sample in healthy volunteers. The use of tPA for assessment of fibrinolytic activity is of clinical importance, because patients with the hyperfibrinolytic phenotype have free average tPA levels in the 50 ng/mL range compared with the shutdown phenotype, wherein the majority of tPA is complexed with plasminogen activator inhbitor 1 (PAI-1), resulting in undetectable free tPA levels (unpublished data)
TEG tPA challenge
Blood was collected in 3.3-mL citrated blood tubes. Samples were kept at room temperature and assayed within 2 h after blood draw, in accordance with manufacturer recommendations. Whole blood was mixed with lysed HL or PL to reach a total volume of 500 µL in individual microcentrifuge tubes. Tissue plasminogen activator was added to whole-blood mixtures to reach a concentration of 75 ng/mL and incubated 5 min prior to running the assay. Citrated native TEG assays were recalcified and run according to the manufacturer’s instructions on a TEG 5000 Thrombelastograph Hemostasis Analyzer (Haemonetics, Niles, Ill). The following parameters were recorded from the tracings of the TEG: R time (ACT, s), angle (α, degrees), maximum amplitude (MA, mm), and lysis 30 min after MA (LY30, %). The significance of each parameter listed above has been previously described in the literature (11).
RBC lysate enhancement of fibrinolysis
Whole blood from healthy volunteers was mixed ex vivo with increasing concentrations of HL (0.5%–4% vol/vol) and assayed with the TEG tPA challenge as described above. Whole blood without HL was used for a baseline control. The range of HL concentration used was based on preliminary titrations in which the linear portion of the dose-response curve was determined to be within this range for most subjects. As a confirmation of the presence of plasmin-mediated fibrinolysis, tranexamic acid (TXA) at a concentration of 20 mg/mL was mixed with the 4% HL sample to demonstrate TXA reversibility of the observed fibrinolytic effect.
Platelet lysate inhibition of fibrinolysis
Whole blood from healthy volunteers was replaced with 0.5% to 4% PL in the same manner as for HL as above and assayed using the TEG tPA challenge. Tranexamic acid at a concentration of 20 mg/mL in whole blood was used as a positive control for inhibition of fibrinolysis in the presence of tPA.
Affinity chromatography
Human tPA and plasminogen were covalently coupled to Amino Link Plus Resin according to the manufacturer’s recommendations (Thermo Fisher Scientific, Rockford, Ill). Briefly, for each 1.0-mL bed volume of coupling resin, 0.5 mg of tPA and plasminogen were incubated for 4 h at room temperature in the presence of 50 mM sodium cyanoborohydride. After the incubation step, the resin was washed three times with the coupling buffer by low-speed centrifugation (1,000g, for 1 min, at room temperature). To quench unreacted sites on the coupling resin, 1.0 mL of 1 M Tris, pH 7.4, was added and incubated at room temperature for 30 min in the presence of 50 mM sodium cyanoborohydride. Lysates were loaded and incubated on columns for 20 min at room temperature. After the incubation step, the mixture was centrifuged at 1,000g for 1 min at 4°C, and the supernatant discarded. The resin was washed five times with PBS and two times with a salt wash buffer (500 mM NaCl in PBS). Proteins bound by tPA and plasminogen the resin were eluted using a low-pH buffer (glycine, pH 2.5).
Sample preparation for mass spectrometric analysis
Proteins were digested according to the FASP protocol using a 30-kD-molecular-weight cutoff filter. In brief, samples were mixed in the filter unit within 8 M urea in 0.1 M Tris-HCl, pH 8.5, and centrifuged at 14,000g for 15 min. The proteins were reduced by addition of 100 µL of 10 mM DTT in 8 M urea in 0.1 M Tris-HCl, pH 8.5, and incubated for 30 min at RT, followed by centrifugation. Subsequently, 100 µL of 55 mM iodoacetamide in 8 M urea in 0.1 M Tris-HCl, pH 8.5, was added to the samples, incubation for 30 min at RT in dark followed by centrifugation. Afterward, two washing steps with 100 µL of 8 M urea in 0.1 M Tris-HCl, pH 8.5, were performed, followed by two washing steps with 100 µL of 50 mM ABC buffer. Proteins were digested with trypsin overnight at 37°C. Peptides were recovered from the filter using 30% acetonitrile (ACN). The volume of the eluted sample was reduced to ~2 µL in a vacuum centrifuge and reconstituted to 20 µL with 0.1% formic acid (FA).
Liquid chromatography–tandem mass spectrometry analysis
Samples were analyzed on an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) coupled to an Eksigent nanoLC-2D system through a nanoelectrospray liquid chromatography–tandem mass spectrometry (MS/MS) interface. A volume of 8 µL of sample was injected into a 10-µL loop using the autosampler. To desalt the sample, material was flushed out of the loop and loaded onto a trapping column (ZORBAX 300SB-C18; dimensions 5 × 0.3 mm, 5 µm) and washed with 0.1% FA at a flow rate of 5 µL/min for 5 min. The analytical column was then switched on-line at 600 nL/min over an in-house–made 100-µm-internal-diameter × 200-mm fused silica capillary packed with 4 µm 80 Å Synergi Hydro C18 resin (Phenomex, Torrance, Calif). After 10 min of sample loading, the flow rate was adjusted to 350 nL/min, and each sample was run on a 90-min linear gradient of 5% to 40% ACN with 0.1% FA to separate the peptides. LC mobile phase solvents and sample dilutions used 0.1% FA in water (buffer A) and 0.1% FA in acetonitrile (buffer B) (Chromasolv LC-MS grade; Sigma-Aldrich, St Louis, Mo). Data acquisition was performed using the instrument supplied Xcalibur (version 2.1, ThermoFisher, San Jose, Calif) software. The mass spectrometer was operated in the positive ion mode. Each survey scan of m/z 400 to 2,000 was followed by collision-assisted dissociation MS/MS of 20 most intense precursor ions. Singly charged ions were excluded from CID selection. Normalized collision energies were used using helium as the collision gas.
Database searching, protein identification
Tandem mass spectrometry spectra were extracted from raw data files and converted into mgf files using a PAVA script (UCSF, MSF, San Francisco, Calif). These mgf files were then independently searched against human SwissProt database using an in-house Mascot server (version 2.2.06; Matrix Science, London, UK). Mass tolerances were +/−15 ppm for MS peaks and +/−0.6 D for MS/MS fragment ions. Trypsin specificity was used allowing for one missed cleavage. Met oxidation, protein N-terminal acetylation, and peptide N-terminal pyroglutamic acid formation were allowed for variable modifications, whereas carbamidomethyl of Cys was set as a fixed modification.
Scaffold (version 4.3.2; Proteome Software, Portland, Ore) was used to validate MS/MS-based peptide and protein identifications.
Filtrate of affinity columns was run using the same methods as previously described for TEG tPA challenge. To contrast the changes in fibrinolytic activity, the baseline lysis of tPA mixed with whole blood to filtrate of different columns mixed with whole blood containing the same final concentration of tPA (75 ng/mL). The percentage of lysate to whole blood was based on prior lysate titrations at which there was a statistically different change from baseline LY30 (2% filtrated RBC lysate and 1% platelet lysate).
Statistical analysis
All analyses were performed using SAS 9.3 for Windows (SAS Institute Inc, Cary, NC) and SPSS 22 software (Microsoft, Armonk, NY). TEG parameters R, angle, and MA had a normal distribution. Box-Cox–guided power transformation was used for TEG variable LY30 (λ = 0.25) to achieve normality, used for statistical analysis. Analysis of variance was used to compare groups with a post hoc Dunnett adjustment using whole blood mixed with tPA as the reference group. Dose effects of lysates (HL or PL) on TEG variables were evaluated with Spearman ρ correlation test. All statistical tests were two-tailed with significance declared at P < 0.05. Affinity capture experiments were filtered at a 0.1% false discovery rate. Peptide identifications were accepted if they could be established at greater than 95.0% probability as specified by the Peptide Prophet algorithm. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified unique peptides in the first set of experiments.
RESULTS
RBC lysate
Enhancement of tPA-mediated fibrinolysis
Whole blood mixed with 4% HL had comparable fibrinolysis compared with whole blood without tPA (P = 0.128). The addition of 75 ng/mL of tPA to whole blood without HL increased fibrinolysis from median LY30 of 1.4% (interquartile range [IQR], 0.9%–2.0%) to 9.0% (IQR, 6.5%–11.5%). In the sample containing 4% HL, the addition of 75 ng/mL of tPA increased fibrinolysis to 20.1% (IQR, 13.2%–32.1%), nearly three times the response to tPA seen in whole blood without HL (P = 0.020). The more modest dose of 2% HL in whole blood also statistically increased fibrinolytic activity of tPA compared with whole blood alone (P = 0.021). Enhancement of tPA-mediated fibrinolysis demonstrated a dose-dependent response to the concentration of added HL (R2 = 0.668, P < 0.001). Notably, even the highest level of enhancement of tPA-mediated fibrinolysis by 4% HL was completely inhibited by TXA, confirming that the observed effect is mediated by the plasmin system (Fig. 1A).
Fig. 1. A, Hemolyzed RBCs enhance tPA-mediated fibrinolysis.
WB indicates whole blood; %, percent lysate replacing whole blood. *P < 0.05 compared with tPA group. Box in upper left corner represents the Spearman ρ R2 value with associated P value. A demonstrates tPA enhancement of fibrinolysis by RBC lysate. The y axis represents the median percent fibrinolysis 30 min after clots reaches their MA. At 2% replacement of whole blood with lysed RBCs, there is an increase in fibrinolysis compared with whole blood with tPA alone. This enhancement had a positive correlation on fibrinolysis with increasing the percentage of platelet lysate. Red blood cells enhanced fibrinolysis is completely reversible with tranexamic acid. B demonstrates tPA inhibition of fibrinolysis by platelet lysate. The y axis represents the median percent fibrinolysis 30 min after clots reaches their MA. At 1% replacement of whole blood with platelet lysate, RBCs decreased fibrinolysis compared with whole blood with tPA alone. This inhibition had a negative correlation on fibrinolysis with increasing the percentage of platelet lysate.
A fast-forming clot prone to fibrinolysis
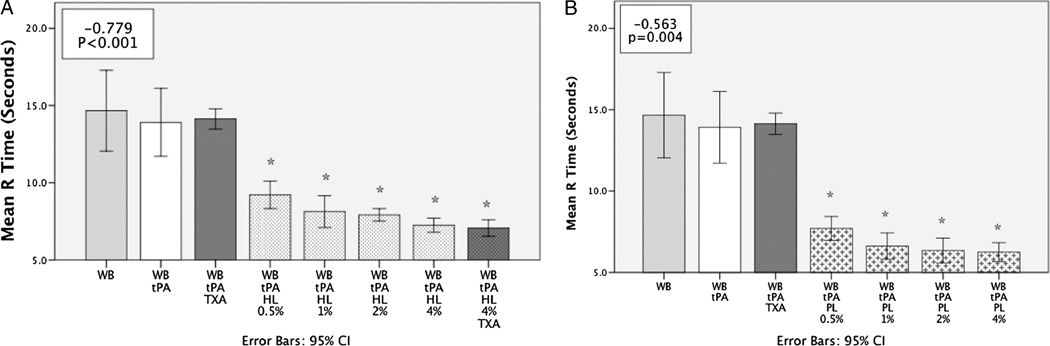
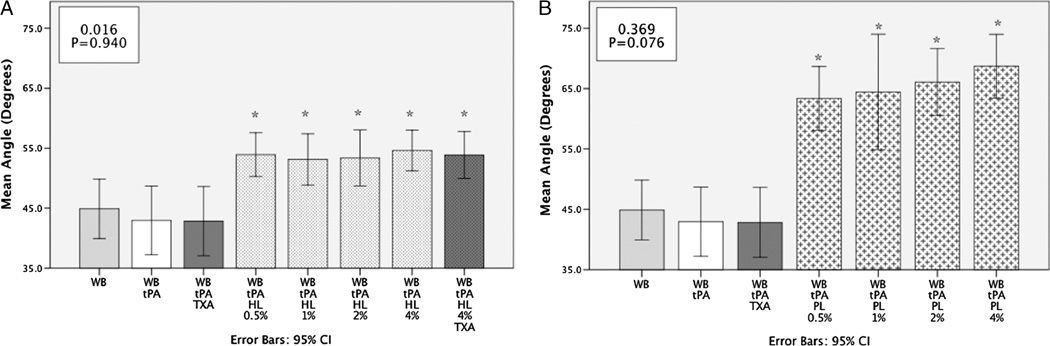
Whole blood with tPA had a longer clotting time (R) compared with 4% HL with tPA (13.9 s [95% CI, 11.7–16.1 s) vs 7.3 s [95% CI, 6.8–7.7 s], P < 0.001). The percent replacement of whole blood with HL had a strong negative correlation with clotting time (Fig. 2A). Angle was increased by HL (53.9 degrees [95% CI, 51.2–58 degrees] vs 43.0 degrees [95% CI, 37.2–48.7 degrees]) but did not demonstrate a dose-response effect (Fig. 3). Whole blood with tPA had a similar clot strength (TEG MA) to 4% HL with tPA (54.4 mm [95% CI, 50.6–58.2 mm] vs 56.4 mm [95% CI, 51.7–61.1 mm]; P = 0.917), but with the addition of TXA clot strength was significantly higher (61.2 mm [95% CI, 58.8–64.1 mm]; P = 0.006). However, at the lowest concentration of HL tested (0.5%) without TXA, clot strength was increased (63.8 mm [95% CI, 60.1–67.4 mm]; P < 0.001). Increasing HL lysate concentration from this lower percentage had a negative correlation with clot strength (Fig. 4A).
Fig. 2. A, Red blood cell lysate reduces clotting time.
WB indicates whole blood; %, percent lysate replacing whole blood. *P < 0.05 compared with tPA group. Box in upper left corner represents the Spearman ρ R2 value with associated P value. A demonstrates that lysate of RBCs decreases clotting time represented by the TEG parameter R. The y axis represents the mean R time in seconds. This decrease in clotting time was significant at all percentage of whole blood replaced with HL. This reduction in R time had a negative correlation with increasing the percentage of HL. This was not TXA reversible. B, Platelet lysate inhibits tPA-mediated fibrinolysis. *P < 0.05 compared with tPA group. Box in upper left corner represent the Spearman ρ R2 value with associated P value. B, Platelet lysate reduces clotting time. Box in upper left corner represents the Spearman ρ R2 value with associated P value. B demonstrates that lysate of platelets decreases clotting time represented by the TEG parameter R. The y axis represents the mean R time in seconds. This decrease in clotting time was significant at all percentage of whole blood replaced with PL. This reduction in R time had a negative correlation with increasing the percentage of PL.
Fig. 3. A, Red blood cell lysate increases angle in non–dose-dependent manner.
WB indicates whole blood; %, percent lysate replacing whole blood. *P < 0.05 compared with tPA group. Box in upper left corner represents the Spearman ρ R2 value with associated P value. A demonstrates that HL increases the rate of clot strengthening measured by the TEG parameter Angle. The y axis represents the mean angle in degrees. All percentage of HL to whole blood significantly increased the angle compared whole blood and tPA. There was not a dose effect and was not reversible by TXA. B, Platelets lysate increases angle in dose-dependent manner. B demonstrates that lysate of platelet increases the rate of clot strengthening measured by the TEG parameter angle. The y axis represents the mean angle in degrees. Platelet lysate increases the angle at all concentrations compared with whole blood and had a dose-like relationship.
Fig. 4. A, Red blood cell lysate and clot strength.
WB indicates whole blood; %, percent lysate replacing whole blood. *P < 0.05 compared with tPA group. Box in upper left corner represent the Spearman ρ R2 value with associated P value. A demonstrates HL relationship with clot strength measured by the TEG parameter MA. The y axis represents the mean MA. At a low dose, HL significantly increases MA compared with whole blood with tPA. Increasing the percentage of HL to whole blood had a negative correlation with clot strength. This was reversible by TXA. B, Platelet lysate increases clot strength. B demonstrates that lysate of platelet increases clot strength measured by the TEG parameter MA. The y axis represents the mean MA. Platelet lysate significantly increases clot strength compared with control at all doses. There was a positive correlation with PL dose and clot strength.
Platelet lysate
Shutdown of tPA-mediated fibrinolysis
Whole blood mixed with 4% PL had comparable (minimal) fibrinolysis compared with whole blood and 4% HL (P = 0.128). Platelet lysate decreased tPA-mediated fibrinolysis to 0.0% (0.00–0.2; P = 0.028) compared with 9.0% (IQR, 6.5–11.5) for whole blood and tPA alone. Two percent PL in whole blood statistically decreased the fibrinolytic activity of exogenous tPA (75 ng/mL) compared with whole blood without PL (Fig. 1B; P < 0.002).
A fast-forming stronger clot resistant to fibrinolysis
Four percent PL in whole blood decreased clotting time in the presence of tPA compared with whole blood without PL (R) (6.3 [95% CI, 5.6–8.6] vs 13.9 [95% CI, 11.7–16.1]; P < 0.001). Angle was steeper in the presence of PL (68.7 degrees [95% CI, 63.4–74 degrees] vs 43.0 degrees [95% CI, 37.2–48.7 degrees]; P = 0.0001) and demonstrated a positive dose-response relationship with increasing concentration of PL (Fig. 3B). This relationship was also true with respect to MA and PL (Fig. 4B). The lowest dose of PL replacement (0.5%) was stronger than whole blood in the presence of tPA (MA 64.3 mm [95% CI, 60.7–67.8 mm] vs 54.4 mm [95% CI, 50.6 –58.2 mm]; P < 0.001).
Affinity chromatography
Red blood cell lysate enhances tPA-mediated fibrinolysis through plasminogen interaction
Passage of HL over a plasminogen affinity column depleted the fibrinolytic enhancement activity of HL by 87% compared with 58% for a mock column. This demonstrates that the observed fibrinolysis enhancement of HL is largely attributable to plasminogen ligands that can be removed by substrate-specific binding to plasminogen. Substrate-specific binding to tPA produced a lesser depletion of fibrinolytic enhancement activity (69% compared with 58% for a mock column). Proteins bound to plasminogen from HL identified by proteomics are listed in Table 1.
Table 1.
Platelet proteins captured by TPA affinity chromatography
| Identified proteins | Accession no. |
Molecular weight |
Peptides identified | ||
|---|---|---|---|---|---|
| TPA | Mock | ||||
| 1 | Tissue-type plasminogen activator* | P00750 | 63 kD | 108 | 0 |
| 2 | Peroxiredoxin 4 | Q13162 | 31 kD | 27 | 0 |
| 3 | Fibronectin | P02751 | 263 kD | 25 | 0 |
| 4 | ATP synthase subunit α | P25705 | 60 kD | 23 | 0 |
| 5 | Pleckstrin | P08567 | 40 kD | 21 | 0 |
| 6 | Multimerin 1 | Q13201 | 138 kD | 14 | 0 |
| 7 | Integrin β3 | P05106 | 87 kD | 13 | 0 |
| 8 | Coagulation factor V | P12259 | 252 kD | 13 | 0 |
| 9 | Calmodulin | P62158 | 17 kD | 12 | 0 |
| 10 | LIM and senescent cell antigen-like | P48059 | 37 kD | 11 | 0 |
| 11 | α-Enolase | P06733 | 47 kD | 18 | 4 |
| 12 | SH3 domain-binding glutamic acid-rich. | Q9H299 | 10 kD | 11 | 0 |
| 13 | P-selectin | P16109 | 91 kD | 8 | 0 |
| 14 | Prohibitin 2 | Q99623 | 33 kD | 6 | 0 |
Protein used for affinity chromatography that is released from resin during low-Ph buffer wash. Table 1 represents 14 proteins that bound tPA with higher affinity than mock resin. The proteins’ association number, molecular weight, and relative protein count bound to tPA vs mock bead are listed in rows.
Platelet lysate shut down tPA-mediated fibrinolysis through tPA interaction
Passage of PL over a tPA affinity column removed 99% of its antifibrinolytic activity compared with a 50% reduction in antifibrinolytic efficacy by passage over a mock column. Thus, PL’s antifibrinolytic function appears to be attributable to a direct interaction with tPA (Fig. 5). Proteins bound to tPA from PL identified by proteomics are listed in Table 1.
Fig. 5. Red blood cell lysate indirectly enhances tPA-mediated fibrinolysis versus platelet lysate directly shuts down tPA-mediated fibrinolysis.
HL indicates RBCs from AB donor; PL, pooled platelet lysate. The figure demonstrates the changes in activity of lysate after the use of affinity chromatography to deplete proteins that interact with tPA or plasminogen. The y axis represents the change in fibrinolytic activity between whole blood mixed with tPA. All samples were run with the same final concentration of tPA (75 ng/mL). Mock (underivatized agarose beads) columns showed expected changes in fibrinolytic activity, in which lysed RBCs increased tPA-mediated fibrinolysis, whereas lysed platelets inhibited fibrinolysis. Red blood cell lysate minimally decreased in activity after running through a tPA affinity column in which proteins binding to tPA were depleted. There was a more robust decrease in activity compared with the mock column when RBC lysates were run over a column capturing proteins that bind plasminogen. Red blood cell enhancement of tPA-mediated lysis works through a mechanism that does not require a direct tPA interaction. Conversely, platelet lysates depleted of proteins that bind tPA showed a reduction in fibrinolytic activity compared with the mock column, suggesting platelet lysate directly inhibits tPA-mediated fibrinolysis.
DISCUSSION
The fibrinolytic activity of exogenous tPA in whole blood can be altered in opposing directions by lysed RBCs or platelets. These are the most abundant circulating cells in the vasculature, and small amounts of intravascular lysis may be sufficient to alter the profibrinolytic/antifibrinolytic balance in injured patients. Release of intracellular components of these cells may serve as a danger signal to the coagulation system, as evidence by lysed RBCs and platelets shortening the clotting time (Fig. 2). However, these clots have dichotomous characteristics in the presence of tPA after clot formation. Hemolysis promotes a clot that is more prone to fibrinolysis, whereas plateolysis promotes a stronger clot resistant to fibrinolysis (Fig. 1, A and B). Both hemolysis-enhanced fibrinolysis and the shutdown of fibrinolysis provoked by plateolysis are observed at concentrations of intracellular contents and tPA that are physiologically relevant.
A major product of lysed RBCs is fragmented phospholipid membrane. Exposure of the cytosolic surface puts the circulating blood and endothelium in contact with a higher concentration of phosphatidylserine than the normal contact with the extracellular surface of the RBC membrane. Increasing the surface area of phospholipid with phosphatidylserine in the presence of factor Xa is known to promote the generation of an intermediate thrombin product, meizothrombin, from prothrombin, which increases thrombin generation by five orders of magnitude (11). Meizothrombin production is accelerated in hemolytic states such as sickle cell disease (12), which is well known for being associated with a hypercoagulable state. We observed that the R time, which correlates to clot initiation, in the presence of lysed RBCs was reduced in a dose-dependent fashion (Fig. 2A). It has been previously shown that the addition of RBC microparticles increases thrombin generation (13), which is likely through this mechanism. Perhaps this mechanism serves as an early activator of coagulation to warn the body of impending danger, as the RBC is the body’s transporter of oxygen. Delivery of oxygen to ischemic environments predisposes RBCs to reactive oxygen species, leading to cell membrane instability, protein modification, and cellular lysis (14, 15). It has recently been shown that RBCs during oxidative stress will flip phosphatidylserine to the extracellular membrane representing a precursor event to eryptosis (16).
Platelet lysate also caused a reduction in clotting time in a dose-dependent fashion (Fig. 2B). This was expected, as platelet granules are rich in procoagulant molecules that also recruit and activate platelets (17). However, this change in platelet lysate clot dynamics was of interest as recent studies have shown that the manner in which platelets are activated can alter clot dynamics. For example, at the site of vascular injury on the vessel wall, platelets form a tightly adherent core that is surrounded by a shell of loosely bound platelets (18). The platelets in the core have degranulated and express p-selectin, whereas peripheral platelets are not fully activated and readily separate from the clot. Interestingly, ADP activation of platelets can promote a stable clot that is resistant to deaggregation in the absence of fibrinogen if the ADP activation level is sufficient to cause platelet degranulation (19). The importance of the fibrinogen free clot is the protective environment between platelets, which allow procoagulants and antifibrinolytic proteins to accumulate (20). From our data, it appears that platelet lysate promotes this environment in which platelets are activated to a high degree represented by an increase in angle and clot strength compared with whole blood stimulated by lysed RBCs (Fig. 2, A and B).
Red blood cell lysate forms a weaker clot compared with platelet lysate (Fig. 4A vs 4B). Unlike platelet lysate, HL had a negative dose relationship to clot strength in the presence of tPA. This progressive loss in clot strength was reversible with TXA at the highest concentration and may represent a process of increased fibrinolysis. The MA was the only other TEG value aside from LY30 that statistically changed when TXA was added to whole blood with tPA (Figs. 1A and 4A). These weaker clots may be the result of decreased platelet activation resulting in less degranulation and resulting in a weaker clot, which is susceptible to clot degradation. However, additional intracellular components of RBCs may enhance fibrinolysis. Affinity chromatography using plasminogen as a target demonstrated less fibrinolytic activity compared with the control column and column containing tPA. Plasminogen is known to bind many different cell types and receptors (21). Affinity chromatography using plasminogen bound heat shock protein 70 (HSP70) from our RBC lysate (Table 2). Heat shock protein 70 can induce a hyperfibrinolytic activity in the presence of endothelial cells by interactions with plasminogen and urokinase (22). Heat shock protein 70 has also been shown to stimulate tPA synthesis (23). Heat shock protein 70 plasma levels after trauma correlate with mortality, and patients who died of their head injury had a 14-fold increase in HSP70 levels compared with control subjects (24). We could not find prior publications measuring both HSP70 levels and coagulation in trauma patients. Table 2 provides an additional list of proteins, which may be involved in RBC lysate exacerbation of fibrinolysis.
Table 2.
Erythrocyte proteins captured by plasminogen affinity chromatography
| Peptides identified | |||||
|---|---|---|---|---|---|
| Identified proteins | Accession no. | Molecular weight | PLG | Mock | |
| 1 | Heat shock 70-kD protein 4 | P34932 | 94 kD | 30 | 0 |
| 2 | Translin-associated protein X | Q99598 | 33 kD | 23 | 0 |
| 2 | Translin | Q15631 | 26 kD | 11 | 0 |
| 4 | T-complex protein 1 subunit γ | P49368 | 61 kD | 19 | 0 |
| 5 | 26S proteasome non-ATPase regulatory subunit 3 | O43242 | 61 kD | 16 | 0 |
| 6 | Ubiquitin carboxyl-terminal hydrolase 14 | P54578 | 56 kD | 10 | 0 |
| 7 | Putative deoxyribose-phosphate aldolase | Q9Y315 | 35 kD | 10 | 0 |
| 8 | Plasminogen* | P00747 | 91 kD | 346 | 13 |
| 9 | Tropomodulin 1 | P28289 | 41 kD | 9 | 0 |
| 10 | Histidine triad nucleotide-binding protein 1 | P49773 | 14 kD | 7 | 0 |
| 11 | Protein DDI1 homolog 2 | Q5TDH0 | 45 kD | 7 | 0 |
| 12 | COP9 signalosome complex subunit 4 | Q9BT78 | 46 kD | 7 | 0 |
Protein used for affinity chromatography that is released from resin during low-Ph buffer wash. Table 2 represents 12 proteins that bound plasminogen with higher affinity than mock resin. The proteins’ association number, molecular weight, and relative protein count bound to tPA vs mock bead are listed in rows.
Platelet lysate affinity chromatography, on the other hand, suggested tPA interacted with inhibitors of fibrinolysis (Fig. 5). Fibronectin, such as PAI-1, is stored in α granules, which undergo secretion during platelet activation (25). Increasing plasma concentrations of this protein are associated with increased thrombin generation (26) and can alter the way fibrin polymerizes (27), making fibrin clot resistant to fibrinolysis. We were surprised that coagulation factor V interacted with tPA. Factor V is a particularly interesting protein. The mutant form of factor V (Leiden) is resistant to thombomodulin-mediated activation of protein C degradation and has been implicated in fibrinolysis resistance (28). The mechanism of this resistance to fibrinolysis for resilient factor V is proposed to be through enhanced factor XIII activation (29) and increased antiplasmin incorporation into the fibrin clot (30). Factor V from platelet lysate promotes a stronger clot resistant to fibrinolysis as supported by TEG data (Figs. 1B, 4B). We were unable to identify direct inhibitors of tPA from our proteomic analysis.
Our study is limited to in vitro whole blood from healthy volunteers. This is a response of normal whole blood. Acutely after injury, platelets can become dysfunctional (31) and plasma proteins can increase with antifibrinolytics (32). It is unclear if cell lysate may promote or exacerbate this event. Park et al. (9) demonstrated that trauma patients have a wide variability in cell lysis early after injury via analysis of microparticles. Similar to our bench work, these data support that cell lysis promotes thrombin generation. This group identified a correlation between microparticles and thrombin generation, but did not evaluate fibrinolysis. Our recent study that evaluated severely injured patients showed distinct phenotypes based on fibrinolytic activity that correlated to mortality rate and cause of mortality (8). However, from this clinical study, it was unclear why patients with similar degrees of oxygen debt and similar injury severity could have vastly different levels of fibrinolysis. The evaluation of microparticles and measurement of intracellular proteins concurrently in this patient population could be the missing variable to explain phenotypic differences. Our exploratory proteomic work has also provided a list of candidate proteins in attempt to understand the mechanism for differences in fibrinolytic activity. Our lack of identification of direct tPA and plasmin inhibitors could be due to a single run from pooled platelet donors and unit of AB blood unit, and we can expect this list to continue to grow with the active investigation of the spectrum of fibrinolysis after injury from our group and other investigators.
From this bench work, we have identified that currently unmeasured clinical parameters of hemolysis and platelet lysis modulate the tPA-mediated fibrinolytic system. Cellular lysis from injury may be one of the pathologic drivers leading to an abnormal fibrinolytic response to shock. It has been known for more than 50 years that fibrinolysis has an important physiologic function (33), and more than 13 years since a phase I clinical trial demonstrated fibrinolysis enhancement improves organ function on ventilated trauma patients in the intensive care unit (34). The Miami group recently observed that TXA increases mortality after major trauma (35). While we await the results of prospective trials in the United States, this is an early warning of the potential hazards of the medical shutdown of fibrinolysis after severe injury. Targeting medications to regulate fibrinolysis to a low physiologic range has clinical relevance and likely is superior to blocking plasmin. Understanding the role of cellular lysis and trauma-induced coagulopathy may provide insight to understanding the dysfunction of fibrinolysis in response to injury and shock and create opportunities for therapeutic intervention for to balance fibrinolysis during resuscitation of trauma patients.
Acknowledgments
This study was supported in part by National Institute of Health grants T32-GM008315 (National Institute of General Medical Sciences [NIGMS]), P50-GM0492221 (NIGMS), and UM 1HL120877 (National Heart, Lung, and Blood Institute), and Colorado Clinical Translational Science Institute supported in part by Colorado Clinical and Translational Science Award grant UL1 TR001082 from NCATS. Additional research support was provided by Haemonetics (Inc).
Footnotes
This manuscript was selected as one of the New Investigator Nominees at the 37th Annual Conference on Shock, held in Charlotte, North Carolina, June 7Y10, 2014.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors have no conflicts of interest.
REFERENCES
- 1.Chakrabarti R, Hocking ED, Fearnley GR. Reaction pattern to three stresses—electroplexy, surgery, and myocardial infarction—of fibrinolysis and plasma fibrinogen. J Clin Pathol. 1969;22:659–662. doi: 10.1136/jcp.22.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 3.Kutcher ME, Ferguson AR, Cohen MJ. A principal component analysis of coagulation after trauma. J Trauma Acute Care Surg. 2013;74:1223–1229. doi: 10.1097/TA.0b013e31828b7fa1. discussion 1229–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schochl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. discussion 370. [DOI] [PubMed] [Google Scholar]
- 5.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75:961–967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schochl H, Frietsch T, Pavelka M, Jambor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2013;67:125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 7.Hardaway RM. Disseminated intravascular coagulation with special reference to shock and its treatment. Mil Med. 1965;130:451–460. [PubMed] [Google Scholar]
- 8.Moore HBME, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, physiologic fibrinolysis, fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and the relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014 Jul 21; doi: 10.1097/TA.0000000000000341. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MS, Owen BA, Ballinger BA, Sarr MG, Schiller HJ, et al. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831–836. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327–337. doi: 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bovill EG, Tracy RP, Hayes TE, Jenny RJ, Bhushan FH, et al. Evidence that meizothrombin is an intermediate product in the clotting of whole blood. Arterioscler Thromb Vasc Biol. 1995;15:754–758. doi: 10.1161/01.atv.15.6.754. [DOI] [PubMed] [Google Scholar]
- 12.Whelihan MF, Mooberry MJ, Zachary V, Bradford RL, Ataga KI, et al. The contribution of red blood cells to thrombin generation in sickle cell disease: meizothrombin generation on sickled red blood cells. J Thromb Haemost. 2013;11:2187–2189. doi: 10.1111/jth.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jy W, Johansen ME, Bidot C, Jr, Horstman LL, Ahn YS. Red cell-derived micro-particles (RMP) as haemostatic agent. Thromb Haemost. 2013;110:751–760. doi: 10.1160/TH12-12-0941. [DOI] [PubMed] [Google Scholar]
- 14.Amer J, Weiss L, Reich S, Shapira MY, Slavin S, et al. The oxidative status of blood cells in a murine model of graft-versus-host disease. Ann Hematol. 2007;86:753–758. doi: 10.1007/s00277-007-0321-7. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S, Zingde SM, Gokhale SM. Identification of human erythrocyte cytosolic proteins associated with plasma membrane during thermal stress. J Membr Biol. 2013;246:591–607. doi: 10.1007/s00232-013-9569-0. [DOI] [PubMed] [Google Scholar]
- 16.Lang F, Abed M, Lang E, Foller M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014;21:138–153. doi: 10.1089/ars.2013.5747. [DOI] [PubMed] [Google Scholar]
- 17.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 18.Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattaneo M, Kinlough-Rathbone RL, Lecchi A, Bevilacqua C, Packham MA, et al. Fibrinogen-independent aggregation and deaggregation of human platelets: studies in two afibrinogenemic patients. Blood. 1987;70:221–226. [PubMed] [Google Scholar]
- 20.Brass LF, Zhu L, Stalker TJ. Minding the gaps to promote thrombus growth and stability. J Clin Invest. 2005;115:3385–3392. doi: 10.1172/JCI26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plow EF, Doeuvre L, Das R. So many plasminogen receptors: why? J Biomed Biotechnol. 2012;2012:141806. doi: 10.1155/2012/141806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukao H, Hagiya Y, Ueshima S, Okada K, Takaishi T, et al. Effect of heat shock on the expression of urokinase-type plasminogen activator receptor in human umbilical vein endothelial cells. Thromb Haemost. 1996;75:352–358. [PubMed] [Google Scholar]
- 23.Bendig MM, Stephens PE, Cockett MI, Hentschel CC. Mouse cell lines that use heat shock promoters to regulate the expression of tissue plasminogen activator. DNA. 1987;6:343–352. doi: 10.1089/dna.1987.6.343. [DOI] [PubMed] [Google Scholar]
- 24.da Rocha AB, Zanoni C, de Freitas GR, Andre C, Himelfarb S, et al. Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J Neurotrauma. 2005;22:966–977. doi: 10.1089/neu.2005.22.966. [DOI] [PubMed] [Google Scholar]
- 25.Cho J, Mosher DF. Role of fibronectin assembly in platelet thrombus formation. J Thromb Haemost. 2006;4:1461–1469. doi: 10.1111/j.1538-7836.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 26.Cho J, Mosher DF. Enhancement of thrombogenesis by plasma fibronectin cross-linked to fibrin and assembled in platelet thrombi. Blood. 2006;107:3555–3563. doi: 10.1182/blood-2005-10-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramanathan A, Karuri N. Fibronectin alters the rate of formation and structure of the fibrin matrix. Biochem Biophys Res Commun. 2014;443:395–399. doi: 10.1016/j.bbrc.2013.11.090. [DOI] [PubMed] [Google Scholar]
- 28.Brugge JM, Simioni P, Bernardi F, Tormene D, Lunghi B, et al. Expression of the normal factor V allele modulates the APC resistance phenotype in heterozygous carriers of the factor V Leiden mutation. J Thromb Haemost. 2005;3:2695–2702. doi: 10.1111/j.1538-7836.2005.01634.x. [DOI] [PubMed] [Google Scholar]
- 29.Koncz Z, Bagoly Z, Haramura G, Mezei ZA, Muszbek L. Thrombomodulin-dependent effect of factor V Leiden mutation on factor XIII activation. Thromb Res. 2012;129:508–513. doi: 10.1016/j.thromres.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Koncz Z, Bagoly Z, Haramura G, Mezei ZA, Muszbek L. Thrombomodulin-dependent effect of factor V Leiden mutation on the cross-linking of alpha2-plasmin inhibitor to fibrin and its consequences on fibrinolysis. Thromb Res. 2012;130:528–534. doi: 10.1016/j.thromres.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassis J, Hirsh J, Podor TJ. Evidence that postoperative fibrinolytic shutdown is mediated by plasma factors that stimulate endothelial cell type I plasminogen activator inhibitor biosynthesis. Blood. 1992;80:1758–1764. [PubMed] [Google Scholar]
- 33.Fearnley GR. Fibrinolysis. Ann R Coll Surg Engl. 1967;41:51–54. [PMC free article] [PubMed] [Google Scholar]
- 34.Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, et al. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67:377–382. [PubMed] [Google Scholar]
- 35.Valle EJ, Allen CJ, Van Haren RM, Jouria JM, Li H, et al. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg. 2014;76:1373–1378. doi: 10.1097/TA.0000000000000242. [DOI] [PubMed] [Google Scholar]