Abstract
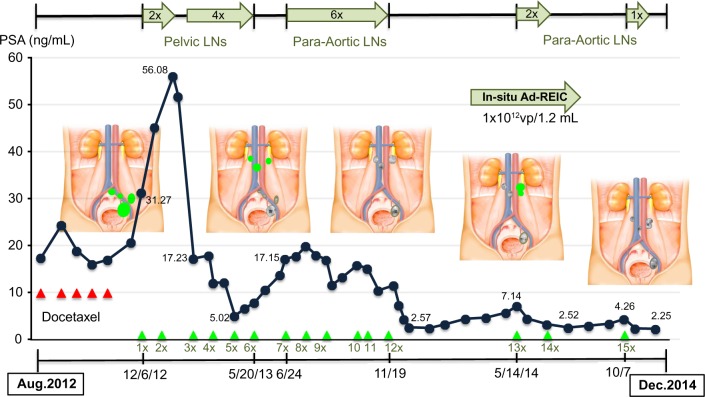
A 63-year-old man with metastatic castration-resistant prostate cancer (CRPC) was successfully treated for two years with in situ gene therapy using an adenovirus vector carrying the human REIC/Dkk-3 gene (Ad-REIC), following chemotherapy. Ad-REIC mediates simultaneous induction of cancer-selective apoptosis and augmentation of antitumor immunity, and a Phase I/IIa clinical study on Ad-REIC has been conducted at Okayama University Hospital since January 2011. At the time of enrollment in December 2012, the patient presented with rapid progression of lymph node (LN) metastases. Two scheduled Ad-REIC injections and 10 additional Ad-REIC injections into metastatic pelvic and para-aortic LNs under CT guidance, with an average four weeks’ interval, exhibited the potent direct and indirect effects of Ad-REIC as a therapeutic cancer vaccine. During the next 12 months, three additional injections into para-aortic LNs showing regrowth achieved adequate control of all metastatic LNs with prostate-specific antigen (PSA) decline, without any particular adverse events.
Keywords: REIC/Dkk-3 gene, cancer gene therapy, cancer vaccine, metastatic castrate-resistant prostate cancer
Introduction
According to a systemic review by Kirby, Hirst, and Crawford, castration-resistant prostate cancer (CRPC) is a common and highly morbid progressive form of prostate cancer, with around 10%–20% of prostatic cancer patients progressing to this state within five years. Metastases are present in more than 84% of CRPC cases, and the reported survival is 9–13 months in patients with metastatic CRPC (mCRPC).1 More recently, significant advances in second-generation hormonal therapy,2,3 immunotherapy,4 bone-targeted therapy,5,6 and chemotherapy7 have become available for the treatment of mCRPC. Despite improvements in patient survival, mCRPC remains incurable, and improvement in the quality of life (QOL) is still a challenge. Therefore, a novel, personalized therapy with a new therapeutic cancer vaccine founded on gene-based medicine is much awaited.
Regarding gene therapy, so far 1,331 cancer gene therapy protocols have been conducted around the world (http://www.wiley.com/legacy/wileychi/genmed/clinical/, updated June 2014). Interestingly, the second highest number of protocols has been for prostate cancer, as the prostate is regarded as an ideal target organ for the development of cancer gene therapy.8 Since 1996, our study group has been taking on challenges to develop cancer gene therapy and the group has conducted several clinical trials on prostate cancer.9–11 These two-way, bench-to-bedside and bedside-to-bench, translational researches clearly indicate that the induction of robust systemic effects through the activation of antitumor immunity is the key to creating an ideal therapeutic strategy for in situ cancer gene therapy. Recently, new therapeutic genes such as MDA-7/IL-24,12 GLIPR1/RTVP-1,13 and REIC/Dkk-3,14 are being investigated, which are multifunctional with selective killing and immunomodulation. In addition, new, sophisticated approaches in the form of armed oncolytic viruses15,16 have also been developed. OncoVexGM-CSF (now, talimogene laherparepvec, or T-VEC) is an oncolytic herpes simplex type 1 virus carrying GM-CSF and the first oncolytic virus found effective in a Phase III clinical trial for unresected stage IIIB/C and IV melanoma.17 An oncolytic virus can replicate only in cancer cells, causing cancer cell death. The virus expressing GM-CSF spreads and initiates an immune response in distant tumors. The new strategy, based on the concept of simultaneous induction of selective killing of cancer cells and augmentation of antitumor immunity, leads to a new generation of cancer vaccines and is expected to become a leading standard in the treatment of most solid cancers, with gene therapy.
The reduced expression in immortalized cells (REIC)/Dickkoph-3 (Dkk-3) gene was isolated and cloned as an immortalization-related gene at Okayama University in 2000,14 and identified as a new tumor suppressor gene.18 This gene is identical to the human Dkk-3, one of the Dkk family genes (hDKK-1, 2, 3, and 4) and has rapidly emerged as a key player in most human cancers.19 The expression of REIC/Dkk-3 is significantly reduced in a wide variety of cancer cells including prostate cancer, and its forced expression using adenovirus vector carrying the human REIC/Dkk-3 gene (Ad-REIC) induces cancer-selective apoptosis as a result of unfolded protein response due to endoplasmic reticulum stress (ER stress).20 Interestingly, ER stress mediates the enhanced IL-7 expression in co-infected normal fibroblasts, resulting in the activation of innate immunity involving natural killer (NK) cells.21 In addition, the secreted REIC protein with immunomodulatory function creates an optimal environment for the activation of host immune cells, inducing cytotoxic T lymphocytes.22,23
In order to develop Ad-REIC as a new form of therapeutic cancer vaccine, the first in-human clinical study, a Phase I/IIa study of in situ Ad-REIC gene therapy for prostate cancer, was initiated at Okayama University from January 2011. The original Ad-REIC, a replication-deficient adenovirus vector, was constructed at Okayama University,18 and its current Good Manufacturing Practice (cGPP) product was supplied by Momotaro-Gene Inc. Two groups of patients were treated: group A consisting of patients with castration-resistant PCa with or without metastasis, and group B consisting of patients with high-risk, localized PCa scheduled to undergo radical prostatectomy. Ad-REIC was injected directly into the prostate or metastatic tumor using four escalating doses of viral particles (vp), starting from 1.0 × 1010 to 3.0 × 1012 vp. In group A, patients received two injections at four-week intervals. In group B, patients received two injections at two-week intervals and underwent a radical prostatectomy six weeks after the second injection. In group A, additional injections were permissible in cases showing positive responses. As of November 2014, 18 scheduled cases in group B were completed, demonstrating the remarkable safety profiles of Ad-REIC and dose-dependent clinical effects without reaching the maximum tolerated dose. Since the maximum feasible dose for intratumoral injection of Ad-REIC was determined, new enrollment in group A was also closed. The entire clinical data on the Phase I/IIa study of in situ Ad-REIC gene therapy for prostate cancer will be published elsewhere. The patient whose case is presented following has given his consent for this publication.
Case Presentation
A 63-year-old man with mCRPC, who showed progression on decetaxel treatment, was referred to our hospital for Ad-REIC gene therapy in November 2012. In 2009, he visited the urology department of a regional general hospital with nocturia, and his prostate-specific antigen (PSA) level was measured for the first time. The PSA level was 483 ng/mL, and prostate biopsy showed an adenocarcinoma having a Gleason score of 9 (4 + 5). After being diagnosed with a locally advanced prostate cancer (stage C), he received combined androgen blockade (CAB) with the nonsteroidal antiandrogen bicalutamide and the LH-RH analog goserelin acetate, showing immediate normalization of the PSA level (0.01 ng/mL). In 2010, after 15 months on CAB, he received TomoTherapy, a form of intensity-modulated radiation therapy, with 74.8 Gy in 34 fractions in combination with CAB, at a regional cancer center. In May 2012, estramustine phosphate was prescribed after evaluating the anti-androgen withdrawal response to bicalutamide, with gradual elevation of the PSA level. Estramustine was unable to lower PSA elevation, and consequent CT, MRI, and PET/CT revealed left obturator LN metastasis. From August 2012, he received five cycles of chemotherapy with decetaxel 75 mg/m2 every 3–4 weeks at another regional core hospital (Fig. 1). PSA response to the last two cycles of docetaxel was not achieved because of bulky pelvic LN metastases. The patient suffered severe peripheral neuropathy, in the form of tingling sensations in the hands.
Figure 1.
Clinical course of in situ Ad-REIC gene therapy. The patient received two scheduled injections into metastatic left obturator LN and additional 13 injections into metastatic LNs using cGMP Ad-REIC (1.0 × 1012 vp/1.2 mL). The procedure was carried out by using a set of 21G long needles and an injection machine that can control the injection speed and solution volume under CT guidance.11 Dynamic changes in metastatic LNs and PSA levels over two years are demonstrated. Green represents viable LNs and gray represents nonviable LNs.
At our hospital, we carefully evaluated the indication for Ad-REIC gene therapy and informed the patient that the gene therapy protocol was approved by the Institutional Review Board (IRB) at Okayama University Hospital and the Japanese Government, although still in the investigational stage. His laboratory data was almost normal except for mild anemia (RBC 3.42 × 106/μL, Hb 40.3 g/dL). He was also taking an oral antidiabetic drug (Metformin), and his HbA1c (6.2%) was under good control. He reviewed the informed consent document and received individual counseling, including a thorough discussion about alternative treatments or the right to decline participation. Subsequently, he underwent a CT-guided needle biopsy of the left obturator LN, which showed a metastatic adenocarcinoma with a Gleason score of 9 (5 + 4) (Fig. 2A), while ultrasound-guided transrectal 10-core prostate biopsy showed no cancer cells. In December 2012, we planned to enroll the patient as the eighth case in group A receiving a dose level 3 of 1 × 1012 vp, and direct injection of Ad-REIC into the left obturator LN under CT guidance was approved by the eligibility monitoring committee of the IRB.
Figure 2.
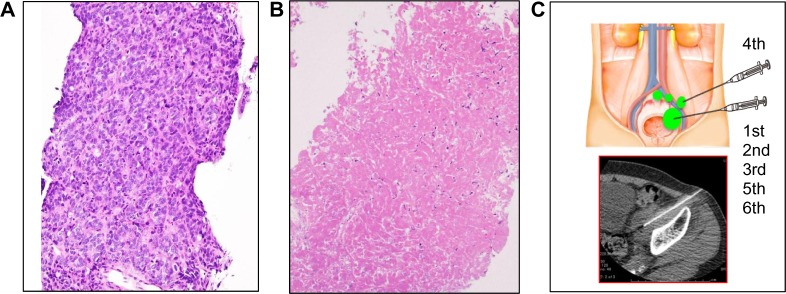
Needle biopsy and Ad-REIC injection under CT guidance. (A) Pretreatment biopsy of left obturator LN, showing a metastatic adenocarcinoma with a Gleason score of 9 (5 + 4), H&E staining, reduced from × 40. (B) Biopsy of left obturator LN after two scheduled Ad-REIC treatments, showing almost complete necrosis (H&E, × 40). (C) Schematic representation and radiological imaging of scheduled and additional Ad-REIC injections into metastatic pelvic LNs.
The clinical course of in situ Ad-REIC is summarized in Figure 1. Ad-REIC (1.0 × 1012 vp/1.2 mL) was directly injected into the left obturator LN (more than 30 mL in volume) under CT guidance. After two scheduled injections, a significant decrease in serum PSA level (31.27→56.08→17.23 ng/mL) and almost complete necrosis within the injected LN was observed by CT and histological examinations (Fig. 2B). Notably, similar remarkable necrotic changes were also seen in other noninjected metastatic pelvic LNs. Based on these clinical findings, an additional four Ad-REIC injections were administered (Fig. 2C), leading to complete control of pelvic LNs with further PSA decline (5.02 ng/mL), as shown in Figures 1 and 3. On the other hand, newly developed multiple para-aortic LNs metastases progressed with gradual PSA re-elevation (17.15 ng/mL). Next, additional six Ad-REIC injections were administered into para-aortic metastatic LNs (Figs. 1 and 4), resulting in remarkable PSA decline (2.57 ng/mL), and necrotic changes in the targeted LNs were detected by CT and histological examination (Fig. 5). Consequently, the patient was treated successfully for one year by 12 injections at an average four weeks’ interval. In the following year, three therapeutic Ad-REIC injections were administered into newly enlarged, gourd-shaped left para-aortic LNs, after a biopsy specimen from the upper part of the left para-aortic LNs showed clusters of viable cancer cells (Fig. 6A). Two injections into the upper part and one into lower part of the LNs resulted in adequate control of the whole of the metastatic LNs (Figs. 3 and 6B) with PSA decline (2.25 ng/mL). No bone or visceral metastases and no particular adverse events including immune-related response were observed, besides mild transient fever associated with 15 Ad-REIC injections during the 24-month treatment period.
Figure 3.
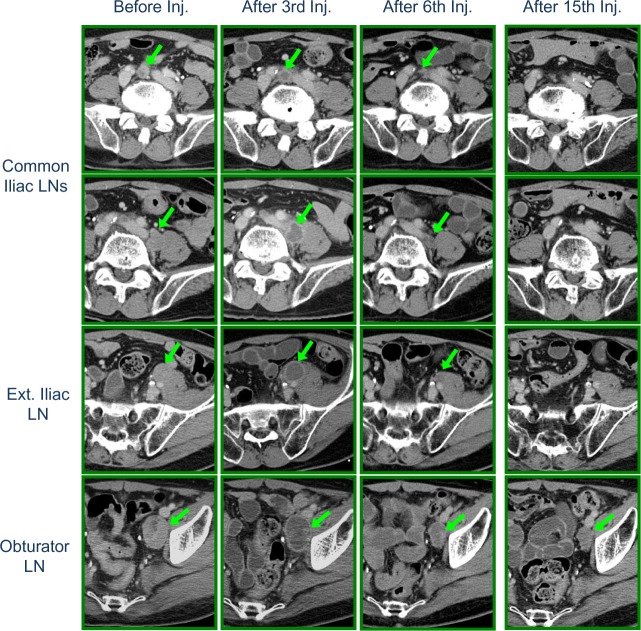
Changes in CT findings of pelvic LN metastases. Before injection, multiple metastatic pelvic LNs were observed. After the third injection, almost complete necrosis of the injected lt. obturator LN was observed, illustrating Ad-REIC induced powerful direct effects. Similar necrotic changes of non-injected external and common iliac LNs were observed, illustrating the induction of strong indirect effects of Ad-REIC. After the sixth injection, cytoreductive effects were clearly seen and external and common iliac LNs were not detected after the 15th injection.
Figure 4.
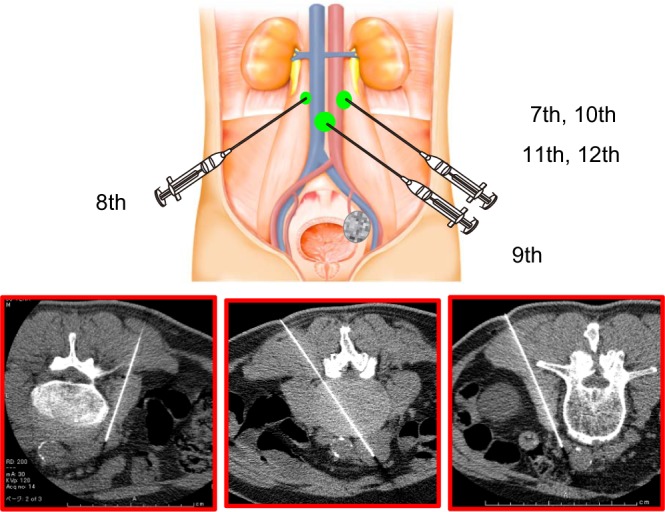
Schematic representation and radiological imaging of Ad-REIC injections into para-aortic metastatic LNs. In the treatment of metastatic para-aortic LNs, three main LNs were treated separately and four injections were administered into lt. para-aortic node.
Figure 5.
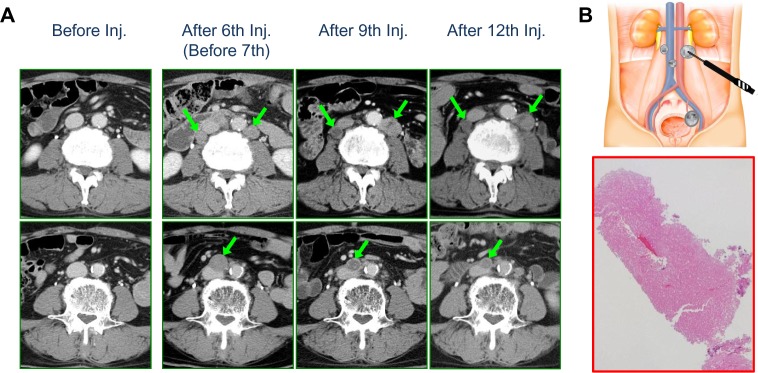
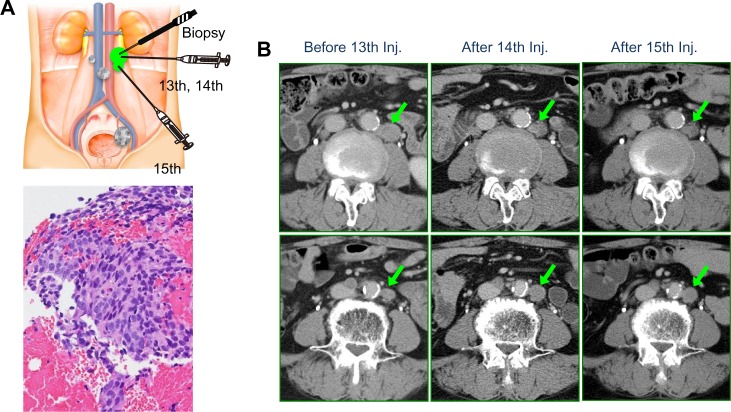
Changes in CT findings of para-aortic LN metastases including histological examination. (A) Before Ad-REIC injection, no para-aortic LN metastases were detected. Before the seventh injection, apparent LN metastases were observed, as indicated by arrows. After the ninth injection, right and central para-aortic LNs showed necrotic changes, but left para-aortic LN (LPN) showed enlargement. After the 12th injection, LPN showed cytoreductive effects with necrotic change. (B) Schematic representation of biopsy procedure and histology of LPN showing complete necrosis after 12th injection (H&E, ×40).
Figure 6.
Histological examination and CT changes of re-enlarged LPN. (A) Before the 13th injection, biopsy of LPN showed clusters of viable cancer cells (H&E, ×100). (B) LPN appeared to have gourd-shaped growth. After the 14th injection, the upper part showed lobulated shrinkage with necrosis but the lower part showed enlargement. After the 15th injection, the lower part ceased to grow.
Discussion
In 1996, when the first suicide gene therapy with an adenovirus vector carrying HSV-tk gene (Ad-HSV-tk) for prostate cancer was conducted at Baylor College of Medicine,24 our research group had started international collaborative studies to develop cancer gene therapy at Okayama University. From March 2001 to July 2005, we completed the first clinical study on suicide gene therapy for prostate cancer in Japan, demonstrating the safety, gene expression, and clinical effects of Ad-HSV-tk gene therapy for locally advanced CRPC.11 Our initial development strategy on in situ gene therapy for prostate cancer was to combine suicide gene therapy with immune gene therapy using interleukin (IL)-12 (Ad-IL-12), in order to augment direct and indirect “bystander” effects.25 As for Ad-IL-12, a Phase I/IIa dose escalation study, starting from 1.0 × 1010 vp and reaching 5.0 × 1012 vp for locally advanced CRPC and mCRPC, was conducted at Okayama University from May 2008, following approval from the Japanese Government. In March 2010, the 10th patient was enrolled into this clinical study, as the first patient in dose level 4 of 5 × 1011 vp. This patient showed clear objective responses, with gradual PSA decline and disappearance of a single metastatic internal iliac LN, after three scheduled monthly injections into prostatic lesions. However, adverse events, such as high fever (grade 2–3) and liver dysfunction (grade 2), were also noted. The patient refused to continue treatment after two additional monthly injections, probably due to high fever following each injection. Therefore, this study was put on hold until we could obtain sufficient evidence on the safety of Ad-IL-12 from other investigational studies.
Contrary to Ad-IL-12, Ad-REIC showed remarkable safety profiles, although we used the same replication-deficient, the so-called “first-generation” adenovirus type 5, vectors. In fact, the present patient received 15 repeated injections with 1 × 1012 vp for two years and showed only transient fever, treatable with antipyretics. The first-generation adenovirus vectors are reported to have two major limitations: first, these vectors usually mediate a short-term gene expression, and, second, these vectors tend to elicit relatively strong immune and inflammatory responses.26 In terms of drug development, we already have a huge amount of long-term accumulated data on safety and pharmacology derived from various clinical trials with first-generation adenoviruses. As for type-specific neutralizing antibodies raised against adenovirus vector particles, it was reported as early as 1999, by Swisher et al, that even with high titers, antibodies in blood would not reduce gene transfer by repeated intratumoral injections, as shown in their initial study on adenovirus-mediated p53 gene transfer (Ad-p53) in advanced non-small-cell lung cancer.27 In addition, many thousands of patients have received Ad-p53 gene therapy in clinical trials, mostly in the USA and China, with some remarkable clinical outcomes. Unfortunately, Ad-p53 (Advexin®) did not receive the U.S. Federal Drug Administration (FDA) approval.28 On the other hand, in 2003, Ad-p53, trademarked as Gendicine, was approved by the Chinese State Food and Drug Administration, and Gendicine has been used to treat head and neck squamous cell carcinoma and other cancers, without any serious adverse events related to the first-generation adenovirus vectors.29 Therefore, the next challenge for in situ gene therapy using the first-generation adenovirus vectors is to find ways to improve direct and indirect “bystander” effects with these well-characterized vectors.
In an orthotopic prostate cancer model with pre- established lung metastases using RM-9 mouse prostate cancer cells, in situ Ad-mouse (m)IL-12 gene therapy clearly suppressed pre-established lung metastases.25 Although the combination of Ad-HSV-tk plus ganciclovir and Ad-mIL-12 gene therapy inhibited local tumor growth more than single treatment protocols, combination therapy did not suppress pre-established lung metastases more than Ad-mIL-12 gene therapy alone. Combination therapy also did not achieve significantly better animal survival compared to single treatment protocols, probably due to complex cooperative therapeutic activities and host immunological responses.30 In the same orthotopic mouse model, however, Ad-mREIC as well as Ad-REIC significantly suppressed local tumor growth and pre-established lung metastases, and prolonged mice survival.22,31 These animal studies clearly demonstrate that, in terms of the induction of direct and indirect “bystander” effects, Ad-REIC monotherapy is extremely simple and effective, in comparison to combination with Ad-HSV-tk plus ganciclovir and Ad-IL−12. Moreover, the results mentioned above indicate that the powerful “bystander” effects medicated by Ad-REIC might overcome several unresolved problems related to conventional adenovirus vectors, including the inability to infect every cell in the tumor, short-term gene expression, and inability of systemic targeted delivery.
More recently, we have reviewed previous fundamental studies and summarized the anticancer mechanisms of in situ Ad-REIC gene therapy, featuring the characteristic synergistic effects between cancer-selective apoptosis and augmentation of antitumor immunity.23 Paul Ehrlich’s “Magic Bullet” concept based on selective toxicity has inspired many scientists to develop innovative therapeutics for more than 100 years.32 As for selective toxicity, Ad-REIC offered a new, sophisticated way to induce cancer-selective killing according to the differences in Ad-REIC-induced unfolded protein responses (UPR) between cancer cells and normal cells. Since REIC/Dkk-3 expression is significantly down regulated in a broad range of human cancer cells18–20,33–38 while being expressed ubiquitously in normal cells,39 these endogenous expression statuses of REIC/Dkkk-3 proteins appear to define the sensitivity to Ad-REIC-induced UPR. As a new “Magic Bullet” for cancer gene therapy, ER-stress-induced apoptosis due to sustained overactivation of UPR was mediated in Ad-REIC-infected cancer cells, while ER-stress-induced overproduction of IL-7 was mediated in co-infected normal cells including cancer-associated fibroblasts.21 In addition to the fact that IL-7 excessively produced by Ad-REIC activates innate immunity involving NK cells, the secreted REIC proteins with potent immunomodulatory functions augment antitumor immunity. As demonstrated in Figure 7, dendritic cells, induced by secreted REIC proteins, acquire cancer antigens from apoptotic cancer cells and induce class 1-restricted CD8+ cytotoxic T lymphocytes (CTLs).22,23 These CTLs play a major role in systemic antitumor immunity of in situ Ad-REIC as a personalized therapeutic cancer vaccine.
Figure 7.
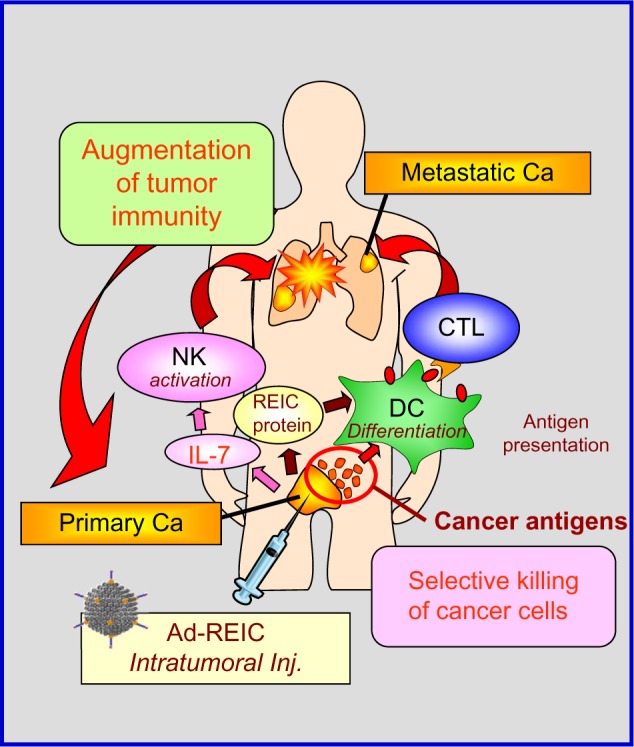
Schematic representation of the mechanism of action of in situ Ad-REIC.23 Intratumoral injection of Ad-REIC induces massive apoptosis of cancer cells and provides an ideal presentation of cancer antigens to the host immune system. Secreted REIC protein at the tumor site creates an optimum environment, mediating tumor-associated, antigen-specific cytotoxic T cells. In addition, overproduction of IL-7 by cancer-associated fibroblasts activates NK cells.21
In the current patient, two initial injections of Ad-REIC, of as little as 1.2 mL of vector solution, induced almost complete degeneration of the injected metastatic obturator lymph node of more than 30 mL in volume. This suggests that, in an optimum environment, the direct effects of Ad-REIC are strong enough to overcome the inability of the adenovirus to infect all targeted cancer cells. Furthermore, similar degeneration developed in distant, noninjected metastatic common and external iliac LNs, indicating the induction of robust systemic antitumor immunity by in situ Ad-REIC. Although complete control of metastatic pelvic LNs was achieved following several boosting injections into pelvic LNs, newly developed multiple para-aortic LN metastases progressed in spite of the presence of Ad-REIC-mediated systemic antitumor immunity. For this progression, there are two possible interpretations: first, heterogenic clones of cancer cells developed with tumor- associated antigens significantly different from those of primary metastatic cancer cells, and, second, same clones of primary metastatic cancer cells developed under a different immunosuppressive tumor microenvironment. The latter is supported by the recent concept of “cancer immunoediting” and findings related to immunosuppressive networks and checkpoints controlling antitumor immunity.40,41 In the treatment of para-aortic LNs, since each injection showed a remarkable effect only on the injected LN, injections into every metastatic LNs including re-enlarged LNs were necessary for sufficient control of metastatic LNs. Probably, the induced systemic effects were compromised by an immunosuppressive microenvironment established in each metastatic LN.
Recently, it has been elucidated that blocking immunosuppressive networks and immune checkpoints realizes a successful immunotherapeutic modality for the treatment of cancer.42–45 Therefore, a combination of Ad-REIC with inhibition of immune checkpoint and/or suppressor cells will offer a more promising strategy for the next generation of cancer immunotherapy. It has been reported that the two main immune checkpoint pathways involve signaling through cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) or programmed cell death protein 1 (PD-1). The CTLA-4 pathway is more important in the early phase of the immune system activation (priming phase), while the PD-1 pathway is more important in the tumor microenvironment during the effector phase.46 A combination of Ad-REIC with PD-1 pathway blockade seems to be more appropriate, since Ad-REIC generates a strong antitumor immunity, as demonstrated in this patient. One of the most important findings (from early clinical trials of PD-1 pathway blockade for advance solid cancers) reveals that programmed death-ligand 1 (PD-L1) expression on tumor cells reflects an immune-active microenvironment and is the single factor most closely correlated with response to anti-PD-1 blockade.43,47 Therefore, in our future study it is crucial to investigate PD-L1 expression status in tumors, before and after Ad-REIC as well in metastatic tumors, if recurrence occurs. In terms of blocking immunosuppressive networks, targeted therapies and cytotoxic agents also modulate immune responses.44 Among them, sunitinib, vascular endothelial growth factor A (VEGF-A), and low-dose cyclophosphamide are possible candidates to combine with Ad-REIC in order to improve clinical outcomes in the treatment of CRPC.
Apart from this combination strategy, the second generation of Ad-REIC using a super gene expression (SGE) system (Ad-SGE-REIC) has already been developed in order to augment the therapeutic effects of in situ Ad-REIC.48 The SGE system is a new plasmid vector, developed by placing three enhancers in tandem after poly A to realize extremely high expression of the targeted REIC gene.49 A Phase I/IIa clinical trial of Ad-SGE-REIC for localized prostate cancer is being conducted at two institutions in the United States (https://clinicaltrials.gov/). Similarly, a Phase I/IIa clinical trial for malignant pleural mesothelioma will be initiated in the near future at three institutions in Japan. In addition, preclinical studies of Ad-REIC on various intractable solid cancers including pancreatic cancer,36 lung cancer,37 and malignant glioma38 have been conducted successfully.
Conclusion
A 63-year-old man with mCRPC after docetaxel failure was successfully treated for two years with in situ Ad-REIC gene therapy. Repeated injections of Ad-REIC into metastatic LNs showed remarkable safety profiles and induced potent direct and indirect antitumor effects, thus paving the way for a new, future cancer therapeutic vaccine against a variety of intractable solid cancers.
Acknowledgments
We thank Dr. Sabina Mahmood, Dr. Shigeru Kobayashi (Okayama University Graduate School, Okayama, Japan), and Mr. Hitoshi Shiomi (Momotaro-Gene Inc., Okayama, Japan) for providing valuable suggestions and help with the preparation of this manuscript.
Footnotes
ACADEMIC EDITOR: William C. S. Cho, Editor in Chief
FUNDING: This research was funded by a grant-in-aid for the “Creation of Innovation Centers for Advanced Interdisciplinary Research Areas”, a research project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan. Okayama University received research funds for joint research in cooperation with Momotaro-Gene Inc. The authors confirm that the funders had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Momotaro-Gene Inc. holds the patents of the Ad-REIC agent and develops the agent as a cancer therapeutic medicine. HK, MW, and YN demonstrated the utility of the agent and also own stocks in Momotaro-Gene Inc. HK is the Chief Scientific Officer of Momotaro-Gene Inc. Okayama University and Momotaro-Gene Inc. are working together on the development of the Ad-REIC agent. Okayama University received the GMP-grade Ad-REIC agent from Momotaro-Gene Inc. to perform clinical trials for the treatment of cancer patients. Outside the submitted work, HK, YN, MW, SK, TH, SE, YA and KS report grants from MEXT of Japan. HK, YN, MW, SK, TH report grants from the Ministry of Health, Labour and Welfare of Japan. HK, YN and MW report grants from the Japan Science and Technology Agency. HK, YN and MW, as indicated following the patent numbers, disclose the following patents, all licensed to Momotaro-Gene Inc: WO 2001/038528 (HK), WO 2006/098074 (HK, YN), WO 2009/060982 (HK, YN, MW), WO 2009/119874 (HK, YN, MW), WO 2010/013846 (HK, YN, MW), WO 2011/062298 (HK, YN, MW), WO 2012/161352 (HK, YN, MW).
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the study: HK, MW, YN. Performed medical operations: HK, KS, YA, TS, HY, MW. Analyzed the data: HK, KS, YA. Wrote the first draft of the manuscript: HK. Contributed to the writing of the manuscript: HK, KS, YA, TS, MW. Agree with manuscript results and conclusions: HK, KS, YA, SE, TH, SK, HY, MW, YN. Jointly developed the structure and arguments for the paper: HK, KS, YA, MW, YN. Made critical revisions and approved final version: HK, HY, MW, YN. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–92. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Logothetis CJ, Molina A, et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–1995. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, et al. AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-timmunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker C, Nilsson S, Heinrich D, et al. ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 7.Paller CJ, Antonarakis ES. Cabazitaxel: a novel second line treatment for metastatic castration-resistant prostate cancer. Drug Des Devel Ther. 2011;5:117–24. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasu Y, Djavan B, Marberger M, Kumon H. Prostate cancer gene therapy: outcome of basic research and clinical trials. Tech Urol. 1999;5:185–90. [PubMed] [Google Scholar]
- 9.Kumon H. Gene therapy in the 21st century. Mol Urol. 2001;5:45–6. doi: 10.1089/109153601300177538. [DOI] [PubMed] [Google Scholar]
- 10.Saika T, Kusaka N, Mouraviev V, et al. Therapeutic effects of adoptive splenocyte transfer following in situ AdIL-12 gene therapy in a mouse prostate cancer model. Cancer Gene Ther. 2006;13:91–8. doi: 10.1038/sj.cgt.7700872. [DOI] [PubMed] [Google Scholar]
- 11.Nasu Y, Saika T, Ebara S, et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther. 2007;15:834–40. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- 12.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–38. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 13.Ren C, Ren CH, Li L, Goltsov AA, Thompson TC. Identification and char-acterization of RTVP1/GLIPR1-like genes, a novel p53 target gene cluster. Genomics. 2006;88:163–72. doi: 10.1016/j.ygeno.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem Biophys Res Commun. 2000;268:20–4. doi: 10.1006/bbrc.1999.2067. [DOI] [PubMed] [Google Scholar]
- 15.Hermiston TW, Kuhn I. Armed therapeutic viruses: strategies and challenges to arming oncolyticviruses with therapeutic genes. Cancer Gene Ther. 2002;9:1022–35. doi: 10.1038/sj.cgt.7700542. [DOI] [PubMed] [Google Scholar]
- 16.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–47. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 17.Goins WF, Huang S, Cohen JB, Glorioso JC. Engineering HSV-1 vectors for gene therapy. Methods Mol Biol. 2014;1144:63–79. doi: 10.1007/978-1-4939-0428-0_5. [DOI] [PubMed] [Google Scholar]
- 18.Abarzua F, Sakaguchi M, Takaishi M, et al. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res. 2005;65:9617–22. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- 19.Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys Acta. 2012;1825:18–28. doi: 10.1016/j.bbcan.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Kashiwakura Y, Ochiai K, Watanabe M, et al. Down-regulation of inhibition of differentiation-1 via activation of activating transcription factor 3 and Smad regulates REIC/Dickkopf-3-induced apoptosis. Cancer Res. 2008;68:8333–41. doi: 10.1158/0008-5472.CAN-08-0080. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi M, Kataoka K, Abarzua F, et al. Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7. J Biol Chem. 2009;284:14236–44. doi: 10.1074/jbc.M808002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe M, Kashiwakura Y, Huang P, et al. Immunological aspects of REIC/Dkk-3 in monocyte differentiation and tumor regression. Int J Oncol. 2009;34:657–63. doi: 10.3892/ijo_00000191. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe M, Nasu Y, Kumon H. Adenovirus-mediated REIC/Dkk-3 gene therapy: development of an autologous cancer vaccination therapy (review) Oncol Lett. 2014;7:595–601. doi: 10.3892/ol.2013.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman JR, Adler HL, Aguilar-Cordova E, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther. 1999;10:1239–49. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- 25.Nasu Y, Bangma CH, Hull GW, et al. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6:338–49. doi: 10.1038/sj.gt.3300834. [DOI] [PubMed] [Google Scholar]
- 26.Breyer B, Jiang W, Cheng H, et al. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149–62. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- 27.Swisher SG, Roth JA, Nemunaitis J, et al. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:763–71. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- 28.Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol. 2010;2:a001222. doi: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Zheng D. An update on gene therapy in China. Curr Opin Mol Ther. 2009;11:547–53. [PubMed] [Google Scholar]
- 30.Nasu Y, Bangma CH, Hull GW, et al. Combination gene therapy with adenoviral vector-mediated HSV-tk+GCV and IL-12 in an orthotopic mouse model for prostate cancer. Prostate Cancer Prostatic Dis. 2001;4:44–55. doi: 10.1038/sj.pcan.4500494. [DOI] [PubMed] [Google Scholar]
- 31.Edamura K, Nasu Y, Takaishi M, et al. Adenovirus-mediated REIC/Dkk-3 gene transfer inhibits tumor growth and metastasis in an orthotopic prostate cancer model. Cancer Gene Ther. 2007;14:765–72. doi: 10.1038/sj.cgt.7701071. [DOI] [PubMed] [Google Scholar]
- 32.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–80. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 33.Kurose K, Sakaguchi M, Nasu Y, et al. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–8. doi: 10.1097/01.ju.0000101047.64379.d4. [DOI] [PubMed] [Google Scholar]
- 34.Tanimoto R, Abarzua F, Sakaguchi M, et al. REIC/Dkk-3 as a potential gene therapeutic agent against human testicular cancer. Int J Mol Med. 2007;19:363–8. [PubMed] [Google Scholar]
- 35.Kawasaki K, Watanabe M, Sakaguchi M, et al. REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 2009;16:65–72. doi: 10.1038/cgt.2008.58. [DOI] [PubMed] [Google Scholar]
- 36.Uchida D, Shiraha H, Kato H, et al. Potential of adenovirus-mediated REIC/Dkk-3 gene therapy for use in the treatment of pancreatic cancer. J Gastroenterol Hepatol. 2014;29:973–83. doi: 10.1111/jgh.12501. [DOI] [PubMed] [Google Scholar]
- 37.Shien K, Tanaka N, Watanabe M, et al. Anti-cancer effects of REIC/Dkk-3-encoding adenoviral vector for the treatment of non-small cell lung cancer. PLoS One. 2014;9:e87900. doi: 10.1371/journal.pone.0087900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimazu Y, Kurozumi K, Ichikawa T, et al. Integrin antagonist augments the therapeutic effect of adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma. Gene Ther. 2014;22(2):146–54. doi: 10.1038/gt.2014.100. [DOI] [PubMed] [Google Scholar]
- 39.Zhang K, Watanabe M, Kashiwakura Y, et al. Expression pattern of REIC/Dkk-3 in various cell types and the implications of the soluble form in prostatic acinar development. Int J Oncol. 2010;37:1495–501. doi: 10.3892/ijo_00000802. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 41.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33:4623–31. doi: 10.1038/onc.2013.432. [DOI] [PubMed] [Google Scholar]
- 42.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marabelle A, Kohrt H, Sagiv-Barfi I, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–63. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe M, Sakaguchi M, Kinoshita R, et al. A novel gene expression system strongly enhances the anticancer effects of REIC/Dkk-3-encoding adenoviral vector. Oncol Rep. 2014;31:1089–95. doi: 10.3892/or.2013.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi M, Watanabe M, Kinoshita R, et al. Dramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol Biotechnol. 2014;56:621–30. doi: 10.1007/s12033-014-9738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]