Abstract
Background
In this two-site randomized trial, we investigated the effect of antiseptic drain care on bacterial colonization of surgical drains and infection after immediate prosthetic breast reconstruction.
Methods
With IRB approval, we randomized patients undergoing bilateral mastectomy and reconstruction to drain antisepsis (treatment) for one side, with standard drain care (control) for the other. Antisepsis care included both: 1) chlorhexidine disc dressing at drain exit site(s), and 2) irrigation of drain bulbs twice daily with dilute sodium hypochlorite solution. Cultures were obtained from bulb fluid at one week and at drain removal, and from the subcutaneous drain tubing at removal. Positive cultures were defined as ≥1+ growth for fluid and >50 CFU for tubing.
Results
Cultures of drain bulb fluid at one week (the primary endpoint) were positive in 9.9% of treatment sides (10/101) versus 20.8% (21/101) of control sides (p=0.02). Drain tubing cultures were positive in 0 treated drains versus 6.2% (6/97) of control drains (p=0.03). Surgical site infection occurred within 30 days in 0 antisepsis sides versus 3.8% (4/104) of control sides (p = 0.13), and within 1 year in 3/104 (2.9%) of antisepsis sides versus 6/104 (5.8%) of control sides (p = 0.45). Clinical infection occurred within one year in 9.7% (6/62) of colonized sides (tubing or fluid) versus 1.5% (2/136) of non-colonized sides (p = 0.03).
Conclusions
Simple and inexpensive local antiseptic interventions with a chlorhexidine disc and hypochlorite solution reduce bacterial colonization of drains, and reduced drain colonization was associated with fewer infections.
Introduction
Surgical site infection (SSI) rates after mastectomy with immediate prosthetic reconstruction are overall low, in the range of 5%.1, 2 However, SSI in this setting is often devastating, with the majority resulting in implant loss.1, 2 Obesity and smoking are well-known risk factors for SSI in breast surgery;1, 3, 4 surgical drains and prolonged use are also associated with increased infection risk.5–7
We have previously shown that antiseptic treatment of surgical drains after mastectomy without reconstruction reduces bacterial colonization.8 In that study, women undergoing immediate breast reconstruction were excluded due to common utilization of prolonged postoperative antibiotics, which might impact bacterial colonization in drains. Considering the risk of implant loss associated with SSI, we performed a prospective, surgeon-blinded, randomized controlled trial to assess effects of simple local antisepsis measures on bacterial colonization of drains and SSI after mastectomy with immediate prosthetic breast reconstruction.
Methods
Study population
Following Institutional Review Board approval at both Mayo Clinic and University of California San Francisco, eligible subjects were recruited prospectively from the breast surgical practices between May 2011 and June 2013. Individuals were included if undergoing bilateral mastectomy with reconstruction (either tissue expander or implant) for benign or malignant disease. Ineligibility criteria included: pregnancy, antibiotics within 14 days of surgery, history of breast/chest wall radiation, allergy to chlorhexidine, or autologous tissue reconstruction. A data safety monitoring board reviewed progress and adverse events.
Randomization
Because a paired study design was used (only bilateral procedures), each subject served as her own control. Randomization assigned which side (right or left) would receive the antisepsis interventions, and the contralateral side received standard drain care. Randomization included cancer versus prophylaxis as a stratification factor, in order to balance the proportion of breasts with cancer between the two treatments.
Perioperative standardization
All subjects received weight-based IV antibiotics within 30 minutes of incision, with appropriate intraoperative redosing. ChloraPrep® (CareFusion Corporation, San Diego, CA) skin prep was used for all subjects. Oral antibiotics were continued postoperatively until drains were removed. Showers were encouraged after 48 hours.
Drain care regimens
Study subjects and family members received personal instruction on drain care on the first postoperative day by the study coordinator and were advised to keep the surgical team blinded to interventions. Drain care instructions are previously described, with antisepsis measures including a chlorhexidine disc- Biopatch® (Ethicon, Inc., Somerville, NJ) application to drain sites every three days and twice daily irrigation of the drainage bulb with dilute Dakin's solution.8 Dakin's concentration was 0.0125% buffered sodium hypochlorite for the first 44 subjects and commercially available 0.125% for the remaining 60 subjects due to ease in procurement. All drains on a surgical side were treated per assigned study arm.
Follow-up visits and cultures
A standardized data collection form was completed at follow-up visits, assessing drainage volume, erythema/skin changes, evidence of infection, and compliance with interventions. Study coordinators removed dressings to maintain surgeon blinding. Patients returned for a mandatory clinical visit and culture of drain bulb fluid at approximately one week [postoperative day (POD) 6–10]. Drains were removed when clinically appropriate. On the day of drain removal, both subcutaneous drain tubing and bulb fluid were obtained aseptically for cultures, as previously described.8 Clinical infections were treated per routine clinical care. Information on late infections was captured with medical record review and telephone follow-up at one year.
Microbiology
Cultures were performed as previously described.8 Growth in bulb fluid was reported as: negative, broth only, or plate growth of 1+, 2+, 3+, 4+. Drain tubing isolates were reported semiquantitatively in colony forming units (CFU) as: <10, 10–19, 20–50, 51–100 or >100. All isolates were identified.
Endpoints and statistical power
The primary endpoint was bacterial growth (1+ or greater) in drainage bulb fluid at POD6-10. The initially planned sample size was 75 patients (150 breasts) to provide 80% power to detect a difference of 23% versus 7% colonization in drain bulb fluid at POD6-10 between antisepsis and control sides (two-sided McNemar's test of paired proportions, alpha=0.05). After a priori planned interim sample size re-estimation, targeted enrollment was increased to 97 patients (194 breasts). Sample size estimates were augmented by ~10% to allow for attrition.
Drain tubing colonization, defined as >50 CFU, was a secondary endpoint.8, 9 Given the potential limitation of using pre-specified cutoffs to define a positive culture, we also performed ordinal quantification analyses for both drain fluid and tubing cultures. In samples colonized with multiple organisms, the highest degree of quantification across all organisms was used to classify the sample for analysis. SSIs included any of the following within 365 days after operation: purulent drainage, positive aseptically collected culture from the wound, signs of inflammation with opening of incision and absence of a negative culture, or physician diagnosis of infection (which could include cellulitis). Cases of SSI were decided by consensus of the research team without knowledge of the treatment arm. Patients were censored for SSI at the time of additional breast surgical procedure (including tissue expander exchange).
Statistical Analysis
Analysis was modified intent-to-treat (ITT); patients who withdrew or were screen failures before study intervention were excluded from analysis. All patients who completed were included in the analysis. The primary analysis was a paired comparison of binary colonization and SSI endpoints between antisepsis and control sides within patient. A side was positive for colonization if any drain on that side was positive. Comparison of paired proportions was performed using McNemar's test or the exact sign test when there were < 20 discordant pairs.10 A secondary per-drain analysis was performed using generalized linear mixed effects models (binomial distribution, logit link) with a fixed effect for intervention (antisepsis or control) and random subject intercepts to account for multiple correlated drains within patient; per-drain models also allowed adjustment for factors that could vary among drains within patient. Analysis of the ordinal degree of colonization was performed using Wilcoxon signed-rank tests to compare maximum quantification between antisepsis and control side. Tests were two-sided with alpha=0.05 significance level. Analysis was performed using SAS (Version 9.3). Study data were collected and managed using REDCap electronic data capture tools.11
Results
Subjects
A total of 110 patients were enrolled and randomized. Six patients withdrew or were screen failures prior to intervention and were excluded from the modified ITT sample (N=104, Figure 1); 101 (97%) had data on the primary endpoint. Most subjects had unilateral cancer (69%), with 8% bilateral cancer and 23% without cancer. Acellular dermal matrix was utilized in 74% of subjects. The paired study design ensured that patient-specific factors (e.g., BMI) were the same for control and antisepsis sides, while randomization resulted in balance across side-specific factors, including acellular dermal matrix (Table 1).
Figure 1.
CONSORT diagram
Table 1.
Patient and clinical characteristics in n = 104 patients in the modified ITT analysis.
| Patient-specific factors | N = 104 |
|---|---|
|
| |
| Age, years, median (range) | 46 (25–67) |
|
| |
| BMI, median (range) | 23.8 (17–45.1) |
|
| |
| ASA class | |
| I | 19 (18.3%) |
| II | 77 (74.0%) |
| III | 8 (7.7%) |
|
| |
| Operative time, hours, median (range) | 5.1 (2.9–9.3) |
|
| |
| Smoking within 4 weeks preop1, n (%) | 4 (3.9%) |
|
| |
| Diabetes, n (%) | 3 (2.9%) |
|
| |
| Neoadjuvant chemotherapy, n (%) | 32 (30.8%) |
|
| |
| Indication for surgery, n (%) | |
| Unilateral cancer with CPM | 72 (69.2%) |
| Bilateral prophylactic mastectomy | 24 (23.1%) |
| Bilateral cancer | 8 (7.7%) |
|
| |
| Type of preoperative antibiotic, n (%) | |
| Cefazolin | 92 (88.5%) |
| Clindamycin | 6 (5.8%) |
| Levofloxacin | 3 (2.9%) |
| Vancomycin | 3 (2.9%) |
| Side-specific factors | Antisepsis | Control |
|---|---|---|
|
| ||
| N = 104 | N = 104 | |
|
| ||
| Type of operation, n (%) | ||
| Mastectomy only | 58 (55.8%) | 61 (58.7%) |
| Mastectomy+SLNB | 35 (33.7%) | 32 (30.8%) |
| Mastectomy+ALND | 11 (10.6%) | 11 (10.6%) |
|
| ||
| Type of mastectomy, n (%) | ||
| Skin-sparing Mastectomy | 37 (35.6%) | 36 (34.6%) |
| Nipple-sparing Mastectomy | 67 (64.4%) | 68 (65.4%) |
|
| ||
| Indication for mastectomy, n (%) | ||
| Cancer | 46 (44.2%) | 42 (40.4%) |
| Risk-reducing | 58 (55.8%) | 62 (59.6%) |
|
| ||
| Number of drains, n (%) | ||
| 1 | 52 (50.0%) | 54 (51.9%) |
| 2 | 47 (45.2%) | 42 (40.4%) |
| 3 | 5 (4.8%) | 8 (7.7%) |
|
| ||
| Type of reconstruction, n (%) | ||
| Tissue expander | 95 (91.4%) | 95 (91.4%) |
| Direct-to-implant | 9 (8.7%) | 9 (8.7%) |
|
| ||
| Acellular dermal matrix used, n (%) | 75 (72.1%) | 76 (73.1%) |
|
| ||
| Intraoperative fill volume2, mL, median (range) | 150 (0–800) | 150 (0–800) |
|
| ||
| Number of lymph nodes removed3, median (range) | 3 (1–33) | 4 (1–36) |
|
| ||
| Maximum4 drain duration, days, median (range) | 13 (6–50) | 13 (6–34) |
|
| ||
| Adjuvant radiation therapy5, n (%) | 7 (6.7%) | 8 (7.7%) |
Smoking within 4 weeks unknown in n = 1 patient.
Intraoperative fill volume available in n = 101 patients and missing in n = 3.
Among sides with either SLNB or ALND, n = 46 antisepsis and n = 43 control.
Maximum duration across all drains on a given side.
Adjuvant radiation therapy within one year but prior to tissue expander exchange.
ALND= axillary lymph node dissection, SLNB= sentinel lymph node biopsy
Colonization of drain fluid
Cultures of drain bulb fluid at one week showed significantly less bacterial colonization from antisepsis sides compared to control sides (Table 2). Using the cutoff of ≥1+ growth for drain fluid, fewer antisepsis sides were positive (10/101=10%), compared to 21% (21/101) of control sides (p=0.02). The per drain analysis showed similar results where 11/157 antisepsis drains (7%) showed colonization compared to 25/160 control drains (16%), p=0.02 in both adjusted and unadjusted analysis. Analysis of the maximum ordinal quantification of bacterial growth at one week showed 85%, 5%, 8%, and 2% of antisepsis sides in categories of no growth, broth only, 1+/2+ growth, and 3+/4+ growth, respectively, as compared to 71%, 8%, 19%, and 2% on control sides (p=0.009).
Table 2.
Outcome comparisons between antisepsis and control sides.
| Antisepsis | Control | p-value3 | |
|---|---|---|---|
|
| |||
| Per patient comparison between sides | |||
|
| |||
| Primary endpoint | |||
|
| |||
| Drain bulb fluid colonization at POD 6-101 | 9.9% (10/101) | 20.8% (21/101) | 0.02 |
|
| |||
| Secondary endpoints | |||
|
| |||
| Drain tubing colonization at removal | 0% (0/97) | 6.2% (6/97) | 0.03 |
|
| |||
| Drain bulb fluid colonization at removal2 | 19.4% (14/72) | 38.9% (28/72) | 0.003 |
|
| |||
| Surgical site infection within 30 days | 0 | 3.8% (4/104) | 0.13 |
|
| |||
| Surgical site infection within 1 year | 2.9% (3/104) | 5.8% (6/104) | 0.45 |
| Antisepsis | Control | |||
|---|---|---|---|---|
|
| ||||
| Per drain analysis | p-value4 | |||
|
| ||||
| Primary endpoint | Unadjusted | Adjusted5 | ||
|
| ||||
| Drain bulb fluid colonization at POD6-101 | 7.0% (11/157) | 15.6% (25/160) | 0.02 | 0.02 |
|
| ||||
| Secondary endpoints | ||||
|
| ||||
| Drain tubing colonization at removal | 0% (0/151) | 3.9% (6/154) | 0.0046 | N/A |
|
| ||||
| Drain bulb fluid colonization at removal2 | 16.5% (14/85) | 37.5% (33/88) | 0.003 | 0.003 |
POD6-10 was the per protocol timeframe for the approximately 1 week culture; although 94% of visits occurred within this protocol range, the actual visit dates ranged from POD4-11.
Reported here only in those where drain removal was later than primary endpoint collection.
P-value from McNemar's test for paired proportions or the exact sign test.
P-value from generalized linear mixed effects model accounting for correlation among multiple drains from the same patient.
Adjusted for side- and drain-specific variables: indication (cancer or prophylaxis), operation (mastectomy only, mastectomy+SLNB, mastectomy+ALND), and drain duration.
Due to zero events in the antisepsis arm for this endpoint, p-value was derived from likelihood-ratio test comparing the intercept only model to the model with intercept and treatment side included.
From drains removed after the first visit (n = 173), a second culture was obtained at drain removal (median 14 days, range: 9–50). Later cultures showed more colonization than POD6-10 cultures for both antisepsis (16% vs 6%, p=0.02) and control sides (38% vs 16%, p=0.0003), and antisepsis sides had persistently less colonization than controls after POD6-10 (16% vs 38%, p=0.003). The mid-study modification to use commercially available Dakin's solution did not affect bulb fluid colonization at POD6-10: 9.5% with dilute Dakin's versus 10.2% with commercially available Dakin's (p=0.91).
Colonization of drain tubing
Among 97 subjects with drain tubing cultures, colonization (>50 CFU) was significantly reduced with antisepsis treatment. No antisepsis-treated drains had a positive tubing culture, compared to 6.2% of control sides (per patient analysis) or 3.9% of control drains (per drain analysis), p-values 0.03 and 0.004 respectively. Treating maximum degree of colonization as an ordinal variable also showed a significant difference between antisepsis and control sides at POD6-10 (p=0.0006), with frequencies as follows for CFU categories of 0, 1–19, 20–50, 51–100, and >100: 86%, 11%, 3%, 0%, and 0% for antisepsis sides, and 69%, 23%, 2%, 4%, and 2% for control sides.
Colonization and drain duration
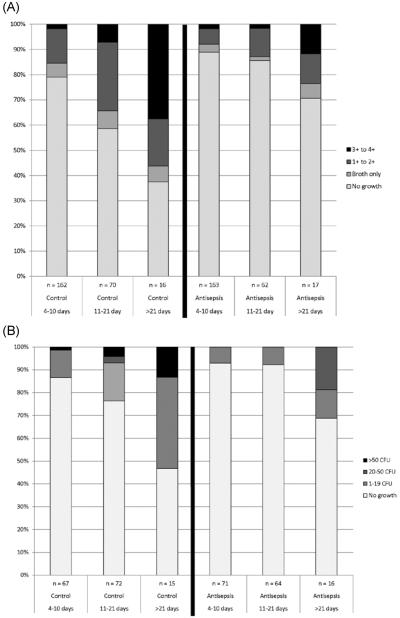
Bacterial colonization increased in frequency and degree with longer drain duration, both for bulb fluid and drain tubing (Figure 2). Increased colonization over time was observed for both control and antisepsis sides, but antisepsis sides demonstrated less frequency and degree of colonization at all timepoints compared to control sides.
Figure 2.
Ordinal quantification of bacterial growth over time for A) 490 drain bulb fluid cultures, and B) 305 drain tubing cultures.
Surgical site infections
SSI involved 9 sides in 8 subjects with one year of follow-up; 4 occurred within 30 days. SSIs were less frequent in the 104 antisepsis-treated sides compared to 104 control sides, both within 30 days (0% vs 3.8%) and within one year (2.9% vs 5.8%), although not statistically significant and limited by the small number of events.(Table 2) The four infected sides within 30 days were all control sides; 3 were limited to cellulitis and the fourth was a deep infection requiring tissue expander (TE) removal.(Table 3) Of these four with early SSI, two had colonization of both fluid and tubing, one had colonization of tubing only, and one had colonization of fluid but was missing tubing cultures. Five additional SSIs (3 antisepsis side, 2 control side) in four patients were observed within 365 days (but prior to any censoring second surgery); four were deep SSIs requiring expander removal.
Table 3.
Details of surgical site infections per operated side within one year of index operation (includes only SSI cases that occurred before a secondary operation such as tissue expander/ implant exchange).
| Pt # | SSI side | POD of SSI onset | Severity of SSI | # of Drains on side of SSI |
Study Bulb Fluid Culture Results at “One Week (POD 6-10) and at Drain Removal | POD of Drain Removal and Study Tubing Culture Results | Clinical Culture Results & Comments | |
|---|---|---|---|---|---|---|---|---|
| Mastx | Axillary | |||||||
|
| ||||||||
| 1 | Control | POD 12 | Cellulitis | 1 | 0 | One week: 1+ CoNS | POD9: >100 CFU CoNS | Not obtained; seen at outside hospital |
|
| ||||||||
| 2 | Control | POD 14 | Cellulitis | 2 | 0 | One week: No growth either drain POD14: Mastx Drain1 No growth POD17: Mastx Drain2 No growth |
POD14: Mastx Drain1 18 CFU CoNS POD17: Mastx Drain2 1 CFU CoNS |
Not obtained; cellulitis only |
|
| ||||||||
| 3 | Control | POD 14 | Cellulitis | 2 | 0 | One week: Mastx Drain 1 1 + Stomatococcus mucilaginosus VGS with unknown quantification |
Cultures missed due to protocol deviation | Not obtained; cellulitis only |
| One week: Mastx Drain 2 Broth only GPB (not Clostridium or Propionibacterium species) | ||||||||
|
| ||||||||
| 4 | Control | POD 30 | Deep SSI requiring TE removal | 2 | 0 | One week: No growth either drain | ||
| POD26: Mastx Drain1 4+ Pseudomonas stutzeri 1+ CoNS |
POD26: Mastx Drain1 51–100 CFU GPB Mycobacterium fortuitum with unknown quantification |
POD30 abscess fluid M fortuitum | ||||||
| POD29: Mastx Drain2 4+ Pseudomonas stutzeri 4+ Acinetobacter species 2+ Enterococcus species |
POD29: Mastx Drain2 No growth |
POD42 TE explant M fortuitum | ||||||
|
| ||||||||
| 5 | Antisepsis | POD 34 | Cellulitis | 1 | 0 | N/A – Patient's drain fell out | N/A – Patient's drain fell out | Not obtained; cellulitis only |
|
| ||||||||
| 6 | Antisepsis | POD 89 | Deep SSI requiring TE removal | 2 | 1 | POD4: Mastx Drain1 No growth One week: No growth Mastx Drain2 or Axillary Drain POD23: Mastx Drain2 No growth POD29: Axillary Drain No growth |
POD4: Mastx Drain1 No growth POD23: Mastx Drain2 No growth POD29: Axillary Drain No growth |
POD89 Cultures: 2+ MSSA (breast swab) 3+ MSSA (breast tissue) 1+ MSSA (deep breast tissue) |
|
| ||||||||
| 7 | Antisepsis | POD 165 | Deep SSI requiring TE removal | 2 | 0 | One week (POD7): No growth either drain | POD7: Mastx Drain2 No growth | Not obtained |
| POD9: Mastx Drain1 1 + Propionibacterium acnes | POD9: Mastx Drain1 No growth | |||||||
|
| ||||||||
| 7 | Control | POD 165 | Deep SSI requiring TE removal | 2 | 1 | One week (POD7): No growth any drain POD13: Axillary drain 2+ VGS 1+ CoNS POD16: Mastx Drain2 1+ CoNS 1+ Staphylococcus pasteuri 1+ Citrobacter freundii complex 1+ GPB |
POD7: Mastx Drain1 1 CFU nonsporeforming GPB 1 CFU Streptococcus mitis POD13: Axillary drain No growth POD16: Mastx Drain2 No growth |
Not obtained; SSI occurred after PMRT |
|
| ||||||||
| 8 | Control | POD 230 | Deep SSI requiring TE removal | 1 | 0 | One week: 1+ VGS POD22: Mastx Drain 3+ Enterococcus species 2+ Corynebacterium species 2+ Staphylococcus haemolyticus 1+ Acinetobacter species |
POD22: Mastx Drain No growth | POD231: Breast swab No growth POD232: Breast fluid 1 colony MSSA Breast capsule 1 colony MSSA SSI occurred after PMRT |
Abbreviations: CoNS—coagulase-negative Staphylococcus species; GPB—gram-positive bacillus; Mastx—mastectomy; MSSA-methicillin sensitive Staphylococcus aureus; PMRT—post-mastectomy radiotherapy; POD—postoperative day; SSI – surgical site infection, VGS–viridans group Streptococcus species
Correlation of SSI and drain colonization
Sides with colonization (per predetermined cutoffs) of either tubing or bulb fluid showed an SSI rate of 9.7% (6/62) at one year, compared to 1.5% (2/136) on sides without colonization of bulb fluid or tubing (p=0.03), indicating that bacterial colonization of drain sites is significantly associated with infection. This analysis excluded one SSI in a patient without study cultures (drain fell out before POD6-10). Correlation of organisms at colonization and infection was limited, with clinical infection cultures available in only 3 of 9 SSIs.(Table 3) In these three cases, the organism matched study drain culture results in one, which grew Mycobacterium fortuitum in drain tubing at removal (POD26) and the same organism in breast abscess culture (POD30) and in expander explant (POD42). The other two infections with positive clinical cultures occurred after POD30 (both methicillin-susceptible Staphylococcus aureus).
Microbiology of colonization
Microbial isolates are listed in Supplemental Table 1. The most common organism type was coagulase negative Staphylococcus species (43% of fluid cultures and 55% of tubing cultures).
Intervention toxicity and compliance
Contact dermatitis attributable to chlorhexidine disc was observed in 7/104 patients (6.7%, 95% CI: 3.3–13.2%); all resolved after discontinuing the disc (subsequent to primary endpoint collection in each case). Compliance with the protocol was generally excellent with 95/104 (91%) patients having no protocol deviations deemed substantial enough to affect the primary endpoint. The nine substantial protocol deviations included: non-compliance >1 day with any part of study protocol (n=7) and drain inadvertently came out before POD6-10 culture (n=2).
Discussion
In this study, local antiseptic measures significantly reduced bacterial colonization of surgical drains after mastectomy with implant reconstruction. Bacterial colonization of drains was linked to clinical infection, with significantly fewer SSIs in sides without colonization. This study was not powered to show a difference in SSI, however fewer SSIs occurred in sides treated with antisepsis measures, both within 30 days and one year, although these differences were not statistically significant. A prior study has also demonstrated bacterial colonization of drain fluid after mastectomy, with 33% of drains colonized at one week, and strong concordance of microorganisms across colonization and infection cultures.12 Overall, these findings strongly implicate a causal relationship between SSI and bacterial colonization of drains, as well as an opportunity for SSI reduction, in women undergoing implant reconstruction.
SSI prevention has gained national attention due to its cost and morbidity. Infection rates after mastectomy and implant reconstruction range from 5–19% in recent literature, high for what is considered a “clean” case and calling for improvement.1, 2, 13–15 Our findings were similar: 3.8% within 30 days and 7.7% within one year. Although a 5% SSI rate may appear low, the potential result of expander removal due to infection is devastating to the patient. Intravenous catheter related infections are also rare but are reduced with use of a chlorhexidine disc dressing.16, 17 Some cellulitis cases after implant reconstruction may resolve with treatment, but they can predispose to subsequent explantation (ie reconstruction failure). In a study of 1952 implant-based reconstructions, cellulitis occurred in 5% of patients, and 75% of cellulitis cases required explantation.1 Therefore, even small reductions in SSI are valuable if achievable at acceptable cost. Based on drain numbers and duration in our study, median drain antisepsis costs were $108 per side. If antisepsis reduces SSI by half, then 40 breast reconstructions would require treatment (estimated cost of $4320) to prevent one SSI. The attributable cost of SSI after mastectomy has been estimated as $4091,13 an underestimate as it did not include the substantial cost of salvage reconstruction. Thus cost-effectiveness of antisepsis measures seems likely but remains unproven.
One strategy commonly utilized to reduce SSI after implant reconstruction is prolonged use of postoperative antibiotics,18, 19 but this strategy is unproven and has other risks.20, 21 In contrast, drain antisepsis side effects are infrequent and self-limiting. In comparing the present study to our similar prior study of mastectomy without reconstruction, the key differences are the placement of a prosthetic device and use of postoperative antibiotics. With prolonged antibiotics in the current study, fewer control sides showed colonization at the one week timepoint (21%) compared to the prior study (65%), suggesting antibiotics may reduce colonization. However, antisepsis-treated sides had >2-fold reduced colonization in both studies. Also, colonization of both fluid and tubing increased with longer drain duration in both studies, underscoring the importance of removing drains at the earliest possible time. These findings are consistent with prior breast surgery investigations confirming increased infection risk with surgical drains and prolonged duration.5–7
In summary, we found that local antisepsis using a chlorhexidine disc to each drain site and drain bulb irrigation with Dakin's after mastectomy and implant reconstruction reduces bacterial colonization of drain bulb fluid and tubing. Reduced colonization of drains was associated with decreased frequency of SSI, demonstrating that local antisepsis has potential to reduce infections. The interventions are simple, have little toxicity, and can be adopted after mastectomy with implant reconstruction to lower bacterial colonization. A larger study with SSI as the primary endpoint is needed to confirm efficacy of drain antisepsis toward SSI reduction, its cost-effectiveness, and effects of each individual component (Biopatch® and Dakin's irrigation).
Supplementary Material
Acknowledgements
Funds for this study were provided by Ethicon, Inc. This project was also supported by NIH Center for Translational Science Activities grant support (UL1 TR000135). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Sincere thanks to Jill Randolph R.Ph. and Sue McCluskey R.Ph. for preparation of dilute Dakin's solution, Lisa M. Nyre, Sherry M. Ihde, and David T. Lynch in Microbiology, and to Marilyn Churchward for assistance with manuscript preparation. Most importantly, this study was made possible by the individuals who elected to participate in this research study.
ClinicalTrials.gov Identifier: NCT 01286168
References
- 1.Reish RG, Damjanovic B, Austen WG, Jr, et al. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plast Reconstr Surg. 2013;131(6):1223–30. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- 2.Leyngold MM, Stutman RL, Khiabani KT, et al. Contributing variables to post mastectomy tissue expander infection. Br J. 2012;18(4):351–6. doi: 10.1111/j.1524-4741.2012.01253.x. [DOI] [PubMed] [Google Scholar]
- 3.Olsen MA, Lefta M, Dietz JR, et al. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207(3):326–35. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Blacam C, Ogunleye AA, Momoh AO, et al. High body mass index and smoking predict morbidity in breast cancer surgery: a multivariate analysis of 26,988 patients from the national surgical quality improvement program database. Ann Surg. 2012;255(3):551–5. doi: 10.1097/SLA.0b013e318246c294. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Compte D, Jacquemin B, Robles-Vidal C, et al. Surgical site infections in breast surgery: case-control study. World J Surg. 2004;28(3):242–6. doi: 10.1007/s00268-003-7193-3. [DOI] [PubMed] [Google Scholar]
- 6.Rey JE, Gardner SM, Cushing RD. Determinants of surgical site infection after breast biopsy. Am J Infect Control. 2005;33(2):126–9. doi: 10.1016/j.ajic.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Rotstein C, Ferguson R, Cummings KM, et al. Determinants of clean surgical wound infections for breast procedures at an oncology center. Infect Control Hosp Epidemiol. 1992;13(4):207–14. doi: 10.1086/646511. [DOI] [PubMed] [Google Scholar]
- 8.Degnim AC, Scow JS, Hoskin TL, et al. Randomized controlled trial to reduce bacterial colonization of surgical drains after breast and axillary operations (in press) Ann Surg. 2013 doi: 10.1097/SLA.0b013e31828c0b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977;296(23):1305–9. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 10.Rosner B. Fundamentals of Biostatistics. 4th Edition Wadsworth Publishing Company; Belmont: 1995. [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomet Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felippe WA, Werneck GL, Santoro-Lopes G. Surgical site infection among women discharged with a drain in situ after breast cancer surgery. World J Surg. 2007;31(12):2293–9. doi: 10.1007/s00268-007-9248-3. discussion 2300-1. [DOI] [PubMed] [Google Scholar]
- 13.Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143(1):53–60. doi: 10.1001/archsurg.2007.11. discussion 61. [DOI] [PubMed] [Google Scholar]
- 14.Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249(1):26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 15.Peled AW, Foster RD, Garwood ER, et al. The effects of acellular dermal matrix in expander-implant breast reconstruction after total skin-sparing mastectomy: results of a prospective practice improvement study. Plast Reconstr Surg. 2012;129(6):901e–908e. doi: 10.1097/PRS.0b013e31824ec447. [DOI] [PubMed] [Google Scholar]
- 16.Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–41. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 17.Levy I, Katz J, Solter E, et al. Chlorhexidine-impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. Pediatr Infect Dis J. 2005;24(8):676–9. doi: 10.1097/01.inf.0000172934.98865.14. [DOI] [PubMed] [Google Scholar]
- 18.Brahmbhatt RD, Huebner M, Scow JS, et al. National practice patterns in preoperative and postoperative antibiotic prophylaxis in breast procedures requiring drains: Survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips BT, Wang ED, Mirrer J, et al. Current practice among plastic surgeons of antibiotic prophylaxis and closed-suction drains in breast reconstruction: experience, evidence, and implications for postoperative care. Ann Plast Surg. 2011;66(5):460–5. doi: 10.1097/SAP.0b013e31820c0593. [DOI] [PubMed] [Google Scholar]
- 20.Landes G, Harris PG, Lemaine V, et al. Prevention of surgical site infection and appropriateness of antibiotic prescribing habits in plastic surgery. J Plast Reconstr Aesthet Sur. 2008;61(11):1347–56. doi: 10.1016/j.bjps.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Throckmorton AD, Hoskin T, Boostrom SY, et al. Complications associated with postoperative antibiotic prophylaxis after breast surgery. Am J Surg. 2009;198(4):553–6. doi: 10.1016/j.amjsurg.2009.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.