Abstract
Background & Aims
Hydrogen sulphide (H2S), nitric oxide (NO), and carbon monoxide (CO) are involved in transitional microvascular tone dysregulation in the preterm infant; however there is conflicting evidence on the interaction of these gasotransmitters, and their overall contribution to the microcirculation in newborns is not known. The aim of this study was to measure the levels of all 3 gasotransmitters, characterise their interrelationships and elucidate their combined effects on microvascular blood flow.
Methods
90 preterm neonates were studied at 24h postnatal age. Microvascular studies were performed by laser Doppler. Arterial COHb levels (a measure of CO) were determined through co-oximetry. NO was measured as nitrate and nitrite in urine. H2S was measured as thiosulphate by liquid chromatography. Relationships between levels of the gasotransmitters and microvascular blood flow were assessed through partial correlation controlling for the influence of gestational age. Structural equation modelling was used to examine the combination of these effects on microvascular blood flow and derive a theoretical model of their interactions.
Results
No relationship was observed between NO and CO (p = 0.18, r = 0.18). A positive relationship between NO and H2S (p = 0.008, r = 0.28) and an inverse relationship between CO and H2S (p = 0.01, r = -0.33) exists. Structural equation modelling was used to examine the combination of these effects on microvascular blood flow. The model with the best fit is presented.
Conclusions
The relationships between NO and H2S, and CO and H2S may be of importance in the preterm newborn, particularly as NO levels in males are associated with higher H2S levels and higher microvascular blood flow and CO in females appears to convey protection against vascular dysregulation. Here we present a theoretical model of these interactions and their overall effects on microvascular flow in the preterm newborn, upon which future mechanistic studies may be based.
Introduction
Endogenous hydrogen sulphide (H2S) is associated with microvascular tone regulation at 24h postnatal age in the preterm infant and production appears to be affected by both gestational age and sex [1]. Nitric oxide (NO) and carbon monoxide (CO) also play a crucial role in the transitional circulation of preterm neonates [2,3]. NO is proposed to play a central role in the maintenance of vascular homeostasis in the perinatal period, however urinary excretion of NO metabolites do not correlate with early changes in microvascular blood flow in the preterm neonate [4]. It has been hypothesised that the rate of NO production in the endothelium of peripheral microvessels (via endothelial nitric oxide synthase (eNOS)) is lower than would be required to activate the downstream sGC pathway in vascular smooth muscle cells responsible for the excessive vasodilatation seen in premature neonates. This has led to the speculation that other mechanisms may be involved in both the production of NO in the microvasculature and its vasoactive effects on vascular smooth muscle cells during the transition from fetal to neonatal circulatory systems, with NO contributing to the maintenance of background tone throughout this period [5,6]. CO levels, on the other hand, correlate with both gestational age and microvascular blood flow at 24h postnatal age, suggesting that CO production by very preterm neonates may contribute to their increased risk of microvascular dysfunction and physiological instability [4].
The interaction of these gasotransmitters may account for a large proportion of their action. For example, NO and CO interact in the neonatal cerebral vasculature to regulate vascular tone—acute elevation in CO produces vasodilatation, yet prolonged production inhibits NO production, causing cerebrovascular constriction. Knecht et al. [7], hypothesised this interaction between CO and NO may form the basis of a negative feedback system in the control of cerebrovascular tone. However, the interaction between NO and CO, and between these two systems and H2S may not be as simple as this. A number of studies examining the relationship between the gasotransmitters have been published, with conflicting results. The different findings reported by these various studies may be due to differences in the tissues studied, the animal studied and/or their developmental stage and the methods used [8,9,10]. Some of these findings are summarised in Table 1. Whilst this is not an exhaustive list of all studies examining the interactions between the gasotransmitters, it gives some idea of the wide-ranging results observed in a number of discrete studies.
Table 1. Published Interactions of the Gasotransmitters.
| Effector | Interaction | Tissue | Species | Developmental Stage | Reference(s) |
|---|---|---|---|---|---|
| Nitric Oxide | |||||
| ↑ HO-1 expression (protein) | Aortic endothelial cells | Bovine | [11] | ||
| ↑ CO production and action | Cerebral vessels (pial arterioles) | Pig | Neonatal | [12,13,14] | |
| ↑ CO action (permissive enabler) | Retina | Salamander | [15] | ||
| ↑ CO, ↑ HO-1 expression (protein, mRNA) | Aortic smooth muscle cells | Rat | [16] | ||
| ↑ CO, ↑ HO-1 expression | Mesangial cells | Rat | [17] | ||
| ↑ CO, ↑ HO-1 expression | Fibroblasts (from lung) | Human | Embryonic | [18] | |
| ↑ CO, ↑ HO-1 expression | Kidney epithelial cells (cell line: LLC-PKI) | Pig | Juvenile (male) | [19] | |
| ↑ CO, ↑ HO-1 expression | Macrophages (cell line: RAW264.7)1 | Mouse | [20] | ||
| ↓ CO (via HO-1 inhibition) | Purified proteins | Human | [21] | ||
| ↓ HO activity | Aortic endothelial cells (cell line: AG08472) | Pig | [22] | ||
| ↓ HO-2 activity | Purified proteins | Rat | [23] | ||
| ↑ CSE expression | Peritoneal macrophages | Mouse | Adult (male) | [24] | |
| ↑ H2S, ↑ CSE expression | Aorta | Rat | Adult (male) | [25] | |
| ↓ CBS activity | Purified Proteins | Human | [26,27] | ||
| Carbon Monoxide | |||||
| ↑ NO release | Pulmonary artery endothelial cells | Bovine | [28] | ||
| ↑ NO release (at low concentrations of CO) | Renal arteries | Rat | Adult (male) | [29] | |
| ↓ NO, ↓ eNOS (at high concentrations of CO) | Renal arteries | Rat | Adult (male) | [29] | |
| ↓ NO (via NOS inhibition) after prolonged elevation of CO | Cerebral vessels (pial arterioles) | Pig | Neonatal | [7] | |
| ↓ NO (via NOS inhibition) | HO-1, HO-2 constructs | Rat | [23] | ||
| ↓ NOS | Cerebellum (granule cells) | Rat | Neonate | [30] | |
| ↓ iNOS activity, ↓ nNOS activity | Macrophages (iNOS), cerebellum (nNOS) | Rat | [31] | ||
| ↓ iNOS expression (transcriptional level) | Astrocytes | Human | Fetal | [32] | |
| ↓ nNOS activity | Cerebellum (granule cells) | Rat | Neonatal | [33] | |
| ↓ H2S (via CBS inhibition) | Astrocytes | Mouse | Neonatal | [34] | |
| ↓ H2S, ↓ CSE expression | Aortic smooth muscle cells | Rat | Juvenile (male) | [35] | |
| ↓ H2S (via CSE inhibition) | Carotid body | Mouse, rat | Adult (male) | [36] | |
| Hydrogen Sulphide | |||||
| ↑ NO release | Brain homogenates | Rat | [37] | ||
| ↑ NO action (permissive enabler) | Ileum, aorta | Guinea Pig, Rat | Juvenile | [38,39] | |
| ↓ NO effect | Aorta | Rat | [40] | ||
| ↓ NO | Retina | Salamander | [15] | ||
| ↓ NO activity | Aorta | Rat | Adult (male) | [41] | |
| ↓ NO, ↓ iNOS | Macrophages (cell line: RAW264.7; lipopolysaccharide exposed)1 | Mouse | [42] | ||
| ↓ eNOS | Aorta | Mouse, rat | Juvenile (male) | [43] | |
| ↓ eNOS, ↓ nNOS, ↓ iNOS | Recombinant proteins | [44] | |||
| ↑ CO, ↑ HO-1 expression (protein, mRNA) | Pulmonary arteries (with induced hypoxic pulmonary hypertension) | Rat | Juvenile (male) | [45] | |
| ↓ CO, ↓ HO-1 expression (protein) | Aortic smooth muscle cells | Rat | Juvenile (male) | [35] | |
HO heme oxygenase; CSE cystathionine-γ-lyase; CBS cystathionine-β-synthase; NOS nitric oxide synthase (eNOS endothelial isoform, iNOS inducible isoform, nNOS neuronal isoform). 1leukaemic monocyte macrophage cell line.
The synergistic effect of the interactions between these gasotransmitters arises from their different signalling cascades and their ability to enhance or diminish the effects of one or more of the others. The aim of the present study was to establish theoretical models of the interactions of the gasotransmitters and their combined effects on blood flow in the preterm newborn. This would provide a framework for establishing and testing current and future mechanistic hypotheses in this population. In order to achieve this, we measured the levels of all three gasotransmitters in the one neonatal population and characterised the interrelationships between NO, CO and H2S using structural equation modelling. As the differences in CO and H2S independently only account for a proportion of the vascular dysfunction observed in preterm neonates at 24h postnatal age we hypothesised that the interactions of NO, CO and H2S would account for a greater proportion of the microvascular tone dysregulation observed in the preterm newborn than the investigation of each of these molecules in isolation.
Methods
Subjects
Neonates 24–36 weeks’ gestation (n = 96) were studied at 24h postnatal age as part of the Cardiovascular Adaptation of the Newborn Study 2 (CANS2). These neonates form part of the cohort reported on previously [1,46]. Hypoxic ischemic encephalopathy, congenital malformations, chromosomal disorders or known congenital infection excluded admission to this study. The study protocol was approved by the human ethics committees at John Hunter Hospital and the University of Newcastle. Parental informed, written consent was obtained prior to investigation.
Microvascular studies
Investigations were performed at 24h postnatal age with a Periflux 5001 laser Doppler (Perimed AB, Jarfalla, Sweden) with a temperature-regulated probe (Probe 457, Perimed) set at 36°C sited on the lateral aspect of the neonates’ lower limb as previously described [46].
Clinical Illness severity
Clinical illness severity was evaluated using the Clinical Risk Index for Babies (CRIB) II scoring system [47].
Carbon monoxide measurement
CO binds competitively to haemoglobin, in preference to oxygen, to form carboxyhaemoglobin (COHb), which represents an in vivo sink for CO. Arterial COHb levels were determined at 24h postnatal age through spectrophotometry by using an ABL700 blood gas analyzer (Radiometer, Copenhagen, Denmark) and expressed as a proportion of total haemoglobin concentration as previously described [4].
Urine collection
24-hour urine samples were collected on day 2 of postnatal life as previously described [48]. Exact 24-hour urinary output was calculated by weighing diapers before and after use. As humidity can contribute to diaper weight, the degree and length of time in humidity were recorded and adjustments were made to calculate “true” increase as previously described [49].
Nitrate/nitrite measurement
NO has a short physiological half-life, making it difficult to measure directly. In order to assess total body turnover of NO, the more stable end products of NO oxidation, nitrate and nitrite, were measured in urine using a commercially available colorimetric assay according to manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, USA). Nitrate/nitrite levels were adjusted for 24h output and body weight to give a measure of total body output/24h (nmol/24h/kg).
Thiosulphate measurement
Thiosulphate, a stable urinary metabolite of H2S was used to assess total body turnover of H2S, due to the short half-life and volatile nature of the gas. Thiosulphate was measured by reversed-phase liquid chromatography as previously described [1,50]. Thiosulphate levels were adjusted for 24h output and body weight to give a measure of total body output/24h (nmol/24h/kg).
Statistical methods
Stata 13 for MacOSX (StataCorp LP, Texas, USA) was used for statistical analyses and structural equation modelling. Stata 13 and Prism 6 for MacOSX (GraphPad Software Inc., La Jolla, CA) were used for generation of figures. Data are presented as median (range) or mean and SEM where appropriate. Differences between groups were analysed by Mann-Whitney U-test unless otherwise stated. The relationships between levels of CO, NO, H2S and microvascular blood flow were assessed through partial correlation controlling for the influence of gestational age as in our previous studies [1,4]. Structural equation modelling was then performed in order to examine the combination of these effects on microvascular blood flow.
Structural equation modelling allows the examination of complex causal hypotheses on a set of intercorrelated non-experimental data and can be used for both exploration and confirmation of theoretical models [51]. For an exploratory approach such as that presented in the current study, a detailed model specifying the relationships among variables is not made a priori. This approach is considered superior over other correlational methods such as regression as multiple variables are analysed simultaneously, and latent factors reduce measurement error. When used as an exploratory or confirmatory approach, structural equation modelling provides information about the complex nature of disease and health behaviours. This is achieved by the examination of both direct and indirect, and unidirectional and bidirectional relationships between measured and latent variables [52]. In our particular construct, this was the interaction between the three gasotransmitters and their individual and combined effects on microvascular blood flow. All possible models were manually constructed for our three input (NO, CO and H2S) and one output (microvascular blood flow) variables. These models were then tested and assessed for suitability by χ2 Goodness of Fit and root mean square error of approximation (RMSEA). Lower χ2 values represent a better predicted model, whilst an RMSEA of below 0.06 shows a good fit [53]. RMSEA also allows for the calculation of a confidence interval (CI) around the predictive value of the model [54].
Results
Of the 96 preterm neonates in the cohort, 6 neonates did not have complete data and could not be included in the model. Physical and clinical characteristics, including microvascular blood flow measurements for the remaining 90 neonates included in the model are reported in Table 2.
Table 2. Physical Characteristics of Neonates.
| Female (n = 43) | Male (n = 47) | |
|---|---|---|
| Gestational Age (weeks) | 28 (24–35) | 29 (24–35) |
| Birth Weight (kg) | 1.06 (0.45–2.38) | 1.27 (0.56–2.76) |
| Microvascular Blood Flow (PU) | 43.4 (4.7–266.8) | 40.4 (6.5–216.64) |
| Completed Antenatal Glucocorticoids (n, %) | 31 (72%) | 36 (77%) |
| APGAR score at 5 min | 8 (4–10) | 9 (4–10) |
| Clinical Risk Index for Babies II score | 8 (0–15) | 5 (0–16) |
| Mean Blood Pressure at 24h | 37.5 (24–68) | 38 (26–81) |
| Small for Gestational Age | 1 (2%) | 6 (13%) |
| Significant patent ductus arteriosus | 11 (26%) | 12 (26%) |
| Intraventricular haemorrhage, grade ≥2 | 2 (5%) | 3 (6%) |
| Sepsis | 11 (26%) | 13 (28%) |
| Died | 4 (9%) | 4 (9%) |
Data presented as median (range) or number (percentage) as appropriate. PU laser Doppler perfusion units
Consistent with our previously reported observations in this cohort of neonates, which included the 96 preterm neonates (as well as 42 term neonates)) [46], microvascular blood flow at 24h postnatal age correlated with gestational age in this subset of neonates (all neonates p<0.0001, r = -0.54; females p = 0.009, r = -0.41; males p<0.0001, r = -0.64). There was no effect of birth weight on baseline microvascular blood flow when gestational age was accounted for (all neonates p = 0.82, r = -0.03; females p = 0.36, r = -0.15; males p = 0.66, r = 0.07). Detailed microvascular blood flow data for this cohort of neonates has been previously published [46].
Gasotransmitter measurement
NO levels were higher in females than males (females 21.4(4.6–37.1)nmol/24h/kg vs males 20.1(0.9–56.7)nmol/24h/kg, p = 0.058). No differences in CO levels between sexes were observed (females 1.5(0.9–4.1)% vs. males 1.4(0.9–2.2)%, p = 0.29). Thiosulphate levels have been previously reported for this cohort [1].
Interactions of the Gasotransmitters and their relationship with microvascular blood flow
Gestational age at birth was related to total gasotransmitter levels: gestational age was inversely correlated with NO (p = 0.03, r = -0.24), with no differences observed between males and females. Gestational age was also inversely correlated with CO (p = 0.0003, r = -0.45) and H2S (p = 0.02, r = -0.25). As urinary nitrates and thiosulphate are standardised to body weight, we could not examine the relationship between birth weight and NO and H2S. For CO, there was no effect of birth weight when gestational age was accounted for (all neonates p = 0.21, r = -0.17; females p = 0.14, r = -0.17; males p = 0.42, r = -0.15).
As gestational age is strongly related to the level of gasotransmitters, the relationships between levels of CO, NO, H2S and microvascular blood flow were assessed through partial correlation, controlling for the influence of gestational age. We observed a significant positive relationship between NO levels and microvascular blood flow for males but not females (p = 0.03, r = 0.38; females p = 0.91, r = -0.02). Furthermore, there was a significant positive relationship between CO levels and microvascular blood flow for males but not females (males p = 0.03, r = 0.38; females p = 0.88, r = -0.03) and H2S and flow (males p = 0.05, r = 0.29; females p = 0.72, r = 0.06).
Again, using partial correlations in order to account for differences associated with gestational age, we found no relationship between NO and CO (p = 0.18, r = 0.18); however, we observe a positive relationship between NO and H2S (p = 0.008, r = 0.28) and an inverse correlation between CO and H2S (p = 0.01, r = -0.33).
Structural equation modelling
Based on the results of the present study, several theoretical models were computed (S1 Data). The overall model with the best fit (χ2 = 1.02; RMSEA = 0.017(CI 0.00–0.28)) is presented in Fig. 1. This model had a better Goodness of Fit in females (χ2 = 0.03; RMSEA<0.0001(CI 0.00–0.21)) than males (χ2 = 1.88; RMSEA = 0.137(CI 0.00–0.44)). In this model, NO promotes H2S production (overall p = 0.002, z = 3.05; males p = 0.06, z = 1.88; females p<0.0001, z = 4.53), whilst CO inhibits H2S (overall p = 0.18, z = -1.34; males p = 0.84, z = -0.20; females p<0.0001, z = -5.39). As described above, NO levels were higher in females than males and no differences in CO levels between sexes were observed. The net result was a slightly enhanced positive relationship of all vasodilators acting on the microvasculature in males (p = 0.006, z = 2.74) compared to the effect of H2S on microvascular blood flow in isolation (model constructed without inclusion of other gasotransmitters; p = 0.008, z = 2.67). In females, the model predicted a lower contribution of H2S on microvascular blood flow (p = 0.905, z = -0.12) compared to the effect of H2S in isolation (p = 0.753, z = 0.31). The model predicted covariance in the levels of NO and CO despite a lack of any direct effect of one on the other (p = 0.362, z = 0.91), this may reflect an effect of gestational age. CO had no direct effect on microvascular blood flow in the model presented, however inclusion of this pathway improved goodness of fit compared to the same model minus this interaction (overall with CO effect χ2 = 1.02 vs without χ2 = 2.34, females with CO effect χ2 = 0.03 vs without χ2 = 0.29, males with CO effect χ2 = 1.88 vs without χ2 = 2.67). Additionally, the inclusion of a direct effect of CO on microvascular blood flow increased the effect of H2S on blood flow in the overall model (with CO effect p = 0.012, z = 2.52 vs. without p = 0.019, z = 2.34) and in males (with CO effect p = 0.006, z = 2.74 vs. without p = 0.008, z = 2.67).
Fig 1. Structural equation model of predicted interactions of the gasotransmitters and their contribution to the regulation of microvascular blood flow at 24h postnatal age in the preterm human.
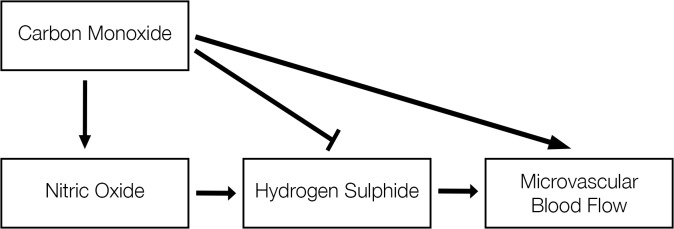
The overall model (males and females combined) is presented and has a Goodness of Fit of χ2 = 1.02 and RMSEA value of 0.017 (CI 0.00–0.28). Structural equation modelling examines linear causal relationships among variables, while simultaneously accounting for measurement error. The measurement error, or variance, determined in the model is 0.66 for microvascular blood flow, 0.77 for hydrogen sulphide, 0.24 for nitric oxide and 0.07 for carbon monoxide. NO was positively correlated with H2S (p = 0.002, z = 3.05). There was an inverse correlation between CO and H2S (p = 0.18, z = -1.34). There was a significant relationship between H2S and microvascular blood flow (p = 0.012, z = 2.52) when the input of NO and CO to H2S was included in the model.
Alternate models were tested and are presented in S1 Data. None of these models had a more acceptable χ2 or RMSEA value and CI, thus the selection of the model presented in Fig. 1.
Discussion
Structural equation modelling is sometimes referred to as “causal modelling”. However, a number of recent publications highlight that caution must be taken when interpreting the results as causation rather than association. Beran and Vialato [52] proposed that for causation to be determined via structural equation modelling the following criteria must be met: 1) there must be an empirical association between the variables, i.e. they are significantly correlated; 2) a common cause of the two variables must have been ruled out; 3) the two variables have a theoretical connection; and 4) that one variable precedes the other, and if the preceding variable changes, the outcome variable also changes (and not vice versa). These requirements are unlikely to be satisfied using non-experimental data, thus, causation cannot be definitively demonstrated. Rather, causal inferences that inform future experimental work may be drawn. The work presented here, and the final model proposed, in fact satisfies the majority of the criteria for causation as set out by Beran and Vialato.
Firstly, the gasotransmitters and microvascular blood flow are inter-correlated: as we have shown previously, CO [4] and H2S [1] were associated with higher microvascular blood flow in male preterm neonates. Contrary to our previous findings, we observed a significant, positive relationship between NO and microvascular blood flow in male preterm neonates. Furthermore, NO was positively correlated with H2S, whilst CO was inversely correlated with H2S. Thus, criteria 1 is met. Secondly, the variables have a theoretical connection (criteria 3): the gasotransmitters have known vasodilatory actions, and high microvascular blood flow in the neonate is assumed to relate to a loss of peripheral vascular tone. More specifically, a number of studies have now shown interactions between the three gasotransmitters (Table 1). Finally, criteria 4 specifies that one variable precedes the other; in the studies presented here, the testing of alternate models (see S1 Data) suggests that changes in CO and NO precede changes in H2S, and not vice versa; however, experimental studies need to be performed in order to confirm this directionality, especially considering the volume of experimental data that supports an effect of H2S on NO (Table 1 and [55]), as well as an effect of NO on H2S.
Thus, we present here a theoretical model, supported by our human observational studies, for the regulation of microvascular tone in the preterm newborn by the action and interaction of the gasotransmitters, which provides a construct from which future experimental studies may work in order to understand the development of circulatory compromise in this vulnerable population.
Interactions of the Gasotransmitters and their relationship with microvascular blood flow
We observed a significant positive relationship between NO and H2S. Previous studies have reported that NO inhibits H2S production via CBS [26,27] but induces CSE expression, and consequently, H2S production via that pathway [25]. This may suggest that in the human preterm newborn, CSE expression is significantly modulated by NO. We have evidence from our animal model that increases in H2S associated with microvascular dysregulation are driven by CSE-dependent mechanisms [56]. The inhibition of CSE prevents the increased H2S production observed at 24h postnatal age in the preterm guinea pig pup, and CSE-dependent, but not CSE-independent H2S production is associated with increased microvascular blood flow. The relationship between NO and CSE/H2S needs to be investigated further, particularly as this appears to be associated with higher microvascular blood flow as measured by laser Doppler. Contrary to our previous findings [4], we observed a significant, positive relationship between NO and microvascular blood flow at 24h postnatal age in male neonates. One source of these differing results may be the use of different methodology—in our previous papers NO metabolites were standardised to creatinine to allow for comparisons between time points and subjects. It has been shown, however, that creatinine may not be the best molecule for this purpose in the neonate as levels change significantly in the transitional period [57,58].
In females, a lower contribution of H2S to microvascular tone regulation was predicted when the other gasotransmitters were added into the model. This suggests that the effect of either NO, CO, or both, negates the effect of H2S to such a degree that there is no net effect on vascular tone. This may be primarily due to CO, which is inversely correlated with H2S and may reflect an inhibitory action of CO on H2S, in line with published reports that have demonstrated that CO decreases the production and action of H2S [34,35,36]. This is of particular interest in this cohort, as females and males had comparable levels of CO, suggesting some protective role of this molecule against inappropriate vasodilation in the female. The findings of our current study are discrepant with our previous studies, which showed that males had higher levels of CO and that this was associated with inappropriate peripheral microvascular dilatation and physiological instability in the first few days of life [4]. There are a number of possible explanations for these differences. Firstly, the infants in our original studies were younger (median age 1 week older in the present study, with neonates up to 35 weeks included compared to an upper age of 32 weeks in the previous study) and therefore, sicker, than the neonates in the present study. Secondly, there was a much higher rate of antenatal glucocorticoid exposure in the present study (74% in the current study compared to 59% in the previous study).
Limitations and future research
The present study does not provide direct confirmation of the mechanisms of action, the expression of gasotransmitter-producing enzyme/s or feedback of the gaseous molecule on the producing and/or releasing pathways. Rather, the aim of this study was to establish a theoretical model of gasotransmitter interactions in the preterm newborn, and the potential effect of these interactions on microvascular blood flow. Given the evidence of interactions between the three gasotransmitters in the preterm newborn population presented here, we propose future mechanistic studies should not focus solely on one of these gasotransmitters as driving dysfunction, but rather investigate the interactions among CO, NO and H2S in this context.
It is not possible to experimentally test these interactions within the sick human preterm infant population studied here, however the results of the present study can be used to inform future studies in relevant animal models [59,60,61] in order to elucidate the mechanisms underlying these correlations.
Future research should also investigate the mechanisms that give rise to the different interactions and effects of the gasotransmitters in male versus female preterm neonates. As many of the steroid hormone receptors (such as those for progesterone, estrogen and testosterone) are located within the endothelium and smooth muscle layers of blood vessels, the sex hormones may have influence over these vasoactive substances and the downstream signalling mechanisms involved in microvascular dilatation in a sex-specific manner.
We hypothesised that rates of antenatal glucocorticoids may contribute to the differences in CO levels and effect on blood flow observed in the present study compared to our previous studies in a similar cohort [4]. Glucocorticoids, such as antenatally administered betamethasone can modulate blood pressure, vascular reactivity and the production and action of vasoconstrictors and vasodilators, such as the gasotransmitters [62,63]. In mice, administration of glucocorticoids reduces eNOS levels (through decreased transcription and increased degradation) in aorta, liver and kidney [64,65,66,67]. Dexamethasone is known to reduce the release of NO from the endothelium and completely suppresses the inducible form of NOS [68,69,70,71]. Dexamethasone also downregulates HO-1 expression in models of systemic inflammation [72] and suppresses CSE expression, reducing H2S production, both directly through regulation of transcription and through inhibition of NO production, which is known to drive CSE expression [24]. Glucocorticoids are also known to decrease other vasodilators, including prostaglandins and enhance the effects of vasoconstrictors such as Angiotensin II [73,74] and norepinephrine [63,75,76]. The effect of glucocorticoids on the levels of individual vasoactive molecules, and overall vascular tone regulation, needs to be studied further in order to determine if antenatal glucocorticoid exposure effects gasotransmitters production and/or action in the preterm newborn. This is particularly relevant in the context of sex differences in gasotransmitters-related regulation of vascular tone, as it is well characterised that males and females metabolise and respond to glucocorticoid exposure differently [48,77].
Future studies should also consider the effect of a range of other vasoactive inputs, such as the sympathetic nervous system and the renin-angiotensin system and the newly identified fourth gasotransmitter, ammonium [78]. The action of these pathways and their interaction with the gasotransmitter system presented here may contribute to overall vascular tone regulation in this vulnerable population.
Conclusions
We identified significant correlations between the gasotransmitters NO, CO and H2S and microvascular blood flow in preterm neonates. This allowed us to produce a theoretical model for the regulation of microvascular tone in the preterm newborn by the action and interaction of the gasotransmitters. The results of the present study suggest that CO may confer some protective advantage in the female preterm neonate whilst in the male neonate, H2S production may be aberrantly modulated by NO, likely through changes in CSE expression. This hypothesis is supported by the results of the present study, previous studies by others (see Table 1) and those of ourselves—we have shown that CSE production is upregulated in the preterm newborn male and that H2S produced via CSE (but not CSE-independent pathways) correlates with microvascular tone dysregulation [56]. The relationship between NO and CSE/H2S is associated with higher microvascular blood flow and may be of particular interest given the wealth of literature surrounding an interaction between these two molecules (and their production pathways); further work is required in order to confirm this. We present a theoretical model built on observations within a human population which provides evidence of gasotransmitters interactions in the preterm newborn. This model provides a framework for establishing and testing current and future mechanistic hypotheses within this population.
Supporting Information
Alternate models were tested and are presented here. As in the manuscript, the interaction between the three gasotransmitters and their individual and combined effects on microvascular blood flow were assessed. All models were manually constructed and presented here for our three input (NO, CO and H2S) and one output (microvascular blood flow) variables. These models were then tested and assessed for suitability by χ2 Goodness of Fit and root mean square error of approximation (RMSEA). Lower χ2 values represent a better predicted model, whilst an RMSEA of below 0.06 shows a good fit. RMSEA also allows for calculation of a confidence interval (CI) around the predictive power of the model. None of these models had a more acceptable χ2 or RMSEA value and CI, thus the selection of the model presented within the manuscript.
(PDF)
Acknowledgments
The authors would like to acknowledge the parents of the neonates enrolled in the 2CANS study for their participation, the staff of the Kaleidoscope Neonatal Intensive Care Unit at the John Hunter Children’s Hospital, and Kimberly-Clark Australia for providing the diapers used in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by a National Health and Medical Research Council Project Grant awarded to IMRW (ID#569285; https://www.nhmrc.gov.au/) and John Hunter Hospital Charitable Trust Project Grants awarded to IMRW, HKP and RMD. RMD was supported by the Hunter Children’s Research Foundation. The HPLC work performed by GC and RG was supported by the University of Lodz. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dyson RM, Palliser HK, Latter JL, Chwatko G, Glowacki R, et al. (2014) A role for H2S in the microcirculation of newborns: the major metabolite of H2S (thiosulphate) is increased in preterm infants. PloS one 9: e105085 10.1371/journal.pone.0105085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krediet TG, Valk L, Hempenius I, Egberts J, van Bel F (2002) Nitric oxide production and plasma cyclic guanosine monophosphate in premature infants with respiratory distress syndrome. Biol Neonate 82: 150–154. [DOI] [PubMed] [Google Scholar]
- 3. Farkas I, Maroti Z, Katona M, Endreffy E, Monostori P, et al. (2008) Increased heme oxygenase-1 expression in premature infants with respiratory distress syndrome. Eur J Pediatr 167: 1379–1383. 10.1007/s00431-008-0673-6 [DOI] [PubMed] [Google Scholar]
- 4. Stark MJ, Clifton VL, Wright IM (2009) Carbon monoxide is a significant mediator of cardiovascular status following preterm birth. Pediatrics 124: 277–284. 10.1542/peds.2008-0877 [DOI] [PubMed] [Google Scholar]
- 5. Chen K, Popel AS (2006) Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free radical biology & medicine 41: 668–680. [DOI] [PubMed] [Google Scholar]
- 6. Kavdia M, Popel AS (2004) Contribution of nNOS- and eNOS-derived NO to microvascular smooth muscle NO exposure. Journal of applied physiology 97: 293–301. [DOI] [PubMed] [Google Scholar]
- 7. Knecht KR, Milam S, Wilkinson DA, Fedinec AL, Leffler CW (2010) Time-dependent action of carbon monoxide on the newborn cerebrovascular circulation. Am J Physiol Heart Circ Physiol 299: H70–H75. 10.1152/ajpheart.00258.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554. [DOI] [PubMed] [Google Scholar]
- 9. Wu L, Wang R (2005) Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev 57: 585–630. [DOI] [PubMed] [Google Scholar]
- 10. Carson RJ, Seyffarth G, Mian R, Maddock H (2004) Interactions Between Gasotransmitters In: Wang R, editor. Signal Transduction and the Gasotransmitters: NO, CO, and H2S in Biology and Medicine. 2004 ed. Totowa, NJ: Humana Press Inc. [Google Scholar]
- 11. Foresti R, Hoque M, Bains S, Green CJ, Motterlini R (2003) Haem and nitric oxide: synergism in the modulation of the endothelial haem oxygenase-1 pathway. Biochem J 372: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leffler CW, Nasjletti A, Johnson RA, Fedinec AL (2001) Contributions of prostacyclin and nitric oxide to carbon monoxide-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol 280: H1490–1495. [DOI] [PubMed] [Google Scholar]
- 13. Leffler CW, Fedinec AL, Parfenova H, Jaggar JH (2005) Permissive contributions of NO and prostacyclin in CO-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol 289: H432–438. [DOI] [PubMed] [Google Scholar]
- 14. Leffler CW, Balabanova L, Fedinec AL, Parfenova H (2005) Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol 289: H1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pong WW, Eldred WD (2009) Interactions of the gaseous neuromodulators nitric oxide, carbon monoxide, and hydrogen sulfide in the salamander retina. J Neurosci Res 87: 2356–2364. 10.1002/jnr.22042 [DOI] [PubMed] [Google Scholar]
- 16. Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI (1997) Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res 80: 557–564. [DOI] [PubMed] [Google Scholar]
- 17. Datta PK, Lianos EA (1999) Nitric oxide induces heme oxygenase-1 gene expression in mesangial cells. Kidney Int 55: 1734–1739. [DOI] [PubMed] [Google Scholar]
- 18. Bouton C, Demple B (2000) Nitric oxide-inducible expression of heme oxygenase-1 in human cells. Translation-independent stabilization of the mRNA and evidence for direct action of nitric oxide. J Biol Chem 275: 32688–32693. [DOI] [PubMed] [Google Scholar]
- 19. Liang M, Croatt AJ, Nath KA (2000) Mechanisms underlying induction of heme oxygenase-1 by nitric oxide in renal tubular epithelial cells. Am J Physiol Renal Physiol 279: F728–735. [DOI] [PubMed] [Google Scholar]
- 20. Alcaraz MJ, Habib A, Creminon C, Vicente AM, Lebret M, et al. (2001) Heme oxygenase-1 induction by nitric oxide in RAW 264.7 macrophages is upregulated by a cyclo-oxygenase-2 inhibitor. Biochim Biophys Acta 1526: 13–16. [DOI] [PubMed] [Google Scholar]
- 21. Wang R (2002) Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 22. Motterlini R, Foresti R, Intaglietta M, Winslow RM (1996) NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol 270: H107–114. [DOI] [PubMed] [Google Scholar]
- 23. Ding Y, McCoubrey WK Jr, Maines MD (1999) Interaction of heme oxygenase-2 with nitric oxide donors. Is the oxygenase an intracellular 'sink' for NO? Eur J Biochem 264: 854–861. [DOI] [PubMed] [Google Scholar]
- 24. Zhu XY, Liu SJ, Liu YJ, Wang S, Ni X (2010) Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages. Cell Mol Life Sci 67: 1119–1132. 10.1007/s00018-009-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao W, Zhang J, Lu Y, Wang R (2001) The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taoka S, Ohja S, Shan X, Kruger WD, Banerjee R (1998) Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. J Biol Chem 273: 25179–25184. [DOI] [PubMed] [Google Scholar]
- 27. Taoka S, Banerjee R (2001) Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem 87: 245–251. [DOI] [PubMed] [Google Scholar]
- 28. Thom SR, Xu YA, Ischiropoulos H (1997) Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem Res Toxicol 10: 1023–1031. [DOI] [PubMed] [Google Scholar]
- 29. Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS (1999) Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol 277: F882–889. [DOI] [PubMed] [Google Scholar]
- 30. Ingi T, Cheng J, Ronnett GV (1996) Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron 16: 835–842. [DOI] [PubMed] [Google Scholar]
- 31. White KA, Marletta MA (1992) Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry 31: 6627–6631. [DOI] [PubMed] [Google Scholar]
- 32. Sheng WS, Hu S, Nettles AR, Lokensgard JR, Vercellotti GM, et al. (2010) Hemin inhibits NO production by IL-1beta-stimulated human astrocytes through induction of heme oxygenase-1 and reduction of p38 MAPK activation. J Neuroinflammation 7: 51 10.1186/1742-2094-7-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMillan K, Bredt DS, Hirsch DJ, Snyder SH, Clark JE, et al. (1992) Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A 89: 11141–11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, et al. (2012) Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A 109: 1293–1298. 10.1073/pnas.1119658109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin HF, Du JB, Li XH, Wang YF, Liang YF, et al. (2006) Interaction between hydrogen sulfide/cystathionine gamma-lyase and carbon monoxide/heme oxygenase pathways in aortic smooth muscle cells. Acta Pharmacol Sin 27: 1561–1566. [DOI] [PubMed] [Google Scholar]
- 36. Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, et al. (2010) H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A 107: 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ondrias K, Stasko A, Cacanyiova S, Sulova Z, Krizanova O, et al. (2008) H(2)S and HS(-) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Arch 457: 271–279. 10.1007/s00424-008-0519-0 [DOI] [PubMed] [Google Scholar]
- 38. Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531. [DOI] [PubMed] [Google Scholar]
- 39. Teague B, Asiedu S, Moore PK (2002) The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol 137: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao W, Wang R (2002) H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474–480. [DOI] [PubMed] [Google Scholar]
- 41. Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, et al. (2006) Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol 149: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, et al. (2006) Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41: 106–119. [DOI] [PubMed] [Google Scholar]
- 43. Kubo S, Doe I, Kurokawa Y, Nishikawa H, Kawabata A (2007) Direct inhibition of endothelial nitric oxide synthase by hydrogen sulfide: contribution to dual modulation of vascular tension. Toxicology 232: 138–146. [DOI] [PubMed] [Google Scholar]
- 44. Kubo S, Kurokawa Y, Doe I, Masuko T, Sekiguchi F, et al. (2007) Hydrogen sulfide inhibits activity of three isoforms of recombinant nitric oxide synthase. Toxicology 241: 92–97. [DOI] [PubMed] [Google Scholar]
- 45. Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, et al. (2004) Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun 317: 30–37. [DOI] [PubMed] [Google Scholar]
- 46. Dyson RM, Palliser HK, Lakkundi A, De Waal K, Clifton VL, et al. (2014) Early microvascular changes in the preterm neonate: a comparative study of the human and guinea pig. Physiol Rep 2: e12145 10.14814/phy2.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parry G, Tucker J, Tarnow-Mordi W, UK Neonatal Staffing Study Collaborative Group (2003) CRIB II: an update of the clinical risk index for babies score. Lancet 24: 1789–1791. [DOI] [PubMed] [Google Scholar]
- 48. Stark MJ, Hodyl NA, Wright IM, Clifton V (2011) The influence of sex and antenatal betamethasone exposure on vasoconstrictors and the preterm microvasculature. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 24: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 49. Amey M, Butchard N, Hanson L, Kinross D, Mannion M, et al. (2008) Cautionary tales from the neonatal intensive care unit: diapers may mislead urinary output estimation in extremely low birthweight infants. Pediatr Crit Care Med 9: 76–79. 10.1097/01.PCC.0000298550.29453.7D [DOI] [PubMed] [Google Scholar]
- 50. Chwatko G, Bald E (2009) Determination of thiosulfate in human urine by high performance liquid chromatography. Talanta 79: 229–234. 10.1016/j.talanta.2009.03.040 [DOI] [PubMed] [Google Scholar]
- 51. Bentler PM, Stein JA (1992) Structural equation models in medical research. Stat Methods Med Res 1: 159–181. [DOI] [PubMed] [Google Scholar]
- 52. Beran TN, Violato C (2010) Structural equation modeling in medical research: a primer. BMC Res Notes 3: 267 10.1186/1756-0500-3-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu LT, Bentler PM (1999) Cutoff criteria for Fit Indexes in Covariance Structure Analysis: conventional criteria versus new alternatives. Structural Equation Modeling 6: 1–55. [Google Scholar]
- 54. MacCallum RC, Browne MW, Sugawara HM (1996) Power analysis and determination of sample size for Covariance Structure Modeling. Psychological Methods 1: 130–149. [Google Scholar]
- 55. Altaany Z, Ju Y, Yang G, Wang R (2014) The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Science signaling 7: ra87 10.1126/scisignal.2005478 [DOI] [PubMed] [Google Scholar]
- 56. Dyson RM, Palliser HK, Ni X, Wright IMR (2014) Cystathionine-γ-lyase: a potential target for treatment of microvascular dysregulation following preterm birth? (PS334). Journal of Pediatrics and Child Health 50 (S1): 40–64. [Google Scholar]
- 57. Applegarth DA, Hardwick DF, Ross PM (1968) Creatinine excretion in children and the usefulness of creatinine equivalents in amino acid chromatography. Clin Chim Acta 22: 131–134. [DOI] [PubMed] [Google Scholar]
- 58. Applegarth DA, Ross PM (1975) The unsuitability of creatinine excretion as a basis for assessing the excretion of other metabolites by infants and children. Clin Chim Acta 64: 83–85. [DOI] [PubMed] [Google Scholar]
- 59. Dyson RM, Palliser HK, Kelleher MA, Hirst JJ, Wright IM (2012) The guinea pig as an animal model for studying perinatal changes in microvascular function. Pediatric research 71: 20–24. 10.1038/pr.2011.9 [DOI] [PubMed] [Google Scholar]
- 60. Polglase GR, Hooper SB, Kluckow M, Gill AW, Harding R, et al. (2012) The cardiopulmonary haemodynamic transition at birth is not different between male and female preterm lambs. Reproduction, fertility, and development 24: 510–516. 10.1071/RD11121 [DOI] [PubMed] [Google Scholar]
- 61. Eiby YA, Wright LL, Kalanjati VP, Miller SM, Bjorkman ST, et al. (2013) A pig model of the preterm neonate: anthropometric and physiological characteristics. PloS one 8: e68763 10.1371/journal.pone.0068763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grunfeld JP (1990) Glucocorticoids in blood pressure regulation. Horm Res 34: 111–113. [DOI] [PubMed] [Google Scholar]
- 63. Baum M, Moe OW (2008) Glucocorticoid-mediated hypertension: does vascular smooth muscle hold all the ansmwers? JASN 19: 1251–1253. 10.1681/ASN.2008040410 [DOI] [PubMed] [Google Scholar]
- 64. Wallerath T, Witte K, Schafer SC, Schwarz PM, Prellwitz W, et al. (1999) Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci U S A 96: 13357–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitworth JA, Schyvens CG, Zhang Y, Andrews MC, Mangos GJ, et al. (2002) The nitric oxide system in glucocorticoid-induced hypertension. J Hypertens 20: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 66. Wen C, Li M, Whitworth JA (2000) Role of nitric oxide in adrenocorticotrophin-induced hypertension: L-arginine effects reversed by N-nitro-L-arginine. Clin Exp Pharmacol Physiol 27: 887–890. [DOI] [PubMed] [Google Scholar]
- 67. Wallerath T, Godecke A, Molojavyi A, Li H, Schrader J, et al. (2004) Dexamethasone lacks effect on blood pressure in mice with a disrupted endothelial NO synthase gene. Nitric Oxide 10: 36–41. [DOI] [PubMed] [Google Scholar]
- 68. Kleinert H, Euchenhofer C, Ihrig-Biedert I, Forstermann U (1996) Glucocorticoids inhibit the induction of nitric oxide synthase II by down-regulating cytokine-induced activity of transcription factor nuclear factor-kappa B. Mol Pharmacol 49: 15–21. [PubMed] [Google Scholar]
- 69. Simmons WW, Ungureanu-Longrois D, Smith GK, Smith TW, Kelly RA (1996) Glucocorticoids regulate inducible nitric oxide synthase by inhibiting tetrahydrobiopterin synthesis and L-arginine transport. J Biol Chem 271: 23928–23937. [DOI] [PubMed] [Google Scholar]
- 70. Singh K, Balligand JL, Fischer TA, Smith TW, Kelly RA (1995) Glucocorticoids increase osteopontin expression in cardiac myocytes and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. J Biol Chem 270: 28471–28478. [DOI] [PubMed] [Google Scholar]
- 71.Blecharz KG, Burck M, Bauersachs J, Thum T, Tsikas D, et al. (2014) Inhibition of proteosome-mediated glucocorticoid recepter degredation restores nitric oxide bioavailability in myocardial endothelial cells in vitro. Biology of the Cell Accepted manuscript online, 18 Apr 2014. [DOI] [PubMed]
- 72. Soriano RN, Ravanelli MI, Batalhao ME, Carnio EC, Branco LGS (2013) Glucocorticoids downregulate systemic nitric oxide synthesis and counteract overexpression of hepatic heme oxygenase-1 during endotoxin tolerance. CJPP 91: 861–865. 10.1139/cjpp-2013-0028 [DOI] [PubMed] [Google Scholar]
- 73. Uno S, Guo DF, Nakajima M, Ohi H, Imada T, et al. (1994) Glucocorticoid induction of rat angiotensin II type 1A receptor gene promoter. Biochem Biophys Res Commun 204: 210–215. [DOI] [PubMed] [Google Scholar]
- 74. Sato A, Suzuki H, Nakazato Y, Shibata H, Inagami T, et al. (1994) Increased expression of vascular angiotensin II type 1A receptor gene in glucocorticoid-induced hypertension. J Hypertens 12: 511–516. [PubMed] [Google Scholar]
- 75. Handa M, Kondo K, Suzuki H, Saruta T (1984) Dexamethasone hypertension in rats: role of prostaglandins and pressor sensitivity to norepinephrine. Hypertension 6: 236–241. [PubMed] [Google Scholar]
- 76. Saruta T (1996) Mechanism of glucocorticoid-induced hypertension. Hypertens Res 19: 1–8. [DOI] [PubMed] [Google Scholar]
- 77. Stark MJ, Wright IM, Clifton VL (2009) Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. American journal of physiology Regulatory, integrative and comparative physiology 297: R510–514. 10.1152/ajpregu.00175.2009 [DOI] [PubMed] [Google Scholar]
- 78. Wang R (2014) Gasotransmitters: growing pains and joys. Trends in biochemical sciences 39: 227–232. 10.1016/j.tibs.2014.03.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alternate models were tested and are presented here. As in the manuscript, the interaction between the three gasotransmitters and their individual and combined effects on microvascular blood flow were assessed. All models were manually constructed and presented here for our three input (NO, CO and H2S) and one output (microvascular blood flow) variables. These models were then tested and assessed for suitability by χ2 Goodness of Fit and root mean square error of approximation (RMSEA). Lower χ2 values represent a better predicted model, whilst an RMSEA of below 0.06 shows a good fit. RMSEA also allows for calculation of a confidence interval (CI) around the predictive power of the model. None of these models had a more acceptable χ2 or RMSEA value and CI, thus the selection of the model presented within the manuscript.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


