Abstract
Previous studies suggest an interdependent relationship between liver and intestine for cholesterol elimination from the body. We hypothesized that a combination of ursodiol (Urso) and ezetimibe (EZ) could increase biliary secretion and reduce cholesterol reabsorption, respectively, to promote cholesterol excretion. Treatment with Urso increased hepatic ABCG5 ABCG8 (G5G8) protein and both biliary and fecal sterols in a dose-dependent manner. To determine whether the drug combination (Urso-EZ) further increased cholesterol excretion, mice were treated with Urso alone or in combination with two doses of EZ. EZ produced an additive and dose-dependent increase in fecal neutral sterol (FNS) elimination in the presence of Urso. Finally, we sequentially treated wide-type and G5G8-deficient mice with Urso and Urso-EZ to determine the extent to which these effects were G5G8 dependent. Although biliary and FNS were invariably lower in G5G8 KO mice, the relative increase in FNS following treatment with Urso alone or the Urso-EZ combination was not affected by genotype. In conclusion, Urso increases G5G8, biliary cholesterol secretion, and FNS and acts additively with EZ to promote fecal sterol excretion. However, the stimulatory effect of these agents was not G5G8 dependent.
Keywords: bile, bile acids and salts/biosynthesis, biliary cholesterol secretion, cholesterol 7-alpha hydroxylase, cholesterol/biosynthesis, high density lipoprotein, nonalcoholic fatty liver disease, reverse cholesterol transport, ursodeoxycholic acid, ATP binding cassette transporter G5, ATP binding cassette transporter G8
Elevated cholesterol is an important risk factor for cardiovascular disease, and strategies to promote cholesterol elimination have long been used to reduce the risk of atherosclerosis. An emerging body of literature suggests that cholesterol also plays an active role in the development of nonalcoholic fatty liver disease (NAFLD) and its progression to nonalcoholic steatohepatitis (NASH). Adding cholesterol to a high-fat (HF) diet in C57BL/6J mice leads to significantly more profound hepatosteatosis, inflammation, and fibrosis resembling human NASH (1). Similarly, in LDL receptor-deficient mice, adding cholesterol to an HF, high-sucrose diet exacerbates the development of insulin resistance and steatosis resulting in NASH (2). In a mouse model of Alström syndrome, an elevation in hepatic free cholesterol due to an increase in hepatic uptake and a decrease in biliary elimination is thought to play a contributing role in the development of liver disease (3). Strategies that promote hepatic cholesterol elimination are likely to have therapeutic benefit in NAFLD.
The ABCG5 ABCG8 (G5G8) heterodimer is the primary mediator of hepatobiliary elimination, accounting for 70% to 90% of biliary cholesterol secretion (4). In addition, it also opposes phytosterol absorption in the proximal small intestine (4, 5). We previously reported that G5G8 plays an essential role in the development of diet-induced obesity phenotypes independent of its role in opposing phytosterol accumulation. The absence of G5G8 and reduced biliary cholesterol secretion resulted in hepatic cholesterol accumulation, acceleration of obesity and insulin resistance, and worsening of NAFLD in mice challenged with a plant sterol-free (PSF) HF diet (6). Conversely, increasing biliary cholesterol secretion by adenoviral expression of G5G8 restored glycemic control, improved hepatic insulin signaling, and lowered plasma TGs in genetically obese, db/db mice (7).
Increased biliary and fecal sterol elimination following adenoviral G5G8 would presumably lower plasma cholesterol levels in db/db mice. However, a significant portion of the secreted cholesterol was reabsorbed, resulting in a paradoxical increase in plasma cholesterol despite increased biliary output. This effect of adenoviral G5G8 can be overcome by coadministration of ezetimibe (EZ), a potent inhibitor of cholesterol absorption that blocks Niemann-Pick C1-like 1 (NPC1L1) activity in the intestine (7, 8). Similar observations were made in atherosclerosis studies in which a G5G8 transgene expressed in both liver and intestine lowered LDL cholesterol and reduced lesion area, whereas a liver specific transgene was ineffective in the absence of EZ (9, 10). These observations indicate an interdependent relationship between biliary secretion and intestinal absorption for effective fecal cholesterol elimination. Consequently, therapeutic approaches to promote cholesterol elimination by targeting the hepatobiliary pathway are likely to be limited by intestinal reabsorption of secreted cholesterol. Thus, a combination therapy that increases biliary cholesterol secretion and simultaneously reduces intestinal absorption is likely to act additively in the elimination of cholesterol from the body.
While EZ is effective in reducing cholesterol absorption, no currently available therapeutics directly target the G5G8 sterol transporter to increase biliary cholesterol secretion. However, we previously published that tauroursodeoxycholic acid (TUDCA) increases G5G8 and biliary cholesterol in both lean and obese, db/db mice (11). TUDCA is a first-pass metabolite of ursodiol (Urso). Studies in mice have shown that TUDCA and its unconjugated precursor, Urso, have a number of beneficial effects on liver function including alleviation of endoplasmic reticulum stress, improved insulin sensitivity, and reduced lipogenesis (11–13). However, the effect of Urso on increasing G5G8 and promoting biliary secretion of cholesterol has not been characterized.
In the present study, we confirmed that Urso had similar effects on G5G8 as TUDCA. Urso increased hepatic G5G8 and dose dependently accelerated both biliary and fecal sterol excretion. We then tested whether an Urso-EZ combination treatment would act additively to promote cholesterol elimination. We treated mice with a constant dose of Urso or Urso in combination with two doses of EZ. EZ produced an additive effect for fecal sterol excretion in the presence of Urso. Although biliary and fecal neutral sterols (FNSs) were invariably lower in G5G8 KO mice, we observed an increase in FNS following Urso alone or Urso-EZ combination treatments. The magnitude of this increase was not affected by genotype suggesting that there may be a G5G8-independent pathway for cholesterol elimination stimulated by Urso.
MATERIALS AND METHODS
Chemicals, reagents, and antibodies
General chemicals were purchased from Sigma, immunoblotting reagents from Thermo/Pierce, real-time PCR reagents from Applied Biosystems. TUDCA, sodium salt (580549-5GM) was purchased from Calbiochem. Urso capsules, USP (NDC 42806-503-01) were purchased from Epic Pharma LLC, and Zetia (EZ) tablets (NDC 66582-414-54) from Merck and Co. Inc. Urethane, bromodeoxyuridine (BrdU), and the silylation reagent N,O-bis (trimethylsilyl) trifluoroacetamide were purchased from Sigma-Aldrich. The chicken anti-G5 polyclonal antibody and the monoclonal antibody directed against G8 were previously reported (6, 14). The β-actin antibody was purchased from Sigma. Anti-ABCA1, [26, 26, 26, 27, 27, 27-2H6]cholesterol and [5, 6, 22, 23-2H4]sitostanol were generous gifts from Ryan Temel (University of Kentucky) (15). The anti-CD3 monoclonal antibody (clone 145-2C11) was purified over protein G beads (Amersham Pharmacia Biotech, Piscataway, NJ). Anti-BrdU was purchased from MPL International (Woburn, MA).
EZ- and/or Urso-supplemented diets
Powdered rodent chow diet (T.2018M.15) was purchased from Harlan Laboratories. Custom formulated pellet (D10040301) and powdered (D10040301M) PSF diets were purchased from Research Diets Inc. Macronutrient composition and sterol content were previously described (6). There was no added cholesterol in the diet. Both Urso and EZ were ground into a fine powder and then thoroughly mixed with control diet (T.2018M.15 or D10040301M) to obtain the desired concentrations. Powdered diets were provided to mice in glass feeding jars and replaced daily.
Animal husbandry
C57BL/6J (Stock #000664) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice homozygous for the Abcg5 and Abcg8 mutant alleles (KO) and their WT littermates were obtained from heterozygous, trio matings as previously described (6). Mice were housed in individually ventilated cages in a temperature-controlled room with a 14:10 light:dark cycle and provided with enrichment in the form of acrylic huts, wood chew sticks, and nesting material. Mice were adapted to powdered diet for a period of 7 days prior to initiation of studies. The diet was then changed to those containing various concentrations of Urso and EZ. All animal procedures conform to Public Health Service policies for humane care and use of laboratory animals and were approved by the institutional animal care and use committee at the University of Kentucky. All surgery was performed under anesthesia, and all efforts were made to minimize suffering.
Animal experiments
Male C57BL/6J mice (n = 6/group) were fed chow (control), chow supplemented with 0.4% TUDCA (w/w), or Urso at concentrations of 0.1%, 0.3%, or 1% (w/w) for 7 days. Mice were housed (2 per cage) in individually ventilated cages. Mice were placed in clean cages to collect feces for 3 days prior to termination. On the final day of the study, mice were transferred to clean cages and fasted for 4 h. The bile duct was ligated, and the gallbladder cannulated for basal bile collection (30 min) under urethane (1 g/kg body weight) anesthesia. Bile flow was determined gravimetrically assuming a density of 1 g/ml. Mice were then exsanguinated, and tissues dissected, frozen in liquid nitrogen, and stored at −80°C until analysis. Another cohort of male C57BL/6J mice (n = 7) were fed chow (control), chow supplemented with 0.3% Urso or 0.3% Urso combined with 0.001% or 0.005% EZ for 14 days and were housed individually. Feces, basal bile, and other tissues were collected as described above.
G5G8 KO mice (n = 4 both male and female) and their WT littermates (n = 6 male and 7 female) were weaned between 18 and 21 days onto a pellet PSF diet to prevent the development of sitosterolemia (6). When maintained on this diet, serum levels of phytosterols are <10% of those observed in G5G8 KO mice maintained on chow diet and do not differ between genotypes (supplementary Fig. 1). Prior to initiation of the study, mice were adapted to a powdered PSF (control) diet for 7 days. Then, mice were fed a control diet for 10 days (phase I) followed by 0.3% Urso for 7 days (phase II). All mice were then treated with 0.3% Urso combined with 0.005% EZ for 14 days (phase III). Feces were collected the final 3 days of each phase. Blood samples were collected by cheek bleed at the final day of each phase. At termination following phase III, basal bile and tissues were harvested as described above.
Another cohort of male and female G5G8 KO and WT mice (n = 3 for each gender and genotype) treated with 0.3% Urso or a PSF control diet for 7 days were used to measure both fractional cholesterol absorption and intestinal epithelial cell sloughing as described with modifications (16, 17). Mice were individually housed and adapted to a powdered PSF (control) diet for 7 days prior to the experiment in wire-bottom cages. Each mouse was gavaged with 50 μl of deuterated sterol/stanol-oil mixture in the amounts described in the supplementary Methods. Feces were collected for 3 days after oral gavage. To determine the relative rates of intestinal epithelial cell proliferation and turnover, each mouse was injected ip with 1 mg BrdU 2 h before tissue dissection. Blood samples were collected, and three segments of the small intestine were harvested and fixed in 10% neutral buffered formalin for immunohistochemistry. Additional details are available in the supplementary Methods.
Immunoblot and quantitative real-time PCR
The preparations of proteins, SDS-PAGE, and immunoblotting were conducted as previously described (6, 7). Total RNAs were extracted from each liver using the RNA STAT-60 (Tel-Test Inc.) and subjected to cDNA synthesis with iScript cDNA Synthesis Kit (BIO-RAD, Hercules, CA). To determine relative abundance, RT-PCR was conducted using SYBRGreen detector on Applied Biosystem 7900HT fast-Real Time PCR System (Carlsbad, CA) (6).
Hepatic, serum, and biliary lipid analysis
Hepatic lipids were extracted by using Folch reagents as previously described (7). Total and nonesterified hepatic and serum cholesterol, as well as cholesterol and phospholipids in gallbladder bile, were determined using commercial colorimetric-enzymatic assays (Wako Chemicals, Richmond, VA). The quantitation of total bile acids (BAs) in bile was performed enzymatically by measuring 3α-hydroxy BAs as previously described (18). Serum was fractionated by fast-protein liquid chromatography (FPLC), and fractions were analyzed for total cholesterol content as previously described (6). The concentrations of lathosterol and phytosterols in serum were measured by LC/MS/MS and GC/MS, respectively, using modifications of previously published methods (19–22). Details are available in the supplementary Methods.
FNSs
FNSs were analyzed as previously described with minor modifications (7). Briefly, total feces from the 72 h period were collected, dried at 37°C, weighed, and ground to powder. An aliquot of 0.30 g of feces was placed into a glass tube with 2.5 ml of ethanol and 0.5 ml of 10 N NaOH. Lipids were saponified at 72°C in a water bath for 2 h and extracted (water-ethanol-petroleum ether, 1:1:1, v/v/v); 0.12 mg of 5α-cholestane (1 μg/μl) was used as the internal standard. Following extraction, the organic phase was dried under nitrogen gas and solubilized in hexane. The amount of neutral sterols (cholesterol, coprostanol, and cholestanol) was quantified by GC/MS.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism. Data are expressed as mean ± SEM. Data shown in Figs. 1, 2 were analyzed by two-tailed t-test or one-way ANOVA followed by Dunnett’s post hoc comparisons. Data shown in Figs. 3, 4, 5 were analyzed by one-way ANOVA followed by Bonferroni post hoc tests. Data shown in Fig. 6 were analyzed by a two-way ANOVA or a repeated measure two-way ANOVA using genotype and treatment as factors. Post hoc comparisons were made using Bonferroni tests. Differences were considered significant at P < 0.05. Where genotype and treatment by sex interactions were not significant, data were analyzed independent of sex.
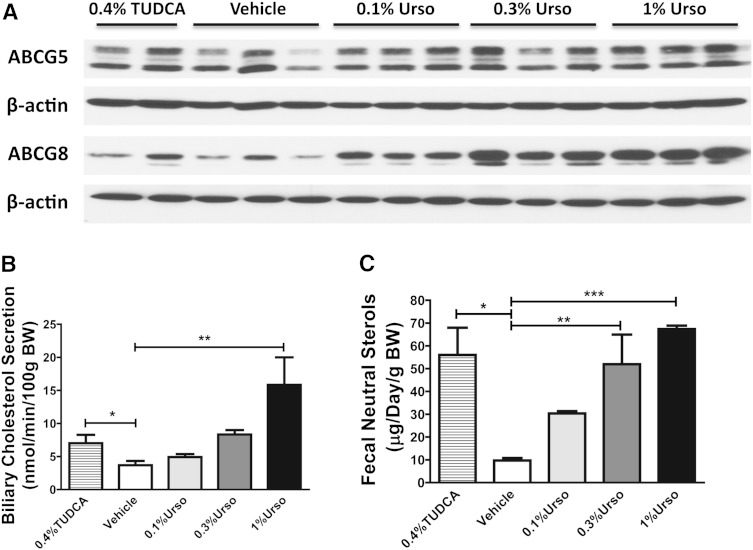
Fig. 1.
Urso increases G5G8 and both biliary and FNS in a dose-dependent manner. Male C57BL/6 mice were fed with control, 0.4%TUDCA, or three doses of Urso for 7 days. A: Hepatic levels of ABCG5 and ABCG8 protein expression were determined by immunoblotting. Membrane preparations were blotted for β-actin as controls. B: Hepatobiliary cholesterol secretion rates under basal conditions. Gallbladder was cannulated, and basal bile was collected for 30 min. n = 6 for each group. C: FNS was determined by GC/MS. n = 3 for each group. Data are presented as mean ± SEM. Differences between TUDCA and control were determined by two-tailed t-test. Differences between control and three doses of Urso were determined by one-way ANOVA followed by Dunnett’s tests. * P < 0.05, ** P < 0.01, *** P < 0.001.
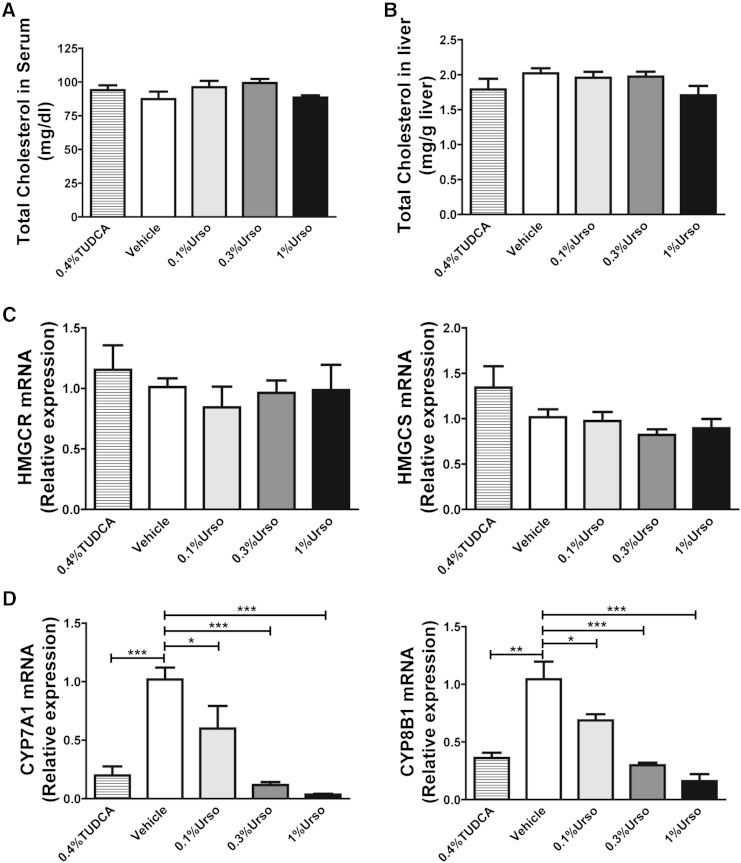
Fig. 2.
Urso suppressed BA synthesis but had no effect on cholesterol levels in liver or serum. A: Total cholesterol concentrations in serum. n = 6 for each group. B: Hepatic total cholesterol per gram of wet tissue weight. n = 6 for each group. The mRNA expression for de novo cholesterol synthetic genes hydroxymethylglutaryl-CoA reductase (HMGCR) and hydroxymethylglutaryl-CoA synthase (HMGCS) (C) and BA synthetic genes CYP7A1 and CYP8B1 (D) was determined by RT-PCR. n = 4–5 for each group. Data are mean ± SEM. Differences between TUDCA and control were determined by two-tailed t-test. Differences between control and three doses of Urso were determined by one-way ANOVA followed by Dunnett’s tests. * P < 0.05, ** P < 0.01, *** P < 0.001.
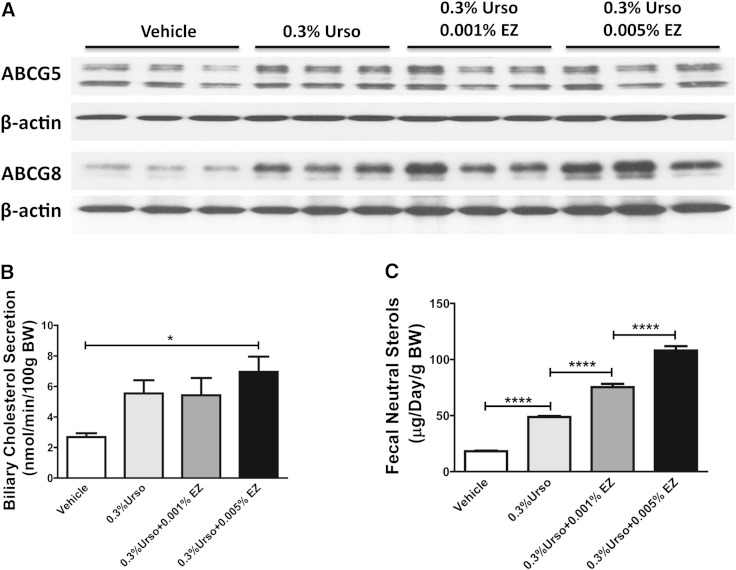
Fig. 3.
EZ produced an additive effect for fecal sterol elimination. Male C57BL/6 mice were fed with control, 0.3% Urso, or 0.3% Urso combined with a low or high dose of EZ for 14 days. A: Hepatic levels of ABCG5 and ABCG8 protein expression were determined by immunoblotting. B: Hepatobiliary cholesterol secretion rates under basal conditions. n = 5–7 for each group. C: FNS was determined by GC/MS. n = 7 for each group. Data are presented as mean ± SEM. Differences were determined by one-way ANOVA followed by Bonferroni post hoc tests. * P < 0.05, **** P < 0.0001.
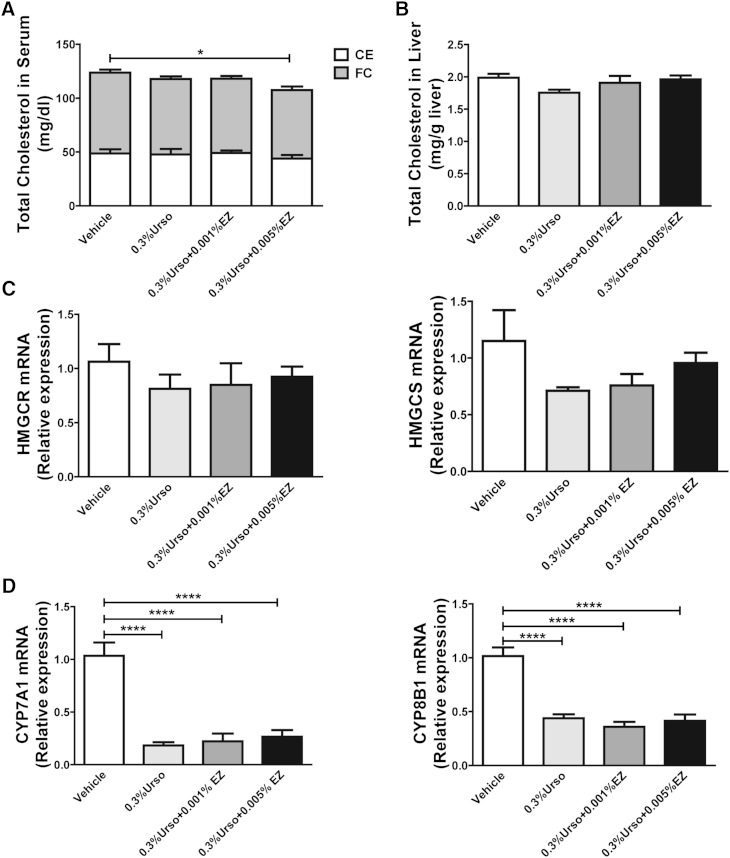
Fig. 4.
EZ reduced free cholesterol in serum but had no additive effect on the biosynthesis of BAs and cholesterol in liver. A: Total cholesterol and nonesterified cholesterol (FC) were determined by Wako enzymatic-colorimetric kits. Cholesteryl esters (CEs) were calculated from the difference in total and free cholesterol. n = 7 for each group. B: Hepatic total cholesterol per gram of wet tissue weight. n = 7 for each group. The mRNA expression for de novo cholesterol synthetic genes HMGCR and HMGCS (C) and BA synthetic genes CYP7A1 and CYP8B1 (D) was determined by RT-PCR. n = 6 for each group. Data are mean ± SEM. Differences were determined by one-way ANOVA followed by Bonferroni post hoc tests. * P < 0.05, **** P < 0.0001.
Fig. 5.
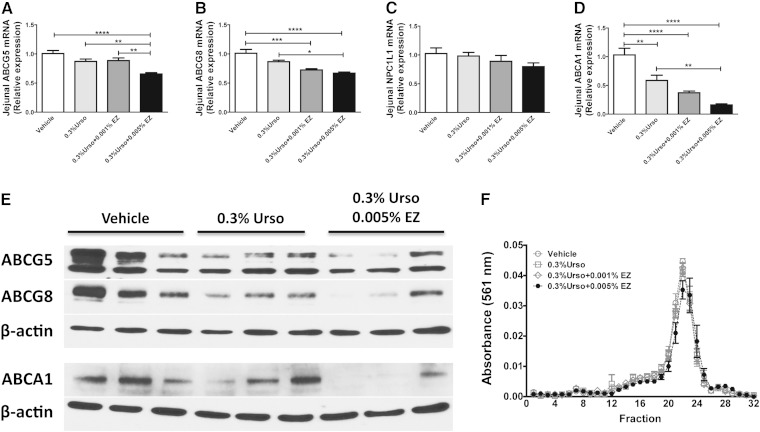
EZ reduced intestinal G5G8 and ABCA1. A–D: The mRNA expression for jejunum ABCG5, ABCG8, NPC1L1, and ABCA1 was determined by RT-PCR. n = 6 for each group. E: Immunoblot analysis of jejunum ABCG5, ABCG8, and ABCA1. F: Serum (60 μl) from 4 mice in each group was fractionated by FPLC and analyzed for the distribution of cholesterol among lipoproteins. Horizontal bars indicated elution fractions for lipoproteins. Data are mean ± SEM. Differences were determined by one-way ANOVA followed by Bonferroni post hoc tests. * P < 0.05, ** P < 0.01 *** P < 0.001, **** P < 0.0001.
Fig. 6.
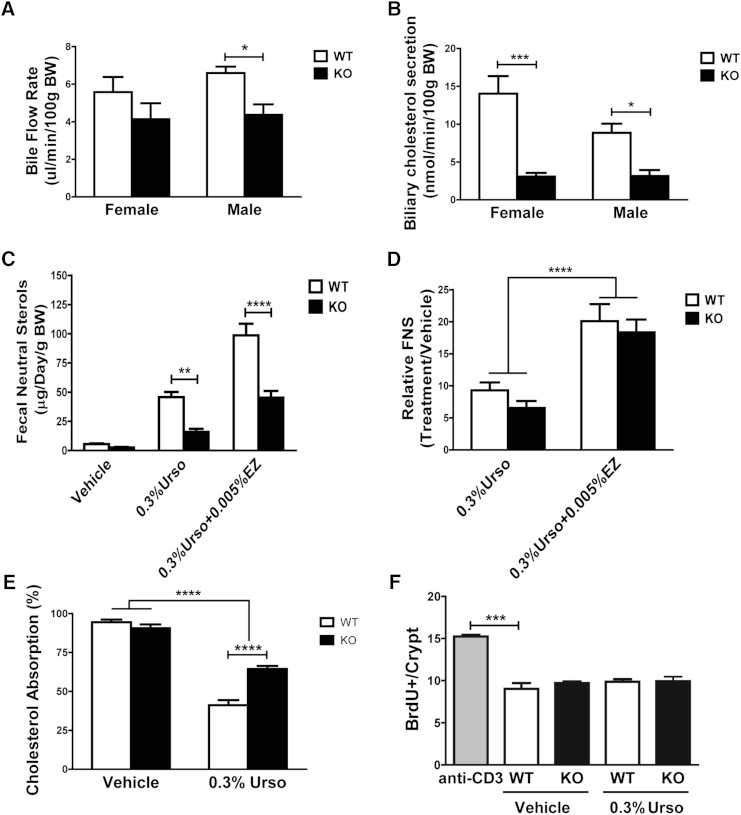
Urso-EZ-induced increase in FNS does not require G5G8. G5G8 KO mice (n = 4 both sexes) and their WT littermates (n = 6 male and 7 female) were sequentially fed with control, 0.3% Urso, and 0.3% Urso plus 0.005% EZ for 14 days. A, B: Bile flow and biliary cholesterol secretion rates by sex. C, D: FNS and the relative difference in fecal sterol loss normalized to controls. Another cohort of male and female KO and WT mice (n = 3 of each gender and genotype) were fed a PSF diet or 0.3% Urso for 7 days. E: The fractional cholesterol absorption was measured by stable dual isotope method and represented irrespective of sex. F: The relative rates of intestinal epithelial cell proliferation and turnover were determined by BrdU staining. Sections were scored, 20 villi/crypts per mouse, 3 mice per group, and the number of cells proliferating in jejunal crypts was determined. Data are expressed as mean ± SEM. Differences were determined by two-way ANOVA followed by Bonferroni posttests (A, B, and E), a repeated measure two-way ANOVA using diet and genotype as factors followed by Bonferroni post hoc tests (C, D), and one-way ANOVA followed by Dunnett’s post hoc comparisons (F). * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
RESULTS
Urso increases G5G8 and both biliary and FNS in a dose-dependent manner
To determine whether Urso could increase G5G8 and biliary cholesterol secretion and elimination, mice were fed chow (control) or chow supplemented with 0.1%, 0.3%, or 1% (w/w) Urso. TUDCA (0.4%, equal molar ratio to 0.3% Urso) was used as a positive control as it was previously shown to increase G5G8 and biliary cholesterol secretion. The abundance of hepatic G5 and G8 was evaluated by immunoblot analysis (Fig. 1A). Urso increased hepatic G5G8 to a similar level at all tested doses. Its effects were equal to, or slightly greater, than TUDCA, particularly for G8. Biliary cholesterol and FNS were used as indirect measures of G5G8 activity. Hepatobiliary cholesterol secretion rates under basal conditions were calculated from the product of bile flow and cholesterol concentration. Urso dose-dependently increased both biliary cholesterol secretion rates and FNS (Fig. 1B, C). Similarly, Urso increased biliary secretions of both phospholipids and BAs in a dose-dependent manner (supplementary Fig. 2A, B).
Urso suppressed BA synthesis but had no effect on cholesterol levels in liver or serum
Urso had no effect on either serum or hepatic total cholesterol at any of the doses examined (Fig. 2A, B). To determine whether the increased FNS was due to increased cholesterol synthesis, we measured the mRNA expression level of HMGCR and HMGCS and observed no differences (Fig. 2C). To investigate the effects of Urso on BA biosynthesis, the mRNA expression level of cholesterol 7α-hydroxylase (CYP7A1), sterol 12-alpha-hydroxylase (CYP8B1), 25-hydroxycholesterol 7-alpha-hydroxylase (CYP7B1), and sterol 27-hydroxylase (CYP27A1) was quantified by RT-PCR (Fig. 2D). TUDCA significantly decreased both CYP7A1 and CYP8B1, but not CYP7B1 and CYP27A1 (not shown). Similarly, both CYP7A1 and CYP8B1 decreased in a dose-dependent manner following Urso treatment. As with TUDCA, Urso had no effect on CYP7B1 and CYP27A1 expression levels (not shown).
EZ produced an additive effect for fecal sterol elimination
To determine whether EZ plays an additive role for accelerating cholesterol elimination, we treated mice with 0.3% Urso combined with two doses of EZ for 14 days. As with 7 day treatment, 0.3% Urso increased G5G8 protein levels in liver. This effect was maintained in the presence of both low and high doses of EZ (Fig. 3A). The presence of EZ had no effect on biliary secretion rates of cholesterol as well as phospholipids and BAs following 2 weeks of treatment (Fig. 3B; supplementary Fig. 3A, B). However, EZ dose dependently increased FNS in the presence of 0.3% Urso (Fig. 3C).
EZ reduced free cholesterol in serum but had no additive effect on the biosynthesis of BAs and cholesterol in liver
EZ at a concentration of 0.005% slightly reduced serum total cholesterol. This was predominantly due to the significant reduction of free cholesterol (Fig. 4A). No differences were observed in hepatic free (not shown) or total cholesterol (Fig. 4B). Although there was a tendency toward suppression of cholesterol synthetic genes at the mRNA level, none achieved statistical significance (Fig. 4C). We confirmed the repression of CYP7A1 and CYP8B1 at the mRNA level in mice treated with Urso. EZ had no additional effect on their mRNA levels (Fig. 4D). This effect of Urso is likely due to the stimulation of ileal fibroblast growth factor 15 (FGF15), which feeds back to repress hepatic BA synthesis (supplementary Fig. 4A). To determine whether extrahepatic sources might contribute to the increased FNS in response to Urso-EZ, we examined the mRNA expression level of HMGCR and HMGCS in adrenal glands, epididymal adipose tissues, and jejuna as well as circulating lathosterol, an indicator of whole body cholesterol synthesis (supplementary Figs. 5 and 6) (23). Urso did not alter the expression level of HMGCR and HMGCS in tested tissues. However, the Urso-EZ combination increased HMGCS mRNA expression in both adrenal glands and jejuna (supplementary Fig. 5B, F). Circulating levels of lathosterol tended to increase but did not reach statistical significance (supplementary Fig. 6).
EZ reduced intestinal G5G8 and ABCA1
With recent studies characterizing transintestinal cholesterol excretion (TICE) as an alternative route for cholesterol elimination, the role of the intestine has regained attention (24). G5G8 is also abundantly expressed at the intestine to oppose cholesterol and phytosterol absorption. Therefore, we measured the jejunum expression of G5G8 at both mRNA and protein levels as well as intestinal ABCA1, which contributes to intestinal HDL biogenesis (25). Urso alone tended to decrease jejunum G5G8 at the mRNA level but did not reach statistical significance (Fig. 5A, B). However, immunoblot analysis indicated a marked reduction in G5G8 protein levels (Fig. 5E). The combination of 0.005% EZ further reduced G5G8 both at mRNA and protein levels. We did not observe any change in NPC1L1 at the mRNA level (Fig. 5C). The combination of Urso and EZ also significantly decreased intestinal ABCA1 at both the mRNA and protein levels (Fig. 5D, E). FPLC fractionation of serum indicated a reduction in HDL cholesterol and a modest shift toward smaller HDL particles (Fig. 5F). Thus, the reduction in serum cholesterol concentrations is likely attributed to reduced ABCA1 and intestinally derived HDL.
Urso-EZ-induced increase in FNS does not require G5G8
To determine the extent to which the effect of Urso and Urso-EZ treatments were G5G8 dependent, WT and KO mice were maintained on a control diet, followed by Urso (0.3%) and then Urso-EZ (0.3%–0.005%). Each mouse served as its own control. Three-day fecal samples and blood were collected at the end of each treatment period. G5G8 KO mice presented slightly lower bile flow rates, but invariably lower rates of biliary cholesterol secretion (Fig. 6A, B). In the absence of drugs, FNSs were reduced by 52% in G5G8 KO mice compared with WT littermates (Fig. 6C). Urso increased FNSs in both WT and G5G8 KO mice by 900% and 700%, respectively (Fig. 6D). EZ produced a further 2-fold increase in both genotypes (Fig. 6D). Thus, while FNSs were invariably lower in G5G8 KO mice, the drug-induced increase remained largely constant (Fig. 6D). Differences were not observed in serum cholesterol (not shown).
Possible factors contributing to FNS excretion include intestinal cholesterol absorption and epithelial cell sloughing. There was no cholesterol added to the PSF diet. Under this condition, fractional cholesterol absorption approached 90% in both WT and KO mice (Fig. 6E). Urso reduced absorption in both genotypes. This may reflect the role of G5G8 in cholesterol absorption or the dilution of the cholesterol isotope associated with increased biliary cholesterol output. Effects were virtually identical in male and female mice (supplementary Fig. 7).
The effects of Urso on intestinal epithelial cell proliferation and turnover are not known. To understand Urso’s effect in this process, we performed a BrdU incorporation study (17). Anti-CD3 monoclonal antibody was used as a positive control by stimulating T-cell-induced epithelial cell proliferation (18 h) and increased crypt depth/villus blunting. In contrast, G5G8 deficiency failed to alter epithelial cell responses to Urso (Fig. 6F; supplementary Fig. 8). These findings indicate that drug-induced cholesterol elimination is independent of G5G8.
DISCUSSION
The major findings of the present study are that Urso increases G5G8 and that Urso-EZ acts in an additive fashion to promote fecal sterol excretion in mice. While G5G8 is the primary route for biliary cholesterol secretion, our studies reveal a G5G8-independent pathway for cholesterol elimination stimulated by Urso and Urso-EZ combination treatments. Whether this is attributed to other biliary or nonbiliary pathways, such as TICE, remains to be determined.
Biliary cholesterol secretion represents an essential step of the reverse cholesterol transport (RCT) process, which involves the transport of cholesterol from peripheral cells to the liver for secretion into bile and subsequent elimination in feces (26). Accelerating RCT has long been a therapeutic goal in the treatment of atherosclerosis (27, 28). However, the role of biliary cholesterol secretion in the development and severity of NAFLD is a relatively recent discovery. We have published the only reports detailing G5G8 and biliary cholesterol secretion in the context of NAFLD (6, 7). Consequently, studies to date have not examined accelerated biliary secretion as a therapeutic strategy in either preclinical models or humans in the treatment of NAFLD. Although G5G8 has been known to increase cholesterol excretion for more than a decade, there has been little interest in drug development for this target. This is mainly because increasing biliary cholesterol secretion is expected to raise the cholesterol saturation index of bile and the risk for cholesterol gallstones. However, Urso is a hydrophilic BA with choleretic, cytoprotective, and antiapoptotic properties (29, 30). It was originally used for cholesterol gallstone dissolution mainly because it reduces hydrophobicity of the BA pool and increases bile flow, two factors that oppose gallstone formation. Therefore, increasing G5G8 with Urso to increase biliary cholesterol secretion in the absence of increased risk of gallstone formation may be a viable therapeutic strategy to accelerate RCT.
A number of preclinical studies and clinical trials have evaluated either Urso or EZ in the treatment of NAFLD or NASH. Results with Urso were mixed. Low-dose Urso (13–15 mg/kg/day) reduced some markers of inflammation including serum alanine transaminase (ALT) but failed to significantly improve NASH in two separate studies (31, 32). High-dose Urso (28–32 mg/kg/day) failed to reduce ALT at either 3 or 6 months in one trial (n = 12); in a separate trial, high-dose Urso lowered ALT levels by an average of 40% at 3, 6, 9, and 12 months and normalized ALT values in 25% of patients compared with no reductions or normalizations in the placebo group (33, 34). Although the numbers of studies and patients were limited, EZ monotherapy showed promise in the treatment of NAFLD. In a 6 month pre-/posttreatment open-label trial in 10 patients, EZ reduced serum ALT, γ-glutamyl transpeptidase, plasma TGs, and hepatic fat, but measures of insulin resistance were unchanged (35). In the largest study to date (n = 45) in a Japanese population, EZ reduced ALT at 12 months and resulted in modest, but significant reductions in steatosis, ballooning, and other indices of NAFLD by 24 months (36). However, neither monotherapy is currently indicated in the treatment of NAFLD. The limited benefit may be due to the interdependent nature of biliary secretion and intestinal absorption with respect to cholesterol elimination. Therefore, a combination therapy that simultaneously increases biliary secretion and reduces cholesterol absorption may provide greater therapeutic benefit compared with Urso or EZ monotherapies.
It was expected that Urso-EZ combination treatment would further stimulate fecal sterol loss and create a negative sterol balance or “cholesterol drain.” In the steady state, the extent of cholesterol loss is directly reflected by the rate of cholesterol synthesis. In the present study, no evidence supports an increase in whole body cholesterol synthesis except a modest increase in adrenal and jejunal HMGCS gene expression. In addition, feeding an Urso-containing diet led to a robust repression of hepatic CYP7A1 and CYP8B1. Thus, the observed increase in FNS may reflect diversion of cholesterol away from BA synthesis into the G5G8 accessible pool, rather than the establishment of a cholesterol drain.
The mechanism(s) by which Urso simultaneously increases G5G8 and suppresses BA synthesis is not known. It may be due to the stimulation of ileal FGF15, which acts on Fibroblast growth factor receptor 4/β-Klotho receptor complexes in liver to repress BA synthesis (37). FGF15 also induces the protein synthesis in liver, but the effect of FGF15 on G5G8 is not known (37, 38). Alternatively, recent studies suggest that the α5β1-integrin is a sensor for TUDCA that promotes choleresis (39, 40). However, the effect of integrin signaling on G5G8 is not known. Therefore, it remains unclear whether the suppression of BA synthesis and the stimulation of G5G8 in response to Urso utilize common or independent mechanisms. If the mechanisms are independent, there may be a therapeutic window whereby biliary cholesterol secretion could increase to a greater extent than BA suppression in order to promote RCT. If not, other therapeutic approaches aimed to increase G5G8 and biliary cholesterol secretion would need to be developed in combination with EZ to accelerate RCT. However, such an approach may increase the risk of gallstone formation.
Another intriguing observation in our present studies was that Urso and Urso-EZ similarly increased FNS in G5G8 KO mice as in their WT littermates. This could be partially attributed to the most studied nonbiliary route, TICE, which may account for ∼33% of total fecal sterol loss in mice and is now considered as an essential alternative route to the hepatobiliary pathway (24). We did not simultaneously measure biliary and intestinal cholesterol secretion in our studies, nor did we evaluate to what extent biliary versus nonbiliary pathways contribute to the total fecal sterol loss. However, Urso and Urso-EZ in G5G8-deficient mice may be useful tools in identifying novel biliary and nonbiliary pathways for cholesterol elimination.
Supplementary Material
Acknowledgments
The authors thank Alan Daugherty and Deborah Howatt within the Saha Cardiovascular Research Center, University of Kentucky for assistance with FPLC analysis of serum; Joseph Chappell and Chase Kempinski within the Department of Pharmaceutical Sciences for assistance with GC/MS analyses; Wendy Katz within the Graduate Center for Nutritional Sciences for assistance with processing intestinal tissues for immunohistochemistry and histological analysis; and Jeannie Haak for assistance with manuscript preparation.
Footnotes
Abbreviations:
- ALT
- alanine transaminase
- BA
- bile acid
- BrdU
- bromodeoxyuridine
- CYP7A1
- cholesterol 7α-hydroxylase
- CYP8B1
- sterol 12-alpha-hydroxylase
- CYP7B1
- 25-hydroxycholesterol 7-alpha-hydroxylase
- CYP27A1
- sterol 27-hydroxylase
- EZ
- ezetimibe
- FGF15
- fibroblast growth factor 15
- FNS
- fecal neutral sterol
- FPLC
- fast-protein liquid chromatography
- G5G8
- ABCG5 ABCG8
- HF
- high-fat
- HMGCR
- hydroxymethylglutaryl-CoA reductase
- HMGCS
- hydroxymethylglutaryl-CoA synthase
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NPC1L1
- Niemann-Pick C1-like 1
- PSF
- plant sterol-free
- RCT
- reverse cholesterol transport
- TICE
- transintestinal cholesterol excretion
- TUDCA
- tauroursodeoxycholic acid
- Urso
- ursodiol
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK080874 (G.A.G.) and 2R01DK095662-06A1 (T.A.B.), the National Institute of General Medical Sciences (8 P20 GM103527-05) of the National Institutes of Health, the Center for Pharmaceutical Science and Innovation (CPRI), and the National Center for Advancing Translational Sciences (UL1TR000117). Tissue processing was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (8 P20 GM103527-05). The authors have no conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures and supplementary Methods.
REFERENCES
- 1.Savard C., Tartaglione E. V., Kuver R., Haigh W. G., Farrell G. C., Subramanian S., Chait A., Yeh M. M., Quinn L. S., Ioannou G. N. 2013. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 57: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramanian S., Goodspeed L., Wang S., Kim J., Zeng L., Ioannou G. N., Haigh W. G., Yeh M. M., Kowdley K. V., O’Brien K. D., et al. 2011. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 52: 1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rooyen D. M., Larter C. Z., Haigh W. G., Yeh M. M., Ioannou G., Kuver R., Lee S. P., Teoh N. C., Farrell G. C. 2011. Hepatic free cholesterol accumulates in obese, diabetic mice and causes non-alcoholic steatohepatitis. Gastroenterology. 141: 1393–1403.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabeva N. S., Liu J., Graf G. A. 2009. The ABCG5 ABCG8 sterol transporter and phytosterols: implications for cardiometabolic disease. Curr. Opin. Endocrinol. Diabetes Obes. 16: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775. [DOI] [PubMed] [Google Scholar]
- 6.Su K., Sabeva N. S., Liu J., Wang Y., Bhatnagar S., van der Westhuyzen D. R., Graf G. A. 2012. The ABCG5 ABCG8 sterol transporter opposes the development of fatty liver disease and loss of glycemic control independently of phytosterol accumulation. J. Biol. Chem. 287: 28564–28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su K., Sabeva N. S., Wang Y., Liu X., Lester J. D., Liu J., Liang S., Graf G. A. 2014. Acceleration of biliary cholesterol secretion restores glycemic control and alleviates hypertriglyceridemia in obese db/db mice. Arterioscler. Thromb. Vasc. Biol. 34: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis H. R., Jr., Zhu L. J., Hoos L. M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S. P., Lam M. H., Lund E. G., et al. 2004. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279: 33586–33592. [DOI] [PubMed] [Google Scholar]
- 9.Wilund K. R., Yu L., Xu F., Hobbs H. H., Cohen J. C. 2004. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/− mice. J. Lipid Res. 45: 1429–1436. [DOI] [PubMed] [Google Scholar]
- 10.Basso F., Freeman L. A., Ko C., Joyce C., Amar M. J., Shamburek R. D., Tansey T., Thomas F., Wu J., Paigen B., et al. 2007. Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J. Lipid Res. 48: 114–126. [DOI] [PubMed] [Google Scholar]
- 11.Sabeva N. S., Rouse E. J., Graf G. A. 2007. Defects in the leptin axis reduce abundance of the ABCG5-ABCG8 sterol transporter in liver. J. Biol. Chem. 282: 22397–22405. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchida T., Shiraishi M., Ohta T., Sakai K., Ishii S. 2012. Ursodeoxycholic acid improves insulin sensitivity and hepatic steatosis by inducing the excretion of hepatic lipids in high-fat diet-fed KK-Ay mice. Metabolism. 61: 944–953. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z., Hotamisligil G. S. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 313: 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf G. A., Yu L., Li W. P., Gerard R., Tuma P. L., Cohen J. C., Hobbs H. H. 2003. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278: 48275–48282. [DOI] [PubMed] [Google Scholar]
- 15.Rayner K. J., Esau C. C., Hussain F. N., McDaniel A. L., Marshall S. M., van Gils J. M., Ray T. D., Sheedy F. J., Goedeke L., Liu X., et al. 2011. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 478: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu L., von Bergmann K., Lutjohann D., Hobbs H. H., Cohen J. C. 2005. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J. Lipid Res. 46: 1739–1744. [DOI] [PubMed] [Google Scholar]
- 17.Cliffe L. J., Humphreys N. E., Lane T. E., Potten C. S., Booth C., Grencis R. K. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 308: 1463–1465. [DOI] [PubMed] [Google Scholar]
- 18.Talalay P. 1960. Enzymic analysis of steroid hormones. Methods Biochem. Anal. 8: 119–143. [DOI] [PubMed] [Google Scholar]
- 19.Yu L., Hammer R. E., Li-Hawkins J., Von Bergmann K., Lutjohann D., Cohen J. C., Hobbs H. H. 2002. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA. 99: 16237–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Mitsche M. A., Luetjohann D., Cohen J. C., Xie X. S., Hobbs H. H. 2015. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 56: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald J. G., Smith D. D., Stiles A. R., Russell D. W. 2012. A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J. Lipid Res. 53: 1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda A., Miyazaki T., Ikegami T., Iwamoto J., Yamashita K., Numazawa M., Matsuzaki Y. 2010. Highly sensitive and specific analysis of sterol profiles in biological samples by HPLC-ESI-MS/MS. J. Steroid Biochem. Mol. Biol. 121: 556–564. [DOI] [PubMed] [Google Scholar]
- 23.Kempen H. J., Glatz J. F., Gevers Leuven J. A., van der Voort H. A., Katan M. B. 1988. Serum lathosterol concentration is an indicator of whole-body cholesterol synthesis in humans. J. Lipid Res. 29: 1149–1155. [PubMed] [Google Scholar]
- 24.van der Veen J. N., van Dijk T. H., Vrins C. L., van Meer H., Havinga R., Bijsterveld K., Tietge U. J., Groen A. K., Kuipers F. 2009. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284: 19211–19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glomset J. A. 1968. The plasma lecithins:cholesterol acyltransferase reaction. J. Lipid Res. 9: 155–167. [PubMed] [Google Scholar]
- 27.Ohashi R., Mu H., Wang X., Yao Q., Chen C. 2005. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM. 98: 845–856. [DOI] [PubMed] [Google Scholar]
- 28.Spady D. K. 1999. Reverse cholesterol transport and atherosclerosis regression. Circulation. 100: 576–578. [DOI] [PubMed] [Google Scholar]
- 29.Amaral J. D., Viana R. J., Ramalho R. M., Steer C. J., Rodrigues C. M. 2009. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J. Lipid Res. 50: 1721–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angulo P. 2002. Use of ursodeoxycholic acid in patients with liver disease. Curr. Gastroenterol. Rep. 4: 37–44. [DOI] [PubMed] [Google Scholar]
- 31.Lindor K. D., Kowdley K. V., Heathcote E. J., Harrison M. E., Jorgensen R., Angulo P., Lymp J. F., Burgart L., Colin P. 2004. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 39: 770–778. [DOI] [PubMed] [Google Scholar]
- 32.Dufour J. F., Oneta C. M., Gonvers J. J., Bihl F., Cerny A., Cereda J. M., Zala J. F., Helbling B., Steuerwald M., Zimmermann A. 2006. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 4: 1537–1543. [DOI] [PubMed] [Google Scholar]
- 33.Ratziu V., de Ledinghen V., Oberti F., Mathurin P., Wartelle-Bladou C., Renou C., Sogni P., Maynard M., Larrey D., Serfaty L., et al. 2011. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J. Hepatol. 54: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 34.Adams L. A., Angulo P., Petz J., Keach J., Lindor K. D. 2010. A pilot trial of high-dose ursodeoxycholic acid in nonalcoholic steatohepatitis. Hepatol. Int. 4: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoneda M., Fujita K., Nozaki Y., Endo H., Takahashi H., Hosono K., Suzuki K., Mawatari H., Kirikoshi H., Inamori M., et al. 2010. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: an open-label, pilot study. Hepatol. Res. 40: 566–573. [DOI] [PubMed] [Google Scholar]
- 36.Park H., Shima T., Yamaguchi K., Mitsuyoshi H., Minami M., Yasui K., Itoh Y., Yoshikawa T., Fukui M., Hasegawa G., et al. 2011. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J. Gastroenterol. 46: 101–107. [DOI] [PubMed] [Google Scholar]
- 37.Potthoff M. J., Kliewer S. A., Mangelsdorf D. J. 2012. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 26: 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kir S., Beddow S. A., Samuel V. T., Miller P., Previs S. F., Suino-Powell K., Xu H. E., Shulman G. I., Kliewer S. A., Mangelsdorf D. J. 2011. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 331: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beuers U. 2013. beta1 integrin is a long-sought sensor for tauroursodeoxycholic acid. Hepatology. 57: 867–869. [DOI] [PubMed] [Google Scholar]
- 40.Gohlke H., Schmitz B., Sommerfeld A., Reinehr R., Haussinger D. 2013. α5 β1-integrins are sensors for tauroursodeoxycholic acid in hepatocytes. Hepatology. 57: 1117–1129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.