Abstract
Regenerative medicine holds great promise in replacing tissues and organs lost to degenerative disease and injury. Applying principles of cellular reprogramming for the treatment of cancer, however, are not well established. Here we present an overview of cell-based reprogramming techniques (i.e. lineage reprogramming and stimulus-triggered acquisition of pluripotency) used in regenerative medicine, and within this context, envision how the scope of regenerative medicine may be expanded to treat metastatic cancer by revitalizing an exhausted and senescent immune system.
Regenerative medicine as a therapy for cancer
Harnessing the therapeutic potential of cellular reprogramming techniques for the treatment of sick and injured patients is theoretically straightforward in some cases. With regard to heart disease, for example, one simply observes the ability of a fish to mend a broken heart: the zebrafish can completely regenerate its heart following amputation of 20% of its ventricle (1–2). Credit for this remarkable ability to reconstitute ventricular tissue was previously attributed to a putative cardiac stem cell progenitor, but recent evidence suggests healing is mediated by cellular reprogramming of mature cardiomyocytes adjacent to the injury (3).
But stealing a page from nature’s playbook to harness the tremendous therapeutic potential of cellular reprogramming is not as forthcoming for the treatment of other major human maladies such as cancer. Apart from regenerating whole organs for transplantation—say regeneration of a liver to replace one riddled with hepatocellular carcinoma—it is difficult to conceptualize how principles of cellular reprogramming can be harnessed to treat patients with advanced malignancy. That is, in the context of systemic cancer, which cells would most benefit from reprogramming?
The ability of tumor cells to evade immune destruction is an emerging hallmark of cancer (4). The theory of immune surveillance posits that an ever-vigilant immune system eliminates the bulk of nascent cancer cells (5). Tumor-specific T cells can become exhausted and senescent with chronic antigen challenge (see Box 1), however, allowing malignant cells to persist and develop into invasive and widespread cancer. Immune-based approaches such as adoptive cellular immunotherapy (ACT) help to overcome T cell exhaustion and senescence by surgically isolating T cells from the tumor microenvironment and expanding them ex vivo prior to adoptive transfer into autologous patients (6). ACT is emerging as a potentially curative therapy for patients with advanced cancer, but one of the main limitations to improving the efficacy of ACT is to ensure that T cells maintain the capacity for self-renewal and are able to continually produce progeny capable of eradicating tumor after adoptive transfer into patients (7).
Box 1. Exhaustion and Senescence of T cells.
A hallmark of adaptive cellular immunity is the ability of T cells to undergo a robust clonal response with secondary antigen challenge (86). Repeated and chronic antigenic stimulation in the tumor microenvironment seems to attenuate this response as T cells become increasingly exhausted and senescent (38). Senescence defines a loss of replicative capacity that is associated with DNA damage and telomere erosion (87–88). Exhaustion refers to compromised functional capability of T cells (89). Traditionally considered to be passive phenomena that weaken an immune response, there is now increasing evidence that both exhaustion and senescence are distinct processes controlled by active molecular pathways (90).
Exhaustion was first described in mice with chronic infection of lymphocytic choriomeningitis virus (LCMV) and later validated in models of human T lymphotropic virus 1 (HTLV1), HIV, hepatitis B virus (HBV), simian immunodeficiency virus (SIV), and hepatitis C virus (HCV) (90). Exhaustion of T cells in mice and humans with high tumor burden have also been observed (39).
Exhausted CD8+ T cells in mice and humans are characterized by attenuated expression of receptors for IL-15 and IL-7, CC-chemokine receptor 7 (CCR7), and L-selectin (also known as CD62L) consistent with an effector memory T cell phenotype (39). Interestingly, exhaustion occurs in distinct stages of functional impairment: IL-2 production is initially lost, followed by TNF expression, and finally IFN-γ in the most severe state of exhaustion (91).
Cellular senescence was first recognized when Hayflick observed a limitation to the replicative capacity of fibroblasts that was later found to be due to shortening of telomeres and triggering of the DNA damage response (DDR) (92). Senescent T cells are characterized by a shortening of telomeres, decreased expression of telomerase, and increased expression of killer cell lectin-like receptor subfamily G, number 1 (KLRG1) (39). Reversal of senescence in fibroblasts by antagonizing the cell cycle arrest protein checkpoint kinase 2 homologue (CHK2) and key mediators such as p21, p53, and p38 (93) suggest it may be possible to reverse or delay senescence in T cells. For an excellent review on T cell exhaustion in the tumor microenvironment see (94).
Herein we envision how reprogramming techniques developed in stem cell biology may be used to treat metastatic cancer by revitalizing an exhausted and senescent immune system. Applying techniques of cellular reprogramming may endow features of stemness to adoptively-transferred T cells—namely enhanced self-renewal and multipotency to produce a continual supply of cytolytic effector progeny—thereby improving the ability of anti-tumor T cells to sustain a prolonged attack on advanced cancer. This review will have a three-prong focus: first, we will offer an overview of cell-based reprogramming techniques to provide a conceptual framework and vocabulary that can be used to understand approaches in regenerative medicine. A discussion of mechanisms underlying exhaustion and senescence of the immune system will follow. Finally, we will sharpen our focus to explore regenerative medicine techniques that may revitalize an exhausted immune response and have the potential to enhance the antitumor efficacy of cell-based immunotherapy.
The language of plasticity
Although the field of regenerative has deep historical roots, there are often conflicting definitions regarding terms of cellular reprogramming (see Table 1 Language of Plasticity). What is at stake, however, is clear: the plasticity of a cell. Plasticity is the ability of a cell to convert from one cell type into another and ultimately reconstitute tissues. This definition of plasticity rests on at least two assumptions. First, that there are discrete cell types (or discrete cell lineages), and second, that a differentiated cell can alter its phenotype, whether within a lineage or between lineages (8).
Table 1.
The Language of Plasticity
| stem cell | Cell with enhanced properties of self-renewal and potency |
| lineage | Cells of same developmental origin with common phenotype and function. |
| differentiation | The process by which a cell loses its potency and capacity for self-renewal and ultimately becomes a mature and discrete cell type within a discrete lineage. |
| pluripotent reprogramming | Reprogramming of a cell to a pluripotent state. Techniques include somatic cell transfer, cell-cell fusion, and direct reprogramming—the common denominator being a reversion to a pluripotent cell |
| lineage reprogramming | Conversion of a cell from one type to another in the same lineage or different lineages without reversion to pluripotency. Techniques include dedifferentiation, transdifferentiation, and transdetermination. |
| dedifferentiation | The process by which a cell reverts to a less specialized progenitor state within a discrete lineage. |
| transdifferentiation | Switch from one cell lineage to another without moving through a dedifferentiated or pluripotent intermediate. |
| nuclear transfer | Transplantation of a nucleus from a somatic cell to an enucleated oocyte where the somatic cell nucleus is reprogrammed in the environment of the oocyte |
| plasticity | Ability of a cell to convert from one discrete cell type or lineage into another. |
| totipotency | Ability of cell to produce all differentiated cells in an organism (including extraembryonic tissue) |
| pluripotency | Capacity to give rise to any of the three germ layers: endoderm, mesoderm, ectoderm. |
| multipotency | Capacity to give rise to cells of multiple lineages or cell subsets. |
| senescence | A growth-arrest program that limits the lifespan of mammalian cells and prevents unlimited cell proliferation. |
| cell fusion | Occurs when two distinct cell types combine to form a single entity. The only form of nuclear reprogramming observed in nature. |
| exhaustion | T cell exhaustion is a state of T cell dysfunction that arises during chronic infection and cancer. It is defined by poor effector function, sustained expression of inhibitory receptors and a transcriptional program distinct from that of functional effector or memory T cells. |
| transdetermination | Dedifferentiation of cell to less committed progenitor state which switches lineages to redifferentiate to a cell type in a new lineage. |
In 1957 Conrad Waddington conceptualized the process of cellular differentiation as a ball (representing a cell) placed at the top of a hill (9). Using the Waddington model, the plasticity of a cell can be conceptualized with reference to its lineage. Totipotent stem cells reside at the top peak with the ability to differentiate into any cell type or extraembryonic tissue (10). As a cell begins to travel from its undifferentiated state, a series of extracellular cues and gene expression programs determines the cell’s path until it arrives at a differentiated valley, representing a distinct cellular lineage (Figure 1). The prevailing paradigm is that somatic cells become increasingly, and irreversibly, committed to their somatic fate and lose potency as they travel down the hill. That is, a mature skin cell, at least in the physiologic setting, cannot give rise to a heart cell, and vice-versa (11).
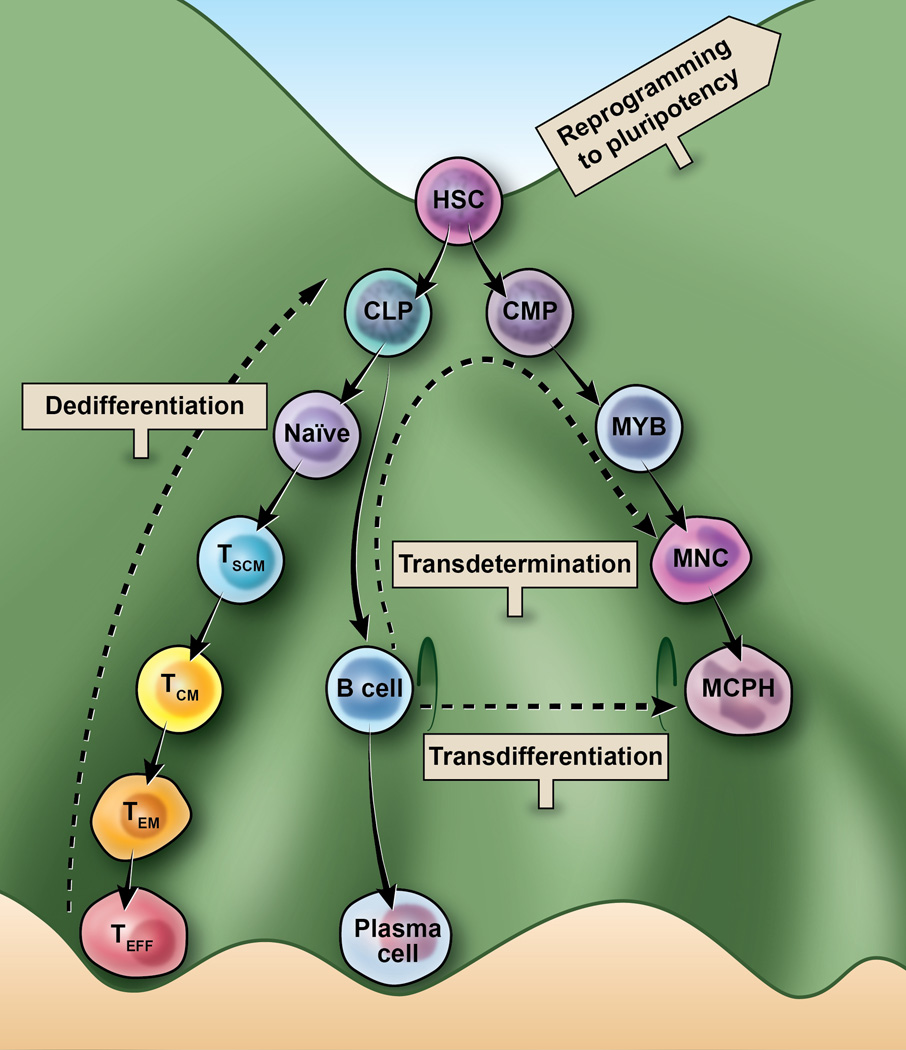
Figure 1. Lineage reprogramming of Waddington’s epigenetic landscape.
The biologic development of a multipotent hematopoietic stem cell (HSC) is represented here as a cell at the top of a hill. HSC can develop into common myeloid progenitors (CMP) or common lymphoid progenitors (CLP) and subsequently develop into several lineages (not all represented). The three approaches of lineage reprogramming illustrated here include: A) dedifferentiation in which a cell reverts to a less specialized progenitor state within a discrete lineage B) transdetermination in which a cell dedifferentiates to a less committed progenitor state and then switches lineages to redifferentiate to a cell type in a new lineage and C) transdifferentiation in which a cell moves directly from one lineage to another without moving through a dedifferentiated or pluripotent intermediate. A sign labeled “to pluripotent reprogramming” points to a higher peak and represents the greater potency associated with this approach. The myeloid lineage includes: MYB (myeloblast), MNC (monocyte), and MCPH (macrophage). Naïve T (Naïve) cells differentiate after antigen stimulation into following T cells subsets: TSCM (stem-cell memory), TCM (central memory), TEM (effector memory), and cytolytic TEFF (effectors).
There are several experimental techniques in regenerative medicine, however, that can induce plasticity and alter the fate of cells that would otherwise be subject to physiologic dictates. These cellular reprogramming techniques can be grouped into two broad approaches: pluripotent reprogramming and lineage reprogramming (10). Pluripotent reprogramming includes cell fusion, somatic cell nuclear transfer, induction of pluripotency by ectopic gene expression, and stimulus-triggered acquisition of pluripotency—the common denominator being a reversion to a pluripotency(12). Lineage reprogramming encompasses approaches of dedifferentiation, transdifferentiation, and transdetermination and refers to conversion of a cell from one type to another in the same lineage or different lineages without reversion to pluripotency (13).
The process of dedifferentiation occurs when a terminally-differentiated cell reverts to a less-differentiated precursor within its own lineage (13). The Waddington “ball”, so to speak, rolls back up the hill, but not all the way to the top (to pluripotency). Ectopic expression of Lin-28 homolog B (Lin28), for example, has been shown to reprogram adult hematopoietic stem/progenitor cells (HSPC) into fetal-like hematopoietic stem cells that have enhanced capacity for multi-lineage reconstitution (14). Another example of dedifferentiation within the lymphoid lineage was observed when conditional deletion of paired box gene 5 (Pax5) in mice enabled mature B cells from peripheral lymphoid organs to dedifferentiate in vivo to early uncommitted progenitors in the bone marrow and ultimately rescue T lymphopoiesis in the thymus of T cell-deficient mice. The B-cell-derived T cells showed evidence of immunoglobin gene rearrangement and maintained the capacity to form germinal centers in immunized mice (15).
Transdetermination is similar to dedifferentiation, but the proverbial Waddington ball does not roll back to the same valley from whence it came. The ball rolls down a different valley. In other words, it dedifferentiates to an earlier progenitor (without a pluripotent intermediate) and then switches lineages to differentiate to a cell of a distinct lineage. An impressive example of transdetermination was demonstrated when human dermal fibroblasts were converted to multilineage blood progenitors by ectopic expression of octamer-binding transcription factor 4 (OCT4) in addition to specific cytokine treatment (16). The fibroblast-derived cells expressed the pan-leukocyte marker CD45 and gave rise to erythroid, megakaryocytic, monocytic, and granulocytic lineages that maintained the capacity for in vivo engraftment. Notably, the adult hematopoetic program was activated by OCT4 in fibroblasts without traversing through a pluripotent state.
There are reported cases of experimental lineage reprogramming that do not seem to require a stepwise dedifferentiation of the primary cell into a less-differentiated intermediate cell type. Conversion of one mature cell type to another, without a dedifferentiated or pluripotent intermediate, is called transdifferentiation. Transdifferentation of cells seems to be mediated by a downregulation of one genetic program and concomitant upregulation of a new genetic program (13) and is demonstrated in the experimental conversion of B cells to macrophages mediated by the transcription factors CCAAT-enhancer-binding protein-α (CEBPα) and CEBPβ (17). Induction of transdifferentiation by CEBPα and CEBPβ is thought to downregulate B cell-specific genes (such as Cd19) and simultaneously activate macrophage-specific genes (such as Macrophage-1 antigen) (13, 17).
The process of dedifferentiation is designated by a different term altogether, called pluripotent reprogramming, if a cell reverts back to pluripotency. There are several types of pluripotent reprogramming—all defined by the mechanism utilized to achieve pluripotency. Cell fusion occurs when two distinct cell types combine to form a single entity (18). The trans-acting transcription factors of the more pluripotent cell typically dominate the more terminally-differentiated cell and induce pluripotency in the resulting hybrid or heterokaryon cell that is no longer diploid (19).
Induced pluripotency can also be achieved experimentally using somatic cell nuclear transfer (SCNT), which involves transplanting the nucleus of a somatic cell to an enucleated oocyte where the somatic cell nucleus is reprogrammed in the environment of the oocyte (20). This was first demonstrated in pioneering experiments in amphibians in the 1950s in which nuclei from the intestinal epithelium of the South African frog Xenopus laevis were transplanted into enucleated eggs and produced normal and fertile adult frogs (21). SCNT was subsequently successful in mammals with the derivation of Dollly the sheep (22). The transcription factors that mediate reversion of the differentiated nucleus in SCNT to its more pluripotent state remained obscure, however, until 2006 when Takahashi and Yamanaka discovered that retroviral expression of four genes (Oct4, Klf4, Sox2, and c-Myc, hereafter referred to as OSKM factors) converted somatic cells into a pluripotent state (23). These pluripotent cells resemble embryonic stem cells in terms of their gene expression profiles, epigenetic status, and their ability to contribute to all cell lineages when transplanted to immunotolerant host embryos. This novel approach of transcription-factor reprogramming to a pluripotent state (cells are called induced pluripotent stem cells, iPSCs) has been demonstrated in several human somatic cell types including blood cells, keratinocytes, and dermal fibroblasts (24–26). It was subsequently shown that iPSCs could be derived from human neural cells and hair follicle dermal papilla cells by mere ectopic expression of one transcription factor (Oct4) (27–28). The rapidity at which cellular reprogramming techniques is evolving is evidenced by two recently published approaches that induce pluripotency in mature somatic cells in the absence of direct genetic manipulation. Hou et al showed that a cocktail of seven small-molecule compounds was sufficient to induce pluripotency in mouse embryonic fibroblasts (referred to as chemically induced pluripotent stem cells, or CiPSCs) (29). More recently, Obokata et al has shown that strong external stimuli such as transient exposure to a low-pH environment reprogrammed lineage-committed murine CD45+ T cells into pluripotent stem cells (30).
An exhaustive review of cellular reprogramming techniques is difficult given rapid progress in the field, but examples of several approaches were discussed here to provide some conceptual framework for an understanding of regenerative medicine. Subsequent discussion of regenerative medicine techniques will take place in the context of cellular reprogramming of T cells in adoptive cellular immunotherapy (ACT) for the treatment of cancer. As further elucidated below, cell-based immunotherapy is particularly well-positioned to profit from recent advances in regenerative medicine simply because ACT allows for the direct ex vivo manipulation of cells prior to adoptive transfer into patients (7).
Adoptive cellular immunotherapy for cancer
Adoptive cellular immunotherapy (ACT) is an experimental therapy for metastatic cancer and viral-associated diseases that takes advantage of the inherent capacity of T cells to recognize and eliminate malignant and infected cells in the body (7). In the context of cancer, there are two main approaches of ACT: (i) autologous lymphocytes are obtained from excised tumors, expanded ex vivo, and infused back into the patient (ii) peripheral lymphocytes of cancer patients are isolated and genetically-modified to express either chimaeric antigen receptors (CARs) or anti-tumor T-cell receptors specific for cancer-associated antigens (31). ACT coupled with a maximum lymphodepleting conditioning regimen, resulted in durable and complete eradication of advanced melanoma in 20 out of 93 patients (22%) (32–33).
Factors associated with an objective response to ACT (as measured by radiographic Response Evaluation Criteria In Solid Tumors (RECIST)) in humans include longer telomeres of the infused cells, the number of CD8+ T cells infused expressing the memory-marker CD27, and the persistence of the cells in the circulation one month after transfer (32, 34). This suggests that transfer of cells with a minimally-differentiated phenotype that maintain replicative capacity and persistence are associated with a greater likelihood of objective response to ACT. This notion has also been corroborated in a tumor-specific T-cell receptor transgenic murine model (Pmel-1) in which there is a progressive loss of anti-tumor function as T-cells mature towards terminal differentiation (35).
The robust clinical response associated with minimally-differentiated anti-tumor T cells suggests that the efficacy of ACT may be improved with transfer of T cells exhibiting features of stemness—namely maintenance of replicative capacity and multipotency (36). Tumor-infiltrating lymphocytes (TILs) harvested for ACT, however, are characterized by a terminally-differentiated phenotype (CD62L-, CD27-, CD28-, perforin+, eomes+, KLRG1+) that is associated with diminished anti-tumor activity (37– 38). In addition, protocols for culturing TILs or T cells genetically-modified to express a TCR or CAR against tumor-associated antigens often involve multiple replications to get an adequate number of cells for treatment (39). The repeated replication of T cells in ACT results in terminally-differentiated cells that become both senescent and exhausted.
The rationale for reprogramming tumor-specific T cells is also based on preclinical evidence that minimally-differentiated T cells subsets, such as naïve (TN) and central memory (TCM) T cells, display greater efficacy in abolishing tumor than their terminally-differentiated counterparts (39). Our current understanding of peripheral CD8+ T cell ontogeny suggests that CD8+ T cells, upon activation with their cognate peptide:MHC complex, undergo a program of differentiation into various subsets characterized by differences in surface phenotype, metabolism, capacity for homeostatic proliferation and recall potential, and cytolytic function(40–41). Prior to activation by antigen presenting cells, CD8+ T cells are designated as naïve and maintained in a quiescent state (42). After activation several subsets of CD8+ T cells develop including central memory T cells (TCM), effector memory T cells (TEM), and effector T cells (TEFF) (43).
More recently another subset was identified with stem cell-like qualities, aptly named stem cell memory T cells (TSCM), which have the ability for prolonged replicative potential and multipotency to produce diverse progeny with potent effector function (44–47). TSCM have been identified in mice, nonhuman primates, and humans and are characterized by expression of CD62L, CCR7, IL2RB, BCL2, and CXCR3 (44, 48). Functionally, TSCM maintain robust replicative capacity and are multipotent in their ability to give rise to cytolytic effector and memory progeny. When transferred into tumorbearing mice, TSCM mediate a superior anti-tumor response compared to other CD8+ T cell subsets (45).
An increasingly sophisticated understanding of the ontogeny of peripheral T cells and advances in cellular reprogramming techniques have now led to the possibility of inducing plasticity in T cells to expand the pool of TSCM and TCM and enrich for populations of cells with increased capacity for self-renewal and multi-potency to produce a continual supply of cytolytic effector progeny (49–50).
Strategies to induce stemness in T cells and enhance anti-tumor immunity
Here we highlight two regenerative medicine approaches—pluripotent and lineage reprogramming—that may be used to reprogram exhausted and terminally-differentiated anti-tumor T cells. Pluripotent and lineage reprogramming are especially relevant for TILs isolated from the tumor microenvironment because TILs often display a terminally-differentiated phenotype and functional exhaustion (37–38). These approaches are also important for T cells that are genetically-modified to express either anti-tumor CARs or TCRs. T cells transduced with a tumor-specific CAR or TCR are largely naïve at the beginning of an ex vivo expansion, but progressively differentiate during the course of the lengthy culture that is required to obtain a therapeutic yield of cells (ex vivo expansion of cells is typically a thousand-fold) (51). Cellular reprogramming techniques to endow enhanced self-renewal properties and multipotency to produce cytolytic effector progeny may significantly improve the antitumor efficacy of adoptively-transferred T cells.
Pluripotent reprogramming of T cells
Previous studies support the feasibility of in vitro generation of T lymphocytes from human embryonic stem cells (hESCs) and iPSCs, though major challenges remain in further developing this approach (52–53). To date, generation of bona-fide hematopoietic stem cells (HSC) from mouse or human iPSCs has not been successful, though a recent report shows limited T cell lymphopoesis can occur in human iPSCs with OP9-DL4 stimulation (53).
Another major concern in deriving T cells from pluripotency lies in the observation that in vitro-generated T cells have a seemingly unpredictable T-cell receptor (TCR) repertoire because of VDJ gene rearrangements that are selected by unclear mechanisms (52). To overcome this limitation, tumor specificity of iPSC-derived T cells can be conferred by transducing with a TCR or CAR specific for tumorassociated antigen. Human iPSC-derived T cells have been generated by introducing a CAR specific for CD19, an antigen expressed by malignant B cells (54). These cells had the ability to infiltrate solid tumors and delay tumor progression in a xenograft model (54).
Another approach to maintain anti-tumor specificity of reprogrammed cells is to derive iPSC from tumor-infiltrating lymphocytes that preserve their capacity to recognize tumor targets. We have recently demonstrated that antigen-experienced tumor-infiltrating lymphocytes (TILs) specific for the melanoma antigen recognized by T-cells 1 (MART-1) isolated from a patient with metastatic melanoma can successfully be reprogrammed to pluripotency (55). Reprogramming of the TILs to iPSC was accomplished using ectopic expression of transcription factors in a non-genomic integrating manner using Sendai virus. Succesful reprogramming was evidenced by endogenous expression of c-MYC, KLF4, SOX2, and OCT3/4, disappearance of T cell markers CD3 and CD8, ESC-like morphology, and capacity for teratoma formation. Redifferentiation to CD8+ T cells was accomplished by co-culture with OP9 feeder cells expressing DL-1, and when cocultured with EBV-lymphoblastoid dells (CIRA0201) pulsed with MART-1-peptide, the redifferentiated T cells secreted IFN-gamma, demonstrating maintenance of TCR specificity and functional integrity (55–56).
A key challenge in future efforts will be to evaluate whether the reprogramming process not only maintains functional integrity, but also confers greater replicative capacity to T cells as well as enhanced potency to differentiate into cytolytic and memory T cells subsets. One major limitation to maintaining potency in the redifferentiation process remains the inability to produce HSC from iPSC (57). An alternative to HSC may be the use of human T cell progenitors which can be generated in vitro from iPSC, and upon adoptive transfer, can migrate to the thymus and differentiate into naïve T cells (58–59). This raises the possibility that iPSC derived from tumor-specific TILs can be differentiated into T cell progenitors and transferred into autologous patients to generate large numbers of cytotoxic T cells against metastatic tumors.
Lineage reprogramming of T cells
It has been suggested that the measure of complete reprogramming is whether a cell can form a fertile adult animal containing functional cells of every kind (totipotency) (11). As far as therapy is concerned, however, totipotency (or even pluripotency) may not be a desirable attribute. In the context of reprogramming dysfunctional anti-tumor cells for the treatment of advanced cancer, it would not necessarily be useful, for example, to derive all hematopoietic lineages. In addition, the length of time to reprogram cells to pluripotency and redifferentiate to naïve-like T cells may not be suitable for patients with metastatic cancer.
Although it has not yet been proven experimentally, the possibility that terminally-differentiated and exhausted T cells can be reprogrammed into young T cells with greater enhanced self-renewal and the ability to form cytolytic progeny could potentially improve the efficacy of ACT. This approach may be adapted to efficiently revitalize exhausted and senescent T cells by enforced expression of transcription factors (e.g. lymphoid enhancer binding factor 1 (LEF1) and transcription factor 7 (TCF7)) that are differentially-expressed in naïve or memory T cells (60). In addition to the existing literature of T cell ontogeny, candidate transcription factors that may effectuate reprogramming can potentially be identified by using microarray data of T-cells isolated in distinct states of differentiation (available in supplementary data (44)).
Safety, efficiency, and immunogenicity of reprogrammed T cells
Despite the therapeutic potential of cellular reprogramming techniques to improve the efficacy of ACT for metastatic cancer, there are at least three areas of concern that need to be addressed prior to clinical translation. First, the efficiency of reprogramming using OSKM factors has traditionally been very low (less than 1% of cells) and seemingly stochastic (20). In addition, it typically takes 3–6 weeks for successful reprogramming which is particularly problematic for patients with stage IV cancer with a very limited life expectancy. More recently, however, Rais et al have reported that OSKM factor transduction coupled with depletion of Mbd3 (methyl-CpG binding domain protein 3) resulted in nearly 100% reprogramming efficiency in mouse and human somatic cells within 7 days (61). Although not yet reproduced in humans, STAP-reprogrammed mouse cells also demonstrated rapid and relatively high efficiency of induced pluripotency (30).
Another concern in treating patients with reprogrammed cells is the reprogramming process can induce an aberrant methylome (62), chromosomal aneuploidy (63), and point mutations (64) in iPSC. Of particular concern is the potential for functional mutations in oncogenes or tumor suppressors that results in malignant transformation of the anti-tumor cells (65). In an attempt to diminish the risk of genetic and epigenetic disruption, several insertion-less techniques using adenovirus vectors, transposons, plasmids, recombinant proteins, episomal vectors, Sendai virus vectors, and modified RNA are actively being developed (see review by (66)) (67–74). Although not yet studied extensively, reprogramming techniques that do not rely on ectopic expression of transcription factors (e.g. STAP and CiPSCs) may also improve the safety profile of reprogrammed cells (29–30).
Finally, it has been reported that abnormal gene expression in mouse iPSCs can induce a T-cell-dependent immune response against the iPSCs after transfer into syngeneic hosts (75). Subsequent studies examining the immunogenicity of tissues derived from mouse iPSCs, however, found no evidence of increased T cell proliferation in vitro, an antigen-specific secondary immune response, or rejection of syngeneic iPSC-derived tissue after transplantation (76–78).
Concluding Remarks
Regenerative medicine is an emerging and rapidly progressing field that will likely have significant implications in both our understanding of immunology and our ability to use the immune system therapeutically to treat cancer and pathogen-associated disease. The discovery of techniques to induce the formation of pluripotent stem cells, in particular, has been a paradigm-shifting advance that has had implications for all disciplines of biology, and the field of immunology is no exception.
Despite the therapeutic potential of regenerative medicine, it has proved difficult to translate findings from bench to bedside. Utilizing principles of regenerative medicine to revitalize an exhausted immune system has a unique feature that makes this approach readily translatable to patients for the treatment of cancer, namely that there are no anatomical obstacles to overcome in cell-based immunotherapy. Several reports have provided a remarkable proof-of-principle in producing bioartificial heart (79), liver (80), and lung (81–82) using decellularized tissue scaffolds grown in bioreactors. Enthusiasm is somewhat tempered by disappointing clinical trials of transplantation of bone marrow mononuclear cells (83–84) and skeletal myoblasts (85) for treatment of myocardial infarction and is a reminder that there are many obstacles to effective stem cell therapy for disease of solid organs.
In contrast to reprogrammed cardiomyocytes, for example, that rely on engrafting on a cellular matrix, a reprogrammed T cell product is simply infused into a patient. The T cells have an inherent capacity to extravasate from the circulation and home towards tumor targets. Reprogramming of the immune system, therefore, may present a more tractable approach compared to reprogramming of other tissues, for translating the promise of regenerative medicine techniques into concrete therapeutic applications for patients.
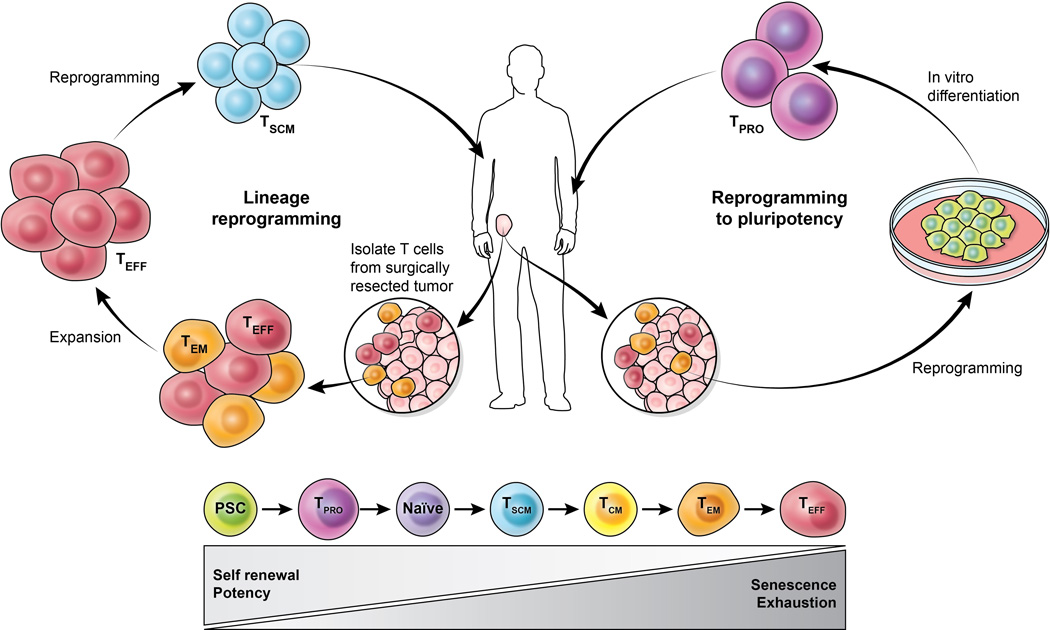
Figure 2. Establishing a culture of potency and self-renewal in adoptive cellular immunotherapy.
There are two main therapies of ACT in either isolating autologous tumor-infiltrating lymphocytes (TILs) from resected tumors of cancer patients or obtaining peripheral lymphocytes that are genetically modified to express antitumor Tcell receptors or chimeric antigen receptors. Both approaches rely on ex vivo expansion of cells that can lead to T cell exhaustion and senescence. Here we illustrate the use of two cellular reprogramming approaches—lineage and pluripotent reprogramming—that may enhance potency and self-renewal in TILs. Lineage reprogramming involves (after adequate expansion) dedifferentiation of TILs from an effector population to a stem cell memory T cell (Tscm) population. In the case of pluripotent reprogramming, TILs carrying an inherent antigen-specificity for tumor can be reprogrammed to iPSCs with subsequent differentiation into early T cell progenitors (TPRO) for adoptive transfer to patients. Pluripotent stem cells (PSC) can give rise to early T cell progenitors (TPRO) and ultimately naïve T cells that differentiate after antigen stimulation into following T cells subsets: TSCM (stem-cell memory), TCM (central memory), TEM (effector memory), and cytolytic TEFF (effectors).
Highlights.
We review cellular reprogramming techniques with applications in regenerative medicine
Reprogramming approaches may be harnessed to revitalize an exhausted immune response
Cellular reprogramming may enhance efficacy of anti-tumor immunity
Regenerative medicine may be applied to treating advanced cancer
Acknowledgements
We apologize to those investigators whose work was not cited due to space constraints. We wish to thank Ethan Tyler and Alan Hoofring of the National Institutes of Health Medical Arts Design Section for their beautiful rendering of the figures for this manuscript. Finally, we are grateful to the National Institutes of Health Center for Regenerative Medicine (NIH CRM) and the National Institutes of Health National Cancer Institute (NIH/NCI) for their support of research in our laboratory. JG Crompton also acknowledges funding support from the Wellcome Trust Translational Medicine and Therapeutics Programme. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. (Translated from eng) Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. (in eng). [DOI] [PubMed] [Google Scholar]
- 2.Raya A, et al. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. (Translated from eng) Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11889–11895. doi: 10.1073/pnas.1834204100. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. (Translated from eng) Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. (Translated from eng) Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. (in eng). [DOI] [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. (Translated from eng) Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. (in eng). [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. (Translated from eng) Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. (Translated from eng) Immunol Rev. 2014;257(1):264–276. doi: 10.1111/imr.12135. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. (Translated from eng) Nat Rev Genet. 2011;12(4):243–252. doi: 10.1038/nrg2938. (in eng). [DOI] [PubMed] [Google Scholar]
- 9.Waddington CH. The strategy of the genes; a discussion of some aspects of theoretical biology. London: Allen & Unwin; 1957. p. 262. ix. [Google Scholar]
- 10.Graf T, Enver T. Forcing cells to change lineages. (Translated from eng) Nature. 2009;462(7273):587–594. doi: 10.1038/nature08533. (in eng). [DOI] [PubMed] [Google Scholar]
- 11.Gurdon JB, Melton DA. Nuclear reprogramming in cells. (Translated from eng) Science. 2008;322(5909):1811–1815. doi: 10.1126/science.1160810. (in eng). [DOI] [PubMed] [Google Scholar]
- 12.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. (Translated from eng) Nature. 2010;465(7299):704–712. doi: 10.1038/nature09229. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. (Translated from eng) Nat Rev Mol Cell Biol. 2011;12(2):79–89. doi: 10.1038/nrm3043. (in eng). [DOI] [PubMed] [Google Scholar]
- 14.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. (Translated from eng) Science. 2012;335(6073):1195–1200. doi: 10.1126/science.1216557. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. (Translated from eng) Nature. 2007;449(7161):473–477. doi: 10.1038/nature06159. (in eng). [DOI] [PubMed] [Google Scholar]
- 16.Szabo E, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. (Translated from eng) Nature. 2010;468(7323):521–526. doi: 10.1038/nature09591. (in eng). [DOI] [PubMed] [Google Scholar]
- 17.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. (Translated from eng) Cell. 2004;117(5):663–676. doi: 10.1016/s0092-8674(04)00419-2. (in eng). [DOI] [PubMed] [Google Scholar]
- 18.Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. (Translated from eng) Nat Rev Mol Cell Biol. 2005;6(7):567–575. doi: 10.1038/nrm1678. (in eng). [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Melton DA. Extreme makeover: converting one cell into another. (Translated from eng) Cell Stem Cell. 2008;3(4):382–388. doi: 10.1016/j.stem.2008.09.015. (in eng). [DOI] [PubMed] [Google Scholar]
- 20.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. (Translated from eng) Cell. 2010;143(4):508–525. doi: 10.1016/j.cell.2010.10.008. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. (Translated from eng) Genes Dev. 2010;24(20):2239–2263. doi: 10.1101/gad.1963910. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. (Translated from eng) Nature. 1997;385(6619):810–813. doi: 10.1038/385810a0. (in eng). [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. (Translated from eng) Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. (in eng). [DOI] [PubMed] [Google Scholar]
- 24.Haase A, et al. Generation of induced pluripotent stem cells from human cord blood. (Translated from eng) Cell Stem Cell. 2009;5(4):434–441. doi: 10.1016/j.stem.2009.08.021. (in eng). [DOI] [PubMed] [Google Scholar]
- 25.Lowry WE, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. (Translated from eng) Proc Natl Acad Sci U S A. 2008;105(8):2883–2888. doi: 10.1073/pnas.0711983105. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aasen T, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. (Translated from eng) Nat Biotechnol. 2008;26(11):1276–1284. doi: 10.1038/nbt.1503. (in eng). [DOI] [PubMed] [Google Scholar]
- 27.Kim JB, et al. Direct reprogramming of human neural stem cells by OCT4. (Translated from eng) Nature. 2009;461(7264):649–643. doi: 10.1038/nature08436. (in eng). [DOI] [PubMed] [Google Scholar]
- 28.Tsai SY, et al. Single transcription factor reprogramming of hair follicle dermal papilla cells to induced pluripotent stem cells. (Translated from eng) Stem Cells. 2011;29(6):964–971. doi: 10.1002/stem.649. (in eng). [DOI] [PubMed] [Google Scholar]
- 29.Hou P, et al. Pluripotent stem cells induced from mouse somatic cells by smallmolecule compounds. (Translated from eng) Science. 2013;341(6146):651–654. doi: 10.1126/science.1239278. (in eng). [DOI] [PubMed] [Google Scholar]
- 30.Obokata H, et al. Stimulus-triggered fate conversion of somatic cells into pluripotency. (Translated from eng) Nature. 2014;505(7485):641–647. doi: 10.1038/nature12968. (in eng). [DOI] [PubMed] [Google Scholar]
- 31.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. (Translated from eng) Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. (Translated from eng) Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. (Translated from eng) Nat Rev Clin Oncol. 2011;8(10):577–585. doi: 10.1038/nrclinonc.2011.116. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). (Translated from eng) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. (in eng). [DOI] [PubMed] [Google Scholar]
- 35.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. (Translated from eng) J Clin Invest. 2005;115(6):1616–1626. doi: 10.1172/JCI24480. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. (Translated from eng) Nat Rev Immunol. 2006;6(5):383–393. doi: 10.1038/nri1842. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu R, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. (Translated from eng) Cancer J. 2012;18(2):160–175. doi: 10.1097/PPO.0b013e31824d4465. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. (Translated from eng) J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. (Translated from eng) Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukumar M, et al. (Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. (Translated from eng) J Clin Invest. 123(10):4479–4488. doi: 10.1172/JCI69589. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaech SM, Cui W. (Transcriptional control of effector and memory CD8+ T cell differentiation. (Translated from eng) Nat Rev Immunol. 12(11):749–761. doi: 10.1038/nri3307. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. (Translated from eng) Immunity. 2012;36(3):374–387. doi: 10.1016/j.immuni.2012.01.015. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. (Translated from eng) Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. (in eng). [DOI] [PubMed] [Google Scholar]
- 44.Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. (Translated from eng) Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. (Translated from eng) Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. (Translated from eng) Nat Med. 2005;11(12):1299–1305. doi: 10.1038/nm1326. (in eng). [DOI] [PubMed] [Google Scholar]
- 47.Lugli E, et al. (Superior T memory stem cell persistence supports long-lived T cell memory. (Translated from eng) J Clin Invest. 123(2):594–599. doi: 10.1172/JCI66327. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lugli E, et al. (Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. (Translated from eng) Nat Protoc. 8(1):33–42. doi: 10.1038/nprot.2012.143. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramesh R, et al. Pro-inflammatory human Th17 cells selectively express Pglycoprotein and are refractory to glucocorticoids. (Translated from eng) J Exp Med. 2014;211(1):89–104. doi: 10.1084/jem.20130301. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. (Translated from eng) Sci Transl Med. 2009;1(11) doi: 10.1126/scitranslmed.3000302. 11ps12 (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radvanyi LG, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. (Translated from eng) Clin Cancer Res. 2012;18(24):6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timmermans F, et al. Generation of T cells from human embryonic stem cellderived hematopoietic zones. (Translated from eng) J Immunol. 2009;182(11):6879–6888. doi: 10.4049/jimmunol.0803670. (in eng). [DOI] [PubMed] [Google Scholar]
- 53.Kennedy M, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. (Translated from eng) Cell Rep. 2012;2(6):1722–1735. doi: 10.1016/j.celrep.2012.11.003. (in eng). [DOI] [PubMed] [Google Scholar]
- 54.Themeli M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. (Translated from eng) Nat Biotechnol. 2013;31(10):928–933. doi: 10.1038/nbt.2678. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vizcardo R, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. (Translated from eng) Cell Stem Cell. 2013;12(1):31–36. doi: 10.1016/j.stem.2012.12.006. (in eng). [DOI] [PubMed] [Google Scholar]
- 56.Crompton JG, Rao M, Restifo NP. Memoirs of a reincarnated T cell. (Translated from eng) Cell Stem Cell. 2013;12(1):6–8. doi: 10.1016/j.stem.2012.12.009. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sturgeon CM, Ditadi A, Clarke RL, Keller G. Defining the path to hematopoietic stem cells. (Translated from eng) Nat Biotechnol. 2013;31(5):416–418. doi: 10.1038/nbt.2571. (in eng). [DOI] [PubMed] [Google Scholar]
- 58.Meek B, et al. In vitro-differentiated T/natural killer-cell progenitors derived from human CD34+ cells mature in the thymus. (Translated from eng) Blood. 2010;115(2):261–264. doi: 10.1182/blood-2009-05-223990. (in eng). [DOI] [PubMed] [Google Scholar]
- 59.Awong G, et al. Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. (Translated from eng) Blood. 2013;122(26):4210–4219. doi: 10.1182/blood-2012-12-472803. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willinger T, et al. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. (Translated from eng) J Immunol. 2006;176(3):1439–1446. doi: 10.4049/jimmunol.176.3.1439. (in eng). [DOI] [PubMed] [Google Scholar]
- 61.Rais Y, et al. Deterministic direct reprogramming of somatic cells to pluripotency. (Translated from eng) Nature. 2013;502(7469):65–70. doi: 10.1038/nature12587. (in eng). [DOI] [PubMed] [Google Scholar]
- 62.Lister R, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. (Translated from eng) Nature. 2011;471(7336):68–73. doi: 10.1038/nature09798. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayshar Y, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. (Translated from eng) Cell Stem Cell. 2010;7(4):521–531. doi: 10.1016/j.stem.2010.07.017. (in eng). [DOI] [PubMed] [Google Scholar]
- 64.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. (Translated from eng) Nature. 2011;471(7336):63–67. doi: 10.1038/nature09805. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker DE, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. (Translated from eng) Nat Biotechnol. 2007;25(2):207–215. doi: 10.1038/nbt1285. (in eng). [DOI] [PubMed] [Google Scholar]
- 66.Okano H, et al. Steps toward safe cell therapy using induced pluripotent stem cells. (Translated from eng) Circ Res. 2013;112(3):523–533. doi: 10.1161/CIRCRESAHA.111.256149. (in eng). [DOI] [PubMed] [Google Scholar]
- 67.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. (Translated from eng) Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okita K, Hong H, Takahashi K, Yamanaka S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. (Translated from eng) Nat Protoc. 2010;5(3):418–428. doi: 10.1038/nprot.2009.231. (in eng). [DOI] [PubMed] [Google Scholar]
- 69.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. (Translated from eng) Nature. 2009;458(7239):766–770. doi: 10.1038/nature07863. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. (Translated from eng) Science. 2008;322(5903):945–949. doi: 10.1126/science.1162494. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lohle M, et al. Differentiation efficiency of induced pluripotent stem cells depends on the number of reprogramming factors. (Translated from eng) Stem Cells. 2012;30(3):570–579. doi: 10.1002/stem.1016. (in eng). [DOI] [PubMed] [Google Scholar]
- 72.Pera MF. Stem cells: Low-risk reprogramming. (Translated from eng) Nature. 2009;458(7239):715–716. doi: 10.1038/458715a. (in eng). [DOI] [PubMed] [Google Scholar]
- 73.Kaji K, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. (Translated from eng) Nature. 2009;458(7239):771–775. doi: 10.1038/nature07864. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ban H, et al. Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. (Translated from eng) Proc Natl Acad Sci U S A. 2011;108(34):14234–14239. doi: 10.1073/pnas.1103509108. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. (Translated from eng) Nature. 2011;474(7350):212–215. doi: 10.1038/nature10135. (in eng). [DOI] [PubMed] [Google Scholar]
- 76.Thanasegaran S, Cheng Z, Ito S, Nishio N, Isobe K. No immunogenicity of IPS cells in syngeneic host studied by in vivo injection and 3D scaffold experiments. (Translated from eng) Biomed Res Int. 2013;2013:378207. doi: 10.1155/2013/378207. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. (Translated from eng) Cell Stem Cell. 2013;12(4):407–412. doi: 10.1016/j.stem.2013.01.006. (in eng). [DOI] [PubMed] [Google Scholar]
- 78.Araki R, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. (Translated from eng) Nature. 2013;494(7435):100–104. doi: 10.1038/nature11807. (in eng). [DOI] [PubMed] [Google Scholar]
- 79.Ott HC, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. (Translated from eng) Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. (in eng). [DOI] [PubMed] [Google Scholar]
- 80.Uygun BE, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. (Translated from eng) Nat Med. 2010;16(7):814–820. doi: 10.1038/nm.2170. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. (Translated from eng) Science. 2010;329(5991):538–541. doi: 10.1126/science.1189345. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ott HC, et al. Regeneration and orthotopic transplantation of a bioartificial lung. (Translated from eng) Nat Med. 2010;16(8):927–933. doi: 10.1038/nm.2193. (in eng). [DOI] [PubMed] [Google Scholar]
- 83.Lunde K, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. (Translated from eng) N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. (in eng). [DOI] [PubMed] [Google Scholar]
- 84.Janssens S, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. (Translated from eng) Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. (in eng). [DOI] [PubMed] [Google Scholar]
- 85.Menasche P, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. (Translated from eng) Circulation. 2008;117(9):1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. (in eng). [DOI] [PubMed] [Google Scholar]
- 86.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. (Translated from eng) Nat Rev Immunol. 2012;12(4):306–315. doi: 10.1038/nri3173. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akbar AN, Vukmanovic-Stejic M. Telomerase in T lymphocytes: use it and lose it? (Translated from eng) J Immunol. 2007;178(11):6689–6694. doi: 10.4049/jimmunol.178.11.6689. (in eng). [DOI] [PubMed] [Google Scholar]
- 88.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. (Translated from eng) Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. (in eng). [DOI] [PubMed] [Google Scholar]
- 89.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. (Translated from eng) Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. (in eng). [DOI] [PubMed] [Google Scholar]
- 90.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? (Translated from eng) Nat Rev Immunol. 2011;11(4):289–295. doi: 10.1038/nri2959. (in eng). [DOI] [PubMed] [Google Scholar]
- 91.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. (Translated from eng) J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. (Translated from eng) Nat Rev Cancer. 2008;8(7):512–522. doi: 10.1038/nrc2440. (in eng). [DOI] [PubMed] [Google Scholar]
- 93.Passos JF, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. (Translated from eng) Mol Syst Biol. 2010;6:347. doi: 10.1038/msb.2010.5. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. (Translated from eng) Trends Immunol. 2014;35(2):51–60. doi: 10.1016/j.it.2013.10.001. (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]