Abstract
Alpha and beta cells act in concert to maintain blood glucose. Alpha cells release glucagon in response to low glucose to stimulate glycogenolysis in the liver. In contrast, beta cells release insulin in response to elevated glucose to stimulate peripheral glucose disposal. Despite these opposing roles in glucose homeostasis, alpha and beta cells derive from a common progenitor and share many proteins important for glucose sensing and hormone secretion. Recent work has underlined these similarities between the two cell types by showing that beta-to-alpha as well as alpha-to-beta transdifferentiation can take place under certain experimental circumstances. These exciting findings highlight unexpected plasticity in adult islets and offer hope of novel therapeutic paths to replenish beta cells in diabetes. In this review we focus on the transcription factor networks that establish and maintain pancreatic endocrine cell identity and how they may be perturbed to facilitate transdifferentiation.
Keywords: alpha cell, beta cell, transdifferentiation, dedifferentiation, ARX, PAX4, PDX1, NKX2.2, FOXO1, diabetes
Introduction
The pancreatic islets consist of several endocrine cell types including insulin-producing beta cells, glucagon-producing alpha cells, somatostatin-producing delta cells, pancreatic polypeptide-producing PP cells and ghrelin-producing epsilon cells. Beta cells are active under conditions of nutrient excess, particularly high blood glucose levels, and secrete insulin whose peripheral actions on liver, muscle and fat tissues reduce blood glucose (Leto and Saltiel 2012; Lin and Accili 2011; Thorel, et al. 2010). Insulin is functionally opposed by glucagon, which promotes liver glycogenolysis to prevent hypoglycemia during fasting or exercise (Ramnanan, et al. 2011). Beta cell products inhibit alpha cell secretion (Franklin, et al. 2005), while alpha cell derived glucagon, paradoxically, activates beta cells to secrete insulin (Kawai, et al. 1995; Samols, et al. 1965). Delta cells are generally considered the brake on islet secretion as somatostatin inhibits the release of both insulin and glucagon (Hauge-Evans, et al. 2009; Strowski, et al. 2000), thereby preventing large swings in blood glucose levels. It was generally thought that the endocrine cell types present in the islet were distinct and terminally differentiated populations. However, recent observations suggest that under certain conditions pancreatic cells of one type can transdifferentiate to another. This review will focus on such interconversions between the various islet endocrine cells and will not discuss in detail the ability of other pancreatic cells, such as acinar cells, to transdifferentiate into beta cells following viral transduction (Zhou, et al. 2008) or in response to soluble factors (Baeyens, et al. 2014). Two apparently distinct pathways have been described by which transdifferentiation of islet endocrine cells takes place, i.e. where a hormone expressing islet cell converts into a hormone expressing islet cell of a different type. One is direct transdifferentiation that proceeds via a double hormone positive intermediate cell (Lu, et al. 2010; Papizan, et al. 2011; Thorel et al. 2010; Yang, et al. 2011). The other involves loss of hormone expression during a process of reversion to a precursor-like state prior to differentiation into a hormone positive cell of a different type (Jonas, et al. 1999; Talchai, et al. 2012; Wang, et al. 2014). Whether these different phenotypes represent mechanistically distinct transdifferentiation processes, or merely represent different gradations of a single transdifferentiation spectrum is not clear at this time and cannot always be distinguished by lineage tracing approaches that are best-suited to evaluate the end result of the transdifferentiation process. It is clear however that the process of transdifferentiation of islet endocrine cells has generated considerable excitement as a potentially novel strategy for treating for diabetes. While diabetes is traditionally cast as a disease of relative (Type 2) or absolute (Type 1) insufficiency in beta cell mass, it is less appreciated that hyperglucagonemia secondary to the loss of the normal inhibitory tone on alpha cells from beta cells contributes to the etiology of diabetes by aggravating hyperglycemia (Franklin et al. 2005; Unger and Cherrington 2012). Despite their opposing roles in the maintenance of a dynamic equilibrium of blood glucose levels, alpha and beta cells derive from a common progenitor. They continue to share expression of many transcription factors and genes for glucose uptake and glycolysis, stimulus secretion coupling and hormone exocytosis (Benner, et al. 2014). This resemblance makes non-beta endocrine cells the cell types that are most closely related to beta cells and makes them prime candidates to target for conversion into beta cells (Fig. 1). Here we will provide an overview of the recent literature on the differentiation and transdifferentiation of beta and alpha cells, based largely on experimental observations in transgenic and knockout mouse models, with emphasis on the transcription factors that are implicated in transdifferentiation of beta and alpha cells, before we address the application of transdifferentiation in managing diabetes.
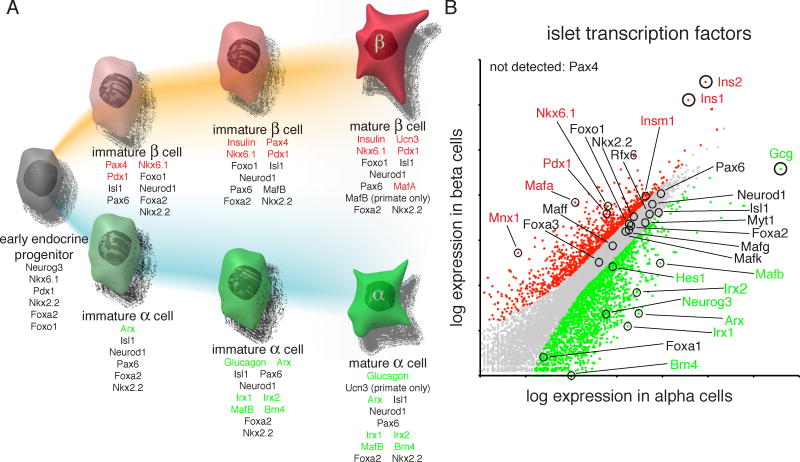
Figure 1.
Development and maintenance of alpha and beta cells from a common progenitor (A) requires intricate networks of transcription factors. Transcription factors and hormones specific to beta (red) and alpha (green) cells are indicated. Despite their opposing functions in the regulation of blood glucose levels, alpha and beta cells continue to share the expression of many transcription factors (black). Specificity of gene expression was determined by significant (p<10−7) enrichment in one cell type over the other (B). This figure was adapted with permission from (Benner et al. 2014).
Transcription factors in early pancreas formation
The pancreas is first identified by the transcription factor pancreatic and duodenal homeobox 1 (PDX1), which marks the pancreatic anlage starting at embryonic day 8.5 (E8.5) in the mouse (Guz, et al. 1995; Ohlsson, et al. 1993). The absolute requirement of PDX1 for pancreas development is evident from global loss of PDX1, which results in the absence of a pancreas at birth (Jonsson, et al. 1994; Offield, et al. 1996). A hypomorphic allele of PDX1, created by the deletion of some regulatory sequences for PDX1 expression, results in a remnant pancreas with ductal and acinar cells, but a relative lack of endocrine cell development, with alpha cells least-affected (Fujitani, et al. 2006). PDX1 expression is quickly followed by expression of the basic helix–loop–helix protein pancreas specific transcription factor 1a (Ptf1a) (Hald, et al. 2008; Krapp, et al. 1996), forkhead box O1 (FOXO1) (Kitamura, et al. 2009), NK2 homeobox 2 (NKX2.2) (Sussel, et al. 1998) and NK6 homeobox 1 and 2 (NKX6.1 (Sander, et al. 2000) and NKX6.2 (Henseleit, et al. 2005), respectively). Ptf1a is initially expressed in progenitors comprising the early pancreatic epithelium but subsequently becomes restricted to the exocrine acinar cells and their precursors (Hald et al. 2008; Kawaguchi, et al. 2002; Krapp et al. 1996; Pan, et al. 2013). Ptf1a expression is repressed by the NKX6 proteins and Ptf1a conversely represses expression of the NKX6 genes, thereby segregating the acinar versus ductal/endocrine lineages (Schaffer, et al. 2010). FOXO1 and PDX1 continue to be expressed in the same pancreatic cells, but either one or the other is localized to the nucleus (Kitamura et al. 2009; Kitamura, et al. 2002). FOXO1 inhibits PDX1 function (Kitamura et al. 2002), yet the expression of FOXO1 is important for maintenance of normoglycemia as animals age (Talchai et al. 2012). The transcription factor that marks the earliest progenitor exclusive to all endocrine cells within the pancreas is the basic helix-loop-helix transcription factor neurogenin 3 (NGN3) (Gu, et al. 2002). NGN3 is required for endocrine cell formation (Gradwohl, et al. 2000) and initiates a new set of developmental programs as expression of many transcription factors that are very important for endocrine cell lineage differentiation and maintenance depend on NGN3. These include the LIM homeobox protein islet-1 (Isl1), paired box 4 and 6 (PAX4 and PAX6), aristaless related homeobox (ARX) and neuronal differentiation 1 (NEUROD), which are all lost in NGN3 deficient mice (Collombat, et al. 2003; Gradwohl et al. 2000). The competency of progenitors changes with time such that NGN3+ cells that arise early in development preferentially differentiate into glucagon+ cells while later-born NGN3+ cells give rise to insulin+ or somatostatin+ cells (Johansson, et al. 2007). The number of differentiating NGN3+ cells is limited by lateral inhibition via Notch signaling (Apelqvist, et al. 1999; Murtaugh, et al. 2003). After endocrine cell fate is specified by NGN3, PDX1, becomes restricted to beta cells and a subset of delta cells by E13.5 (Ohlsson et al. 1993; Peshavaria, et al. 1994) and helps maintain beta cell mass (Ahlgren, et al. 1998). NKX2.2 and NKX6.1 expression too become progressively restricted to the endocrine compartment (Sander et al. 2000; Sussel et al. 1998). NKX2.2 marks most endocrine cells with the exception of delta cells. Deletion of NKX2.2 causes major changes in the endocrine makeup of the islets, as alpha and PP cells are reduced in number (Sussel et al. 1998), while beta cells are replaced by ghrelin cells (Prado, et al. 2004). Within the islet, NKX6.1 functions downstream of NKX2.2 and is both necessary and sufficient for beta cell neogenesis during the secondary transition, a time of further differentiation and major expansion of the endocrine cell mass. By E15.5 NKX6.1 is found exclusively in beta cells and scattered ductal and periductal cells (Sander et al. 2000; Schaffer, et al. 2013). While NKX2.2 and NKX6.1 (and in its absence NKX6.2) are required for early endocrine cell development, including alpha cells (Henseleit et al. 2005; Sussel et al. 1998), NKX6.1 expression is later lost from the developing alpha cell. In fact, continued expression of both NKX2.2 and NKX6.1 in the beta cell actually represses alpha cell fate via repression of ARX (see also below) to maintain beta cell identity (Papizan et al. 2011; Schaffer et al. 2013). These observations point to a central role of the PDX1, NKX2.2 and NKX6.1 transcription factors in the early development of beta cells.
Transcription factors later in beta cell differentiation
The transcription factor PAX4 is induced later than NKX2.2 and NKX6.1 and acts downstream of NGN3 (Gradwohl et al. 2000). PAX4 is necessary for normal beta and delta cell development, as alpha and ghrelin cell numbers are increased in its absence at the expense of beta and delta cells. This suggests a fate switch of endocrine progenitors in the absence of PAX4 from beta/delta to alpha/epsilon cells (Prado et al. 2004; Sosa-Pineda, et al. 1997).
During the later stages of beta cell differentiation, the MAFB and MAFA transcription factors play a prominent role. MAFB expression in beta cells precedes an increase in PDX1 expression that marks the start of insulin transcription. In the absence of MAFB fewer alpha and beta cells are present, although total endocrine cell mass is not changed (Artner, et al. 2007). MAFA expression follows shortly after insulin expression (Artner, et al. 2006; Nishimura, et al. 2006) and persists in adult beta cells (Fig. 2). MAFB expression is lost post-natally from mouse (Artner, et al. 2010) but not human (Dai, et al. 2012; Riedel, et al. 2012) beta cells. In MAFB deficient mice, insulin expression is reduced and delayed until MAFA becomes expressed, but the resulting insulin+ cells lack PDX1, NKX6.1 and GLUT2 (Artner et al. 2007) and are therefore unlikely to represent true beta cells. In MAFA deficient pancreata, about one third of beta cells continue to express MAFB post-natally, which may partially compensate for MAFA loss (Artner et al. 2010). Nevertheless these mice display reduced expression of several genes involved in glucose stimulated insulin secretion and have reduced glucose tolerance (Artner et al. 2010; Hang, et al. 2014; Zhang, et al. 2005). Upon beta cell specification, the transcription of insulin is initiated and maintained by MAFB (Matsuoka, et al. 2003), PDX1 (Ohlsson et al. 1993), NEUROD1 (Naya, et al. 1995), PAX6 (Sander, et al. 1997), and MAFA (Kataoka, et al. 2002; Matsuoka et al. 2003; Olbrot, et al. 2002). Additionally, PAX4 (until expression is lost in adult, Fig. 2) and NKX6.1 and PDX1 inhibit glucagon expression (Ritz-Laser, et al. 2002; Schisler, et al. 2005) thereby preventing the expression of glucagon by beta cells.
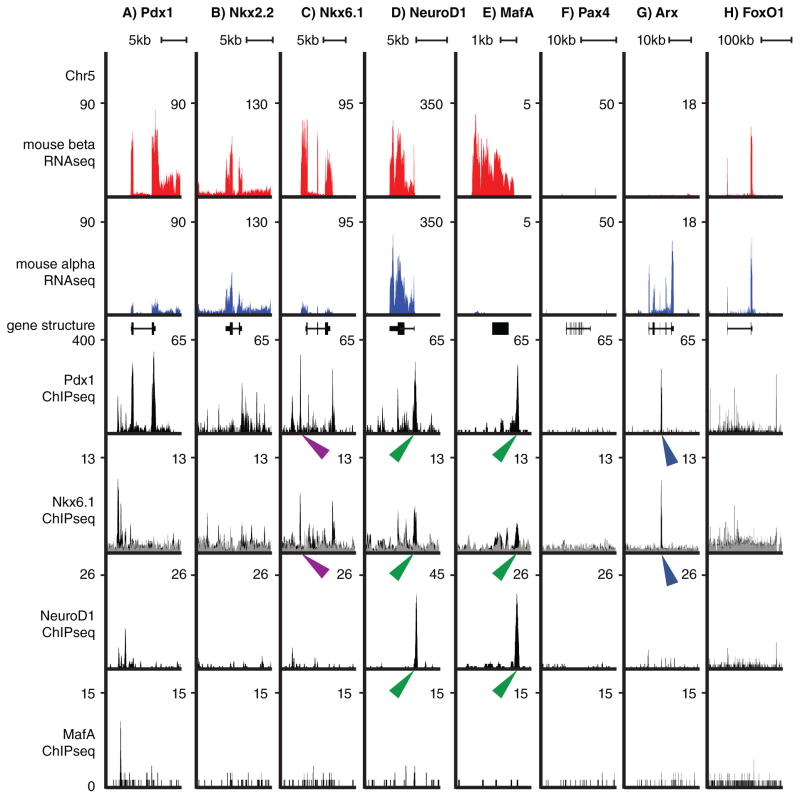
Figure 2.
Illustration of the pancreatic transcription networks in alpha and beta cells. Expression of transcription factors PDX1(A), NKX2.2 (B), NKX6.1 (C), NEUROD1 (D), MAFA (E), PAX4 (F), ARX (G) and FOXO1 (H) in beta and alpha cells by whole transcriptome sequencing (RNA-Seq), combined with chromatin immuno-precipitation data (ChIP-Seq) for PDX1 (Khoo, et al. 2012), NKX6.1 (Taylor et al. 2013) and NEUROD1 and MAFA (Tennant, et al. 2013). Purple arrowheads indicate binding of NKX6.1 and PDX1 to the same TAAT sequence in the NKX6.1 gene, green arrow heads point to closely spaced but non-identical binding sites for NEUROD1, NKX6.1 and PDX1 on the NEUROD1 and MAFA genes. Blue arrow heads indicate the PDX1 and NKX6.1 binding site on the ARX gene. NKX6.1 inhibits ARX expression via this interaction (Schaffer et al. 2013).
Transcription factors that define the alpha cell
ARX is one of the first known transcription factors to mark the alpha cell lineage (Fig. 2) and is required for alpha cell development (reviewed in (Bramswig and Kaestner 2011)). In the absence of ARX, no alpha cells develop and the number of beta and delta cells increases without affecting total endocrine cell number. This suggests that precursors alter their fate from alpha to beta and delta (Collombat et al. 2003). This phenotype is exactly the converse of that seen in PAX4-deficient mice described above. Indeed, a competitive reciprocal interaction between ARX and PAX4 is involved in the decision of cell fate between alpha and beta cell (Collombat, et al. 2005; Collombat et al. 2003). In the absence of ARX, PAX4 expression in early development takes over the ARX domain, leading to increased numbers of beta/delta cells at the expense of the alpha cell population. Conversely, in the absence of PAX4, ARX expression in early development expands, resulting in the opposite phenotype. When both ARX and PAX4 are lost, both alpha and beta cells are lost, while the delta cell population persists (Collombat et al. 2005). The same holds true for mice in which both ARX and NKX2.2 are lost (Kordowich, et al. 2011; Mastracci, et al. 2011). From these observations, it follows that ARX is required to generate the alpha cell phenotype, while NKX2.2 and PAX4 are required for the beta cell phenotype and are permissive of, but not required for delta cell formation. While ARX is necessary for the early specification of the alpha cell and directly maintains alpha cell mass (Courtney, et al. 2013), it is not directly involved in glucagon expression (Gosmain, et al. 2011).
Transcription factors later in alpha cell differentiation
The PAX4-related factor PAX6 is expressed by both alpha and beta cells. PAX6-deficient mice lack alpha cells (St-Onge, et al. 1997). In addition to its role in insulin transcription in the beta cell, PAX6 also coordinates glucagon transcription in the alpha cell directly by binding to the glucagon promoter (Sander et al. 1997) and indirectly by inducing expression of other transcription factors such as c-Maf, MAFB and NEUROD1, which also activate glucagon expression (Artner et al. 2006; Dumonteil, et al. 1998; Gosmain et al. 2011). Moreover, PAX6 forms heterodimers with c-Maf on the glucagon promoter (Gosmain, et al. 2007). A third factor that is crucial for alpha cell development is Foxa2. Foxa2-deficient pancreata show normal expression levels of ARX and PAX6, suggesting Foxa2 to play a role later in alpha cell development downstream of these transcription factors. Indeed Foxa2-deficient pancreata display a lack of mature alpha cells while alpha cell precursors are present (Lee, et al. 2005). Upon successful specification of the alpha cell lineage, pre-pro-glucagon expression is further promoted by Foxa1 (Kaestner, et al. 1999), Brn4 (Hussain, et al. 1997), and Isl-1 (Du, et al. 2009; Wang and Drucker 1995). Of all these transcription factors that can promote glucagon expression, expression of only two, Brn4 and MAFB, is enriched in adult mouse alpha cells compared to beta cells, while transcripts for PAX6, Foxa2, Foxa1 and Isl-1 are expressed at comparable levels in mouse alpha and beta cells (Benner et al. 2014).
Redundant transcription factor networks
As just described, the identity of the different pancreatic lineages is coordinated by a complex network of transcription factors that first establishes and then maintains differentiated cell identity. The transcription factor networks in the endocrine pancreas in particular are self-reinforcing, which is illustrated by key transcription factors binding regulatory elements in proximity to their own genes, as well as those of other transcription factors (Pasquali, et al. 2014). They activate lineage-appropriate genes or, equally importantly, repress expression of lineage-inappropriate genes (Collombat et al. 2003; Papizan et al. 2011; Schaffer et al. 2010; Schaffer et al. 2013)). Multiple transcription factors bind the same promoter and the net effect of the individual interactions determines the transcriptional activity of a gene. This principle is illustrated for the NEUROD1, PDX1 and NKX6.1 transcription factors in Fig. 2. NEUROD1, which recognizes a different core motif from NKX6.1 and PDX1 (Seo, et al. 2007), binds in close proximity to PDX1 and NKX6.1 on the promoters for NeuroD1 and MafA (Fig. 2d.e, green arrow heads). Moreover, related transcription factors recognize highly similar consensus binding sites. For example, homeobox containing genes such as PDX1 (Liberzon, et al. 2004) and NKX6.1 (Jorgensen, et al. 1999; Taylor, et al. 2013) recognize a core TAAT sequence, while their preference for the adjacent nucleotides is less stringent. Indeed, super-imposable peaks for PDX1 and NKX6.1 over a single TAAT sequence are evident on the NKX6.1 gene (Fig. 2c, purple arrow heads). Beta cell specific PDX1 and NKX6.1 bind the alpha cell specific ARX gene suggesting an inhibitory effect of this binding (Fig. 2, blue arrow heads). Indeed this is known for the NKX6.1 binding site (Schaffer et al. 2013). The resulting redundancy within the transcriptional networks may help maintain cell identity. Conversely, severe disruptions of the network compromise cell identity and contribute to dedifferentiation and transdifferentiation.
Transdifferentiation of beta to alpha cells
Forcing beta-to-alpha transdifferentiation by overexpression of ARX
One of the first pieces of evidence that suggested beta cells can be transdifferentiated into alpha cells resulted from the forced mis-expression of ARX within the pancreas. Transgenic mice were generated that express ARX as well as beta-galactosidase from the human beta-actin promoter (CAG), but only upon Cre recombinase mediated recombination. When ARX was expressed in all pancreas cells (by PDX1-Cre (Gu et al. 2002)) or all endocrine cells (by PAX6-Cre (Ashery-Padan, et al. 2000)), pancreata showed massive reductions of beta and delta cell numbers and increased alpha and PP cell numbers, predictably resulting in hyperglycemia (Collombat, et al. 2007). The total number of endocrine cells was not altered upon overexpression in the entire pancreas, indicating that ARX is not able to divert pancreatic non-endocrine progenitor cells to an alpha cell fate, but instead acts on endocrine progenitors and/or their offspring. Persistent ARX expression in all beta cells (by rat Ins2-Cre (Herrera 2000)) also resulted in the transdifferentiation of beta cells towards alpha and PP cells (Collombat et al. 2007). Delta cell numbers were unchanged. No double hormone positive cells were reported, suggesting that beta cells first down-regulated insulin before expressing glucagon (Collombat et al. 2007). Taken together, these data indicate that ARX expression not only directs endocrine progenitors towards the alpha and PP cell fate early in development but is able later in development to overcome an established beta cell fate in favor of an alpha cell fate.
The importance of PDX1 for beta cell identity
In addition to the importance of PDX1 for early pancreas specification, several lines of evidence show that PDX1 is also important for subsequent beta cell generation and maintenance of beta cell identity. Forced expression of PDX1 in all NGN3+ cells and their offspring via NGN3-Cre resulted in a reduction of the embryonic alpha cell population with a concomitant increase in the beta cell population (Yang et al. 2011). Deletion of PDX1 slightly later in development, upon insulin expression using Cre recombinase under the control of the Rat insulin1-promoter (RIP1), resulted in the opposite phenotype: reduced beta and increased alpha cell numbers, with many double hormone positive cells as well as overt diabetes by 3–5 months of age (Ahlgren et al. 1998). Cre mediated recombination in this mouse line was inefficient and only became prominent by 3–5 weeks of age. Similar experiments using a more efficient Rat insulin2 promoter driven Cre recombinase (RIP-Cre) (Gannon, et al. 2000; Postic, et al. 1999) showed earlier recombination, but essentially the same phenotype, except in an accelerated fashion and without double-hormone positive cells. Lineage tracing the recombined beta cells using RIP-Cre suggested that alpha cells exhibited an increased proliferation rate, while beta cells decreased proliferation, with no detectable beta-to-alpha transdifferentiation (Gannon, et al. 2008). However, a more recent study using tamoxifen-inducible RIP-CreER (Dor, et al. 2004) to delete Pdx1 in the beta cells of young adult (30-day-old) mice, reaches a different conclusion (Gao et al., 2014). Here, beta cell-specific Pdx1 ablation also resulted in diabetes and increased numbers of alpha cells at the expense of beta cells, but here the mechanism is transdifferentiation of beta to alpha cells (Gao, et al. 2014). It is possible that the difference in outcome between these studies is attributable to the onset of PDX1 ablation, which is commensurate with the onset of insulin expression in immature beta cells during embryonic development using Rip1-Cre (Gannon et al. 2008), but was started at 30 days post parturition using RIP-CreER (Gao et al. 2014) and thus did not occur until most beta cells have matured (van der Meulen, et al. 2012). Moreover, these two stages differ substantially in the way new pancreatic cells can be generated. During embryonic development and up to the first three weeks of postnatal life, islet cells continue to be generated through neogenesis from ductal precursors (Kopp, et al. 2011). In contrast, self-replication is the main mechanism by which beta cell mass is maintained in the adult (Dor et al. 2004). This suggests an age-dependent difference in plasticity between the embryonic pancreas and the pancreas of young adult mice. Pdx1 ablation mediated by RIP-Cre and RIP1-Cre takes place during late embryonic development and can activate compensatory changes in endocrine cell ratios via both neogenesis and proliferation. In contrast, the window for neogenesis has largely closed upon tamoxifen-dependent Pdx1 ablation in RIP-CreER mice at p30 and leads to more pronounced beta to alpha transdifferentiation undiluted by neogenesis. The tamoxifen-inducible RIP-CreER line offers an opportunity to directly compare the effects of PDX1 ablation at different onsets on the ensuing putative alpha to beta cell transdifferentiation.
Maintaining beta cell identity through NKX2.2 expression
A recent study highlights the importance NKX2.2, in particular its tinman domain, for the maintenance of beta cell identity (Papizan et al. 2011). The tinman domain is located outside the homeobox domain and is not directly involved in DNA binding, but instead recruits Groucho corepressors (Muhr, et al. 2001). Deletion of the tinman domain of NKX2.2 (NKX2.2TNmut/TNmut) phenocopies some aspects of total NKX2.2 deficiency, including reduced beta cell mass, but the phenotype diverges in several important aspects from that of global NKX2.2 null mice. First, until E12.5 alpha and beta cell development appear normal (Papizan et al. 2011) while NKX2.2 deficient mice have reduced alpha cell numbers and lack beta cells altogether (Sussel et al. 1998). Second, in contrast to the lower alpha cell numbers in NKX2.2 deficient mice, NKX2.2TNmut/TNmut pancreata show increased alpha cell numbers from E18.5 onward as well as an increase in the number of cells co-expressing glucagon and insulin. Yet, NKX2.2TNmut/TNmut mutants have no overt metabolic phenotype until 3.5 weeks of age when progressive hyperglycemia develops, in accordance with the increase in alpha cell numbers paired with a decrease in beta cells (Papizan et al. 2011). Lineage tracing of the existing beta cells using rat Ins2-Cre (Herrera 2000) suggests that at least a few of the increased population of alpha cells in the NKX2.2TNmut/TNmut mutant mice derive from transdifferentiated beta cells, as >2% of alpha cells carried a beta cell lineage mark (Papizan et al. 2011). Irreversibly labeling beta cells at three weeks of age using a PDX1-CreER (Gu et al. 2002) reveals that mature beta cells continue to transdifferentiate before any metabolic phenotype is evident (Papizan et al. 2011). While these data are very exciting, they should be interpreted with the understanding that the constitutive lack of the tinman domain of NKX2.2 could have affected beta cell development in ways that were not immediately obvious from histological analyses, for example by inhibiting final differentiation steps, potentially making it easier for these beta cells to transdifferentiate, Of note is the fact that deletion of ARX from beta cells in NKX2.2TNmut/TNmut mice prevented beta-to-alpha transdifferentiation (Papizan et al. 2011) as this highlights once more the requirement of ARX for differentiation along the alpha cell lineage. Mechanistically, NKX2.2 achieves inhibition of the Arx promoter by forming a complex with both the de novo methyltransferase 3a (Dnmt3a), which methylates CG dinucleotides to repress transcription, and histone deacetylase 1 (Hdac1) (Papizan et al. 2011). This NKX2.2/Dnmt3a/Hdac1 complex occupies the Arx promoter and inhibits ARX transcription (Papizan et al. 2011). Beta cell-specific deletion of Dnmt3a (Papizan et al. 2011), or of the related Dnmt1 (Dhawan, et al. 2011), predictably resulted in the de-repression of ARX in beta cells followed by beta-to-alpha transdifferentiation. As NKX2.2 as well as the other known components of the ARX repression complex are also expressed in adult ARX+ alpha cells there likely exist additional beta cell-specific factors that aid in ARX repression in the beta cell. In summary, NKX2.2 is required to facilitate endocrine differentiation early in embryonic development. Later in beta cell development, NKX2.2 takes on the role of maintaining beta cell identity and in this capacity prevents transdifferentiation to the alpha cell lineage by epigenetically modifying the Arx promoter.
Maintaining beta cell identity through FOXO1 expression
Another transcription factor that is integral for maintaining beta cell identity is FOXO1. While FOXO1 mRNA is similarly expressed in both alpha and beta cells (Benner et al. 2014), immunofluorescence analysis suggests that FOXO1 protein is more restricted to beta cells (Kitamura et al. 2009). In beta cells, FOXO1 is normally found in the cytoplasm, but mild hyperglycemia causes nuclear translocation of FOXO1 (Talchai et al. 2012), possibly contributing to the maintenance of MAFA and NEUROD1 expression and preservation of beta cell function (Kitamura, et al. 2005). Indeed, ablation of FOXO1 from beta cells via rat Ins2-Cre (Herrera 2000), increases susceptibility of beta cells to metabolic stress (Kobayashi, et al. 2012; Talchai et al. 2012). In younger mice on a db/db background, that is associated with severe obesity, beta cell specific deletion of FOXO1 further impaired glucose tolerance (Kobayashi et al. 2012). Older multiparous females and aging males that lack beta cell FOXO1 expression demonstrate increased alpha cell mass, reduced beta cell mass and reduced glucose tolerance accompanied by concomitant increases in plasma glucagon and decreases in plasma insulin levels (Talchai et al. 2012). Increased alpha cell mass in these mice was not due to increased proliferation (Talchai et al. 2012). Instead, during metabolic stress, FOXO1-deficient beta cells lose MAFA, PDX1 and insulin expression (Talchai et al. 2012), degranulate (Kobayashi et al. 2012; Talchai et al. 2012) and dedifferentiate into progenitor-like cells (Talchai et al. 2012). These dedifferentiated beta cells start to express the endocrine progenitor marker NGN3 (Talchai et al. 2012), possibly owing to reduced expression of the Notch effector and NGN3 repressor HES1 in the absence of FOXO1 (Kitamura, et al. 2007), resulting in de-repression of NGN3 (Lee, et al. 2001). The increase in NGN3 expression was confirmed in db/db and GIRKO mice (Talchai et al. 2012); the latter mouse model lacks insulin receptors in muscle and fat and has a high incidence of diabetes (Lin, et al. 2011). Surprisingly deletion of FOXO1 in the db/db model decreases NGN3 again (Kobayashi et al. 2012). In addition to NGN3, dedifferentiated beta cells express the pluripotency markers octamer-binding protein 4 (OCT4), avian myelocytomatosis viral oncogene homolog (c-MYC) and the homeobox transcription factor nanog (Talchai et al. 2012). Forced expression of c-MYC alone in beta cells is already sufficient to induce dedifferentiation in beta cells, as indicated by the loss of insulin, PDX1 and GLUT2 (Cheung, et al. 2010). Taken together, the expression of NGN3, Oct4, c-MYC and nanog suggests that the progenitor state is a regulated state rather than a regression to a degenerative state (Talchai et al. 2012) and raises the question of whether de-differentiated beta cells can be re-differentiated into mature glucose-responsive beta cells. Indeed, lineage tracing of beta cells showed that after dedifferentiation they can subsequently re-differentiate into alpha, delta and PP cells (Talchai et al. 2012). The authors could not demonstrate re-differentiation of de-differentiated beta cells owing to the limitations of the conventional lineage tracing approaches. However, it is reasonable to expect that de-differentiated beta cells will re-differentiate into beta cells under these conditions as well. Thus, these studies suggest that beta cells lacking FOXO1 under mild metabolic stress associated with ageing dedifferentiate into a progenitor-like cell capable of re-differentiating into any endocrine cell type of the islet, including alpha cells.
Human beta-to-alpha transdifferentiation without genetic manipulation of transcription factor expression
The specific genetic manipulations presented above showcase the importance of transcription factors in the transdifferentiation process. Human beta cells show unexpected plasticity as they too have the capacity to convert into alpha cells, and apparently do so without genetic perturbation of transcription factor networks. This was demonstrated by irreversibly labeling more than 80% of beta cells by transducing dissociated human islets with lentiviral vectors containing a rat Ins2-Cre and a GFP reporter (Russ, et al. 2009), with less than one percent of alpha cells being labeled (Spijker, et al. 2013). Following labeling, the dissociated cells were re-associated into islet-like clusters. Over the course of two weeks the percentage of beta cells in these clusters declined and that of alpha cells increased. Low levels of both apoptosis and proliferation suggested that these processes are not major contributors to the changing cluster composition. Instead, up to 15% of alpha cells showed a beta cell lineage mark two weeks following transduction (Spijker et al. 2013). Generalized inhibition of ARX in the cultures reduced the percentage of alpha cells carrying a beta cell lineage label, while at the same time increasing the number of remaining beta cells with a lineage label. This was interpreted as a reduction in beta-to-alpha transdifferentiation but, given the central role of ARX in the alpha cell, could also be due in part to a loss of alpha cells (Spijker et al. 2013). No cells that co-expressed insulin and glucagon were found. Instead, beta cells degranulated prior to expressing glucagon and did so despite the continued presence of PDX1 and NKX6.1 (Spijker et al. 2013); beta cell-specific transcription factors that inhibit glucagon expression (Schisler et al. 2005). Alpha cell identity persisted for at least two weeks after transplantation into mice (Spijker et al. 2013). These findings suggest that human beta cells, like those in mouse, have the capacity to transdifferentiate into alpha cells, at least under experimental conditions in vitro and dependent on ARX. Whether such plasticity of endocrine cell types exists in the human islet in situ remains to be seen.
Transdifferentiation of alpha to beta cells
Forcing alpha-to-beta transdifferentiation by over-expression of PAX4
As beta cells transdifferentiate into alpha cells by forced ARX expression and with the knowledge that ARX and PAX4 play opposing roles in the differentiation of alpha and beta cells during embryonic development, it follows that the over-expression of PAX4 might promote a beta cell identity. PAX4 is normally expressed only in the subset of endocrine cells that will become beta and delta cells (Sosa-Pineda et al. 1997) and is undetectable in adult mouse islets (Benner et al. 2014; Smith, et al. 1999). Cre recombinase-mediated overexpression of PAX4 in the PDX1 domain (all pancreas cell types), the PAX6 domain (all endocrine cells) or the glucagon domain (alpha cells) invariably reduced alpha cell numbers (Collombat, et al. 2009). Despite decreased alpha cell numbers, blood glucose levels remained normal or were slightly repressed at birth, although glucose clearance was improved in line with reduced glucagon tone (Collombat et al. 2009). Lineage tracing in mice with PAX4 over-expression in the alpha cell revealed that alpha cells were lost through their transdifferentiation into beta cells. An unforeseen effect was the development of progressive hyperglycemia, despite increased beta cell mass and reduced alpha cell numbers (Collombat et al. 2009). This paradoxical observation likely relates to the continued over-expression of PAX4 by beta cells, which is normally absent from adult mouse beta cells Fig. 2 (Benner et al. 2014; Smith et al. 1999). Over-expression of PAX4 in beta cells induces beta cell proliferation via expression of the oncogene c-myc, but impairs glucose stimulated insulin secretion (Brun, et al. 2004), consistent with C-Myc repressing the protein levels of insulin, GLUT2 and PDX1 (Cheung et al. 2010). The combined effects of PAX4 over-expression and c-MYC induction appear to cause partial dedifferentiation and thus explain the phenomenon of beta cell failure despite increased beta cell mass in PAX4 over-expressing beta cells.
Deletion of ARX from alpha cells results in loss of alpha cell phenotype
Alpha to beta transdifferentiation can also be induced independently of PAX4 overexpression in beta cells by deleting ARX from alpha cells via Glucagon-Cre-mediated (Herrera 2000) recombination of a floxed ARX allele (Fulp, et al. 2008). This results in alpha-to-beta transdifferentiation (Courtney et al. 2013; Wilcox, et al. 2013). One group reported continued alpha cell neogenesis from a proposed ductal progenitor and enlarged islets over time (Courtney et al. 2013), while the other observed no differences in total hormone+ cell numbers, possibly due to the early ages of mice examined as well as a much lower observed efficiency of Cre recombination (Wilcox et al. 2013). In contrast to the over-expression of PAX4 in alpha cells, which precipitates hyperglycemia presumably attributable to continuation of forced PAX4 expression upon alpha to beta cell transdifferentiation, deletion of ARX in alpha cells improves glucose tolerance (Courtney et al. 2013). Combined deletion of PAX4 and ARX from the alpha cell produced a phenotype similar to that resulting from ARX deletion alone. This suggests that while PAX4 and ARX transcriptionally repress each other (Collombat et al. 2003) and global deletion of both ARX and PAX4 results in a lack of both alpha and beta cells (Collombat et al. 2005), alpha cells can transdifferentiate into beta cells upon loss of ARX in the absence of PAX4 (Courtney et al. 2013). These data, combined with the above-mentioned roles for ARX, suggest that ARX is a key driver of alpha cell differentiation and maintenance as well as transdifferentiation of beta-to-alpha cells.
Maintaining alpha cell identity through expression of MEN1 tumor suppressor
An interesting example of possible alpha to beta cell transdifferentiation in the human pancreas is found in patients with MEN syndrome, caused by mutations in the MEN1 tumor suppressor gene. MEN1 is important for normal development of all islet cell lineages (Fontaniere, et al. 2008). MEN syndrome patients present with tumors of various endocrine descent (Lemos and Thakker 2008). The development of MEN1 tumors is likely to be a multistep process in which progressive loss of the two alleles of the MEN1 gene precipitates hyperplasia and atypia (with abnormally shaped cells) while the development of full blown adenomas may require additional somatic mutations (Crabtree, et al. 2003). Glucagonomas, while abundant in microadenomas of the pancreas, become increasingly rare as MEN1 deficient tumors grow and insulinomas and mixed islet tumors prevail (Anlauf, et al. 2006; Perren, et al. 2007). The deletion of MEN1 from the alpha cell using glucagon-Cre produced a phenotype that resembles that of patients with MEN syndrome. Alpha cell-specific deletion resulted in glucagonomas, hyperglucagonemia and hyperglycemia as expected. Interestingly, MEN1-deficient insulinomas subsequently developed and became the dominant effectors on blood glucose over time (Lu et al. 2010). Such insulinomas developed via a glucagon-insulin double positive intermediate and the location of the insulin granules within the cell resembled those of glucagon in alpha cells. Indeed, lineage tracing experiments pointed to an alpha cell origin of these insulinoma cells (Lu et al. 2010). Glucagon-insulin double positive cells appeared before islet tumors arose, pointing to the possibility of a direct MEN1 effect on alpha cell maintenance, rather than other somatic mutations that are acquired over time. These data not only indicate that alpha cells in a MEN1-deficient tumor model transdifferentiate into beta cells, but suggest that a similar process of transdifferentiation may take place in human MEN syndrome patients.
Near total beta cell ablation replenishes beta cells through an alpha or delta cell source
Significant loss of beta cell mass is accompanied by beta cell regeneration, achieved largely through self-duplication of existing beta cells in adult mice (Dor et al. 2004). Depletion of about 90% of beta cells has been shown to result in hyperglycemia and to induce a beta cell proliferative response (Cano, et al. 2008). However, recent studies have shown that more acute (>90%) beta cell loss is able to invoke alternative mechanisms in efforts to replenish the beta cell deficit. Even more extreme depletion was achieved in a genetic mouse model in which beta cells express the diphtheria toxin receptor under the rat Ins2 promoter (Thorel et al. 2010). Administration of diphtheria toxin resulted in near-total beta cell ablation (99.6%) (in two month old mice). This extreme loss of beta cells triggered alpha-to-beta cell transdifferentiation and was, over time, able to regenerate sufficient beta cell mass to restore normoglycemia (Thorel et al. 2010). Subsequent work from the same group suggests that near-complete beta cell ablation in the juvenile (two week old) pancreas triggers a rapid transdifferentiation response from delta cells (Chera, et al. 2014). Trans-differentiated beta cells of alpha cell descent expressed insulin and the beta cell transcription factors PDX1 and NKX6.1 and proceed through a transient co-positive stage, followed by the loss of glucagon (Thorel et al. 2010). In contrast, transdifferentiated beta cells of delta cell descent proceed through a hormone negative stage prior to acquisition of insulin expression (Chera et al. 2014). When only 50% of beta cells were depleted, no alpha to beta cell transdifferentiation was observed (Thorel et al. 2010). The authors therefore suggested that transdifferentiation is induced under circumstances of extreme beta cell loss, where beta cell self-replication can no longer restore significant beta cell mass, with the source of transdifferentiation dependent on the postnatal age of the animal. This age-dependence may stem from differences in alpha versus delta cell development. Alpha cells are among the first endocrine cells to differentiate, while the beta and delta lineages appear later and separate later from each other (Ben-Othman, et al. 2013; Habener, et al. 2005). Thus delta and beta cells may be more closely related, as exemplified by the continued expression of Pdx1 in both cell types (Peshavaria et al. 1994). Perhaps the beta and delta cell lineages are still diverging in early postnatal life, enabling the observed rapid transdifferentiation of delta-to-beta cells upon near-total beta cell depletion (Chera et al. 2014). As delta cells mature, they may lose the ability to readily transdifferentiate into beta cells, leaving the slower process of alpha-to-beta cell conversion to emerge as the main source of new beta cells by transdifferentiation.
Partial alpha-to-beta transdifferentiation in human islets by modulation of epigenetic marks
In accordance with the importance of epigenetic modifications for maintenance of beta cell fate, there is evidence from cultured human islets to suggest that the same holds true for alpha cell fate. The epigenome of the alpha cell appears to make them more amenable to transdifferentiation (Bramswig, et al. 2013). This conclusion is based on the interesting observation that many more genes in the alpha cell fraction were associated with both activating (H3K4me3) and inhibiting (H3K27me3) histone modifications when compared to beta and exocrine cells. Resolution of dual marks into a single mark is a common feature during the course of normal differentiation (Mikkelsen, et al. 2007), and the overrepresentation of dual marks in the alpha cell was interpreted to suggest that the alpha cell may be poised to change fate (Bramswig et al. 2013). However, the increased prevalence of bivalently labeled genes in the alpha cell fraction could have been embellished by contaminating beta cells. This possibility is difficult to exclude when applying cell surface markers to purify populations for FACS analysis. Moreover, the study is based on ex vivo conditions which may be far more predisposed to induce transdifferentiation as a culture artifact. Nevertheless, treatment of human islets with the histone methyltransferase inhibitor Adox resulted in mis-expression of PDX1 in alpha cells and the induction of glucagon-insulin co-positive cells (Bramswig et al. 2013). Although ectopic expression of alpha cell transcription factors in beta cells under these conditions was not addressed, these observations once more highlight the surprising plasticity that exist amongst endocrine cells of the mouse and human pancreas.
Concluding remarks
Diabetes is characterized by an absolute or relative deficiency of beta cells. Thus increasing beta cell mass will be instrumental in restoring normoglycemia in these patients. Stimulating human beta cell proliferation has proven challenging, even when results from rodent models seemed promising (Bernal-Mizrachi, et al. 2014; Kulkarni, et al. 2012). Moreover, particularly in later stages of T1D, the dearth of residual beta cells in combination with their notoriously slow proliferation rate makes the restoration of beta cell mass through the stimulation of beta cell proliferation a tall order. Direct transplantation of beta cells in the form of islets or whole pancreas has proven more successful in managing diabetes but is hindered by: 1) a lack of donor material and 2) the requirement for life-long immunosuppression (Shapiro 2012). Efforts to generate mature, functional beta cells from induced pluripotent or embryonic stem cells are in full swing (Kelly, et al. 2011; Pagliuca, et al. 2014; Rezania, et al. 2014), but will have many safety hurdles to overcome. Alternate sources of new beta cells, including ductal, acinar and, most recently, non-beta endocrine cells, provide alternative strategies for treating diabetes (Lysy, et al. 2013). Together these efforts have revealed a remarkable degree of plasticity amongst the different endocrine cells of the islet and have firmly established that transdifferentiation takes place under certain experimental circumstances in mouse models.
The dynamic landscape of identity switches that is now coming to light offers hope for novel therapeutic strategies with which to treat diabetes. Alpha to beta cell transdifferentiation in particular, is not only an avenue for the (partial) restoration of beta cell mass, it simultaneously reduces alpha cell mass and thus potentially restores the balance between insulin and glucagon which is perturbed in diabetes (Unger and Cherrington 2012; Unger and Orci 2010). While non-islet cells far outnumber islet cells as a potential source for new beta cells, non-beta islet cells may be more amenable to transdifferentiation into beta cells than duct or acinar cells because they share a more recent progenitor in development. Moreover, non-beta endocrine cells functionally resemble beta cells in many ways, which is reflected in a high degree of overlap in expression between many of the genes that are required for glucose sensing, stimulus secretion coupling and exocytosis (Benner et al. 2014).
In order to capitalize on the potential for transdifferentiation in the islet, important questions remain unanswered that will undoubtedly be addressed going forward. For example, it remains to be demonstrated that individual transdifferentiated beta cells have fully completed their transdifferentiation and are therefore functionally equivalent to endogenous beta cells. Second, it is imperative to identify molecules that can channel transdifferentiation towards the desired outcome of increased beta cell mass by promoting alpha or delta to beta cell transdifferentiation, while preventing the transdifferentiation or dedifferentiation of beta cells. Finding such molecules and ways to deliver them to the pancreas is essential to replace strategies that relied on genetic manipulation to establish proof-of-principle for transdifferentiation in the islet. Whether these molecules will be as efficient as transcription factors to induce transdifferentiation is a separate question and is related to the question of phenotype stability using these molecules. While the possibility of depleting the islet from alpha and/or delta cells is a potential concern, the more immediate challenge remains the identification of druggable targets that can induce islet cell transdifferentiation, before we worry about the consequences of succeeding at this task. We are encouraged by these new opportunities despite the fact that such key questions regarding transdifferentiation in the islet remain unanswered as yet.
Acknowledgments
Funding
MOH is the recipient of a Career Development Award from the Juvenile Diabetes Research Foundation (JDRF) and received support from grants 17-2012-424 and 2-2013-54 from the JDRF, P01-DK026741 from the NIH/NIDDK and the Clayton Medical Research Foundation, Inc.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest
References
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anlauf M, Schlenger R, Perren A, Bauersfeld J, Koch CA, Dralle H, Raffel A, Knoefel WT, Weihe E, Ruszniewski P, et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560–574. doi: 10.1097/01.pas.0000194044.01104.25. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, Sosa-Pineda B, Stein R. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stange G, Shemer R, Nord C, Scheel DW, Pan FC, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ben-Othman N, Courtney M, Vieira A, Pfeifer A, Druelle N, Gjernes E, Faurite B, Avolio F, Collombat P. From pancreatic islet formation to beta-cell regeneration. Diabetes Res Clin Pract. 2013;101:1–9. doi: 10.1016/j.diabres.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Benner C, van der Meulen T, Caceres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. doi: 10.1186/1471-2164-15-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocana A. Human beta-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest. 2013;123:1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig NC, Kaestner KH. Transcriptional regulation of alpha-cell differentiation. Diabetes Obes Metab. 2011;13(Suppl 1):13–20. doi: 10.1111/j.1463-1326.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol. 2004;167:1123–1135. doi: 10.1083/jcb.200405148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Rulifson IC, Heiser PW, Swigart LB, Pelengaris S, German M, Evan GI, Bluestone JA, Hebrok M. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes. 2008;57:958–966. doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L, Zervou S, Mattsson G, Abouna S, Zhou L, Ifandi V, Pelengaris S, Khan M. c-Myc directly induces both impaired insulin secretion and loss of beta-cell mass, independently of hyperglycemia in vivo. Islets. 2010;2:37–45. doi: 10.4161/isl.2.1.10196. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben–Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, et al. The inactivation of Arx in pancreatic alpha-cells triggers their neogenesis and conversion into functional beta-like cells. PLoS Genet. 2013;9:e1003934. doi: 10.1371/journal.pgen.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JS, Scacheri PC, Ward JM, McNally SR, Swain GP, Montagna C, Hager JH, Hanahan D, Edlund H, Magnuson MA, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23:6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic beta cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell. 2011;20:419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonteil E, Laser B, Constant I, Philippe J. Differential regulation of the glucagon and insulin I gene promoters by the basic helix-loop-helix transcription factors E47 and BETA2. J Biol Chem. 1998;273:19945–19954. doi: 10.1074/jbc.273.32.19945. [DOI] [PubMed] [Google Scholar]
- Fontaniere S, Duvillie B, Scharfmann R, Carreira C, Wang ZQ, Zhang CX. Tumour suppressor menin is essential for development of the pancreatic endocrine cells. J Endocrinol. 2008;199:287–298. doi: 10.1677/JOE-08-0289. [DOI] [PubMed] [Google Scholar]
- Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev. 2006;20:253–266. doi: 10.1101/gad.1360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labosky PA, Golden JA. Identification of Arx transcriptional targets in the developing basal forebrain. Hum Mol Genet. 2008;17:3740–3760. doi: 10.1093/hmg/ddn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon M, Ables ET, Crawford L, Lowe D, Offield MF, Magnuson MA, Wright CV. pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol. 2008;314:406–417. doi: 10.1016/j.ydbio.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis. 2000;26:139–142. doi: 10.1002/(sici)1526-968x(200002)26:2<139::aid-gene12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T, et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014;19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosmain Y, Avril I, Mamin A, Philippe J. Pax-6 and c-Maf functionally interact with the alpha-cell-specific DNA element G1 in vivo to promote glucagon gene expression. J Biol Chem. 2007;282:35024–35034. doi: 10.1074/jbc.M702795200. [DOI] [PubMed] [Google Scholar]
- Gosmain Y, Cheyssac C, Heddad Masson M, Dibner C, Philippe J. Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in alpha-cell differentiation, glucagon biosynthesis and secretion. Diabetes Obes Metab. 2011;13(Suppl 1):31–38. doi: 10.1111/j.1463-1326.2011.01445.x. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- Hald J, Sprinkel AE, Ray M, Serup P, Wright C, Madsen OD. Generation and characterization of Ptf1a antiserum and localization of Ptf1a in relation to Nkx6.1 and Pdx1 during the earliest stages of mouse pancreas development. J Histochem Cytochem. 2008;56:587–595. doi: 10.1369/jhc.2008.950675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang Y, Yamamoto T, Benninger RK, Brissova M, Guo M, Bush W, Piston DW, Powers AC, Magnuson M, Thurmond DC, et al. The MafA transcription factor becomes essential to islet beta-cells soon after birth. Diabetes. 2014;63:1994–2005. doi: 10.2337/db13-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58:403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development. 2005;132:3139–3149. doi: 10.1242/dev.01875. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Lee J, Miller CP, Habener JF. POU domain transcription factor brain 4 confers pancreatic alpha-cell-specific expression of the proglucagon gene through interaction with a novel proximal promoter G1 element. Mol Cell Biol. 1997;17:7186–7194. doi: 10.1128/mcb.17.12.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen MC, Vestergard Petersen H, Ericson J, Madsen OD, Serup P. Cloning and DNA-binding properties of the rat pancreatic beta-cell-specific factor Nkx6.1. FEBS Lett. 1999;461:287–294. doi: 10.1016/s0014-5793(99)01436-2. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Katz J, Liu Y, Drucker DJ, Schutz G. Inactivation of the winged helix transcription factor HNF3alpha affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 1999;13:495–504. doi: 10.1101/gad.13.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem. 2002;277:49903–49910. doi: 10.1074/jbc.M206796200. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kawai K, Yokota C, Ohashi S, Watanabe Y, Yamashita K. Evidence that glucagon stimulates insulin secretion through its own receptor in rats. Diabetologia. 1995;38:274–276. doi: 10.1007/BF00400630. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D’Amour KA, Kroon E, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- Khoo C, Yang J, Weinrott SA, Kaestner KH, Naji A, Schug J, Stoffers DA. Research resource: the pdx1 cistrome of pancreatic islets. Mol Endocrinol. 2012;26:521–533. doi: 10.1210/me.2011-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, DePinho RA, Kitajewski J, Accili D. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–2485. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kitamura YI, Kobayashi M, Kikuchi O, Sasaki T, Depinho RA, Accili D. Regulation of pancreatic juxtaductal endocrine cell formation by FoxO1. Mol Cell Biol. 2009;29:4417–4430. doi: 10.1128/MCB.01622-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, 3rd, Wright CV, White MF, Arden KC, Accili D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kikuchi O, Sasaki T, Kim HJ, Yokota-Hashimoto H, Lee YS, Amano K, Kitazumi T, Susanti VY, Kitamura YI, et al. FoxO1 as a double-edged sword in the pancreas: analysis of pancreas- and beta-cell-specific FoxO1 knockout mice. Am J Physiol Endocrinol Metab. 2012;302:E603–613. doi: 10.1152/ajpendo.00469.2011. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordowich S, Collombat P, Mansouri A, Serup P. Arx and Nkx2.2 compound deficiency redirects pancreatic alpha- and beta-cell differentiation to a somatostatin/ghrelin co-expressing cell lineage. BMC Dev Biol. 2011;11:52. doi: 10.1186/1471-213X-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Knofler M, Frutiger S, Hughes GJ, Hagenbuchle O, Wellauer PK. The p48 DNA-binding subunit of transcription factor PTF1 is a new exocrine pancreas-specific basic helix-loop-helix protein. EMBO J. 1996;15:4317–4329. [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484–495. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lee JC, Smith SB, Watada H, Lin J, Scheel D, Wang J, Mirmira RG, German MS. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- Liberzon A, Ridner G, Walker MD. Role of intrinsic DNA binding specificity in defining target genes of the mammalian transcription factor PDX1. Nucleic Acids Res. 2004;32:54–64. doi: 10.1093/nar/gkh156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Ren H, Samuel VT, Lee HY, Lu TY, Shulman GI, Accili D. Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes. 2011;60:700–709. doi: 10.2337/db10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Herrera PL, Carreira C, Bonnavion R, Seigne C, Calender A, Bertolino P, Zhang CX. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138:1954–1965. doi: 10.1053/j.gastro.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Lysy PA, Weir GC, Bonner-Weir S. Making beta cells from adult cells within the pancreas. Curr Diab Rep. 2013;13:695–703. doi: 10.1007/s11892-013-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci TL, Wilcox CL, Arnes L, Panea C, Golden JA, May CL, Sussel L. Nkx2.2 and Arx genetically interact to regulate pancreatic endocrine cell development and endocrine hormone expression. Dev Biol. 2011;359:1–11. doi: 10.1016/j.ydbio.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J, Andersson E, Persson M, Jessell TM, Ericson J. Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell. 2001;104:861–873. doi: 10.1016/s0092-8674(01)00283-5. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Stellrecht CM, Tsai MJ. Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev. 1995;9:1009–1019. doi: 10.1101/gad.9.8.1009. [DOI] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrot M, Rud J, Moss LG, Sharma A. Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci U S A. 2002;99:6737–6742. doi: 10.1073/pnas.102168499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of Functional Human Pancreatic beta Cells In Vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140:751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papizan JB, Singer RA, Tschen SI, Dhawan S, Friel JM, Hipkens SB, Magnuson MA, Bhushan A, Sussel L. Nkx2.2 repressor complex regulates islet beta-cell specification and prevents beta-to-alpha-cell reprogramming. Genes Dev. 2011;25:2291–2305. doi: 10.1101/gad.173039.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali L, Gaulton KJ, Rodriguez-Segui SA, Mularoni L, Miguel-Escalada I, Akerman I, Tena JJ, Moran I, Gomez-Marin C, van de Bunt M, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46:136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren A, Anlauf M, Henopp T, Rudolph T, Schmitt A, Raffel A, Gimm O, Weihe E, Knoefel WT, Dralle H, et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab. 2007;92:1118–1128. doi: 10.1210/jc.2006-1944. [DOI] [PubMed] [Google Scholar]
- Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright CV, Stein R. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol. 1994;8:806–816. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. 2011;13(Suppl 1):118–125. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Asadi A, Wang R, Ao Z, Warnock GL, Kieffer TJ. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia. 2012;55:372–381. doi: 10.1007/s00125-011-2344-9. [DOI] [PubMed] [Google Scholar]
- Ritz-Laser B, Estreicher A, Gauthier BR, Mamin A, Edlund H, Philippe J. The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia. 2002;45:97–107. doi: 10.1007/s125-002-8249-9. [DOI] [PubMed] [Google Scholar]
- Russ HA, Ravassard P, Kerr-Conte J, Pattou F, Efrat S. Epithelial-mesenchymal transition in cells expanded in vitro from lineage-traced adult human pancreatic beta cells. PLoS One. 2009;4:e6417. doi: 10.1371/journal.pone.0006417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols E, Marri G, Marks V. Promotion of Insulin Secretion by Glucagon. Lancet. 1965;2:415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- Schaffer AE, Freude KK, Nelson SB, Sander M. Nkx6 transcription factors and Ptf1a function as antagonistic lineage determinants in multipotent pancreatic progenitors. Dev Cell. 2010;18:1022–1029. doi: 10.1016/j.devcel.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Taylor BL, Benthuysen JR, Liu J, Thorel F, Yuan W, Jiao Y, Kaestner KH, Herrera PL, Magnuson MA, et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet. 2013;9:e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler JC, Jensen PB, Taylor DG, Becker TC, Knop FK, Takekawa S, German M, Weir GC, Lu D, Mirmira RG, et al. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc Natl Acad Sci U S A. 2005;102:7297–7302. doi: 10.1073/pnas.0502168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012;9:385–406. doi: 10.1900/RDS.2012.9.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Ee HC, Conners JR, German MS. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol Cell Biol. 1999;19:8272–8280. doi: 10.1128/mcb.19.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Spijker HS, Ravelli RB, Mommaas-Kienhuis AM, van Apeldoorn AA, Engelse MA, Zaldumbide A, Bonner-Weir S, Rabelink TJ, Hoeben RC, Clevers H, et al. Conversion of mature human beta-cells into glucagon-producing alpha-cells. Diabetes. 2013;62:2471–2480. doi: 10.2337/db12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4:1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant BR, Robertson AG, Kramer M, Li L, Zhang X, Beach M, Thiessen N, Chiu R, Mungall K, Whiting CJ, et al. Identification and analysis of murine pancreatic islet enhancers. Diabetologia. 2013;56:542–552. doi: 10.1007/s00125-012-2797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci U S A. 2010;107:16009–16012. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T, Xie R, Kelly OG, Vale WW, Sander M, Huising MO. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PLoS One. 2012;7:e52181. doi: 10.1371/journal.pone.0052181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Drucker DJ. The LIM domain homeobox gene isl-1 is a positive regulator of islet cell-specific proglucagon gene transcription. J Biol Chem. 1995;270:12646–12652. doi: 10.1074/jbc.270.21.12646. [DOI] [PubMed] [Google Scholar]
- Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CL, Terry NA, Walp ER, Lee RA, May CL. Pancreatic alpha-cell specific deletion of mouse Arx leads to alpha-cell identity loss. PLoS One. 2013;8:e66214. doi: 10.1371/journal.pone.0066214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]