Abstract
Elevated anxiety symptoms are one of the most common forms of psychopathology to co-occur with autism spectrum disorders (ASD). The purpose of this study was to explore the association between anxiety and ASD symptoms, particularly the degree to which the relationship is explained by Insistence on Sameness (IS) behaviors and/or cognitive ability. The sample included 1429 individuals aged 5:8 to 18:0 years who participated in the Simons Simplex Collection (SSC), a genetic consortium study of ASD. Child Behavior Checklist Anxiety Problems T-scores and Autism Diagnostic Interview-Revised “Insistence on Sameness” item raw totals were treated as both categorical and continuous measures of anxiety and IS respectively. Chronological age, verbal IQ, and a variety of ASD phenotype-related and other behavioral variables were assessed for potential association with anxiety and IS. Anxiety and IS continuous variables were minimally though significantly associated with each other and with chronological age and verbal IQ. Neither anxiety nor IS was associated with other core autism diagnostic scores. Anxiety was associated with a variety of other psychiatric and behavioral symptoms in ASD, including irritability, attention problems, and aggression, while IS was not. Anxiety and Insistence on Sameness appear to function as distinct constructs, each with a wide range of expression in children with ASD across age and IQ levels. Thus, both variables could be of use in ASD behavioral research or in dimensional approaches to genetic exploration. Unlike IS, however, anxiety is related to non-ASD-specific behavioral symptoms.
Keywords: Autism Spectrum Disorders, anxiety, Insistence on Sameness, Simons Simplex Collection, Child Behavior Checklist 6-18 (CBCL), Autism Diagnostic Interview-Revised (ADI-R)
Autism spectrum disorders (ASD) are associated with several behavioral features that have genetic and neurobiological implications, including intellectual disability, hyperactivity, aggression, and anxiety. Understanding how ASD and commonly co-occurring symptoms converge and diverge in terms of behavioral, biological, and genetic profiles should contribute new perspectives to the study of autism phenotypes and etiology. For example, these associated features may provide additional means by which to stratify genetic samples (Cannon et al., 2010; Hus, Pickles, Cook, Risi, & Lord, 2007) or provide insight into basic neurological functioning that could be linked to both ASD and co-occurring disorders. However, before associations with genetic or neurobiological mechanisms can be interpreted, the relationship between behavioral phenotypes of autism and comorbid constructs must be clearly documented in large research samples. The purpose of this study is to explore the association between anxiety and ASD symptoms, particularly the degree to which that relationship is explained by Insistence on Sameness (IS) behaviors and/or cognitive ability.
Anxiety, whether defined as a constellation of DSM-IV or ICD-10 disorders or as a psychological trait, is thought to be one of the most common comorbidities with ASD (Howlin, Mawhood, & Rutter, 2000; Leyfer et al., 2006; Wood et al., 2009). Sukhodolsky and colleagues (2008) reported that 43% of an ASD sample (N=171) from NIH medication trials met a screening cut-off for at least one anxiety disorder. In 177 3-18-year-olds with ASD, 25% received scores in the clinical concern range on the anxiety subscale of the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), compared to 10% of their siblings without ASD (Kanne, Abbacchi, & Constantino, 2009). Additionally, in a study comparing 2323 consecutive referrals to a child psychopharmacology clinic, the 217 children identified with an ASD were at comparable risk for most other psychological disorders, but at significantly increased risk for having two or more anxiety disorders, in comparison with the non-ASD referrals (Joshi et al., 2010).
One explanation for overlap in these symptom families is the hypothesis that common genetic and/or biological factors underlie both ASD and anxiety disorders (e.g., Piven & Palmer, 1999; Gadow, Roohi, DeVincent, & Hatchwell, 2008). Evidence of amygdala dysfunction in individuals with ASD (Baron-Cohen et al., 2000; Sweeten, Posey, Shekhar, & McDougle, 2002) has been suggested as a link to the high rates of anxiety in this population (Amaral, Bauman, & Schumann, 2003). Studies encompassing a variety of foci for knock-out mouse models of autism (including genes involved in serotonin, immunoglobulin, and corticosterone production) have reported both impaired social behavior and increased anxiety-related behavior in affected mice (Takayanagi et al., 2010; Balemans et al., 2010; Benno, Smirnova, Vera, Liggett, & Schanz, 2009; Lesch & Mossner, 1998). Additionally, the elevated rates of anxiety disorders in relatives of individuals with ASD suggest a common genetic link between the two symptom spectra (Piven & Palmer, 1999; Gadow, DeVincent, & Scheider, 2008; Bolton, Pickles, Murphy, & Rutter, 1998; Smalley, McCracken, & Tanguay, 1995; Delong & Dwyer, 1988). Though Hallett and co-authors noted a low level of genetic overlap between anxiety and ASD symptoms in a large study of typically developing twin pairs, they suggest a possible “threshold effect” in which disorders not linked in the general population are linked in clinically affected samples (Hallet, Ronald, & Happe, 2009). The question remains whether shared genetic risk between autism spectrum and anxiety disorders explains the high rates of co-occurrence or suggests that anxiety is an intrinsic feature of ASD, possibly as a result of restricted, compulsive behaviors or unusual responses to sensory aspects of the environment (Green & Ben-Sasson, 2010; Joosten, Bundy, & Einfeld, 2009; Ben-Sasson et al., 2008).
Anxiety and Insistence on Sameness in ASD
From the earliest history of autism as a defined disorder, Kanner suggested that restricted and repetitive behaviors (RRB) were driven by the “anxiously obsessive desire for the maintenance of sameness” (Kanner, 1943, p. 245).
Factor analyses of the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003) and other measures repeatedly yield an “Insistence on Sameness,” or IS, factor (Bishop et al., in press; Szatmari et al., 2006; Cuccaro et al., 2003) within RRB domains. This factor is characterized clinically by behavioral rigidity and resistance to change. As Kanner suggested, IS behaviors are frequently accompanied by obvious symptoms of anxiety surrounding the maintenance of routines or rituals. In fact, one recent report labeled this the “Anxiety and Compulsions” factor (Kamp-Becker, Ghahreman, Smidt, & Remschmidt, 2009). However, rates of overlap in anxiety and IS have not yet been documented and replicated in large samples. At this point, it is not established that “anxious” children with ASD tend to exhibit IS, or conversely, that the majority of children with ASD and IS also exhibit anxiety.
The Role of IQ in Anxiety
Although most of the anxiety-in-ASD literature published to date has been conducted in small samples of individuals with relatively high cognitive abilities, several findings suggest nonetheless that IQ likely plays a significant role in mediating the ASD-Anxiety relationship. Sukhodolsky et al. (2008) observed that children with ASD who had high levels of anxiety on the Child and Adolescent Symptom Inventory (CASI; Silverman, Fleisig, Rabian, & Peterson, 1991) tended to have higher IQs and language abilities, as well as more stereotyped behaviors and social impairments on the ADI-R, than did children with lower anxiety and ASD. Another study found that 36 children with ASD and IQs below 70 were significantly less likely to meet criteria for Generalized Anxiety Disorder (GAD) than were a comparison group of 22 children with ASD and IQs of 70 or greater (Witwer & Lecavalier, 2010). However, the symptom differences appeared to be related to verbally-loaded items; physical symptoms of GAD were endorsed at similar rates across the IQ groups. In contrast to the anxiety-IQ findings in ASD, IS has been reported to have minimal association with IQ (Hus et al., 2007), though Cannon et al. (2010) noted the two were positively correlated when IS factor scores were adjusted to remove the variance shared with Repetitive Sensory Motor behaviors (RSM).
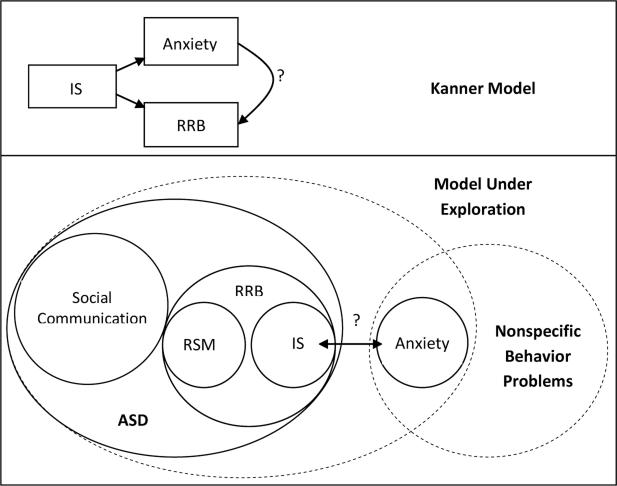
In summary, anxiety and ASD commonly co-occur, but it is not clear to what extent their association is explained by Insistence on Sameness, a core feature of ASD. Thus, the primary aim of this study is to explore the degree to which anxiety and IS are separable dimensions in ASD. Whereas Kanner described IS as leading to both anxiety and to restricted, repetitive behavior, we are starting with the hypothesis that IS and anxiety are separate constructs and testing the association between them to comment on whether anxiety is better described as a component of the ASD phenotype or as a commonly overlapping behavior that is not specific to ASD (see Figure 1).
Figure 1.
Models of Anxiety-Insistence on Sameness Relationship
Note. IS=Insistence on Sameness; RRB=Restricted, Repetitive Behavior; RSM=Repetitive Sensory Motor Behavior
We also aim to evaluate the potential influence of cognitive level on anxiety, IS, and the relationship between them. The use of a large, carefully phenotyped genetic research sample of children with ASD allows us the statistical power to examine the Anxiety-IS association while controlling for age and verbal IQ, with the further benefit of assessing these target variables’ relationships with ASD severity and other behavioral dimensions, such as irritability and aggression.
Methods
Participants
Data were collected as part of the Simons Simplex Collection (SSC), a genetic consortium study focusing on “simplex” families, in which a single child with ASD has no first, second, or third degree relatives with suspected ASD (Fischbach & Lord, 2010). SSC probands recruited from 12 university-based sites all met criteria for ASD on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000), Collaborative Programs for Excellence in Autism (CPEA) ‘ASD’ criteria on the ADI-R (Rutter et al., 2003), and had a nonverbal mental age of at least 18 months (see www.sfari.org for detailed eligibility criteria). Children with marked motor impairments, blindness, deafness, genetic syndromes, or significant medical histories (e.g., very low birth weight) were excluded. Potential probands were also excluded if they had a sibling with language or psychological problems likely related to ASD.
Because the primary focus of the present study is the association between anxiety and IS symptoms, the sample described here was restricted to those SSC participants who had the Child Behavior Checklist 6-18 Anxiety Problems T-score, a normed summary score derived from the raw total of 6 items describing DSM-IV criteria for childhood anxiety disorders (Achenbach & Rescorla, 2001). The final dataset included 1429 individuals aged 5 years, 8 months, to 18 years, 0 months, at the time of the in-person assessment (Mean Age=10 years, 2 months; SD=3 years, 1 month); all children were 6 or older when the CBCL was administered. Mean verbal IQ was 80 (SD=31, Range=5-167) and mean nonverbal IQ was 86 (SD=26, Range=9-161).
Fourteen percent of the sample was female (n=193). Ethnicities included 4% African American (n=50 individuals); 3% Asian American (n=49); 79% Caucasian (n=1135); 8% more than one race (n=114); 5% other (n=60); and 1% not reported (n=20). Ten percent of families reported Hispanic heritage (n=145). Maternal education was a graduate or professional degree for 24% of the sample, 66% bachelor's degree or some college, and 10% high school degree or less.
Procedures and Measures
Children received an ADOS and cognitive testing (see Fischbach & Lord, 2010, for IQ test hierarchy). Parents were interviewed using the ADI-R and the Vineland Adaptive Behavior Scales, Second Edition (Vineland-II; Sparrow, Cicchetti, & Balla, 2005) and completed questionnaires about both the proband and the sibling, including the Social Responsiveness Scale (SRS; Constantino & Gruber, 2005) and the CBCL. Parents provided informed consent, and procedures were approved by Institutional Review Boards at each university.
For a complete listing of SSC measures, see https://sfari.org/ssc-instruments. The primary measures used to group the sample in this study were (1) the ADI-R, a semi-structured parent interview for the purpose of informing diagnosis of ASD, demonstrated to have good to excellent validity and reliability (Rutter et al., 2003). ADI-R items include current (Current) and historical (Ever) scores. Diagnostic algorithm domains of Communication, Reciprocal Social Interaction, and Stereotyped and Repetitive Interests and Behaviors are based on sums of specific item Ever scores. All examiners affiliated with the SSC achieved research reliability on the measure and maintained their reliability with semi-annual checks throughout the data collection period. (2) The CBCL 6-18, a parent-rated questionnaire with demonstrated reliability and validity that assesses a child's current social competence and emotional and behavior problems. A three-point scale (0-2, with higher scores indicating greater symptomatology) is used to rate 118 items. In addition to Internalizing and Externalizing subscales, eight DSM-IV syndrome scales are produced, including a six-item Anxiety Total Problems scale. All subscale raw totals correspond to T-scores based on the child's age and gender. Though the CBCL 6-18 has not been validated for use in the ASD population, its preschool equivalent (CBCL 1.5-5; Achenbach & Rescorla, 2001) was found to have a factor structure and internal consistency similar to general population norms in a well-characterized sample of 128 young children with ASD (Pandolfi, Magyar, & Dill, 2009).
Design and Analysis
CBCL Anxiety Problem T-scores were used to operationalize anxiety, as T-scores allowed sample grouping by clinical cut-offs (see below). Exploratory factor analysis of the ADI-R Stereotyped and Repetitive Behavior items, conducted in SPSS, Version 17 (2008) using promax rotation, confirmed an Insistence on Sameness (IS) factor comprised of the items Difficulty with Minor Changes in Routine, Compulsions/Rituals, Resistance to Trivial Changes in the Environment, Abnormal Response to Specific Sensory Stimuli, Sensitivity to Noise, and Circumscribed Interests (see Supplementary Table 1), as well as a Repetitive Sensory-Motor factor (see Szatmari et al., 2006). Though the Circumscribed Interests item has been interpreted as distinct from IS in previous literature (see Bishop et al., in press), it was included in our IS factor because factor loadings, item-rest correlations, and alpha coefficients for this “Ever” item were similar to those statistics for the other five IS items. Distributions of IS factor scores were nearly identical to those of raw score sums for the IS-loading items, with scores of 2 and 3 collapsed to ‘2’ per accepted use of the measure. A summed IS “Ever” raw total therefore was chosen to operationalize IS behaviors. Note that IS raw totals based on summed Current or Ever items were highly correlated (r=.85); results described below were identical when Current raw totals were used.
Distributions of and correlations between the anxiety and IS variables were generated in this sample of ASD participants. For one analysis only, data from 1184 non-ASD siblings of the present sample participants (who were screened to exclude ASD characteristics but were not excluded on the basis of anxiety symptoms unless they had a history of hospitalization), were used to compare proband and sibling anxiety score distributions. IS data were not available for siblings.
In order to test the relationship between the anxiety and IS variables beyond bivariate correlation, groupings based on these variables were created in the ASD sample and assessed for cell size compared to expectations. Both the CBCL Anxiety T-scores and the IS “Ever” raw totals were divided and cross-tabulated (1) at the sample medians, (2) at the highest and lowest 25% of each variable and the middle 50%, and (3) at the CBCL Anxiety subclinical range of concern (T-scores of 65 and greater) against the 25-50-25 percent split of IS. As all methods yielded almost equivalent results in terms of observed-versus-expected cell size discrepancy, we focus on the last method. The CBCL Anxiety T-score was divided into scores of 50-64 (54%) and 65-80 (46%), and the un-normed IS raw total was divided from 0-4 (26%), 5-8 (50%), and 9-12 (24%). One-way ANOVA with Tukey post-hoc tests were used to assess group differences by chronological age and verbal IQ; linear regression modeling was used to test the effects of these variables on continuous Anxiety T-scores and IS raw totals. For all statistical tests reported here, p-values were compared to a criterion standard of .001 to address multiplicity and to limit the likelihood of over-interpreting small effects in a large sample with many variables tested.
Next, behavioral phenotyping variables were assessed for potential effects of anxiety and IS. Controlling for chronological age and verbal IQ, multivariate General Linear Model analyses were used to assess the effects of the continuous Anxiety T-scores and IS raw totals on continuous and categorical dependent variables, including: (1) selected participant characteristics: gender, familial depression and anxiety disorders, and regression, seizure, speech delay, and current speech status; (2) ASD symptomatology: ADOS and ADI-R domain totals; ADOS severity scores (Gotham, Pickles, & Lord, 2009); ADI-R RSM factor scores; SRS parent and teacher raw totals; Repetitive Behavior Scale-Revised (RBS-R; Bodfish, Symons, Parker, & Lewis, 2000) subtotals; clinician certainty ratings; (3) adaptive behavior: Vineland II domain standard scores; (4) nonspecific behavior and/or psychiatric problems: Aberrant Behavior Checklist (ABC; Aman, Singh, Steward, & Field, 1985) overall total and domain subtotals; CBCL subscale T-scores; ADOS and ADI-R specific item scores regarding aggression, overactivity, and anxiety.
Results
Distributions of IS and Anxiety Scores
Distributions of the variables used to operationalize anxiety and IS are shown in Supplemental Figures 1 and 2 (website). These variables had a small but significant correlation with each other in this ASD sample, r=.28 (p<.001)1. Forty-six percent of probands had CBCL Anxiety Problems T-scores in the subclinical range of concern and above (T-scores>=65), compared to only 9% of siblings who fell in this range, for an odds ratio of 8.7. No other analyses presented here used sibling data.
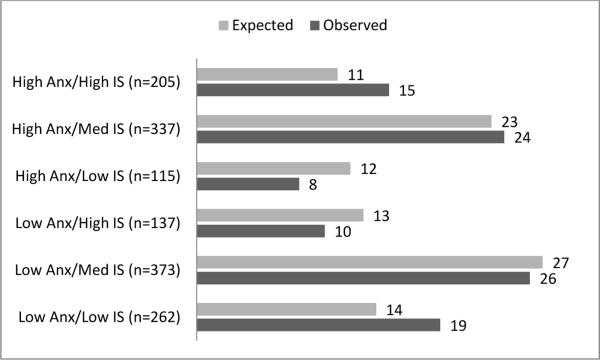
As described above, the clinical vs. non-clinical CBCL Anxiety groups were cross-tabulated by the low, middle, and high IS groupings. All six groups had at least 100 individuals, supporting the finding that, though anxiety and IS scores are correlated, they are relatively independent constructs. The largest discrepancies from expected group size occurred in the extremes: the proportions of children in the Low Anxiety × Low IS group (19%), or High Anxiety × High IS group (15%), were greater than the expected percentages for those groups (14% and 11% respectively). The proportions of children in the Low Anxiety × High IS group (10%), and those in the High Anxiety × Low IS group (8%), were lower than the expected percentages for those groups (13% and 12% respectively). See Figure 2 for a comparison of expected by observed percentages in all groups.
Figure 2.
Expected by Observed Percentages in the Anxiety-Insistence on Sameness Groupings
Note. N's denote number of observed participants in each cell. Numbers following each bar denote the percentage of the total sample in each group (expected versus observed).
Relationship of Covariates Age and Verbal IQ to IS and Anxiety
In the binary CBCL clinical cut by 25-50-25 IS variable groupings, chronological age did not approach significance for any comparison. Verbal IQ was significantly higher in the highest Anxiety × highest IS group (M=89.2, SD=26.0) than it was for the lowest Anxiety × lowest IS group (M=74.3, SD=33.0) and lowest Anxiety × middle IS group (M=76.8, SD=32.3) respectively (F(5,1423)=7.4, p<.001 for omnibus and both post-hoc results reported). Additionally, both chronological age and verbal IQ predicted CBCL Anxiety T-scores (Age: B=.02, SE=.01; VIQ: B=.04, SE=.01) and IS raw totals (Age and VIQ: B=.01, SE=.002) at the p<.001 level, with greater age and VIQ associated with both higher anxiety and IS scores. Despite these significant findings, the effect sizes of age and verbal IQ on the continuous anxiety and IS variables appeared to be negligible (on CBCL Anxiety T-scores: R=.17, R2=.03; on IS: R=.15, R2=.02).
The Role of Anxiety and IS in the ASD Phenotype
Means and standard deviations for all dependent variables tested can be found in Supplementary Table 2 (website). Significant results of the GLM analyses using continuous CBCL Anxiety T-scores and IS raw totals are displayed in Table 1, with a note summarizing the categorical findings.
Table 1.
Variables Significantly Predicted (p<.001) by Continuous Anxiety and/or Insistence on Sameness Scores
| Anxiety T-scores | IS Raw Totals | ||
|---|---|---|---|
| Intercept (SE) | B(SE) | B (SE) | |
| RBS-R Overall | −3.4(10.2) | 0.7(0.2)* | n.s. |
| RBS-R Ritualistic | −3.0(2.4) | 0.2(0.1)^ | n.s. |
| RBS-R Sameness | −5.3(3.6) | 0.2(0.1)* | n.s. |
| ABC Total | −23.3(15.2) | 1.6(0.3)* | n.s. |
| ABC Irritability | −12.8(5.2) | 0.5(0.1)* | n.s. |
| ABC Hyperactivity | −0.03(6.5) | 0.5(0.1)* | n.s. |
| ABC Lethargy | −10.9(4.5) | 0.3(0.1)* | n.s. |
| CBCL Internalizing | 3.6(4.2) | 0.8(0.1)* | n.s. |
| CBCL Externalizing | 19.7(6.4) | 0.7(0.1)* | n.s. |
| CBCL Thought | 47.7(5.1) | 0.3(0.1)* | n.s. |
| CBCL Social Problems | 36.3(4.5) | 0.3(0.1)* | n.s. |
| CBCL Attention | 36.0(6.2) | 0.6(0.1)* | n.s. |
| CBCL Aggressive | 33.6(5.6) | 0.5(0.1)* | n.s. |
| CBCL ADHD | 38.0(5.1) | 0.4(0.1)* | n.s. |
=Significant at p<.001.
=Approaching significance (p=.001).
n.s. =Not significant at p<.001.
Note. Categorical groupings (Anxiety clinical cut by IS 25-50-25 percent splits) had identical significant results with the following exceptions: neither anxiety nor IS categorical level predicted RBS-R Overall or CBCL Thought subscales, and higher IS level significantly predicted RBS-R Ritualistic subscale, Intercept B=5.4(SE=0.5), IS B=1.4(SE=.04).
The nonsignificant findings are of specific interest: IS raw totals were not significantly related to any of the variables assessed. Anxiety, too, had little association with participant demographic characteristics (including gender, race, and nonverbal IQ) or with most ASD diagnostic phenotyping measures (e.g., ADI-R and ADOS domains, SRS scores) or adaptive behavior (e.g., Vineland II domain standard scores) in this sample. However, higher anxiety was associated with higher RBS-R Overall Total and Sameness subscale scores. Anxiety was also a significant predictor of a range of psychiatric and behavioral symptom scores, including ABC Overall, Irritability, Hyperactivity, and Lethargy totals, and CBCL Externalizing, Social Problems, Attention Problems, Thought Problems, and Aggressive Behavior totals. Neither anxiety nor IS was associated with parent diagnoses of anxiety or depressive disorders. No anxiety-by-IS interaction was significant for any dependent variable. Again, findings were similar when “Current” IS raw totals were used in place of “Ever” scores, and when 25-50-25 percent categorical splits were entered for both IS and anxiety variables (rather than the subclinical threshold cut for the CBCL Anxiety T-scores).
Discussion
Results of this study indicate that anxiety and Insistence on Sameness function as distinct constructs, each with a wide range of expression in children with ASD across age and IQ levels. Anxiety was significantly more prevalent in probands with ASD than in the participants’ non-ASD siblings (IS was not measured in these siblings and thus precluded comparison on that variable). Anxiety and IS continuous variables were not strongly associated with each other or with core autism diagnostic scores. Within this large ASD sample, both variables were minimally though significantly associated with participant chronological age and verbal IQ. More importantly, anxiety was associated with a variety of other psychiatric and behavioral symptoms in ASD, including irritability, attention problems, and aggression.
Despite the relatively low correlation between anxiety and IS scores in this sample (r=.28), High/High and Low/Low Anxiety-by-IS groups were slightly more populated than would be expected if there was no relation between the variables. Additionally, anxiety scores were positively associated with the RBS-R Sameness subscale (see Bishop et al., in press, for a discussion of the association between RBS-R and ADI-R RRB domains). Thus, the general conclusion that anxiety and IS are relatively independent within ASD is tempered by the reminder that some relationship exists. The discrepancy could be accounted for by different anxiety types within ASD, such as a heritable, mood-related clinical anxiety versus an RRB-specific ‘tenseness’ associated with Insistence on Sameness. That may suggest the notion of children with both high clinical anxiety and high IS tenseness having a ‘double-hit’ that influences how they respond to their environment, however we found no evidence of an Anxiety-IS interaction in this study. Alternative models may involve mediators of the anxiety-IS relationship, such as level of sensory sensitivity or perseverative patterns to thoughts and behaviors. The relationship between anxiety and RRBs in general continues to warrant further exploration, particularly with regard to shared neurobiological mechanisms (Amaral, Schumann, & Nordahl, 2008) and pharmacological implications.
Like anxiety, Insistence on Sameness was not significantly associated with (other) core autism diagnostic features, such as scores on measures of social affect and reciprocity or repetitive sensory-motor behaviors. Unlike anxiety (and the social-communication and RSM behaviors themselves,based on previous literature; see Hus et al., 2007), IS was also independent from most participant characteristics and nonspecific behaviors tested here. Of particular interest, our findings replicate previous reports on the minimal association between IS and IQ (Cannon et al., 2010; Hus et al., 2007) and underscore the utility of IS behaviors as a means to characterize ASD samples that is independent of mediating variables.
One marked difference between Anxiety and IS in this sample was the preponderance of strong associations between anxiety and other psychiatric and/or behavioral symptoms, such as inattention, hyperactivity, and social withdrawal, despite negligible associations between these symptoms and IS. It is unclear whether this is a meaningful profile or measurement artifact. Because the anxiety groups were defined by a parent-rated CBCL subscale, it is possible that the associations between the anxiety ratings and ABC and CBCL subscales are driven by a parent-report effect, and that the same patterns are not noted in IS or for the core autism measures due to clinician-mediated rating (i.e., ADI-R). However, the parent-rated anxiety scale was not predictive of the majority of parent-rated RBS-R subscales. Thus, anxious children with ASD indeed may have more behavioral problems than non-anxious children with ASD. Recent literature suggests that behavioral problems may be related to certain genetic profiles, such as children with 16p deletions (Hanson et al., 2010); thus anxiety may be of value in genetic and neurobiological subgrouping despite, or in fact because of, these behavioral associations.
Limitations
Though the CBCL has shown some promise for use in ASD, it is not yet well-validated in this population. The CBCL Anxiety scale likely bears some verbal ability effects that may influence these findings; this work should be replicated with specific anxiety measures (e.g., clinical interview or teacher report). Additionally, ADI-R IS “Ever” scores were chosen in order to include all children with a proclivity toward IS; though this represents a comparison of historical IS features with current anxiety symptoms, results were identical when ADI-R IS “Current” scores were used in place of “Ever.” Of note, our IS factor included the item “Circumscribed Interests” (CI), which has not been included in several previous factor analyses of the ADI-R RRB domain (occasionally due to missing data); here, CI was deliberately included both to reflect empirical findings from the factor analysis and to include an item that involves fixed attention, a potential mechanism linking anxiety and IS symptoms. Finally, we chose a stringent significance criterion-value to control for the quantity of statistical comparisons, which may have obscured some meaningful findings.
Conclusions
Parent-rated CBCL Anxiety T-scores and ADI-R Insistence on Sameness raw totals appear to be minimally associated with each other and with chronological age and verbal IQ. Quantitative scores on continuous measures of anxiety and IS are likely to be relatively unrelated in children with ASD, thus anxiety is another dimension on which large ASD samples could be stratified for genetic exploration. Neither anxiety nor IS was linked to (other) core autism features or severity scores. Anxiety was associated with a number of general behavioral symptoms, including attention problems and aggression. Describing research samples in terms of anxiety and IS variables may inform our understanding of mechanisms potentially common to both anxiety and ASD, such as “perseverative attention” in the form of circumscribed interests in ASD and rumination in anxiety (Anholt et al., 2010; Sasson, Elison, Turner-Brown, Dichter, & Bodfish, 2011).
Supplementary Material
Elevated anxiety symptoms are one of the most common forms of psychopathology to co-occur with autism spectrum disorders (ASD). The purpose of this study was to explore the association between anxiety and ASD symptoms, particularly the degree to which the relationship is explained by Insistence on Sameness (IS) behaviors and/or cognitive ability. Within a large sample of children from a genetic consortium study of ASD, Child Behavior Checklist Anxiety Problems T-scores and Autism Diagnostic Interview-Revised “Insistence on Sameness” item raw totals were treated as measures of anxiety and IS respectively; these variables were assessed for potential association with each other and with chronological age, verbal IQ, and a variety of ASD symptom and other behavioral variables. In children with ASD, anxiety and Insistence on Sameness (IS) appear to be relatively independent of each other and of chronological age and IQ. Neither anxiety nor IS was significantly associated with other core autism features or ASD severity scores. Anxiety was associated with a number of general behavioral symptoms, including attention problems and aggression. In contrast, IS was not significantly associated with non-ASD-specific behavioral symptom domains. Describing research samples in terms of anxiety and IS variables may inform our understanding of the behavioral phenotype, biology, and genetics of the autism spectrum.
Acknowledgements
• This work was supported by grants from the Simons Foundation (SFARI) to K.G., A.B., and A.K., NIMH training grant (T32-MH18921) and NICHD Grant P30HD15052, R01MH081873-01A1 and RC1MH089721 to C.L., R01HD065277 to S.B.
• We are grateful to all of the families at the participating SFARI Simplex Collection (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, E. Wijsman).
• We appreciate obtaining access to phenotypic data on SFARI Base. Approved researchers can obtain the SSC population dataset described in this study (https://ordering.base.sfari.org/~browse_collection/archive[sfari_collection_v9_1]/ui:view()) by applying at https://base.sfari.org
• We gratefully acknowledge the support of Ed Cook, Andrew Pickles, Devon Oosting, LeeAnne Green-Snyder, Amy Esler, Fiona Miller, Jennifer Olson, Susan Risi, Kathryn Larson, and Kathy Hatfield.
Footnotes
Conflict of Interest Statement: Dr. C. Lord receives royalties from the publisher of the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS), described in this paper. She donates all profits generated by the Simons Simplex Collection use of these measures to charity. Drs. Gotham and Bishop will receive royalties from the ADOS-2, the second edition of the measure described here; both plan to donate all proceeds from research use to charity. Drs. Huerta, Lund, Buja, Krieger, and Ms. Hus have no conflicts of interest.
A concern in interpreting the correlation of 0.28 between anxiety and Insistence on Sameness as being statistically significant is that this relationship might be driven by the association of other covariates with each of these two variables. In order to partially address this issue we considered the available covariates of verbal IQ, age, and gender. After adjusting for these three covariates, the partial correlation between anxiety and IS was 0.27. Hence the conclusion that there is a modest but statistically significant association between anxiety and Insistence on Sameness remains.
Literature Cited
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles. ASEBA; Burlington,VT: 2001. [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985;89(5):485–491. [PubMed] [Google Scholar]
- Amaral D, Schumann C, Nordahl C. Neuroanatomy of autism. Trends in Neurosciences. 2008;31(3):137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: Implications from non-human primate studies. Genes, Brain, & Behavior. 2003;2(5):295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Anholt GE, Cath DC, Oppen P, Eikelenboom M, Smit JH, Megen H. Autism and ADHD symptoms in patients with OCD: Are they associated with specific OC symptom dimensions or OC symptom severity? Journal of Autism and Developmental Disorders. 2010;40(5):580–9. doi: 10.1007/s10803-009-0922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans M, Huibers M, Eikelenboom N, Kuipers AJ, van Summeren R, Pijpers M. Reduced exploration, increased anxiety, and altered social behavior: Autistic-like features of euchromatin histone methyltransferase 1 heterozygous knockout mice. Behavioral Brain Research. 2010;208(1):47–55. doi: 10.1016/j.bbr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24(3):355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: An unusual behavioral phenotype. Behavioral Brain Research. 2009;197(2):462–5. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, Carter AS. Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry. 2008;49(8):817–25. doi: 10.1111/j.1469-7610.2008.01899.x. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Huerta M, Gotham K, Duncan A, Lord C. Subtypes of restricted and repetitive behaviors in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-012-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish J,W, Symons F,J, Parker DE, Lewis MH. Varieties in repetitive behavior in autism. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: Patterns of familial aggregation. Psychological Medicine. 1998;28(2):385–95. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- Cannon DS, Miller JS, Robison RJ, Villalobos ME, Wahmhoff NK, Allen-Brady K. Genome-wide linkage analyses of two repetitive behavior phenotypes in Utah pedigrees with autism spectrum disorders. Molecular Autism. 2010;1(3):1–13. doi: 10.1186/2040-2392-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber C. The Social Responsiveness Scale. Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-Revised. Child Psychiatry & Human Development. 2003;34(1):3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- DeLong RG, Dwyer JT. Correlation of family history with specific autistic subgroups: Asperger's syndrome and bipolar affective disease. Journal of Autism and Developmental Disorders. 1988;18(4):593–600. doi: 10.1007/BF02211877. [DOI] [PubMed] [Google Scholar]
- Fischbach G, Lord C. The Simons Simplex Collection: A resource for identification of autism genetic risk factors. Neuron. 2010;68(2):192–5. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent C, Schneider J. Predictors of psychiatric symptoms in children with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2008;38(9):1710–20. doi: 10.1007/s10803-008-0556-8. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Roohi J, DeVincent CJ, Hatchwell E. Association of ADHD, tics, and anxiety with dopamine transporter (DAT1) genotype in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2008;49(12):1331–1338. doi: 10.1111/j.1469-7610.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Ben-Sasson A. Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: Is there a causal relationship? Journal of Autism and Developmental Disorders. 2010;40(12):1495–504. doi: 10.1007/s10803-010-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Happé F. Investigating the association between autistic-like and internalizing traits in a community-based twin sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(6):618–27. doi: 10.1097/CHI.0b013e31819f7116. [DOI] [PubMed] [Google Scholar]
- Hanson E, Nasir RH, Fong A, Lian A, Hundley R, Shen Y. Cognitive and behavioral characterization of 16p11.2 deletion syndrome. Journal of Developmental and Behavioral Pediatrics. 2010;31:649–657. doi: 10.1097/DBP.0b013e3181ea50ed. [DOI] [PubMed] [Google Scholar]
- Howlin P, Mawhood L, Rutter M. Autism and developmental receptive language disorder—A follow-up comparison in early adult life. II: Social, behavioural, and psychiatric outcomes. Journal of Child Psychology and Psychiatry. 2000;41(5):561–78. doi: 10.1111/1469-7610.00643. [DOI] [PubMed] [Google Scholar]
- Hus V, Pickles A, Cook E, Risi S, Lord C. Using the Autism Diagnostic Interview--Revised to increase phenotypic homogeneity in genetic studies of autism. Biological Psychiatry. 2007;61(4):438–48. doi: 10.1016/j.biopsych.2006.08.044. [DOI] [PubMed] [Google Scholar]
- Joosten AV, Bundy AC, Einfeld SL. Intrinsic and extrinsic motivation for stereotypic and repetitive behavior. Journal of Autism and Developmental Disorders. 2009;39(3):521–31. doi: 10.1007/s10803-008-0654-7. [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Kotarski M, Walls S, Biederman J. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders. 2010;40:1361–1370. doi: 10.1007/s10803-010-0996-9. [DOI] [PubMed] [Google Scholar]
- Kamp-Becker I, Ghahreman M, Smidt J, Remschmidt H. Dimensional structure of the autism phenotype: Relations between early development and current presentation. Journal of Autism and Developmental Disorders. 2009;39(4):557–71. doi: 10.1007/s10803-008-0656-5. [DOI] [PubMed] [Google Scholar]
- Kanne SM, Abbacchi AM, Constantino JN. Multi-informant ratings of psychiatric symptom severity in children with autism spectrum disorders: The importance of environmental context. Journal of Autism and Developmental Disorders. 2009;39(6):856–64. doi: 10.1007/s10803-009-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- Lesch K, Mossner R. Genetically driven variation in serotonin uptake: Is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biological Psychiatry. 1998;44(3):179–92. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36:849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Pandolfi V, Magyar CI, Dill CA. Confirmatory factor analysis of the Child Behavior Checklist 1.5–5 in a sample of children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(7):986–95. doi: 10.1007/s10803-009-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: Evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry. 1999;156(4):557–63. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised – WPS. WPS ed. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Smalley SL, McCracken J, Tanguay P. Autism, affective disorders, and social phobia. American Journal of Medical Genetics. 1995;60:19–26. doi: 10.1002/ajmg.1320600105. [DOI] [PubMed] [Google Scholar]
- Sasson N, Elison J, Turner-Brown L, Dichter G, Bodfish J. Brief report: Circumscribed attention in young children with autism. Journal of Autism and Developmental Disorders. 2011;41(2):242–7. doi: 10.1007/s10803-010-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Fleisig W, Rabian B, Peterson RA. Child Anxiety Sensitivity Index. Journal of Clinical Child Psychology. 1991;20(2):162–168. [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd ed. American Guidance Service Inc.; Circle Pines, MN: 2005. [Google Scholar]
- SPSS for Windows, Rel. 17.0.1. SPSS Inc.; Chicago: 2008. [Google Scholar]
- Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ. Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology. 2008;36(1):117–28. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Sweeten T, Posey D, Shekhar A, McDougle C. The amygdala and related structures in the pathophysiology of autism. Pharmacology, Biochemistry, and Behavior. 2002;71(3):449–55. doi: 10.1016/s0091-3057(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry. 2006;47(6):582–90. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Fujita E, Yu Z, Yamagata T, Momoi M, Momoi T. Impairment of social and emotional behaviors in Cadm1-knockout mice. Biochemical and Biophysical Research Communications. 2010;396(3):703–8. doi: 10.1016/j.bbrc.2010.04.165. [DOI] [PubMed] [Google Scholar]
- Witwer A, Lecavalier L. Validity of comorbid psychiatric disorders in youngsters with autism spectrum disorders. Journal of Developmental and Physical Disabilities. 2010;22:367–380. [Google Scholar]
- Wood J, Drahota A, Sze K, Har K, Chiu A, Langer D. Cognitive behavioral therapy for anxiety in children with autism spectrum disorders: A randomized, controlled trial. Journal of Child Psychology and Psychiatry. 2009;50(3):224–234. doi: 10.1111/j.1469-7610.2008.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.