Highlights
-
•
Heterogeneity limits our ability to identify the underlying neurobiology of ADHD.
-
•
We used neuroimaging data and community detection to identify subgroups of children.
-
•
We identified three subgroups of children with and without ADHD.
-
•
Atypical connections in ADHD were specific to subgroup membership.
-
•
Differentiation in subgroups is related to delay discounting and activity level.
Keywords: Attention deficit hyperactivity disorder, Community detection, Delay discounting, Nucleus accumbens, Functional connectivity, RDoC
Abstract
One potential obstacle limiting our ability to clarify ADHD etiology is the heterogeneity within the disorder, as well as in typical samples. In this study, we utilized a community detection approach on 106 children with and without ADHD (aged 7–12 years), in order to identify potential subgroups of participants based on the connectivity of the reward system. Children with ADHD were compared to typically developing children within each identified community, aiming to find the community-specific ADHD characteristics. Furthermore, to assess how the organization in subgroups relates to behavior, we evaluated delay-discounting gradient and impulsivity-related temperament traits within each community. We found that discrete subgroups were identified that characterized distinct connectivity profiles in the reward system. Importantly, which connections were atypical in ADHD relative to the control children were specific to the community membership. Our findings showed that children with ADHD and typically developing children could be classified into distinct subgroups according to brain functional connectivity. Results also suggested that the differentiation in “functional” subgroups is related to specific behavioral characteristics, in this case impulsivity. Thus, combining neuroimaging data and community detection might be a valuable approach to elucidate heterogeneity in ADHD etiology and examine ADHD neurobiology.
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent and persistent neurodevelopmental disorder, characterized by excessive behavioral inattention/disorganization and/or hyperactivity-impulsivity (American Psychiatric Association, 2013). The disorder is frequently associated with several comorbid disorders, functional impairment and poor long-term outcomes (Dopheide and Pliszka, 2009, Rommelse et al., 2009). However, despite substantial progress in understanding the related brain systems, small effect sizes and variability of associations with neurobiological correlates limit clinical utility.
1.1. Heterogeneity in ADHD
One obstacle likely limiting our ability to identify the underlying etiology of ADHD is the heterogeneity of the disorder. ADHD subtypes, or presentation specifiers, as defined by the DSM (DSM-IV and DSM-5) are an attempt to deal with clinical heterogeneity; however, even within the same subtype, variations in clinical presentation are remarkable, and variations in etiology and pathophysiology are expected (Fair et al., 2012, Karalunas et al., 2015, Nigg et al., 2005). A major obstacle to applying neurobiological findings to clinical practice is likely related to ineffective separation of patients into appropriate mechanistic subtypes (i.e., diagnostic categories do not reflect biological changes) (Hyman, 2007, Kapur et al., 2012). Considering this problem, the National Institutes of Mental Health launched the Research Domain Criteria (RDoC), a project that proposes a new way of classifying psychopathology, not based on DSM symptom criteria, but instead, based on observable behaviors, genetic traits and neurobiological measures (Insel et al., 2010). This framework may be better suited to clarifying the neurobiology of atypical behaviors, such as impulsivity and other types of ADHD-related behaviors, and provides important context for the current report.
1.2. Reward system impairment in ADHD
Impairment in reward processing is hypothesized as one core dysfunction in ADHD (Nigg, 2005, Sonuga-Barke, 2005). Importantly, neuroimaging studies related to impaired reward processing have found that the nucleus accumbens (NAcc), a key region of the reward system, exhibits atypical functioning or connectivity in individuals with ADHD (Costa Dias et al., 2012, Furukawa et al., 2014, Plichta et al., 2009, Plichta and Scheres, 2014, Scheres et al., 2007, Tomasi and Volkow, 2012). We recently assessed the functional connectivity of the NAcc in children with and without ADHD and found that, on average, in ADHD, NAcc was atypically connected to regions of the default network, cortical regions involved in control processes, posterior insula, and thalamus (Costa Dias et al., 2012). However, only a specific subset of connections (NAcc to anterior PFC and to ventromedial PFC) were related to impulsive decision-making – as measured by delay discounting – in ADHD. Just as importantly, these connections were not necessarily atypical across the entire ADHD population. These findings highlight the current need of using innovative methods to identify biologically based subtypes to assess heterogeneity in ADHD.
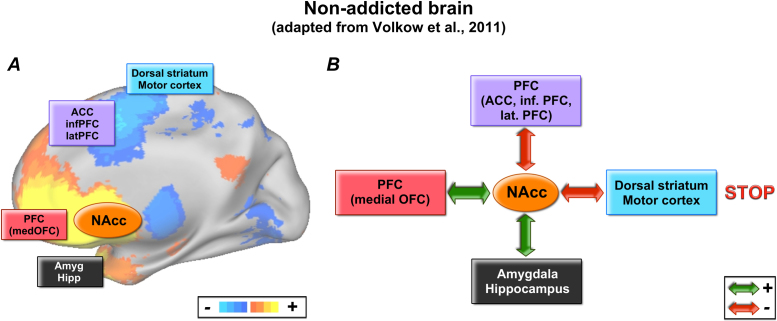
Volkow et al. (2011a) recently proposed a model for impulsive behavior (or atypical sensitivity and reward response) (Fig. 1). The model is presented in the context of addiction, but provides a framework for which to consider our prior findings. It readily applies to ADHD because children with ADHD are at elevated risk of addiction, and both addiction and ADHD have been strongly associated with dysfunction in ascending dopaminergic systems that relate to reward response (Volkow et al., 2009, Volkow et al., 2011a, Volkow et al., 2011b).
Fig. 1.
Model of network underlying impulsive decision-making. Volkow et al., 2011a, Volkow et al., 2011b postulated that multiple networks interact to provide inhibitory control and decision-making. Drug addiction is associated with a disturbance of this system, which may also be involved in other types of impulsive decision-making.
The model is adapted from Volkow et al., 2011a, Volkow et al., 2011b.
At the core, the model illustrates how several unique subcortical and cortical links with the NAcc can contribute to impulsive behavior. The NAcc interacts with conditioning, executive control and motivation systems to regulate decision-making. An imbalance of these interactions results in impaired inhibitory control and, subsequently, a “Go” (instead of a normative “Stop”) response. There are two principles of this model that may assist investigators in characterizing heterogeneity in ADHD. The first principle suggests that there are multiple pathways (i.e. atypical connections) that can lead to the same atypical phenotype (i.e. GO response). For instance, Subject A with ADHD may have typical connections between the NAcc and prefrontal cortex and amygdala, but have an atypical connection between the NAcc and the dorsal striatum/motor cortex, which could lead to impulsive decision-making. Along the same lines, Subject B with ADHD may have atypical connectivity between the accumbens and prefrontal cortex and normative connectivity with the other circuits, and display impulsive decision-making. This would mean that despite differences in the underlying mechanisms, Subject B would be indistinguishable from Subject A in terms of surface behaviors and would hence receive the same clinical diagnosis using current criteria.
The second principle the model highlights is that normative behavior can be equally heterogeneous. In other words, as long as balance is maintained in the system, multiple normative profiles may exist within a typically developing group or atypically developing group. The question is, how might one discern or characterize: (a) whether such heterogeneity exists and (b) to what extent it informs the nature of ADHD?
1.3. Graph theory in combination with rs-fcMRI
Numerous methods exist for mathematically clustering or identifying more homogenous subgroups. One particularly attractive approach derives from graph theory. It is based on the principle that many systems of scientific interest can be represented as networks, which are sets of nodes or vertices joined in pairs by lines or edges. Graph theory has been used to examine the organization of numerous networks including the social networks, metabolic networks, communication lines, distribution networks and brain networks (Bullmore and Sporns, 2009, Newman, 2003). Importantly, most networks have well defined internal structures and can be described or demarcated with graph theoretical analyses (Boccaletti et al., 2006). One area of interest, receiving considerable attention, is the detection and characterization of community structure in networks. Community structure refers to the appearance of densely connected groups of nodes, with only sparse connections between the groups (Newman, 2006). The ability to detect such groups has been of significant practical importance for understanding the nature of complex systems. For example, groups within social networks might correspond to social cliques (see Newman, 2006); or groups of ADHD subjects with similar behavioral or brain properties may group to form specific mechanistic subtypes or neurotypes.
Three recent reports from our team have highlighted the use of community detection as a way to characterize heterogeneity in ADHD (Fair et al., 2012, Gates et al., 2014, Karalunas et al., 2015). Fair and colleagues (Fair et al., 2012) used graph theory and community detection to examine whether a dataset of children with and without ADHD were organized into subtypes (or communities) according to their performance in various neuropsychological domains. The neuropsychological battery included tests measuring the following processes: inhibition, working memory, arousal/activation, response variability, temporal information processing, memory span, and processing speed. The study found that both groups (children with ADHD and typically developing children) could be classified into unique, but similar neuropsychological profiles. Furthermore, children with ADHD from each profile showed atypical scores in very specific neuropsychological measures. Karalunas et al. (2015) applied the same method to classify childhood ADHD based on temperament dimensions. The study found that the sample was organized into communities, which were associated with specific patterns of physiological response, brain connectivity of the amygdala, and, most importantly, long-term outcomes. Gates et al. (2014) utilized simulated imaging data, along with empirical resting-state functional connectivity of the fronto-parietal network to validate the use of community detection in identifying subgroups. Findings from these studies suggest that identifying mechanistic subpopulations across ADHD and typically developing children may inform heterogeneity and the clinical course of a given child.
In this report, we used resting-state functional connectivity MRI (rs-fcMRI) in combination with graph theory to categorize individuals and to inform heterogeneity of the underlying reward circuitry in ADHD and typically developing populations. The aims were: (1) to examine whether distinct subgroups in a sample of children with and without ADHD could be determined based on functional connectivity patterns of the brain reward system; (2) to evaluate whether the categorization into neurotypes was related to behavior in terms of reward valuation and impulsivity.
2. Methods
2.1. Participants and measures
The study included 106 children, aged 7–12 years, with and without ADHD (63 controls, 42 children with ADHD, 1 child with subthreshold ADHD). Children were recruited from families who volunteered in response to mass mailings in the community. Their diagnostic grouping was carefully evaluated in best-estimate, multi-stage case finding procedure that included parent clinical interview using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-E) (Orvaschel, 1995), and parent and teacher standardized rating scales including the Conners’ Rating Scale, 3rd edition (Conners, 2008), ADHD Rating Scale (ADHD-RS, DuPaul et al., 1998), and Strengths and Difficulties Questionnaire (Goodman, 1997). Intelligence was estimated with a three-subtest short form (Block Design, Vocabulary, and Information) of the Wechsler Intelligence Scale for Children, 4th edition (Wechsler, 2003), and academic achievement with word reading and numerical operations subtests of the Wechsler Individual Achievement test. A best-estimate diagnostic team reviewed this information, as well as IQ, academic scores, and observer notes, and independently assigned a diagnosis. Their agreement on ADHD/non-ADHD status was acceptable (k > 0.85 for all diagnoses occurring at base rate > 5% in the sample, including ADHD and ADHD subtype). Disagreements that could not be resolved by discussion would lead to exclusion, but in this study consensus could be achieved in each case, thus no subject had to be excluded for this reason.
Children were excluded if they did not meet criteria for ADHD or non-ADHD groups; if they had evidence of tic disorder, psychotic disorder, bipolar disorder, autism spectrum disorder, or mental retardation; if parent reported history of neurological illness, chronic medical problems, sensorimotor handicap, or significant head trauma (with loss of consciousness); or if they were taking psychotropic medications other than psychostimulants. Children were also excluded if they presented metal in their bodies, which could contra-indicate MRI acquisition or cause imaging artifacts (e.g. dental braces, intracranial aneurysm clips). Additional exclusion criteria for control children were: presence of conduct disorder, major depressive disorder, or ADHD. Only right-handed children were included in the study. Children prescribed psychostimulant medications were scanned after a minimum washout period of five half-lives (i.e. 24–48 h depending on the preparation).
The Human Investigation Review Board at Oregon Health & Science University approved the research. Written informed consent was obtained from respective parents and written assent was obtained from all child participants.
2.2. Impulsivity measures
We used the delay-discounting task to measure impulsive decision-making, and subscales of the Temperament in Middle Childhood Questionnaire to measure different dimensions of impulsivity-related temperament traits.
The delay-discounting task was applied, outside the magnet, to a subset of the sample (101 subjects). The task measures impulsive choice by evaluating the intolerance to delay-of-gratification, or delay discounting, proposed to be a core dysfunction of ADHD (Sagvolden et al., 1998, Sonuga-Barke, 2005). Children performed a computerized task as described in detail by Wilson et al. (Wilson et al., 2011). The task consisted of questions for which the children had to choose between smaller amounts of hypothetical money now and hypothetical $10.00 after a varying delay (Costa Dias et al., 2012, Wilson et al., 2011). Details on the task are provided in the Supplementary Material.
The outcome variable was the discounting gradient (k). k represents the rate of discounting of the delayed outcome and was calculated for each subject as described by Mitchell (1999) and Wilson et al. (2011). Larger k values indicate greater preference for immediate rewards. As k values are not normally distributed, a natural-log transformation was applied and the transformed values (ln(k)) were used for the analyses. Data from 11 participants had to be excluded because of two exclusion criteria: (a) negative k values, which makes it impossible to perform the log-transformations and (b) data deemed unsystematic (i.e. the data pattern indicated the action of processes other than reward/delay evaluation), which was identified as described by Johnson and Bickel (2008). In summary, 90 participants (54 controls, 36 children with ADHD) were included in the analysis of delay discounting.
A parent or legal guardian completed the Temperament in Middle Childhood Questionnaire (TMCQ), which was designed to evaluate temperament in children and consists of 16 subscales (Simonds and Rothbart, 2004). We analyzed scores from four subscales, which evaluate traits related to impulsivity/hyperactivity. The subscales analyzed were: impulsivity (speed of response initiation), inhibitory control (capacity to plan and suppress inappropriate approach response), activity level (motor activity), and activation control (capacity to perform an action that one would tend to avoid).
2.3. MRI acquisition
MRI was acquired using a 3.0 T Siemens Magnetom Tim Trio scanner with a twelve-channel head-coil at the OHSU Advanced Imaging Research Center. We collected one high resolution T1-weighted MPRAGE sequence and BOLD-weighted functional imaging data. The T1-weighted sequence lasted 9 min and 14 s, and had the following parameters: TR = 2300 ms, TE = 3.58 ms, sagittal orientation, 256 × 256 matrix, resolution = 1 mm3. We obtained three runs of BOLD-weighted functional imaging data, of 3.5 min each. Functional data were collected at rest, in an oblique plane (parallel to anterior commissure-posterior commissure plane), using T2*-weighted echo-planar imaging, with: TR = 2500 ms, TE = 30 ms, 90° flip angle, FOV = 240 mm, 36 slices covering the whole brain, slice thickness = 3.8 mm, in-plane resolution = 3.8 mm2. We assumed steady state magnetization after five frames (∼10 s). During resting periods participants were instructed to stay still and fixate their gaze on a standard fixation-cross in the center of the display.
2.4. Imaging processing and movement attenuation
Functional images were preprocessed to reduce artifacts (Miezin et al., 2000). The preprocessing steps included: (i) removal of a central spike caused by MR signal offset, (ii) correction of odd vs. even slice intensity differences attributable to interleaved acquisition without gaps, (iii) correction for head movement within and across runs, and (iv) within-run intensity normalization to a whole brain mode value of 1000. Atlas transformation of the functional data was computed for each individual via the MPRAGE scan. Each run was then resampled in atlas space (Talairach and Tournoux, 1988), combining movement correction and atlas transformation in one interpolation. All subsequent operations were performed on the atlas-transformed volumetric time series.
Connectivity preprocessing followed prior methods, well described and widely used in the imaging literature (Fair et al., 2007, Fair et al., 2008, Fair et al., 2009, Fox et al., 2005). These steps included: (i) a temporal band-pass filter (f < 0.1 Hz), (ii) regression of six parameters obtained by rigid body head motion correction, (iii) regression of the whole brain signal averaged over the whole brain, (iv) regression of ventricular signal averaged from ventricular region of interest (ROI), and (v) regression of white matter signal averaged from white matter ROIs. Regression of first order derivative terms for the whole brain, ventricular, and white matter signals were also included in the correlation preprocessing, and all regressors were bandpass-filtered to the same frequency domain as the original band-pass filter (Hallquist et al., 2013). These preprocessing steps aim to reduce spurious variance unlikely to reflect neuronal activity (Fox and Raichle, 2007).
In this study, we combined two methods for addressing motion. First, as just noted, we applied the traditional motion correction method, which corrects/quantifies head movement using an analysis of head position based on rigid body translation and rotation, relative to a reference frame. We used six motion parameters (along with their first order derivatives) as regressors in preprocessing to remove potential motion-related artifact. Total root mean square (RMS) values were also calculated on a run-by-run basis for each participant. As an initial screen, participants’ BOLD runs with movement exceeding 1.5 mm RMS were not included in the analysis, and participants with two or more runs with excess movement were excluded. This procedure was followed by “scrubbing”, based on framewise displacement (FD), as introduced by Power et al. (2012). FD measures movement in any given frame relative to the previous frame, instead of relative to the reference frame. We used a method of volume censoring, which consisted of removing frames based on the magnitude of FD (Power et al., 2012). We appropriately accounted for potential temporal blurring of the motion-related artifact during the bandpass filter (Power et al., 2012). Details on how the motion correction methods were applied are discussed in the Supplementary Material.
2.5. Region of interest definition
The reward region of interest was obtained using an automated brain-mapping meta-analysis platform (Yarkoni et al., 2011). This platform (NeuroSynth) allows researchers to use terms of interest to identify related activation areas, based on automatically generated maps. Two types of maps may be generated: (a) forward inference maps and (b) reverse inference maps. Forward inference maps show the probability of activation given the presence of the term. Reverse inference maps show the probability of the term given the presence of the activation (Yarkoni et al., 2011). Because we were interested in the brain region most selectively (not just consistently) associated with reward, we used the reverse inference map for the term reward. Peak regions were extracted from the map and the region with highest probability was used as region of interest (Talairach coordinates: −9 +7 −6), which corresponded to the left nucleus accumbens.
2.6. Identification of subgroups using community detection
For each participant the resting BOLD time series for the reward region of interest (ROI) was correlated with all other voxels in the brain, generating voxelwise functional connectivity maps. We computed within-group t-tests to all connections to generate a functional connectivity map for the group of 106 subjects. A high threshold (Z > 10 and Z > −10) was applied to the group map, creating a mask that was applied to each individual's connectivity map, in order to include in the analysis only the voxels that were, on average, strongly connected to the ROI. The z-transformed correlation coefficients in the masked maps were aligned into one-dimensional matrices (i.e. vectors), one per participant.
Community detection analysis was then performed in two steps. First, we used the connectivity vectors to compute correlation coefficients between all possible pairs of participants. This step generated a square matrix (106 × 106) providing distance information (i.e. correlation) between any given subject pair. Second, the community detection algorithm was applied to the distance matrix. The threshold for connected vs. unconnected pairs was based on the maximum threshold where reachability remained equal to 1. Reachability equal to 1 is the maximum threshold where every subject is connected via at least one path to every other subject (no isolates). Thus, the graphs remain sparse, but fully connected (i.e., there are no isolated individuals lacking in any connections). The reachability threshold was at r = 0.52. We ran the community detection analysis both on the full group and on the ADHD and control populations separately, in order to determine whether ADHD status altered the identified community structure.
In order to verify robustness of the community structure, we examined variation of information (VOI). This method consists of randomizing the edges of a network with a probability α of perturbing it, and provides the VOI between the original and perturbed networks over a range of α (Karrer et al., 2008). VOI indicates how much information differs between the two sets of community assignments and varies from 0 (identical community assignments) to 1 (completely different community assignments). The process was repeated 20 times and was also applied on a random network with equivalent degree.
The community detection analysis and related calculations were performed in MATLAB (Mathworks), using scripts provided by Rubinov and Sporns (2010).
2.7. Direct comparison between control and ADHD children
After identifying the communities in the network of children, we performed a direct comparison of connectivity maps between groups (i.e. ADHD and Control) within each community for the reward ROI (−9 +7 −6, left NAcc). We performed two-sample two-tailed t-tests on all potential connections (Fisher z-transformed r-values) to compare ADHD children and controls within each community (unequal variance; p < 0.05). The voxelwise approach provides three different maps for the seed region for each community: an ADHD map, a control map, and a map representing the direct comparison between the groups. To account for multiple comparisons, thresholding based on Monte Carlo simulation was implemented (Forman et al., 1995). To obtain multiple comparisons corrected, p < 0.05 voxel clusters, at threshold of 53 contiguous voxels with a Z-value >2.25, were used.
The same type of analysis was implemented in the entire sample to examine whether differences between controls and children with ADHD were related to the community organization of the sample, that is, whether the atypical connection in ADHD children was specific to the etiological subgroup/neurotype.
2.8. Association between community structure and impulsivity
To test the hypothesis that children in different subgroups exhibit different levels of impulsivity, we compared the discounting gradient (ln(k)) between controls and ADHD children within the identified subgroups, and also scores from the impulsivity-related subscales of the TMCQ. We performed independent sample t-test between groups, and generated boxplots to display the results. We tested whether ln(k) was correlated with other variables and found that it was correlated with age (R = −0.258, p = 0.014), but in this sample it was not correlated with IQ (R = −0.113, p = 0.29) or movement (mean FD of remaining frames [R = 0.028, p = 0.79]). Therefore, we decided to control for age when comparing ln(k) between ADHD and controls.
We also performed ANOVA to compare the subgroups of children with ADHD and the subgroups of controls.
2.9. Association between community structure and clinical outcomes
To evaluate whether community organization was associated with specific clinical characteristics, we compared data on comorbidities, psychostimulant use, ADHD subtype, and ADHD-RS scores (from parent about child) between different subgroups of ADHD children (and different subgroups of controls, when applicable). We performed chi-square test, and ANOVA whenever indicated.
2.10. Mapping findings onto current network definitions
In order to identify which networks were atypically connected to the NAcc in each subgroup of children, we defined brain networks in a sample of adults, using community detection, and used them to identify the systems in which differences between groups coalesced. We used community detection in a separate cohort to define specific brain networks as in Power et al. (2011). Thirty-two adults, aged 19–35 (19 females; average age: 26.5 years), with no psychiatric disorder, underwent an MRI scan at the OHSU Advanced Imaging Research Center. Acquisition parameters can be viewed in Supplementary Material.
Community detection was performed on the preprocessed resting-state data, by way of the Infomap algorithm (Rosvall and Bergstrom, 2008). In this analysis, the aim was to verify whether the network of brain regions was organized into communities and whether the identified communities corresponded to known brain networks. In this analysis, nodes were defined as voxels in the brain (as opposed to subjects), and edges as the connections between pairs of voxels (as opposed to correlations between pairs of subjects). The brain communities identified corresponded to known brain networks (brain communities found in this analysis will be referred to as “brain networks” henceforth). For details on how the analysis was performed, see Supplementary Material and Figure S.1.
In order to identify which networks were atypically connected to the NAcc in each subgroup of children, the maps generated from the Infomap analysis were overlapped with the comparison maps. We generated peak ROIs from the Monte Carlo corrected difference maps, including only voxels that had a z-score above 2.5 (or below −2.5). The produced ROIs were pre-blurred 4 mm FWHM, with peaks at least 10 mm apart (peaks within 10 mm were consolidated). The comparison peaks were then overlapped with the map generated from the Infomap analysis, and were color-coded according to the network with which they had the most overlapping voxels.
3. Results
3.1. Comparing controls and ADHD
Participant characteristics and sample overview are summarized in Table 1. We first examined differences in connectivity across the entire sample (Fig. 2). We found that in both groups the reward region (left NAcc) was strongly functionally connected to the medial prefrontal cortex (PFC; medial frontal gyrus) and temporal lobe bilaterally (middle temporal gyrus). Those regions are part of the default network, a network implicated with self-referential processes, such as daydreaming, past recollection and future planning (Buckner et al., 2008). Also, in both groups, the reward region was negatively connected to the anterior cingulate cortex (ACC), dorso-lateral PFC (middle frontal gyrus), inferior parietal lobule, occipital lobe (lingual gyrus) and thalamus. Those regions have been described as part of a network responsible for cognitive and attention processes (Dosenbach et al., 2007, Fox et al., 2005) (Fig. 2).
Table 1.
|
105 subjects – Group statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Controls |
ADHD |
p | ||||||
| Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | ||
| Age | 9.07 | 7.17 | 12.50 | 1.18 | 9.59 | 7.42 | 12.33 | 1.42 | 0.044a |
| IQ | 117.13 | 94.00 | 148.00 | 13.02 | 110.77 | 82.00 | 144.00 | 15.21 | 0.024a |
| Mean frame-to-frame displacement (FD)j | 0.12 | 0.06 | 0.18 | 0.03 | 0.12 | 0.06 | 0.17 | 0.03 | 0.944a |
| % frames removed | 25.59 | 0.00 | 59.58 | 18.78 | 25.98 | 0.00 | 56.88 | 19.02 | 0.918a |
| ln(k)k | −3.62 | −9.47 | 1.46 | 2.00 | −2.94 | −8.56 | 1.46 | 2.76 | 0.206b,* |
| Inattentive symptoms | 0.38 | 0 | 8 | 1.26 | 6.12 | 1 | 9 | 2.74 | <0.001b |
| Hyperactive/impulsive symptoms | 0.38 | 0 | 6 | 1.10 | 4.71 | 0 | 9 | 3.10 | <0.001b |
| TMCQ subscales | |||||||||
| Impulsivity | 2.40 | 1.46 | 3.92 | 0.56 | 3.58 | 2.00 | 5.00 | 0.76 | <0.001b |
| Inhibitory control | 3.76 | 2.25 | 4.63 | 0.48 | 2.70 | 1.13 | 3.63 | 0.61 | <0.001b |
| Activation control | 3.65 | 2.80 | 4.67 | 0.38 | 3.06 | 1.85 | 3.93 | 0.53 | <0.001b |
| Activity level | 3.88 | 2.00 | 5.00 | 0.64 | 4.10 | 2.56 | 5.00 | 0.66 | 0.001a |
| N | % | N | % | Sig. | |
|---|---|---|---|---|---|
| Gender | 0.015c | ||||
| Male | 31 | 49.2 | 31 | 73.8 | |
| Female | 32 | 50.8 | 11 | 26.2 | |
| Community | 0.088d | ||||
| A | 23 | 36.5 | 10 | 23.8 | |
| B | 29 | 46.0 | 17 | 40.5 | |
| C | 11 | 17.5 | 15 | 35.7 | |
| Comorbid diagnosis (at least one)p | 5 | 7.90 | 14 | 33.30 | 0.002e |
| Stimulant usel | – | – | 15 | 35.70 | – |
| ADHD subtype | – | ||||
| Combined | – | – | 27 | 64.3 | |
| Inattentive | – | – | 14 | 33.3 | |
| Hyperactive | – | – | 1 | 2.4 |
|
Subgroup A – Group statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Controls |
ADHD |
p | ||||||
| Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | ||
| Age | 8.85 | 7.42 | 11.17 | 0.98 | 8.32 | 7.42 | 9.08 | 0.62 | 0.121a |
| IQ | 119 | 96 | 144 | 15.07 | 110.80 | 90.00 | 132.00 | 15.24 | 0.142a |
| Mean frame-to-frame displacement (FD)j | 0.12 | 0.07 | 0.16 | 0.02 | 0.12 | 0.09 | 0.17 | 0.03 | 0.748a |
| % frames removed | 22.83 | 0.00 | 57.92 | 18.60 | 17.17 | 1.67 | 52.50 | 17.03 | 0.417a |
| ln(k)m | −3.19 | −7.75 | 1.46 | 1.99 | −0.69 | −4.10 | 1.46 | 1.74 | 0.005a,** |
| Inattentive symptoms | 0.65 | 0 | 8 | 1.85 | 5.60 | 1 | 9 | 3.37 | 0.001b |
| Hyperactive/impulsive symptoms | 0.61 | 0 | 6 | 1.50 | 5.00 | 1 | 9 | 3.56 | 0.003b |
| TMCQ subscales | |||||||||
| Impulsivity | 2.46 | 1.62 | 3.92 | 0.66 | 3.80 | 2.00 | 5.00 | 0.98 | <0.001a |
| Inhibitory control | 3.63 | 2.25 | 4.43 | 0.56 | 2.74 | 1.13 | 3.63 | 0.74 | 0.001a |
| Activation control | 3.65 | 2.80 | 4.20 | 0.42 | 3.16 | 2.36 | 3.93 | 0.54 | 0.008a |
| Activity level | 3.93 | 2.56 | 5.00 | 0.66 | 4.53 | 3.78 | 5.00 | 0.37 | 0.002b |
| N | % | N | % | Sig. | |
|---|---|---|---|---|---|
| Gender | 0.031f | ||||
| Male | 9 | 39.1 | 8 | 80.0 | |
| Female | 14 | 60.9 | 2 | 20.0 | |
| Comorbid diagnosisq | 2 | 8.7 | 1 | 10.0 | 0.675g |
| Stimulant use | – | – | 3 | 30.0 | – |
| ADHD subtype | – | ||||
| Combined | – | – | 7 | 70.0 | |
| Inattentive | – | – | 2 | 20.0 | |
| Hyperactive | – | – | 1 | 10.0 |
|
Subgroup B – Group statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Controls |
ADHD |
p | ||||||
| Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | ||
| Age | 9.27 | 7.58 | 12.50 | 1.26 | 10.72 | 8.08 | 12.33 | 1.35 | 0.001a |
| IQ | 117 | 96 | 148 | 11.71 | 111.05 | 82.00 | 132.00 | 15.56 | 0.151a |
| Mean frame-to-frame displacement (FD)j | 0.12 | 0.06 | 0.18 | 0.03 | 0.12 | 0.07 | 0.16 | 0.02 | 0.978a |
| % frames removed | 31.42 | 0.00 | 59.58 | 18.38 | 30.16 | 0.00 | 56.88 | 18.59 | 0.825a |
| ln(k)n | −4.31 | −9.47 | −1.72 | 1.73 | −3.74 | −8.56 | −0.64 | 2.30 | 0.386a,*** |
| Inattentive symptoms | 0.24 | 0 | 4 | 0.83 | 7.00 | 1 | 9 | 2.12 | <0.001b |
| Hyperactive/impulsive symptoms | 0.21 | 0 | 2 | 0.56 | 5.59 | 0 | 9 | 3.12 | <0.001b |
| TMCQ subscales | |||||||||
| Impulsivity | 2.38 | 1.54 | 3.54 | 0.44 | 3.63 | 2.15 | 4.77 | 0.72 | <0.001a |
| Inhibitory control | 3.79 | 3.13 | 4.63 | 0.42 | 2.54 | 1.63 | 3.50 | 0.61 | <0.001b |
| Activation control | 3.64 | 3.13 | 4.40 | 0.32 | 2.99 | 1.85 | 3.93 | 0.60 | 0.001b |
| Activity level | 3.91 | 2.00 | 4.78 | 0.61 | 4.01 | 2.56 | 4.67 | 0.70 | 0.634a |
| N | % | N | % | Sig. | |
|---|---|---|---|---|---|
| Gender | 0.526h | ||||
| Male | 16 | 55.2 | 11 | 64.7 | |
| Female | 13 | 44.8 | 6 | 35.3 | |
| Comorbid diagnosisr | 2 | 6.9 | 8 | 47.1 | 0.001i |
| Stimulant use | – | – | 9 | 52.9 | – |
| ADHD subtype | – | ||||
| Combined | – | – | 10 | 58.8 | |
| Inattentive | – | – | 7 | 41.2 | |
| Hyperactive | – | – | 0 | 0.0 |
|
Subgroup C – Group statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Controls |
ADHD |
p | ||||||
| Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | ||
| Age | 9.02 | 7.17 | 11.75 | 1.35 | 9.17 | 8.17 | 11.33 | 0.80 | 0.745b |
| IQ | 113 | 94 | 134 | 11.60 | 110.43 | 84.00 | 144.00 | 15.86 | 0.678a |
| Mean frame-to-frame displacement (FD)j | 0.12 | 0.07 | 0.16 | 0.03 | 0.11 | 0.06 | 0.17 | 0.03 | 0.837a |
| % frames removed | 16.02 | 1.67 | 47.50 | 16.22 | 27.12 | 0.00 | 55.83 | 20.01 | 0.144a |
| ln(k)o | −2.73 | −5.79 | 1.46 | 2.24 | −3.43 | −8.46 | 1.14 | 3.08 | 0.546a,**** |
| Inattentive symptoms | 0.18 | 0 | 1 | 0.40 | 5.47 | 1 | 9 | 2.83 | <0.001b |
| Hyperactive/impulsive symptoms | 0.36 | 0 | 4 | 1.21 | 3.53 | 0 | 9 | 2.53 | <0.001b |
| TMCQ subscales | |||||||||
| Impulsivity | 2.34 | 1.46 | 3.69 | 0.66 | 3.36 | 2.54 | 4.54 | 0.62 | <0.001a |
| Inhibitory control | 3.99 | 3.38 | 4.63 | 0.36 | 2.85 | 1.63 | 3.50 | 0.51 | <0.001a |
| Activation control | 3.69 | 3.13 | 4.67 | 0.46 | 3.07 | 1.93 | 3.73 | 0.47 | 0.003a |
| Activity level | 3.72 | 2.67 | 4.78 | 0.73 | 3.92 | 2.56 | 4.67 | 0.68 | 0.494a |
| N | % | N | % | Sig. | |
|---|---|---|---|---|---|
| Gender | 0.218g | ||||
| Male | 6 | 54.5 | 12 | 80.0 | |
| Female | 5 | 45.5 | 3 | 20.0 | |
| Comorbid diagnosiss | 1 | 9.1 | 5 | 33.3 | 0.197g |
| Stimulant usel | – | – | 3 | 20 | – |
| ADHD subtype | – | ||||
| Combined | – | – | 10 | 66.7 | |
| Inattentive | – | – | 5 | 33.3 | |
| Hyperactive | – | – | 0 | 0.0 |
ln(k), natural log of the discounting gradient.
After controlling for age, p = 0.054.
After controlling for age, p = 0.008.
After controlling for age, p = 0.32.
After controlling for age, p = 0.68.
Equal variances assumed, because Levene's test for equality >0.05.
Equal variances not assumed, because Levene's test for equality <0.05.
X2 = 6.308.
X2 = 4.861.
X2 = 10.967.
X2 = 4.661.
Fisher's exact test was used because one condition for using chi-square test was not met (more than 25% of the cells have expected count less than 5).
X2 = 0.402.
X2 = 10.161.
Mean FD of the remaining frames.
54 controls and 36 ADHD with data.
1 ADHD subject from subgroup C without information about stimulant use.
20 controls and 7 ADHD with data.
25 controls and 14 ADHD with data.
10 controls and 14 ADHD with data.
ncontrols: nADHD/mood - 0:1; anxiety - 3:5; ODD/CD - 1:9; learning 1:0.
ncontrols: nADHD / mood - 0:0; anxiety - 1:0; ODD/CD - 1:1; learning - 0:0.
ncontrols: nADHD / mood - 0:1; anxiety - 1:2; ODD/CD - 0:5; learning - 1:0.
ncontrols: nADHD / mood - 0:0; anxiety - 1:3; ODD/CD - 0:3; learning - 0:0.
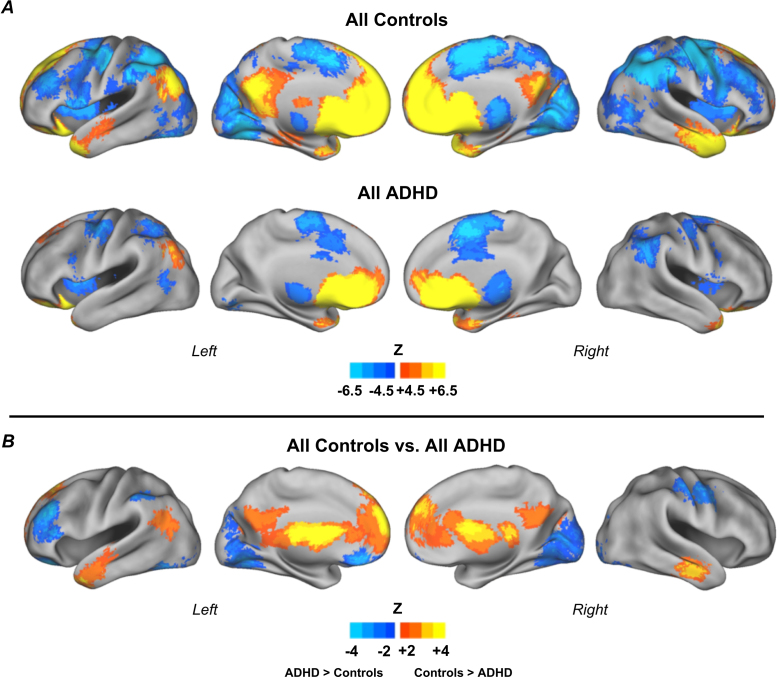
Fig. 2.
Voxelwise resting state functional connectivity maps for the reward ROI. Results for all control children (n = 63) and all children with ADHD (n = 42) (A); and direct comparison between groups (B). Results show atypical connectivity of the reward system in children with ADHD. Monte Carlo simulation was applied to correct for multiple comparisons (Z > 2.25, p < 0.05).
When comparing controls vs. children with ADHD, we found the following connections from the reward region were weaker in ADHD: positive connections to anterior PFC (medial frontal gyrus and superior frontal gyrus), middle temporal gyrus, posterior cingulate cortex (PCC); and negative connections to dorso-lateral PFC (middle frontal gyrus), inferior parietal lobule bilaterally and occipital lobe (lingual gyrus) bilaterally. On the other hand, the ADHD group displayed stronger positive connectivity to left orbito-frontal cortex (OFC; inferior frontal gyrus) and stronger negative connectivity to the thalamus (Fig. 2). Table S.1 (Supplementary Material) displays coordinates and labels of regions generated from the comparison analysis. Structure details were generated with Talairach Client (Lancaster et al., 2000).
Because the ADHD and the control groups differed significantly in gender distribution and age, we performed the same analysis on ADHD and control groups matched by age and gender (ADHD: n = 42; controls: n = 42). The results were very similar and are displayed in the Supplementary Material, Figure S.2. Demographic characteristics of the new matched groups are shown in Table S.2 (Supplementary Material).
3.2. Community detection
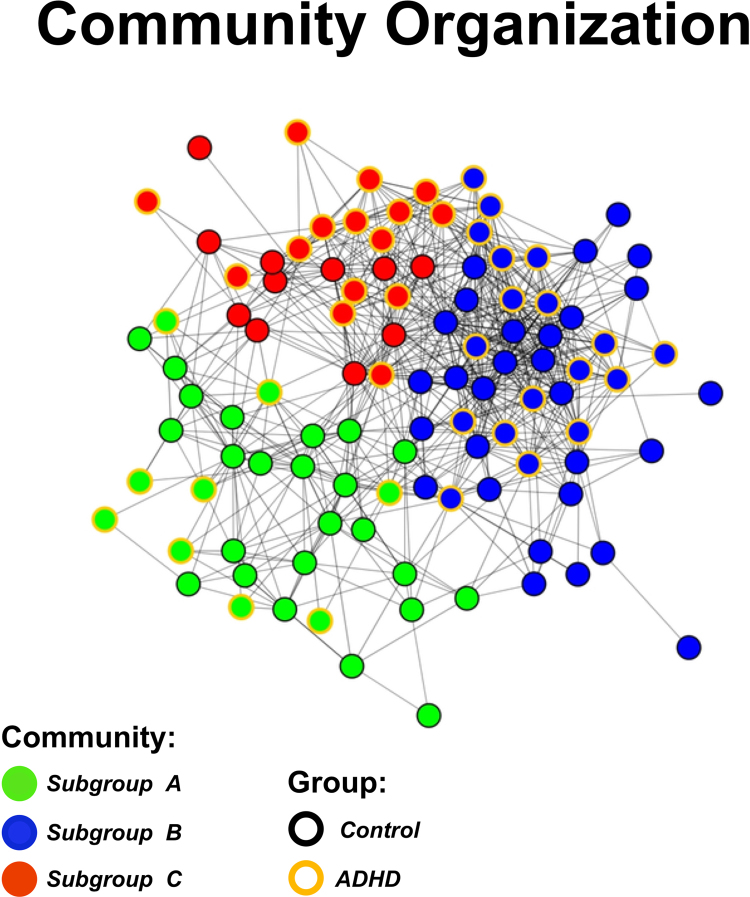
We applied the community detection algorithm to the entire sample and found that the network of children with and without ADHD was organized into three communities, which we called subgroups A (n = 33), B (n = 47) and C (n = 26). The quality index (Q = 0.38) and a secondary analysis utilizing variation of information (Supplementary Material, Figure S.3) showed that the communities identified were highly unlikely to be random, by demonstrating robust community assignment stability. Interestingly, all the communities were comprised of individuals both with and without ADHD. The demographic characteristics of the three communities are summarized in Table 1, Table 2. The spring embedded figure showing how the participants were organized into the three communities is displayed in Fig. 3. We can note that community A is more segregated from the other two groups. We also performed the community detection analysis on the whole-brain data (i.e. on the unthresholded connectivity map), but the analysis did not yield communities. This finding may be due to the inclusion of too much information, which introduced noise to the data, and thus decreased the chance of identifying communities.
Table 2.
| Variable |
A |
B |
C |
p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | ||
| Controls | |||||||||||||
| Age | 8.85 | 7.42 | 11.17 | 0.98 | 9.27 | 7.58 | 12.50 | 1.26 | 9.02 | 7.17 | 11.75 | 1.35 | 0.449 |
| IQ | 119.42 | 96.20 | 144.00 | 15.07 | 116.95 | 96.00 | 148.00 | 11.71 | 112.81 | 94.00 | 134.00 | 11.60 | 0.387 |
| Mean frame-to-frame displacement (FD)k | 0.12 | 0.07 | 0.16 | 0.02 | 0.12 | 0.06 | 0.18 | 0.03 | 0.12 | 0.07 | 0.16 | 0.03 | 0.709 |
| % frames removed | 22.83 | 0.00 | 57.92 | 18.60 | 31.42 | 0.00 | 59.58 | 18.38 | 16.02 | 1.67 | 47.50 | 16.22 | 0.044a |
| ln(k)l | −3.19 | −7.75 | 1.46 | 1.99 | −4.31 | −9.47 | −1.72 | 1.73 | −2.73 | −5.79 | 1.46 | 2.24 | 0.051 |
| Inattentive symptoms | 0.65 | 0 | 8 | 1.85 | 0.24 | 0 | 4 | 0.83 | 0.18 | 0 | 1 | 0.40 | 0.437 |
| Hyperactive/impulsive symptoms | 0.61 | 0 | 6 | 1.50 | 0.21 | 0 | 2 | 0.56 | 0.36 | 0 | 4 | 1.21 | 0.430 |
| TMCQ subscales | |||||||||||||
| Impulsivity | 2.46 | 1.62 | 3.92 | 0.66 | 2.38 | 1.54 | 3.54 | 0.44 | 2.34 | 1.46 | 3.69 | 0.66 | 0.809 |
| Inhibitory control | 3.63 | 2.25 | 4.43 | 0.56 | 3.79 | 3.13 | 4.63 | 0.42 | 3.99 | 3.38 | 4.63 | 0.36 | 0.116 |
| Activation control | 3.65 | 2.80 | 4.20 | 0.42 | 3.64 | 3.13 | 4.40 | 0.32 | 3.69 | 3.13 | 4.67 | 0.46 | 0.939 |
| Activity level | 3.93 | 2.56 | 5.00 | 0.66 | 3.91 | 2.00 | 4.78 | 0.61 | 3.72 | 2.67 | 4.78 | 0.73 | 0.663 |
| N | % | N | % | N | % | Sig. | |
|---|---|---|---|---|---|---|---|
| Gender | 0.012b | ||||||
| Male | 9 | 39.1 | 16 | 55.2 | 6 | 54.5 | |
| Female | 14 | 60.9 | 13 | 44.8 | 5 | 45.5 | |
| Comorbid diagnosis | 2 | 8.7 | 2 | 6.9 | 1 | 9.1 | 0.960c |
| Variable |
A |
B |
C |
p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | Mean | Min. | Max. | SD | ||
| ADHD | |||||||||||||
| Age | 8.32 | 7.42 | 9.08 | 0.62 | 10.72 | 8.08 | 12.33 | 1.35 | 9.17 | 8.17 | 11.33 | 0.80 | <0.001d |
| IQ | 110.80 | 90.00 | 132.00 | 15.24 | 111.05 | 82.00 | 132.00 | 15.56 | 110.43 | 84.00 | 144.00 | 15.86 | 0.994 |
| Mean frame-to-frame displacement (FD)k | 0.12 | 0.09 | 0.17 | 0.03 | 0.12 | 0.07 | 0.16 | 0.02 | 0.11 | 0.06 | 0.17 | 0.03 | 0.673 |
| % frames removed | 17.17 | 1.67 | 52.50 | 17.03 | 30.16 | 0.00 | 56.88 | 18.59 | 27.12 | 0.00 | 55.83 | 20.01 | 0.225 |
| ln(k)m | −0.69 | −4.10 | 1.46 | 1.74 | −3.74 | −8.56 | −0.64 | 2.30 | −3.43 | −8.46 | 1.14 | 3.08 | 0.026e |
| Inattentive symptoms | 5.60 | 1 | 9 | 3.37 | 7.00 | 1 | 9 | 2.12 | 5.47 | 1 | 9 | 2.83 | 0.232 |
| Hyperactive/impulsive symptoms | 5.00 | 1 | 9 | 3.56 | 5.59 | 0 | 9 | 3.12 | 3.53 | 0 | 9 | 2.53 | 0.166 |
| TMCQ subscales | |||||||||||||
| Impulsivity | 3.80 | 2.00 | 5.00 | 0.98 | 3.63 | 2.15 | 4.77 | 0.72 | 3.36 | 2.54 | 4.54 | 0.62 | 0.355 |
| Inhibitory control | 2.74 | 1.13 | 3.63 | 0.74 | 2.54 | 1.63 | 3.50 | 0.61 | 2.85 | 1.63 | 3.50 | 0.51 | 0.369 |
| Activation control | 3.16 | 2.36 | 3.93 | 0.54 | 2.99 | 1.85 | 3.93 | 0.60 | 3.07 | 1.93 | 3.73 | 0.47 | 0.745 |
| Activity level | 4.53 | 3.78 | 5.00 | 0.37 | 4.01 | 2.56 | 4.67 | 0.70 | 3.92 | 2.56 | 4.67 | 0.68 | 0.054f |
| N | % | N | % | N | % | Sig. | |
|---|---|---|---|---|---|---|---|
| Gender | 0.542g | ||||||
| Male | 8 | 80 | 11 | 64.7 | 12 | 80 | |
| Female | 2 | 20 | 6 | 35.3 | 3 | 20 | |
| Comorbid diagnosis | 1 | 10 | 8 | 47.1 | 5 | 33.3 | 0.143h |
| Stimulant usen | 3 | 30 | 9 | 52.9 | 3 | 21.4 | 0.171i |
| ADHD subtype | 0.381*,j | ||||||
| Combined | 7 | 70 | 10 | 58.8 | 10 | 66.7 | |
| Inattentive | 2 | 20 | 7 | 41.2 | 5 | 33.3 | |
| Hyperactive | 1 | 10 | 0 | 0 | 0 | 0 |
ln(k), natural log of the discounting gradient.
Post hoc Tukey's HSD test: B vs. C, p = 0.05.
X2 = 8.798.
X2 = 0.081; chi-square not valid, because more than 25% of the cells have expected count less than 5.
Post hoc Tukey's HSD test: A vs. B, p < 0.001/B vs. C, p < 0.001.
Post hoc Tukey's HSD test: A vs. B, p = 0.028/A vs. C, p = 0.052.
Post hoc Tukey's HSD test: A vs. C, p = 0.056.
X2 = 1.224.
X2 = 3.891.
X2 = 3.533.
X2 = 4.190; chi-square test not valid, because more than 25% of the cells have expected count less than 5.
Mean FD of the remaining frames.
54 subjects (A = 19; B = 25; C = 10) with data.
36 subjects (A = 8; B = 14; C = 14) with data.
1 subject from subgroup C without information about stimulant use.
Chi-square performed on only the combined and inattentive subtypes yielded valid results: p = 0.623; X2 = 0.947.
Fig. 3.
Spring embedding representation of the community organization of the whole sample. Nodes represent subjects and are color coded by their community assignment (node cores) and their group (i.e. ADHD or control; node outlines). Green: subgroup A, blue: subgroup B, red: subgroup C. Yellow outline: ADHD, black outline: control. Connections with r ≥ 0.52 were considered connected. The network of participants was naturally organized into three communities, which are densely connected sets of participants (nodes), with sparser connections between groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Applying community detection to the ADHD and the typically developing groups separately yielded similar results. In the typically developing group, we found three communities (Q = 0.39; A: n = 24/B: n = 28/C: n = 11); only nine participants switched communities, comparing to the whole-sample community organization. In the ADHD group, the results yielded four communities (Q = 0.34; A1: n = 4/A2: n = 5/B: n = 18/C: n = 16). Participants from the original subgroup A split up into two communities (A1 and A2). Only eight subjects switched subgroups. The original community distribution (whole-sample community detection) was used for further analysis.
Table 1, Table 2 summarize demographic and clinical characteristics of the subgroups separated in ADHD and controls, the comparisons between controls and ADHD within each subgroup, and also the comparisons between subgroups within the ADHD and the control samples. ADHD subgroups and control subgroups did not differ in ADHD-RS scores, age, IQ, movement (mean FD), and presence of comorbid diagnosis. ADHD subgroups also did not differ in stimulant use and ADHD subtype (or presentation). Table S.3 (Supplementary Material) summarizes demographic and clinical characteristics of each community (not separating by ADHD vs. controls) and the comparisons between them.
3.3. Comparison between ADHD and typical development within the subgroups
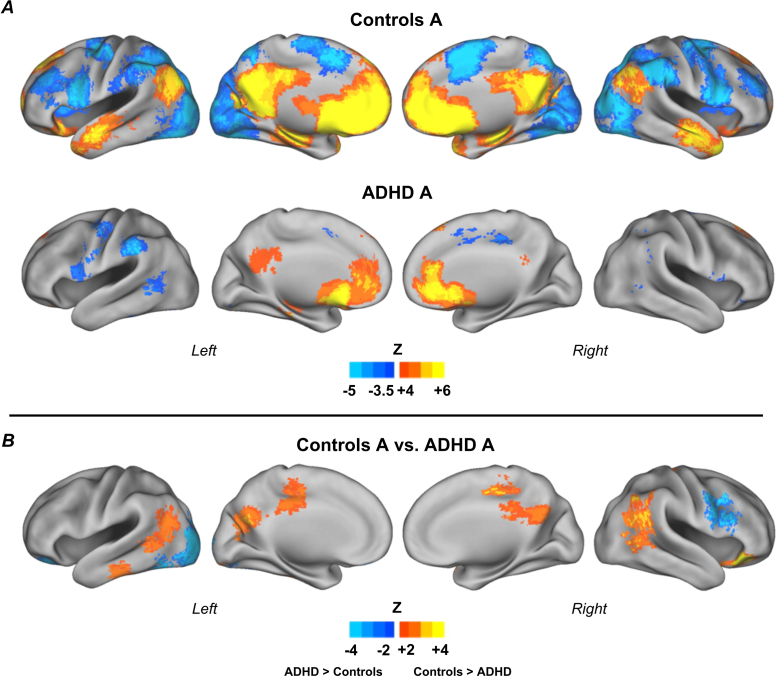
We next found that children with ADHD from different subgroups have distinct patterns of atypical connections when compared to the relative controls (Fig. 4, Fig. 5, Fig. 6). Compared to controls in subgroup A, ADHD children displayed atypical connections from the reward region to the PCC bilaterally, temporal lobes bilaterally (middle temporal gyrus), right middle frontal gyrus/precentral sulcus, inferior frontal gyrus, left occipital lobe (Fig. 4). Coordinates and labels are shown in Table S.1 (Supplementary Material).
Fig. 4.
Connectivity maps for the reward ROI, subgroup A. Results for control children (n = 23) and children with ADHD (n = 10) (A); and direct comparison between groups (B). Results show a specific pattern of atypical connectivity of the reward system in children with ADHD, compared to control children in the same subgroup. Monte Carlo simulation was applied to correct for multiple comparisons (Z > 2.25, p < 0.05).
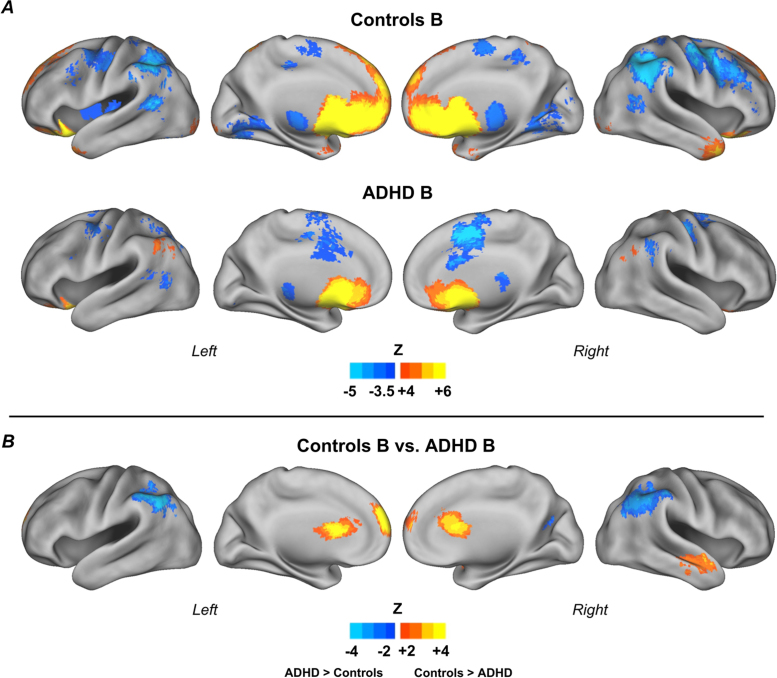
Fig. 5.
Connectivity maps for the reward ROI, subgroup B. Results for control children (n = 29) and children with ADHD (n = 17) (A); and direct comparison between groups (B). Results show a specific pattern of atypical connectivity of the reward system in children with ADHD, compared to control children in the same subgroup. Monte Carlo simulation was applied to correct for multiple comparisons (Z > 2.25, p < 0.05).
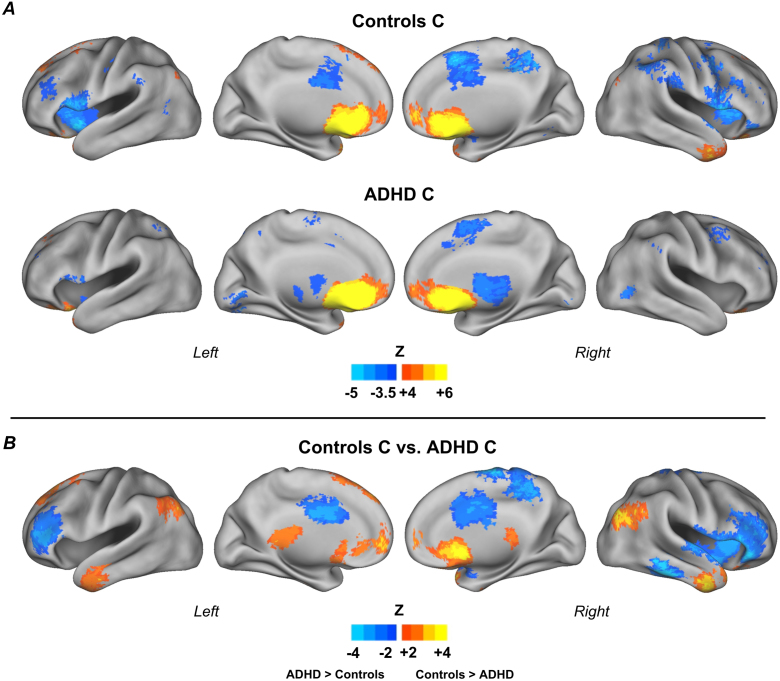
Fig. 6.
Connectivity maps for the reward ROI, subgroup C. Results for control children (n = 11) and children with ADHD (n = 15) (A); and direct comparison between groups (B). Results show a specific pattern of atypical connectivity of the reward system in children with ADHD, compared to control children in the same subgroup. Monte Carlo simulation was applied to correct for multiple comparisons (Z > 2.25, p < 0.05).
Within subgroup B, ADHD children displayed atypical connectivity to the inferior parietal lobule bilaterally, caudate, ACC, right middle temporal gyrus, and superior frontal gyrus (Fig. 5). Because ADHD and controls within subgroup B significantly differed in age, we performed the same analysis on ADHD and control groups matched by age (ADHD: n = 17; controls: n = 17). The results were very similar and are displayed in the Supplementary Material, Figure S.4. The differences in the middle temporal gyrus and in the caudate became weaker after matching the groups, and did not remain after Monte Carlo correction. However, these findings are visible in the uncorrected comparison maps, suggesting the absence of these regions may be due to power, since sample size reduced after matching. Figure S.4 displays the between-group t-test of the matched sample with and without Monte Carlo correction. Demographic characteristics of the new matched groups are shown in Table S.2 (Supplementary Material). See Table S.1 (Supplementary Material) for coordinates and labels.
Finally, children with ADHD in subgroup C, compared to controls in the same subgroup, displayed several atypical connections. The main ones were: middle temporal gyrus bilaterally, right superior temporal gyrus, medial frontal gyrus, ACC bilaterally, right anterior insula, dorso-lateral PFC (middle frontal gyrus) bilaterally, right caudate, right precuneus, right paracentral lobule, inferior frontal gyrus bilaterally (Fig. 6). See Table S.1 (Supplementary Material) for coordinates and labels.
3.4. Mapping findings onto current network definitions
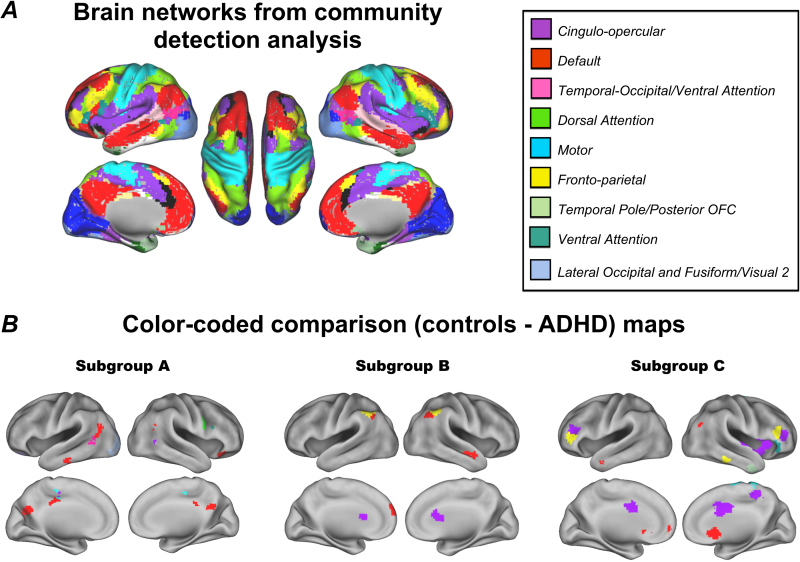
Nineteen communities (or networks), which corresponded to known brain systems, were identified in a sample of typical adults (Supplementary Material, Figure S.1). Of these networks, nine had voxels overlapping with the comparison maps, which included the cingulo-opercular, default, temporal-occipital/ventral attention, dorsal attention, motor, fronto-parietal, temporal pole/posterior OFC, ventral attention, and lateral occipital and fusiform/visual 2. (Fig. 7).
Fig. 7.
Brain networks from community detection analysis and color-coded comparison maps. Community detection was applied to the average functional connectivity map of 32 adults; the community assignments were mapped onto ROIs as colors (A). The nine communities found corresponded to known brain networks. Atypical connections of the NAcc in each subgroup were color coded according to which brain network they had the most voxels overlapping with (B). The legend displays colors and names assigned to the nine networks that overlapped with voxels from the comparison maps.
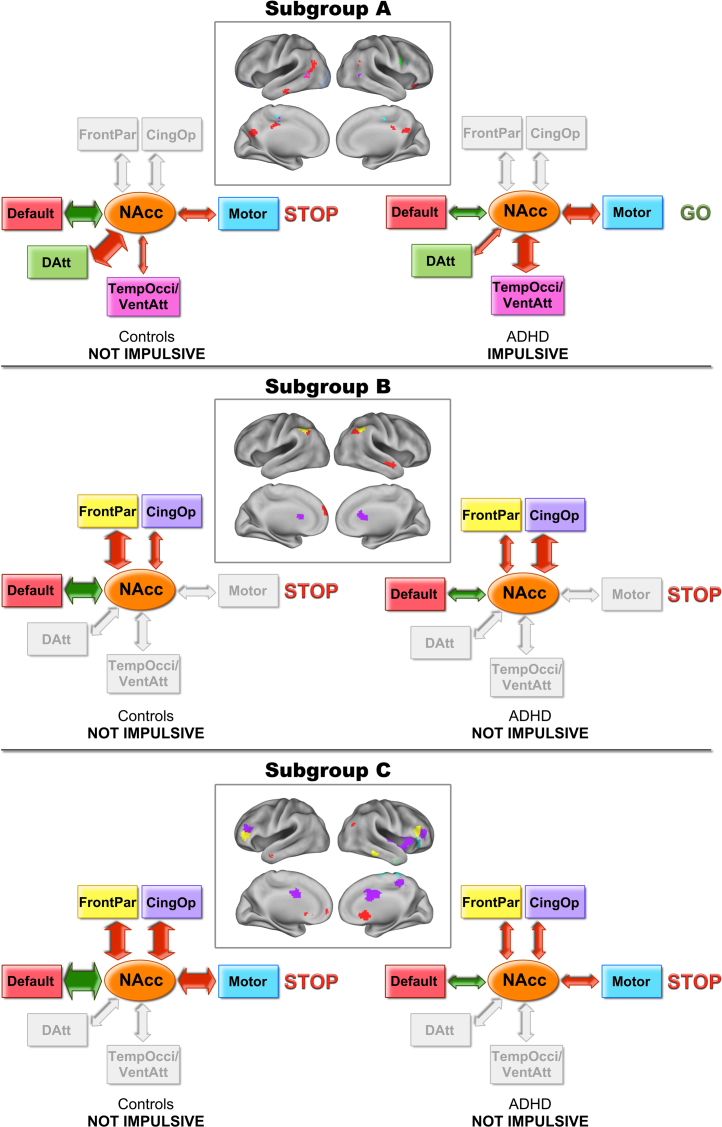
The peak regions from the comparison maps (Fig. 4, Fig. 5, Fig. 6) were color-coded using a “winner take all procedure” for which the region was color coded by its maximally overlapping network (Fig. 7). The ADHD children from each subgroup had atypical connections in different networks. ADHD children in subgroup A showed atypical connections primarily in the default network. In subgroup B atypical connections were identified in the cingulo-opercular, default and fronto-parietal networks. Finally, in subgroup C, ADHD children showed atypical connections to several networks, but most prominently, the cingulo-opercular network.
3.5. Association between community structure and impulsivity
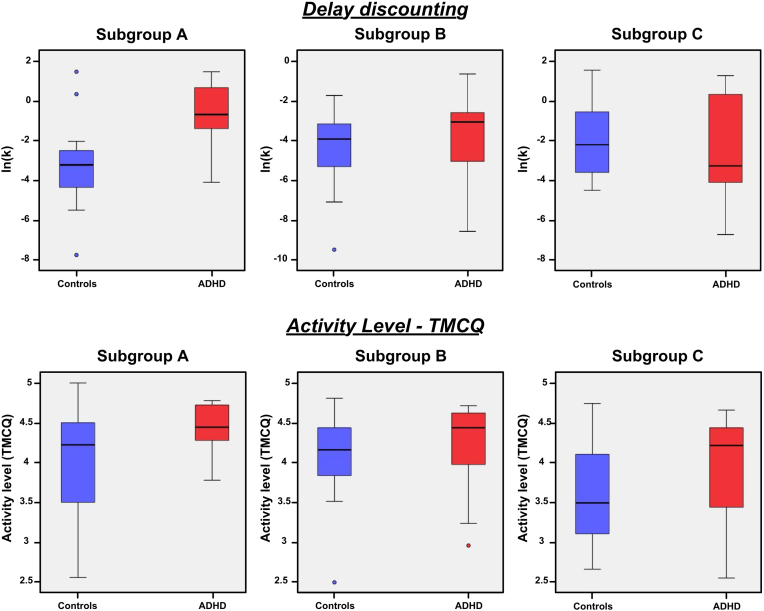
Ln(k) was not significantly different between all control and all children with ADHD (two-tailed, unequal variances, p = 0.206). After controlling for age a trend level difference was identified (F(1,87) = 3.812, p = 0.054), and was consistent with prior reports (Sagvolden et al., 1998, Wilson et al., 2011), with ADHD more impulsive than controls. When we compared controls and ADHD children within each subgroup, we found significant differences in subgroup A (p = 0.005, two-tailed, equal variances; p = 0.008 after controlling for age/n[ADHD] = 7, n[controls] = 20) (Table 1 and Fig. 8). In subgroup A, ln(k) for ADHD children was greater than that for controls, indicating that in this subgroup ADHD children were more impulsive than typically developing children. Within the other subgroups (B and C), ln(k) was not significantly different between control and ADHD children (p = 0.386, p = 0.546, respectively; two-tailed, equal variances/B: n[ADHD] = 14, n[controls] = 25; C: n[ADHD] = 14, n[controls] = 10) (Table 1 and Fig. 8). There were also significant differences between subgroups of ADHD children as determined by one-way ANOVA (F(2,33) = 4.091, p = 0.026), but differences between subgroups of controls were not significant (F(2,51) = 3.161, p = 0.051). Post hoc Tukey's HSD test revealed that ADHD-A had significantly greater ln(k) compared to ADHD-B (p = 0.028). See Table 2.
Fig. 8.
Boxplots of ln(k) and activity level scores (from TMCQ) in controls and ADHD children. Boxplots were generated for each subgroup and emphasize that ln(k) and activity level scores were significantly different between controls and ADHD children only within subgroup A. Blue: controls; red: children with ADHD. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The temperament measures of impulsivity, inhibitory control, and activation control were significantly different between controls and ADHD (Table 1; two-tailed, unequal variances, p < 0.001 for the three subscales), and so was activity level (two-tailed, equal variances, p = 0.001). Items on the impulsivity, inhibitory control, and activity level TMCQ subscales overlap with DSM-IV and DSM-5 symptom criteria; therefore differences between controls and ADHD are expected for these temperament traits. When comparing children with ADHD to controls within each subgroup, ADHD children were significantly different from controls in impulsivity, inhibitory control, and activation control scores in every subgroups, but only ADHD children from subgroup A were significantly different from controls in activity level scores (two-tailed, unequal variances, p = 0.002), with ADHD-A displaying significantly higher levels of activity then controls-A.
There was also a trend toward significance when comparing ADHD subgroups only for activity level scores (F(3,38) = 3.152, p = 0.054); the ADHD-A subgroup displayed higher activity levels compared to the other ADHD subgroups (Table 2).
4. Discussion
Our understanding of the neurobiological underpinnings and etiology of ADHD will likely be limited without the proper characterization of the heterogeneity within the disorder. In recent years this consideration has been recognized with increasing efforts to move away from the DSM categorization – utilizing new ways of classifying and characterizing psychopathology (Fair et al., 2012, Hyman, 2010, Karalunas et al., 2015). This effort has been enhanced by the NIMH's Research Domain Criteria (RDoc) (Insel et al., 2010). In line with this trend, we followed three recent reports (Fair et al., 2012, Gates et al., 2014, Karalunas et al., 2015) that utilized graph theory, and in particular community detection, to unravel ADHD ‘neurotypes’ that could be related to differential behavioral patterns, in this case, delay discounting and impulsivity-related temperament dimensions.
It has been proposed that multifactorial models may assist in explaining heterogeneity in ADHD etiology (Nigg et al., 2005, Nigg and Casey, 2005, Sonuga-Barke, 2005). These theories have been supported by studies using neuropsychological data, which showed that not all individuals with ADHD have deficits in all core neuropsychological domains (Fair et al., 2012, Nigg et al., 2005, Sonuga-Barke et al., 2010). Our data support and add to these theories in several ways. We find that both the ADHD and the typically developing groups are organized into distinct communities/subgroups. These data highlight not only variation in ADHD, but in typically developing children as well (Fair et al., 2012, Gates et al., 2014). The results also suggest that heterogeneity in both groups in some instances will be relevant for investigating the disorder's underlying neurobiology. Comparisons within subgroups identified several brain features of ADHD that were unique to each neurotype. These identifying features were interesting in that they tended to map onto well-known networks important for higher order cognition (Corbetta and Shulman, 2002, Dosenbach et al., 2007, Dosenbach et al., 2008) and introspection (Buckner et al., 2008, Raichle and Snyder, 2007). Just as importantly, behaviorally, only one of these neurotypes showed atypical delay discounting and atypical activity level scores. These findings further highlight the importance of characterizing heterogeneity when attributing dysfunction to children with ADHD.
This study corroborates findings of Karalunas et al. (2015) and Gates et al. (2014). As in those previous studies, we verified the value of community detection as a tool to identify subgroups of children with and without ADHD based on relevant disorder characteristics (temperament measures, functional connectivity of brain networks). It is important to note that we did not necessarily expect that the number of subgroups or the distribution of individuals would be similar in the three studies, because different features of interest were used in each study. There are many valid ways to subdivide populations, and divisions are dependent on the features used to generate similarity (or distance) measurements between individuals. Distinct subdivisions within populations are likely to be meaningful and informative in different ways. In the current report the community organization based on reward connectivity patterns informed specific profiles of atypical reward functional connectivity related to ADHD, which was validated with assessments of impulsive decision-making (via delay discounting) and impulsivity-related temperament dimensions.
4.1. Variation in the reward system functional connectivity
The current report builds on prior reports showing NAcc connectivity to several brain networks either positively or negatively (see Fig. 2 and Costa Dias et al., 2012, Cservenka et al., 2014, Tomasi and Volkow, 2012). These networks included control systems (fronto-parietal, cingulo-opercular networks), attention systems (dorsal attention, temporal-occipital/ventral attention networks), the default network, and motor network.
The default network was positively connected to the reward system. This relationship is consistent with work highlighting the default system's role in assigning value to future rewards, future planning and goal setting (Buckner et al., 2008). On the other hand, the NAcc was negatively connected to several task-control and attention networks. These systems are important for providing task level control and inhibiting inappropriate behaviors (Corbetta and Shulman, 2002, Dosenbach et al., 2008). Importantly, the distinct relationships of connectivity to the default system and/or the task control systems varied according to the community assignment in both children with and without ADHD. In other words the pattern and strength of NAcc connectivity to each of these systems were distinct for each neurotype. This particular finding is similar in theme to that of previous reports (Fair et al., 2012, Gates et al., 2014). It also provides some insight with regard to how the reward circuitry, in particular, might vary from person to person.
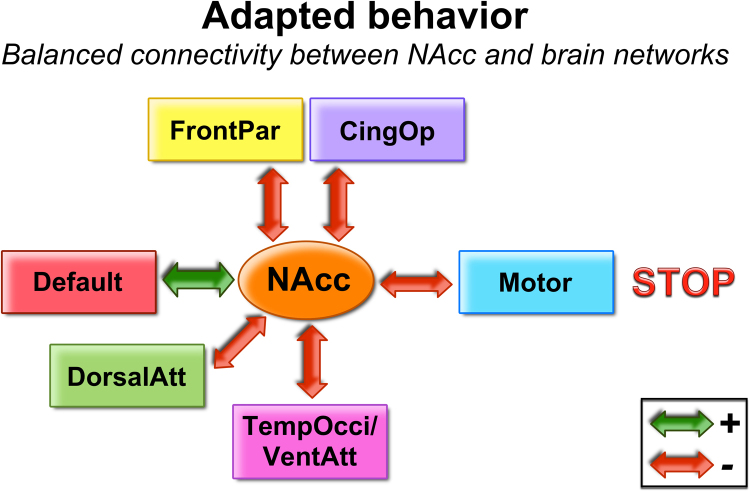
Volkow and colleagues have recently proposed a model of how the interaction of cortical and sub-cortical systems with the NAcc might inform addictive behaviors (Volkow et al., 2011a). The model asserts that a balanced interaction between all of the systems is necessary for adequate inhibitory control and decision-making (see Fig. 1). The interconnected systems are: reward (NAcc, ventral tegmental area), conditioning/memory (amygdala, medial OFC, hippocampus, dorsal striatum), executive control (dorso-lateral prefrontal cortex, anterior cingulate cortex, inferior frontal cortex, lateral orbital frontal cortex), and motivation/drive (medial OFC, ventral ACC, dorsal striatum, substantia nigra, motor cortex, VTA). This model suggests that a balanced interaction of the reward system with other brain networks is essential for adaptive (i.e. not impulsive) decision-making. On the other hand, impulsivity (and other associated behaviors) would be a result of an unbalance of those connections (Volkow et al., 2011a). Relating this model to the current cohort would suggest that in typically developing populations, as well as in disorder populations, the connections of the reward system may diverge from one individual to another; however, if the system remained in balance, the result would be typical (as opposed to atypical) behavior. In the current report we describe our findings in the context of a slightly modified version of this model, which also incorporates emerging information of cortical network structure in adult and developing populations (Fig. 9).
Fig. 9.
Connectivity between NAcc and brain networks. The NAcc is functionally connected to several networks. The negative and positive connections have to be balanced in order to provide adapted behavior (i.e. not impulsive). Some connections may be atypical, but still result in adapted behavior, as long as the balance is maintained. Several possible connection combinations may unbalance the system and result in atypical behavior.
Under this context, an important finding in the current report is the identification of heterogeneity in reward system connectivity in typically developing children. Specifically, we found three communities of typically developing individuals with distinct patterns of functional connectivity of the NAcc. It has long been known that neuropsychological diversity is an important feature of the human behavior. For example, in a recent work, we showed that variation across several neuropsychological measures does not always shape across a continuous dimension (i.e. unimodal distribution), but rather some behavioral features form multiple discrete subgroups (i.e. multimodal distribution) (Fair et al., 2012). The current report (and others, Walhovd et al., 2012) highlights variation in the brain circuitry that may also follow this principle, at least in the case of the reward system. Just as important, the result also suggests that heterogeneity within developmental neuropsychiatric disorders, such as ADHD, might be “nested” within this normal variation. Thus, characterizing variation in the typically developing population is likely important for understanding atypical developmental trajectories.
4.2. Heterogeneity in the ADHD population
The brain is organized into systems (or networks), whose patterns of interaction (within and between systems) underlie behavioral traits. A core system involved in ADHD is the reward system, which interacts with other brain networks to promote decision-making. Here, we found that, as with the typically developing population, discrete subgroups were identified that characterized distinct reward connectivity profiles. Importantly, which connections were atypical relative to the control population were specific to the community membership. For example, in subgroup A atypical connections were primarily identified in the default network; in subgroup B, cingulo-opercular, default and fronto-parietal networks; and in subgroup C, mainly the cingulo-opercular network (Fig. 7). Some of these findings would have otherwise been missed if one were to only consider the control and ADHD populations as homogeneous groups (Fig. 2). Importantly, as each of these systems is likely to underlie unique control processes, it is possible that unique behavioral phenotypes may exist between these ADHD groups. Our examination of delay discounting and impulsivity-related temperament traits across these populations is an early example of a preliminary characterization of these potential behavioral characterizations (discussed below).
Connections to the default network were atypical in ADHD in all the three subgroups, corroborating previous findings of atypical connectivity between the nucleus accumbens and regions of the default network in a group of children with ADHD combined type (which shared some participants with the current study) (Costa Dias et al., 2012), and of atypical integration of this network in ADHD (Castellanos et al., 2008, Fair et al., 2010). However, atypical connectivity in ADHD with the default network was unique in each subgroup with regard to region specificity, further highlighting the heterogeneity in the sample. Atypical connections between NAcc and control/attention networks were also evident in the three subgroups, but involved networks and regions were, again, specific to each subgroup.
Subgroups A and B displayed strong negative connectivity between the NAcc and regions of the control systems (fronto-parietal and cingulo-opercular networks), and the motor system. These control networks are important for moment-to-moment task level control, maintenance of task sets (Dosenbach et al., 2008), as well as inhibitory control (Dosenbach et al., 2007, Dosenbach et al., 2008) – processes likely important for the regulation of reward processing. ADHD children in subgroups A and B were characterized by atypical connectivity between the NAcc and control networks.
In subgroup A, the NAcc was also connected to regions of the attention networks (dorsal attention and temporal-occipital/ventral attention, to the motor region and to a visual network (lateral occipital and fusiform). Children with ADHD within subgroup A displayed specifically atypical connections between the NAcc and regions of the attention networks: stronger negative connectivity with ventral attention network and weaker negative connectivity with dorsal attention network. These attention networks likely support the involvement of moment-to-moment control and shifts of attention related to reward processing and decision-making (Dosenbach et al., 2008), findings consistent with the extant literature on ADHD (Tomasi and Volkow, 2012).
In subgroup C, ADHD children exhibited atypical connectivity between the NAcc and several networks, including: default network, cingulo-opercular, fronto-parietal network and motor region. But, it is noteworthy that a number of key regions of the cingulo-opercular network were involved in ADHD in this subgroup, suggesting that atypical functional connectivity between the reward system and the cingulo-opercular network might be a key dysfunction in this subpopulation.
4.2.1. Relating ADHD heterogeneity to impulsive behavior
In order to link our reward circuitry based neurotypes to behavioral phenomena we examined delay-discounting behavior and impulsivity-related temperament traits across the sub-populations. Only one of the ADHD subgroups had atypical delay discounting and, at the same time, atypical activity level scores (subgroup A). ANOVA between the three ADHD groups confirmed the differences in delay discounting, with ADHD-A significantly more impulsive than ADHD-B (Table 2). The other two subgroups of ADHD children (subgroups B and C) were similar to the related control population with regards to delay discounting and activity level, and the three ADHD subgroups were similarly atypical in impulsivity, inhibitory control and activation control scores (Table 1). It is possible that, in subgroup A, impulsive decision-making – as measured by the delay- discounting task – and activity level traits share similar neurobiological underpinnings. As the delay-discounting task, the TMCQ subscales supported that ADHD neurotypes distinguished by brain connectivity patterns of the reward system may be discriminated from typically developing individuals by specific behavioral characteristics.
We used Volkow et al.’s addiction model (Volkow et al., 2011a) as a framework to characterize these findings with regard to heterogeneity in ADHD. Fig. 10 displays the atypical connections between NAcc and brain networks within each subgroup. This figure illustrates and provides a framework to consider how the system's “balance” may or may not change depending on the atypical connections. The unbalance of the system potentially relates to impulsive behavior – as measured by delay discounting – and higher activity level.
Fig. 10.
Color-coded brain networks and schematic representation of the reward system functional connectivity. Atypical connections of the reward ROI in each subgroup were color-coded according to which brain network they had the most voxels overlapping with. The models schematically display the functional connectivity of the reward system in controls and children with ADHD.
The ADHD children in subgroups B and C did not have atypical delay discounting or activity level relative to their peers. These data suggest that in these ADHD youth, despite having distinct connectivity from their neurotype peers, they maintained a balance to support normative behavior, as measured by the delay-discounting task and activity level. On the other hand our findings suggest that atypical discounting of delayed rewards and atypical activity level may be a result of an unbalanced connectivity of the reward system, which in this case was specific to a subset of individuals (subgroup A). Illustration of this interaction is provided with the hypothetical models in Fig. 10.
With that said, it is important to consider that we were only able to examine one objective task of impulsivity – a measure of impulsive choice. There are several other measures of impulsive decision-making, including delay aversion tasks or other methods of evaluating discounting of delayed rewards (Bitsakou et al., 2006, Knutson et al., 2001, Muller et al., 2006, Rachlin et al., 1991). While we were able to complement this task with temperament traits related to impulsivity and hyperactivity, our findings are presented as an illustrative framework and do not exclude the potential for the ADHD subjects in subgroups B and C to be impulsive in unique ways, measured via different instruments. In fact, ADHD subgroups were significantly more impulsive than the related control subgroups, as measured by three impulsivity related TMCQ subscales. This supports the idea that in each ADHD subgroup, specific brain processing mechanisms may lead to distinctive ways of impulsivity. Different tasks that evaluate impulsivity and delay processing may be related to unique brain processing mechanisms. Thus, while the work here provides some proof of principle, detail characterization of associated behavioral constructs to these groups is still needed.
Our findings of atypical inter-communication between brain networks in ADHD are similar to the framework highlighted by Sonuga-Barke and Fairchild (2012). These authors have hypothesized about interconnected networks influencing on decision-making process in ADHD. They used a Neuroeconomics model of ADHD to postulate three hypotheses regarding the roles of the default network and frontostriatal subsystems on decision-making and how the dysfunction of each system results in differential consequences. One hypothesis is that atypical connectivity between regions of the default network produces inappropriate predictions of outcome value and, therefore, is related to poor goal setting and intention implementation. The second hypothesis states that dysfunctions in the dorsal frontostriatal subsystem (dorsolateral prefrontal cortex and dorsal striatum) result in ineffective comparison between choice options. The third hypothesis postulates on the involvement of the ventral frontostriatal subsystem (orbitofrontal cortex, ventromedial prefrontal cortex, ventral striatum, and amygdala) in ADHD, and proposes that dopamine dysregulation in this system results in atypical valuation of delayed rewards and disrupts learning of prediction of future outcomes. This theory relates to our findings in a few ways. First, our findings corroborate this theory, wherein we identify the involvement of brain networks in ADHD neurobiology, as opposed to isolated brain regions. Indeed the same systems involved in decision-making highlighted in this prior work are identified here. Second, as with this prior theoretical framework, our findings suggest that alterations in a given network may affect a given individual to various degrees.
4.3. Limitations
It is crucial to emphasize that in the addiction model the positive connections mean excitatory connections, while negative mean inhibitory connections. In our model one cannot know whether a connection is excitatory or inhibitory. While we use the addiction model here as a heuristic for our data, we do not interpret the functional connectivity data as revealing information about excitatory or inhibitory synapses. Previous studies suggest systems that are positively correlated may be interpreted as integrated systems, while the ones negatively correlated may be highly segregated (Castellanos et al., 2008, Fox et al., 2005, Fox et al., 2009).
The interpretation of negative correlations is especially controversial because they only become strongly evident after regressing out the global signal; therefore some argue that negative connections in functional data may be purely a statistical artifact (Murphy et al., 2009). Studies have shown that they provide biologically plausible and relevant information (Fox et al., 2009). Other work in animal models highlights the improved correspondence of functional connectivity to secondary measures (e.g. viral tracings of axonal tracts) after the use of global regression (Miranda-Dominguez et al., 2014). Such improvements, also consistent with other reports (Fox et al., 2009, Keller et al., 2013), likely reflect the reduction in shared variance amongst regions due to noise and a true global signal (Scholvinck et al., 2010) – an important consideration for the current report.
We also note that while the size of our overall sample is quite large, the sample size of each subgroup is significantly reduced after applying the community detection procedure (e.g., see subgroup A which was the only subgroup to show effects with delay discounting). Studies with larger sample sizes are needed to support our findings. Second, additional delay tasks or tests of impulsivity would have been useful to further characterize the subgroups behaviorally and to evaluate whether specific patterns of connectivity of the reward ROI are associated with specific types of impairment.
5. Conclusions
Our findings support that combining graph theory with neuroimaging data may represent a valuable approach for classifying children with ADHD and typical development; for informing ADHD heterogeneity; and for investigating ADHD neurobiology. Research examining other brain systems is needed to further validate our approach. Future studies should evaluate how community organization relates to long-term outcomes, such as prognosis and response to treatment. Also, a longitudinal study would be useful to evaluate whether the community organization is stable over time. Similar methods have been used to assess behavioral and temperamental variation in ADHD and typically developing populations (Fair et al., 2012, Gates et al., 2014, Karalunas et al., 2015). In the future, neuroimaging, temperament, neuropsychological, and possibly genetic data should be combined in order to improve the ability of identifying subgroups of individuals with and without ADHD based on neurobiological information.
Financial support
This work was funded by R01 MH096773 (Fair), K99/R00 MH091238 (Fair), and R01 MH086654 (Nigg). The funding sources had no involvement in study design, data collections, analysis and interpretation of data, writing of the report, or in the decision to submit the article for publication.
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
The authors thank Corinne Stevens, David Grayson, and BriaThurlow for their valuable help with data collection and preparation; and Julia Grieser-Painter and Colleen Schmitt for coordinating recruitment and participant scheduling. We also thank the participants of the study and their families.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2014.12.005.
Contributor Information
Taciana G. Costa Dias, Email: tacigcdias@gmail.com, costadias@usp.br.
Damien A. Fair, Email: faird@ohsu.edu, damien.fair@aya.yale.edu.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bitsakou P., Antrop I., Wiersema J.R., Sonuga-Barke E.J. Probing the limits of delay intolerance: preliminary young adult data from the Delay Frustration Task (DeFT) J. Neurosci. Methods. 2006;151(1):38–44. doi: 10.1016/j.jneumeth.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Boccaletti S., Latora V., Moreno Y., Chavez M., Hwang D. Complex networks: structure and dynamics. Phys. Rep. 2006;424(4–5):175–308. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N.Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., Shaw D., Shehzad Z., Di Martino A., Biswal B., Sonuga-Barke E.J., Rotrosen J., Adler L.A., Milham M.P. Cingulate–precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K. 3rd ed. Multi-Health Systems Inc.; Toronto: 2008. Conners Manual. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costa Dias T.G., Wilson V.B., Bathula D.R., Iyer S.P., Mills K.L., Thurlow B.L., Stevens C.A., Musser E.D., Carpenter S.D., Grayson D.S., Mitchell S.H., Nigg J.T., Fair D.A. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A., Casimo K., Fair D.A., Nagel B.J. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221(3):210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide J.A., Pliszka S.R. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29(6):656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top–down control. Trends Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G., Power T., Anastopoulos A., Reid R. Guilford Press; New York: 1998. ADHD Rating Scales-IV: Checklists, Norms and Clinical Interpretation. [Google Scholar]
- Fair D.A., Bathula D., Nikolas M.A., Nigg J.T. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc. Natl. Acad. Sci. U. S. A. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Posner J., Nagel B.J., Bathula D., Dias T.G., Mills K.L., Blythe M.S., Giwa A., Schmitt C.F., Nigg J.T. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2010;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Schlaggar B.L., Cohen A.L., Miezin F.M., Dosenbach N.U., Wenger K.K., Fox M.D., Snyder A.Z., Raichle M.E., Petersen S.E. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa E., Bado P., Tripp G., Mattos P., Wickens J.R., Bramati I.E., Alsop B., Ferreira F.M., Lima D., Tovar-Moll F., Sergeant J.A., Moll J. Abnormal striatal BOLD responses to reward anticipation and reward delivery in ADHD. PLOS ONE. 2014;9(2):e89129. doi: 10.1371/journal.pone.0089129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates K.M., Molenaar P.C., Iyer S.P., Nigg J.T., Fair D.A. Organizing heterogeneous samples using community detection of GIMME-derived resting state functional networks. PLOS ONE. 2014;9(3):e91322. doi: 10.1371/journal.pone.0091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S.E. The diagnosis of mental disorders: the problem of reification. Annu. Rev. Clin. Psychol. 2010;6:155–179. doi: 10.1146/annurev.clinpsy.3.022806.091532. [DOI] [PubMed] [Google Scholar]
- Hyman S.E. Can neuroscience be integrated into the DSM-V? Nat. Rev. Neurosci. 2007;8(9):725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Johnson M.W., Bickel W.K. An algorithm for identifying nonsystematic delay-discounting data. Exp. Clin. Psychopharmacol. 2008;16(3):264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S., Phillips A.G., Insel T.R. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol. Psychiatry. 2012;17(12):1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Karalunas S.L., Fair D.A., Musser E.D., Aykes K., Iyer S.P., Nigg J.T. Toward biologically-based nosology: ADHD subtyping using temperament dimensions. Arch. Gen. Psychiatry. 2015 (in press) [Google Scholar]
- Karrer B., Levina E., Newman M.E. Robustness of community structure in networks. Phys. Rev. E. 2008;77(4 Pt 2):046119. doi: 10.1103/PhysRevE.77.046119. [DOI] [PubMed] [Google Scholar]
- Keller C.J., Bickel S., Honey C.J., Groppe D.M., Entz L., Craddock R.C., Lado F.A., Kelly C., Milham M., Mehta A.D. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J. Neurosci. 2013;33(15):6333–6342. doi: 10.1523/JNEUROSCI.4837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kochunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin F.M., Maccotta L., Ollinger J.M., Petersen S.E., Buckner R.L. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11(6 Pt 1):735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miranda-Dominguez O., Mills B.D., Grayson D., Woodall A., Grant K.A., Kroenke C.D., Fair D.A. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure–function relationships and network topology. J. Neurosci. 2014;34(16):5552–5563. doi: 10.1523/JNEUROSCI.4229-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell S.H. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Muller U.C., Sonuga-Barke E.J., Brandeis D., Steinhausen H.C. Online measurement of motivational processes: introducing the Continuous Delay Aversion Test (ConDAT) J. Neurosci. Methods. 2006;151(1):45–51. doi: 10.1016/j.jneumeth.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M.E.J. Vol. 45. 2003. pp. 167–256. (The Structure and Function of Complex Networks). [Google Scholar]
- Newman M.E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. U. S. A. 2006;103(23):8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol. Psychiatry. 2005;57(11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Casey B.J. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Willcutt E.G., Doyle A.E., Sonuga-Barke E.J. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol. Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Nova Southeastern University; Ft. Lauderdale, FL: 1995. Schedule for Affective Disorders and Schizophrenia for School-Aged Children – Epidemiologic Version 5 (K-SADS-E) [Google Scholar]
- Plichta M.M., Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Vasic N., Wolf R.C., Lesch K.P., Brummer D., Jacob C., Fallgatter A.J., Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2009;65(1):7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H., Raineri A., Cross D. Subjective probability and delay. J. Exp. Anal. Behav. 1991;55(2):233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Snyder A.Z. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Rommelse N.N., Altink M.E., Fliers E.A., Martin N.C., Buschgens C.J., Hartman C.A., Buitelaar J.K., Faraone S.V., Sergeant J.A., Oosterlaan J. Comorbid problems in ADHD: degree of association, shared endophenotypes, and formation of distinct subtypes. Implications for a future DSM. J. Abnorm. Child Psychol. 2009;37(6):793–804. doi: 10.1007/s10802-009-9312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M., Bergstrom C.T. Maps of random walks on complex networks reveal community structure. Proc. Natl. Acad. Sci. U. S. A. 2008;105(4):1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Aase H., Zeiner P., Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav. Brain Res. 1998;94(1):61–71. [PubMed] [Google Scholar]
- Scheres A., Milham M.P., Knutson B., Castellanos F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scholvinck M.L., Maier A., Ye F.Q., Duyn J.H., Leopold D.A. Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. U. S. A. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds J., Rothbart M.K. Paper Presented at Occasional Temperament Conference. 2004. The temperament in middle childhood questionnaire (TMCQ): a computerized self-report measure of temperament for ages 7–10. [Google Scholar]
- Sonuga-Barke E., Bitsakou P., Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(4):345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol. Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol. Psychiatry. 2012;72(2):126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Tomasi D., Volkow N.D. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2012;71(5):443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Tomasi D., Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. U. S. A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Kollins S.H., Wigal T.L., Newcorn J.H., Telang F., Fowler J.S., Zhu W., Logan J., Ma Y., Pradhan K., Wong C., Swanson J.M. Evaluating dopamine reward pathway in ADHD: clinical implications. J. Am. Med. Assoc. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Newcorn J.H., Kollins S.H., Wigal T.L., Telang F., Fowler J.S., Goldstein R.Z., Klein N., Logan J., Wong C., Swanson J.M. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol. Psychiatry. 2011;16(11):1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J., Jr., Roddey J.C., Erhart M., McCabe C., Akshoomoff N., Amaral D.G., Bloss C.S., Libiger O., Schork N.J., Darst B.F., Casey B.J., Chang L., Ernst T.M., Frazier J., Gruen J.R., Kaufmann W.E., Murray S.S., van Zijl P., Mostofsky S., Dale A.M. Pediatric imaging, neurocognition, and genetics study, long-term influence of normal variation in neonatal characteristics on human brain development. Proc. Natl. Acad. Sci. U. S. A. 2012;109(49):20089–20094. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 4th ed. WISC-IV®/Harcourt Assessment; San Antonio, TX: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Wilson V.B., Mitchell S.H., Musser E.D., Schmitt C.F., Nigg J.T. Delay discounting of reward in ADHD: application in young children. J. Child Psychol. Psychiatry. 2011;52(3):256–264. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.