Abstract
Patients with schizophrenia can sometimes report strange face illusions when staring at themselves in the mirror; such experiences have been conceptualized as anomalous self-experiences that can be experienced with a varying degree of depersonalization. During adolescence, anomalous self-experiences can also be indicative of increased risk to develop schizophrenia-spectrum disorders. To date however, the Mirror-Gazing test (MGT), an experimentally validated experiment to evaluate the propensity of strange face illusions in nonclinical and clinical adults, has yet to be investigated in an adolescent sample. The first goal of the present study was to examine experimentally induced self-face illusions in a nonclinical sample of adolescents, using the MGT. The second goal was to investigate whether dimensions of adolescent trait schizotypy were differentially related to phenomena arising during the MGT. One hundred and ten community adolescents (59 male) aged from 12 to 19 years (mean age = 16.31, SD age = 1.77) completed the MGT and Schizotypal Personality Questionnaire. The results yielded 4 types of strange face illusions; 2 types of illusions (slight change of light/color [20%] and own face deformation [45.5%]) lacked depersonalization-like phenomena (no identity change), while 2 other types (vision of other identity [27.3%], and vision of non-human identity [7.3%]) contained clear depersonalization-like phenomena. Furthermore, the disorganization dimension of schizotypy associated negatively with time of first illusion (first press), and positively with frequency of illusions during the MGT. Statistically significant differences on positive and disorganized schizotypy were found when comparing groups on the basis of degree of depersonalization-like phenomena (from slight color changes to non-human visions). Similarly to experimentally induced self-face illusions in patients with schizophrenia, such illusions in a group of nonclinical adolescents present significant associations to schizotypy dimensions.
Key words: schizotypal, schizotypy, visual perception, misattribution of self-agency, strange-face-in-the mirror illusion, self-recognition
Introduction
A number of different theoretical conceptualizations of schizophrenia consider anomalies of subjective experience (ASE) as a central feature of schizophrenia and related spectrum conditions.1–5 ASE encompass a broad range of subtle disturbances in affect, perception processes, thought, experience of cognition, and bodily experiences (eg, somatic depersonalization). In healthy adult samples, self-face illusions can be induced by looking at oneself in the mirror under dimmed light conditions and represent rather typical phenomena associated to sensory deprivation procedures.6,7 Such experiences have been conceptualized as ASE that can be experienced with a varying degree of depersonalization, ie, having the impression of seeing another “identity” than oneself in the mirror. In patients with schizophrenia, day-to-day experience can sometimes be punctuated by self-face illusions when staring at themselves in the mirror.8 Patients that have participated in the experimental task inducing such self-face illusions typically report more frequent and disturbing illusions.8
One important developmental feature of disturbances in self-experience is that they often precede the acute onset of clinically significant psychotic states. Previous empirical studies show that ASE constitute a potential liability phenotype for psychosis in both clinical and genetic high-risk samples,9–11 and in a nonpsychotic clinical population.12 For instance, ASE were associated with increasing schizotypal phenotypic expressivity in nonpsychotic genetically high-risk samples.9 During adolescence and young adulthood, ASE can be indicative of increased risk to develop schizophrenia-spectrum disorder.10 For example, Nelson et al10 found that the presence of anomalous self-experiences were significantly higher in individuals at ultra high risk (UHR) (ages 15–25 y old) compared with the healthy control sample, and the total score of the Examination of Anomalous Self-Experience13 predicted the onset of psychotic disorder. ASE can also be observed among nonpsychotic help-seeking adolescents and moderately associated with prodromal symptoms.14 Recently, experimental designs have been developed to measure potential cognitive components of the basic sense of self in the UHR population.15 For instance, Jia et al15 found that self-face recognition, a cognitive index of basic disturbance and measured by an experimental task, was deficient in individuals at UHR compared with a control group. Similarly to self-face recognition, experimentally induced ASE could represent a proxy measure of proneness to disturbances in self-experience conferring risk to develop schizophrenia-spectrum disorders.
Several experimental procedures inducing aberrant perceptual experiences have been employed in psychosis and in clinical, genetic, and psychometric high-risk samples.6,16–19 Among these, Caputo6,7 developed an experimental task to examine the apparitional experiences and perceptual illusions during mirror gazing, called Mirror-Gazing test (MGT). In a first study including 50 healthy young adult volunteers, most participants engaging in the MGT began to perceive apparitions after about 1 min. Such apparitional experiences were described as “strange-face in the mirror” illusions.6,7 After a 10-min session of self-face mirror gazing, participants tended to report having experienced striking effects, including significant deformations of one’s own face (reported by 66% of individuals); perceiving a relative’s face with the features changed (18%), of whom 8% were still alive and 10% were deceased; an unknown person (28%); an archetypal face, such as that of an old woman, a child, or an image of an ancestor (28%); an animal face such as that of a cat or a pig (18%); and monstrous beings (48%).7 It should be noticed that the sum of percentages is not 100%, because the average number of different strange faces7 was 2.6±0.1 per 10-min sessions. In another study, MGT was administered to patients with schizophrenia and compared with healthy controls. Apparitions of strange faces in the mirror were significantly more frequent and intense in patients with schizophrenia in comparison to controls.8
From the vantage point of research in clinical and personality psychology, investigations on the multidimensional construct of schizotypy have evidenced the presence of phenomena such as magical thinking, psychotic-like hallucinations, or paranoid ideation throughout youth development.20,21 Schizotypal traits, as an indicator of the extended psychosis phenotype, are thought to represent the behavioral expression of latent vulnerability to psychosis.21 Independent follow-up studies show that children and adolescents who report schizotypal traits and/or psychotic-like experiences, compared with those who do not report such experiences, are at greater risk of transition to psychosis or other psychiatric disorders.22–24 Furthermore, healthy adolescents who report schizotypal traits also show subtle emotional, behavioral, cognitive, neuropsychological, and/or social deficits,25–29 similar to those found in patients with psychosis. Particularly, schizotypy was associated with, social cognitive impairments (ie, emotional processing of facial expressions and face recognition), and atypical neural activation during self-evaluations in nonclinical adolescents.30–32 Furthermore, García-Álvarez et al32 found that adolescents with high scores on cognitive-perceptual and disorganization schizotypy dimensions were significantly associated with face recognition difficulties. Such preliminary results suggest that trait schizotypy could already increase the risk of experiencing significant ASE during adolescence. From developmental psychopathology framework, adolescence is an important developmental stage for socioemotional development, but is also marked by the emergence of mental health problems, specifically, psychotic disorders, symptoms, and experiences. Importantly adolescence is the most appropriate time for studying possible risk markers for schizophrenia, as detection and early intervention strategies previous to the development of the psychosis risk syndromes (eg, UHR states, basic symptoms, etc.) could be initiated on the basis of potent risk markers. Moreover, confounding factors such as psychotic illness duration and long-term antipsychotic medication are avoided in nonclinical adolescents.
To summarize, recent research is starting to draw a link between trait schizotypy and ASE, as well as between schizotypy and difficulties in (self) face recognition across different gradients of the psychosis continuum. In this context, evaluating ASE during adolescence could critically contribute to: (1) Evaluating whether schizotypy and ASE are related during adolescence; (2) If so, specify which dimension(s) of schizotypy may specifically relate to ASE during adolescence. We may hypothesize that cognitive-perceptual and disorganization schizotypy would be associated to an increased propensity to experience ASE. One way to examine such an association is to evaluate the relationship between adolescent trait schizotypy and experimentally induced ASE, using the MGT. To date, this experimental task has yet to be investigated in an adolescent sample. Following these considerations, the first goal of the present study was to explore experimentally induced self-face illusions in a sample of nonclinical adolescents using the MGT. We anticipated that similarly to healthy young adults6,7 self-face illusions would be common in adolescents and encompass a varying degree of depersonalization-like phenomena. The second objective was to evaluate the putative relationship between MGT-induced phenomena and self-reported schizotypy scores. Consistent with a previous study involving patients diagnosed with schizophrenia, we predicted that dimensions of schizotypy will be associated with the frequency of illusions during the MGT. Furthermore, we anticipated that severity in depersonalization-like phenomena during the MGT would be associated to the extent of self-reported schizotypy.
Methods
Participants
Participants in this research were French-speaking community adolescents attending schools in the city of Geneva, Switzerland. To be eligible to participate in the study, youths needed to be aged between 12 and 19 years, French-native speakers and receive parental consent. After a telephone contact during which research objectives were explained to parents and potential adolescent participants (n = 131), families decided whether they wished to volunteer for the study; 9 parents declined, 8 adolescents declined, and 4 failed to show up at their scheduled appointments. The final sample encompassed 110 adolescents (mean age = 16.31, SD age = 1.77; 59 boys), including 81.8% white Caucasian, 9.1% other (African, Asian), and 9.1% mixed ethnicity. Participants were primarily from middle socioeconomic status (n = 87). Block design and vocabulary were used as intelligent quotient (IQ) estimation.33 Exclusion criteria were as follows: adolescents in an acute psychotic phase or reporting drug abuse, patients with comorbid autism or pervasive developmental disorder, estimated IQ lower than 80, and adolescents with mental retardation. Each adolescent received financial compensation for completing this study. Written informed consent was received from participants and their parents under protocols approved by the Institutional Review Board of the Department of Psychiatry of the University of Geneva Medical School. The study took place at Office Médico-Pédagogique, in the research department of the local outpatient service for children and adolescents. This study is integrated in a broader research looking at the link between mentalizing skills and personality traits during adolescence.
Instruments
The Schizotypal Personality Questionnaire (SPQ)34 is a self-report instrument made up of 74 items with a dichotomous response format (Yes/No). The instrument was developed for measuring schizotypal traits according to DSM-III-R criteria.35 Items are distributed across 9 subscales: odd beliefs or magical thinking, unusual perceptual experiences, ideas of reference, paranoid ideation/suspiciousness, excessive social anxiety, no close friends, constricted affect, odd or eccentric behavior, and odd speech. The 9 subscales are collapsed in 3 schizotypy dimension: positive (including magical thinking, unusual perceptual experiences, ideas of reference, and paranoid ideation), negative (including excessive social anxiety, constricted affects, and no close friends), and disorganized (namely odd behavior and speech). The psychometric properties of the SPQ have been widely analyzed in both adults and nonclinical adolescents.36–39 A French version of the SPQ validated in adolescents was used.40
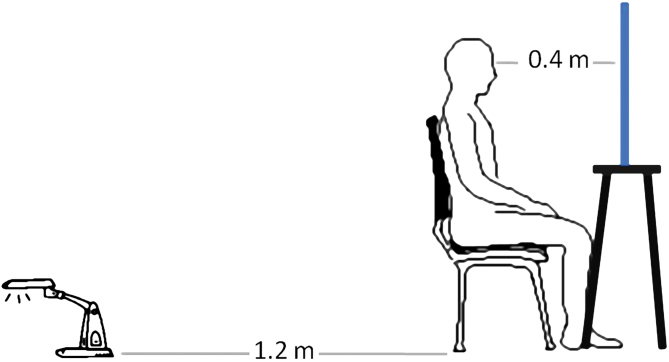
MGT6,7: The MGT was conducted in a darkened room, a portion of which (2 × 3 m) isolated the subject seating in front of a mirror, in between a white wall and a white screen. The large mirror (0.5 × 0.5 m) was mounted on a tripod in the center of the room. The subjects were seated at a distance of 0.4 m in front of the mirror (see figure 1). The room was lit only by a halogen light bulb (12V, 20W). The bulb was mounted on a spotlight placed 1.2 m behind the subjects so that they could not see it. The light bulb beam was directed toward the floor and the distance between bulb and floor was about 5cm. This beam set-up provided diffuse, indirect lighting over the whole room. All the fine facial features could be perceived in detail; colors were attenuated. An experimenter, seated behind the screen, tested participants in random order and blind design conducted the MGT. Before the beginning of the task, the experimenter explained the task: “Your task is to look at your face in the mirror. You should keep staring into your eyes. The task will last 10 minutes.” The experimenter told the participants how to use the keyboard, placed the keyboard on participants’ lap out of view of the specular frame, using the following words: “During the 10 minutes while you are looking at your face in the mirror and staring at your eyes you may or may not notice changes in your face. If you notice a change then press this button and hold it down for as long as the change lasts. If you do not notice any changes then do not press the button.” Subjects were then asked if they understood the task, and after the experimenter had further clarified any ambiguous points, the task began.
Fig. 1.
The set-up for the Mirror-Gazing test (MGT).
*From Caputo et al8, permission granted by Elsevier Limited.
During the MGT the subjects’ perceptions were qualitatively and quantitatively assessed. The number and latency of abnormal perceptions were evaluated by recording event-related responses to apparitional experiences. Every time subjects had an abnormal perception, they had to press a button and their responses were digitally recorded using COGENT software (http://cogent.psyc.bbk.ac.uk). The mirror-gazing session lasted 10 min. When the 10-min MGT ended, the experimenter distributed a post-task–standardized questionnaire to the participants. The following questions were then used in the analyses: During the task, have you: (1) Noticed a change in light, color or contrast? If yes, describe; (2) Did you observe any particular movement? If yes, describe; (3) Did you see another person in the mirror? If yes describe; (4) Please provide a listing of all types of modifications you saw during the task.
Procedure
Participants were administered a battery of self-report questionnaires assessing the expression of schizotypal personality traits and MGT. To ensure that all participants understood the items, trained clinical psychologists (M.D. and D.B.) supervised this process.
Data Analyses
First, we calculated descriptive statistics for the MGT and SPQ dimensions. Four MGT quantitative scores (first onset, frequency, mean duration, and cumulative duration) were used.8 For event-related responses, the mean onset of the first apparition was defined as the first time the subject pressed the button. The frequency of event-related responses was defined as the number of times subjects pressed the response button, averaged per minute. The mean duration was the mean time they held the response button down. The cumulative duration of apparitions was defined as the sum of durations of apparitions in MGT, averaged per minute (the cumulative duration equals the algebraic product of frequency and mean duration). Second, from a phenomenological point of view, all participants were classified into 4 groups on the basis of the increasing number of depersonalization-like phenomena they reported in the questionnaire. The classification provided 4 illusion categories qualitatively varying in degree of depersonalization-like phenomena: (1) slight change of light/color; (2) own face deformation; (3) vision (other facial identity); (4) non-human vision. Individuals were classified into one of these groups on the basis of the most significant (depersonalized) illusion they reported. This kind of classification was done in order to better account for the phenomenology with adolescents and to provide a classification closer to what can be experienced in basic self-disturbances in front of a mirror. It should be noticed that this classification was different to that proposed by Caputo6,7, who did a classification of the contents of self-face illusions; a comparable classification with reports from our adolescent sample is provided in the qualitative description section of the results.
Mean scores of the 4 event-related responses on the MGT groups were compared using MANOVA. Third, in order to examine the relationship between schizotypy dimensions and both MGT scores and illusion categories 2 statistical analyses were performed. On the one hand, multiple hierarchical regression analyses were conducted to assess the relationships between MGT scores and schizotypy dimensions. For each MGT score a regression analysis was performed. MGT scores were used as dependent variables and schizotypy dimensions were used as independent predictor variables. In step 1, we controlled the variance explained by demographic variables (gender and age) before entering in step 2 the 3 schizotypy dimensions (positive, negative, and disorganized). The variance inflation factor was used as an indicator of multicollinearity. On the other hand, we examined mean scores of the MGT groups across the 3 schizotypy dimensions. In order to do this, a MANOVA was conducted, taking the SPQ dimensions as the dependent variables, and MGT groups as the fixed factor. As an estimate of effect size, partial eta squared (partial η2) was employed. For post-hoc tests Bonferroni correction was applied. The statistical analyses were carried out using SPSS 15.0.41
Results
MGT: Quantitative Scores
The mean, SD, skewness, kurtosis, and range for the MGT event-related scores are shown in table 1. A total of 20 adolescents did not report perceptual illusions or apparitional experiences. Statistically significant differences were found by gender on first press time (t (89,68) = 2.107; P = .04), where males pressed the first time later than females. None of the MGT event-related scores was correlated with age.
Table 1.
Descriptive Statistics for the Mirror-Gazing Test (MGT) Scores and Schizotypal Personality Questionnaire (SPQ) Dimensions
| Mean | SD | Skewness | Kurtosis | Range | |
|---|---|---|---|---|---|
| MGT | |||||
| First press | 51.24 | 73.64 | 2.54 | 6.84 | 0–374.7 |
| Frequency press | 16.44 | 16.16 | 1.03 | 0.39 | 0–65 |
| Mean duration | 3.80 | 5.61 | 3.86 | 19.84 | 0–40.7 |
| Cumulative duration | 70.54 | 80.49 | 1.42 | 2.43 | 0–423.1 |
| SPQ | |||||
| Positive | 8.05 | 6.47 | 0.94 | 0.13 | 0–27 |
| Negative | 6.18 | 4.12 | 0.51 | −0.39 | 0–18 |
| Disorganized | 5.61 | 3.91 | 0.47 | −0.59 | 0–16 |
MGT: Qualitative Descriptions
The following types of strange face illusions were found in this sample: 20% (n = 22) reported only slight change of light/color, 45.45% (n = 50) reported own face deformation, 27.27% (n = 30) reported vision, and 7.27% (n = 8) reported non-human vision. No statistically significant differences were found by gender (χ 2 = 0.948; P = .95). The mean comparison between the 4 illusion groups on MGT event-related scores showed statistically significant differences on frequency (F (3,106) = 7.440; P = .001; partial η2 = 0.174), mean duration (F (3,106) = 2.919; P = .037; partial η2 = 0.076), and mean cumulative duration (F (3,106) = 7.904; P = .001; partial η2 = 0.183), but not on first press time (F (3,106) = 2.909; P = .890; partial η2 = 0.006). MGT slight group scored lower than the other 3 groups on frequency (P = .001). MGT non-human vision group scored higher than slight group on mean duration (P = .006). Significant differences were found for mean cumulative duration scores between all MGT groups (non-human vision > vision > own face deformation > slight) (P < .01).
For purpose of comparison with Caputo6,7, we found the following prevalence of each of the following phenomena: significant deformations of one’s own face (79%); perceiving a relative’s face (1.8%); an unknown person (24.5%); an archetypal face, such as that of an old woman, a child, or an image of an ancestor (5.5%); an animal face such as that of a cat or a pig (4.5%); and monstrous beings (3.6%).
MGT and Schizotypy Dimensions
The descriptive statistics of the schizotypy dimensions are shown in table 1. No statistically significant differences were found by gender on the schizotypy dimensions (positive: t (108) = −1.372, P = .173; negative: t (108) = −0.962, P = .338; disorganized: t (108) = −0.582, P = .562). None of the 3 schizotypy dimensions were correlated with age. Regression models revealed that, after controlling for gender and age, the disorganized dimension predicted MGT first time press (R 2 = 0.08; B = −3.606; SE = 1.762; β = −0.191; t = −2.05; P = .014) and frequency (R 2 = 0.06; B = 0.977; SE = 0.391; β = 0.236; t = −2.50; P = .043). Disorganization scores were negatively associated with first press and positively associated with frequency of MGT. None of the 3 schizotypy dimensions predicted mean duration or mean cumulative duration of MGT (all P > .05).
The mean comparison between the 4 groups of MGT and schizotypy dimensions showed statistically significant differences on positive schizotypy (F (3,106) = −2.953; P = .036; partial η2 = 0.077) and disorganized schizotypy (F (3,106) = −4,313; P = .007; partial η2 = 0.109). The mean scores on schizotypy dimensions for the 4 MGT groups are displayed in table 2. Several post-hoc comparisons between the 4 groups in the mean scores of schizotypy dimensions were statistically significant (P < .05). In positive schizotypy dimension own face deformation group scored lower than vision group, and participants from slight group scored lower than vision group. For the disorganized schizotypy dimension, non-human vision group scored higher than own face deformation and slight groups, and vision group scored higher than own face deformation and slight group.
Table 2.
Mean Comparison Mirror-Gazing Test (MGT) Qualitative Groups and Schizotypy Dimensions
| MGT Groups | Schizotypy Dimensions | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | Disorganized | ||||
| Meana | SD | Mean | SD | Meanb | SD | |
| Own face deformation | 6.90 | 5.41 | 5.72 | 3.88 | 4.94 | 3.80 |
| Non-human vision | 10.13 | 9.86 | 7.13 | 4.91 | 8.25 | 4.27 |
| Slight | 6.45 | 5.06 | 5.95 | 3.55 | 4.23 | 2.99 |
| Vision | 10.60 | 7.32 | 6.87 | 4.72 | 7.03 | 3.98 |
Note: aPositive schizotypy: own face deformation < vision; slight < vision.
bDisorganized schizotypy: slight < non-human vision; own face deformation < non-human vision; slight < vision; own face deformation < vision.
P < .05.
Discussion
The present study examined experimentally induced self-face illusions using the MGT in a sample of nonclinical adolescents. We further examined whether dimensions of trait schizotypy were differentially related to phenomena arising during the MGT. The results show that the MGT constitutes a reliable tool to induce strange face illusions during adolescence. We observed 4 types of strange face illusions and discriminated them between reporting no depersonalization phenomena (no identity change) in the specular image vs reporting depersonalization-like phenomena (identity change; vision) in the specular image during the MGT.
The results found in the present study are consistent with those reported in young adults6,7 and in a clinical sample of patients with schizophrenia.8 We observed that 20% of the sample reported slight change of light/color and 45.45% reported own face deformations. In terms of visions, 27.27% reported seeing another identity in the specular image, and 7.27% reported seeing a non-Human vision. In comparison to Caputo7 who administered the MGT to 50 naive young adults, some of the frequencies of apparitions appear reduced in our adolescent sample. While the comparison between seeing another identity in our adolescent sample (27.3%) is comparable to seeing unknown people in the young adult sample (28%), own face deformations and seeing non-human beings are more prevalent in the young adult sample.7 The discrepancy between the findings could be due to age, although we did not find a significant correlation between MGT results and age in our adolescent sample. It is possible that recruitment strategy might have partly contributed to the discrepancy between the 2 samples. Indeed, a number of studies on sensory deprivation suggest that volunteering for such studies as well as prior knowledge on sensory deprivation effects can influence the phenomena experienced in such experimental settings.42,43 Our adolescent sample did not have information concerning the MGT prior to participation, which may have partly contributed to the decreased prevalence of illusions in comparison to the young adult sample. Furthermore, the fact that testing occurred in a service traditionally associated with treatment of psychological problems might have primed the subjects to “inhibit” content that could be perceived as a sign of mental illness.44 However, the fact that we do find a high prevalence of self-face illusions in the current adolescent sample, in association with schizotypal traits in day-to-day settings is consistent with both early and recent findings in adult samples suggesting that illusions during lowered sensory stimulation42,43 are more frequent in individuals reporting schizotypal traits.
When administered in a sample of adults with schizophrenia, the MGT yields more significant illusions in the patient group compared with controls.8 Specifically, patients reported more frequent apparitions, for an increased cumulative duration, a greater variety of types of strange faces; apparitions are reported by patients as more powerfully experienced with a greater sense of reality in comparison to controls. It is noteworthy to observe that similarly to experimentally induced self-face illusions in patients with schizophrenia, such illusions in a group of nonclinical adolescents present significant associations to schizotypy dimensions. More precisely, adolescent disorganized schizotypy correlated negatively with time of first illusion (first press), and positively with frequency of illusions during the MGT. Moreover, statistically significant differences on positive and disorganized schizotypy were also found when comparing groups on the basis of degree in depersonalization-like phenomena (from slight color changes to non-human visions). The patterns of result, in comparison to observations in adults with schizophrenia, suggest that depersonalization-like phenomena experienced during the MGT are significantly related to schizotypy dimensions already in earlier stages of development. Previous studies have shown that schizotypy was associated with ASE during adolescence,30 as well as face recognition difficulties32 and poorer facial emotion recognition.31 Future studies should investigate how self-face illusions are related to deficits in social cognition (eg, self-face recognition), and how they could constitute an indirect indicator of basic disturbances of self.45 The results from this group of adolescents, if replicated, would suggest that one of the pathways through which schizotypy is predictive of full-blown psychosis is by virtue of its association with an increased propensity to experience ASE in low stimulation settings.46 This working hypotheses would benefit from a longitudinal design where other ASE would be periodically evaluated from adolescence to early adulthood in high-risk youths.
Previous studies show that ASE constitute a liability marker for psychosis in high-risk populations,9–11 and in nonpsychotic clinical population.12 Moreover, anomalies of self-experience are related with increasing schizotypal phenotypic expressivity.9 The MGT, when used to experimentally induce self-face illusions, could serve as a proxy measure to test proneness to ASE in developmental periods where interview methods are less reliable. Experimental assessments of induced perceptual aberrations were also reported by Galdos et al16 Using a white noise task, the authors found that the tendency to detect speech illusions in random noise was more prevalent in patients with psychosis, progressively greater across groups with increasing familial risk, and associated with high levels of positive schizotypy in healthy controls and siblings of patients. Overall, these results suggest that aberrant phenomena induced by some experimental tasks can be linked to individual differences in schizotypy on the clinical continuum of psychosis. Further research is required to evaluate the additive value of tasks such as the MGT in improving the accuracy for identification of individuals at risk for developing psychosis. The MGT phenomena may also motivate neuroimaging studies interested in modeling ASE. Several reports have emphasized a specific alteration in self-face recognition in patients with schizophrenia.47 More recently, functional neuroimaging studies have identified regions such as the inferior frontal gyrus and disconnectivity between the right supramarginal gyrus, cuneus, and prefrontotemporal brain areas as sustaining self-face recognition impairments in patients with schizophrenia.48,49 Another line of research currently investigates how subtle impairments in the regulation of excitatory/inhibitory balance in schizophrenic patients may lead to the propagation of delusional experiences, especially in conditions where the individual faces ambiguous stimuli.50 Future studies using the MGT should investigate altered connectivity and current models of neural predictive coding to potentially uncover the neural dynamics of strange-face-in-the-mirror illusions.
The results obtained in the present study must be interpreted in light of the following limitations. First, the developmental stage of adolescence is understood herein as the period from 10 to 19 years of age, acknowledging that characteristics of this stage may extend up to age 24; however, our sample does not cover the entire 10–24 years range of adolescence. The age range used was limited to teenage years and could be broadened to include late childhood and early adulthood. In addition, the present results must be framed in a developmental perspective and further evaluated to understand their natural developmental course. Second, sample characteristics preclude the generalization of the results to other populations of interest. Third, we must not lose sight of the transversal nature of this research, for which it is not possible to establish any cause-effect inferences.
Future studies should examine the MGT in high-risk samples, and perhaps evaluate other variables such as culture. Finally, the neural bases of ASEs during the MGT could be evaluated, with a specific attention to atypical connectivity that might sustain the experiences of strange-face-in-the-mirror illusions. Indeed, several hypotheses are currently being tested.
Conclusions
MGT constitutes a reliable tool to induce strange face illusions during adolescence. Schizotypy dimensions are differentially related to phenomena arising during the MGT. Similarly to experimentally induced self-face illusions in patients with schizophrenia, such illusions in a group of nonclinical adolescents present significant associations to schizotypy dimensions.
Funding
E.F-P. was supported by the Spanish Ministry of Science and Innovation (PSI 2011–28638, PSI 2011–23818) and “Programa Campus de Excelencia Internacional.” M.D. was supported by the Swiss National Science Foundation (100014–135311/1) and the Gertrude Von Meissner Foundation (ME 7871). M.D. and S.E. were supported by the National Center of Competence in Research (NCCR) “SYNAPSY - The Synaptic Bases of Mental Diseases” financed by the Swiss National Research Fund (51AU40-125759).
Acknowledgments
We wish to thank the participants, as well as Matthieu Ischer for his help in setting up the experiment and data collection. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–444. [DOI] [PubMed] [Google Scholar]
- 2. Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44:121–134. [DOI] [PubMed] [Google Scholar]
- 3. Raballo A, Sæbye D, Parnas J. Looking at the schizophrenia spectrum through the prism of self-disorders: an empirical study. Schizophr Bull. 2011;37:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gross G, Huber G, Klosterkötter J, et al. Bonner Skala für die Beurteilung von Basissymptomen. Berlin, Heidelberg: New York Springer-Verlag; 1987. [Google Scholar]
- 5. Nelson B, Parnas J, Sass LA. Disturbance of minimal self (ipseity) in schizophrenia: clarification and current status. Schizophr Bull. 2014;40:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caputo GB. Strange-face-in-the-mirror illusion. Perception. 2010;39:1007–1008. [DOI] [PubMed] [Google Scholar]
- 7. Caputo GB. Apparitional experiences of new faces and dissociation of self-identity during mirror gazing. Percept Mot Skills. 2010;110:1125–1138. [DOI] [PubMed] [Google Scholar]
- 8. Caputo GB, Ferrucci R, Bortolomasi M, et al. Visual perception during mirror gazing at one’s own face in schizophrenia. Schizophr Res. 2012;140:46–50. [DOI] [PubMed] [Google Scholar]
- 9. Raballo A, Parnas J. The silent side of the spectrum: schizotypy and the schizotaxic self. Schizophr Bull. 2011;37:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr Bull. 2012;38:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson B, Fornito A, Harrison BJ, et al. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Biobehav Rev. 2009;33:807–817. [DOI] [PubMed] [Google Scholar]
- 12. Parnas J, Raballo A, Handest P, et al. Self-experience in the early phases of schizophrenia: 5-year follow-up of the Copenhagen Prodromal Study. World Psychiatry. 2011;10:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parnas J, Møller P, Kircher T, et al. EASE: Examination of Anomalous Self-Experience. Psychopathology. 2005;38:236–258. [DOI] [PubMed] [Google Scholar]
- 14. Koren D, Reznik N, Adres M, et al. Disturbances of basic self and prodromal symptoms among non-psychotic help-seeking adolescents. Psychol Med. 2013;43:1365–1376. [DOI] [PubMed] [Google Scholar]
- 15. Jia H, Yang J, Zhu H, et al. Self-face recognition in the ultra-high risk for psychosis population. Early Interv Psychiatry. In press. 10.1111/eip.12097 [DOI] [PubMed] [Google Scholar]
- 16. Galdos M, Simons C, Fernandez-Rivas A, et al. Affectively salient meaning in random noise: a task sensitive to psychosis liability. Schizophr Bull. 2011;37:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffman RE, Woods SW, Hawkins KA, et al. Extracting spurious messages from noise and risk of schizophrenia-spectrum disorders in a prodromal population. Br J Psychiatry. 2007;191:355–356. [DOI] [PubMed] [Google Scholar]
- 18. Vercammen A, Aleman A. Semantic expectations can induce false perceptions in hallucination-prone individuals. Schizophr Bull. 2010;36:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Rijn S, Aleman A, de Sonneville L, et al. Misattribution of facial expressions of emotion in adolescents at increased risk of psychosis: the role of inhibitory control. Psychol Med. 2011;41:499–508. [DOI] [PubMed] [Google Scholar]
- 20. Jardri R, Bartels-Velthuis AA, Debbané M, et al. From phenomenology to neurophysiological understanding of hallucinations in children and adolescents. Schizophr Bull. 2014;40(suppl 4):S221–S232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 22. Welham J, Scott J, Williams G, et al. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol Med. 2009;39:625–634. [DOI] [PubMed] [Google Scholar]
- 23. Dominguez MD, Wichers M, Lieb R, et al. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. 2011;37:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zammit S, Kounali D, Cannon M, et al. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry. 2013;170:742–750. [DOI] [PubMed] [Google Scholar]
- 25. Fonseca-Pedrero E, Paino M, Santarén-Rosell M, et al. Cluster A maladaptive personality patterns in a non-clinical adolescent population. Psicothema. 2013;25:171–178. [DOI] [PubMed] [Google Scholar]
- 26. Debbané M, Badoud D, Balanzin D, Eliez S. Broadly defined risk mental states during adolescence: disorganization mediates positive schizotypal expression. Schizophr Res. 2013;147:153–156. [DOI] [PubMed] [Google Scholar]
- 27. Debbané M, Van der Linden M, Balanzin D, et al. Associations among metacognitive beliefs, anxiety and positive schizotypy during adolescence. J Nerv Ment Dis. 2012;200:620–626. [DOI] [PubMed] [Google Scholar]
- 28. Kelleher I, Keeley H, Corcoran P, et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201:26–32. [DOI] [PubMed] [Google Scholar]
- 29. Kelleher I, Clarke MC, Rawdon C, et al. Neurocognition in the extended psychosis phenotype: performance of a community sample of adolescents with psychotic symptoms on the MATRICS neurocognitive battery. Schizophr Bull. 2013;39:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Debbané M, Vrtička P, Lazouret M, et al. Self-reflection and positive schizotypy in the adolescent brain. Schizophr Res. 2014;152:65–72. [DOI] [PubMed] [Google Scholar]
- 31. Roddy S, Tiedt L, Kelleher I, et al. Facial emotion recognition in adolescents with psychotic-like experiences: a school-based sample from the general population. Psychol Med. 2012;42:2157–2166. [DOI] [PubMed] [Google Scholar]
- 32. García-Álvarez L, Lemos-Giráldez S, Paíno M, et al. Reconocimiento de caras en la esquizotipia [Face recognition in schizotypy]. Anal Psicol. 2013;29:791–799. [Google Scholar]
- 33. Wechsler D. Wechsler Intelligence Scale for Children - Fourth Edition. San Antonio, TX: Harcourt Assessment, Inc; 2003. [Google Scholar]
- 34. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 35. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder. 3rd ed, revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 36. Fonseca-Pedrero E, Fumero A, Paino M, et al. Schizotypal personality questionnaire: new sources of validity evidence in college students. Psychiatry Res. 2014;219:214–220. [DOI] [PubMed] [Google Scholar]
- 37. Wuthrich VM, Bates TC. Confirmatory factor analysis of the three-factor structure of the schizotypal personality questionnaire and Chapman schizotypy scales. J Pers Assess. 2006;87:292–304. [DOI] [PubMed] [Google Scholar]
- 38. Chen WJ, Hsiao CK, Lin CC. Schizotypy in community samples: the three-factor structure and correlation with sustained attention. J Abnorm Psychol. 1997;106:649–654. [DOI] [PubMed] [Google Scholar]
- 39. Fossati A, Raine A, Carretta I, et al. The three-factor model of schizotypal personality: Invariance across age and gender. Pers Individ Diff. 2003;35:1007–1019. [Google Scholar]
- 40. Badoud D, Chanal J, Eliez S, et al. [Validation study of the French schizotypal personality questionnaire in a sample of adolescents: a confirmatory factor analysis]. Encephale. 2011;37:299–307. [DOI] [PubMed] [Google Scholar]
- 41. Statistical Package for the Social Sciences. SPSS Base 15.0 User’s Guide. Chicago, IL: SPSS Inc; 2006. [Google Scholar]
- 42. Jackson CW, Jr, Pollard JC. Some nondeprivation variables which influence the “effects” of experimental sensory deprivation. J Abnorm Psychol. 1966;71:383–388. [DOI] [PubMed] [Google Scholar]
- 43. Pollard JC, Uhr L, Jackson CW., Jr Studies in sensory deprivation. Arch Gen Psychiatry. 1963;8:435–454. [DOI] [PubMed] [Google Scholar]
- 44. Mohr C, Leonards U. Does contextual information influence positive and negative schizotypy scores in healthy individuals? The answer is maybe. Psychiatry Res. 2005;136:135–141. [DOI] [PubMed] [Google Scholar]
- 45. Nelson B, Sass LA, Thompson A, et al. Does disturbance of self underlie social cognition deficits in schizophrenia and other psychotic disorders? Early Interv Psychiatry. 2009;3:83–93. [DOI] [PubMed] [Google Scholar]
- 46. Barrantes-Vidal N, Gross GM, Sheinbaum T, et al. Positive and negative schizotypy are associated with prodromal and schizophrenia-spectrum symptoms. Schizophr Res. 2013;145:50–55. [DOI] [PubMed] [Google Scholar]
- 47. Lee J, Kwon JS, Shin YW, et al. Visual self-recognition in patients with schizophrenia. Schizophr Res. 2007;94:215–220. [DOI] [PubMed] [Google Scholar]
- 48. Backasch B, Sommer J, Klöhn-Saghatolislam F, et al. Dysconnectivity of the inferior frontal gyrus: implications for an impaired self-other distinction in patients with schizophrenia. Psychiatry Res. 2014;223:202–209. [DOI] [PubMed] [Google Scholar]
- 49. Yun JY, Hur JW, Jung WH, et al. Dysfunctional role of parietal lobe during self-face recognition in schizophrenia. Schizophr Res. 2014;152:81–88. [DOI] [PubMed] [Google Scholar]
- 50. Jardri R, Denève S. Circular inferences in schizophrenia. Brain. 2013;136:3227–3241. [DOI] [PubMed] [Google Scholar]