Abstract
This study was designed to explore the protective effect of D4F, an apoA-I mimetic peptide, on oxidized LDL (ox-LDL)-induced endoplasmic reticulum (ER) stress-CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) pathway-mediated apoptosis in macrophages. Our results showed that treating apoE knockout mice with D4F decreased the serum ox-LDL level and apoptosis in atherosclerotic lesions with concomitant downregulation of cluster of differentiation 36 (CD36) and inhibition of ER stress. In vitro, D4F inhibited macrophage-derived foam cell formation. Furthermore, like ER stress inhibitor 4-phenylbutyric acid (PBA), D4F inhibited ox-LDL- or tunicamycin (TM, an ER stress inducer)-induced reduction in cell viability and increase in lactate dehydrogenase leakage, caspase-3 activation, and apoptosis. Additionally, like PBA, D4F inhibited ox-LDL- or TM-induced activation of ER stress response as assessed by the reduced nuclear translocation of activating transcription factor 6 and the decreased phosphorylation of protein kinase-like ER kinase and eukaryotic translation initiation factor 2α, as well as the downregulation of glucose-regulated protein 78 and CHOP. Moreover, D4F mitigated ox-LDL uptake by macrophages and CD36 upregulation induced by ox-LDL or TM. These data indicate that D4F can alleviate the formation and apoptosis of macrophage-derived foam cells by suppressing CD36-mediated ox-LDL uptake and subsequent activation of the ER stress-CHOP pathway.
Keywords: apolipoprotein A-I mimetic peptide, oxidized low density lipoprotein, endoplasmic reticulum stress, C/EBP homologous protein, apoptosis, cluster of differentiation 36
Macrophage apoptosis, a prominent feature of atherosclerotic plaques, occurs throughout all stages of atherosclerosis and plays a crucial role in the formation and development of atherosclerotic lesions (1). Macrophage apoptosis in early lesions, coupled with rapid phagocytic clearance of dead cells (efferocytosis), reduces macrophage burden and slows lesion progression. Whereas in late lesions, macrophage apoptosis, accompanied by defective efferocytosis, promotes the enlargement of the lipid core and results in inflammation, necrosis, and even plaque rupture, which are identified as the causative processes in the small percentage of atherosclerotic lesions that cause acute vascular events such as stroke, acute myocardial infarction, and sudden coronary death (2–4). Therefore, it is believed that the suppression of macrophage apoptosis may be a therapeutic implication for combating plaque instability (3, 5).
An intrinsic pathway mediated by endoplasmic reticulum (ER) stress, mainly involving CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP), caspase-12, and c-Jun N-terminal kinase (JNK) signals, has been defined as the underlying mechanism in macrophage apoptosis. The ER stress-mediated apoptotic pathway is activated when macrophages are exposed to atherogenic factors that trigger ER stress, such as oxidized LDL (ox-LDL), oxidized phospholipids, “free” cholesterol, and hyperglycemia (1, 6). CHOP, a specific pro-apoptotic transcription factor under condition of ER stress, has been confirmed to mediate macrophage apoptosis and contribute to the instability of atherosclerotic plaques, whereas the deficiency of CHOP has been demonstrated to protect macrophages from ER stress-mediated apoptosis in vitro and in advanced atherosclerotic plaques of mice (7–9). Our previous studies have demonstrated that ox-LDL can induce macrophage apoptosis by upregulating CHOP expression during the formation of macrophage-derived foam cells, whereas quercetin protects macrophages from ox-LDL-induced apoptosis by inhibiting CHOP expression (10, 11). Thus the CHOP-mediated ER stress pathway may be a new therapeutic target for atherosclerosis (12, 13).
D4F is an apoA-I mimetic peptide that contains four phenylalanine (F) residues and is synthesized from D-amino acids. It does not possess sequence homology to apoA-I, the major lipoprotein component on HDL, but possesses the secondary structural motif of class A amphipathic helices similar to apoA-I (14–16). Several lines of evidence demonstrate that D4F exhibits an anti-atherogenic effect without changing plasma cholesterol levels in dyslipidemic mouse models (17–20). It has been reported that D4F can increase paraoxonase activity in HDL, induce pre-βHDL formation, improve HDL-mediated reverse cholesterol transport and anti-inflammatory properties, and promote endothelial progenitor cell proliferation, migration, and adhesion, which are responsible for its anti-atherogenic role (21–23). In addition, our recent work has shown that D4F is able to inhibit ox-LDL-induced cytotoxicity on human umbilical vein endothelial cells via preventing the downregulation of pigment epithelium-derived factor (24), consistent with the report that small dense HDL3 potently protects endothelial cells from ox-LDL-induced apoptosis and that apoA-I is pivotal to such protection (25). However, little is known of the impact of D4F on macrophage-derived foam cell apoptosis. In the present study, we investigated the effect of D4F on macrophage apoptotic death, with emphasis on its role in downregulating ER stress-CHOP pathway-mediated apoptosis in vitro and in vivo.
MATERIALS AND METHODS
Reagents
Oil red O, tunicamycin (TM), 4-phenylbutyric acid (PBA), and anti-β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO). DMEM and DiI-ox-LDL were from Gibco (Rockville, MD) and Xiesheng Biotech (Beijing, China), respectively. Rabbit antibody against phospho-double-stranded RNA-activated protein kinase-like ER kinase (p-PERK), anti-cluster of differentiation 36 (CD36) monoclonal antibody (mAb) and rat anti-monocyte/macrophage-specific antibody (MOMA-2) antibody were purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibodies against phospho-eukaryotic translation initiation factor 2α (p-eIF2α), activating transcription factor 6 (ATF6), CHOP, and glucose-regulated protein 78 (GRP78) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 594-labeled donkey anti-rabbit and Alexa Fluor 488-labeled donkey anti-rat antibodies were from Molecular Probes (Eugene, OR). SABC-Cy3 immunohistochemistry kits were obtained from Boshide (Wuhan, China). Annexin V-FITC apoptosis detection kits were obtained from KeyGEN Biotech (Nanjing, China). Polyvinylidene difluoride membranes and ECL kits were obtained from Millipore (Bedford, MA) and Thermo Scientific Pierce (Rockford, IL), respectively. The terminal deoxynucleotidyl transferase-mediated (dUTP) nick end-labeling (TUNEL) assay kit (In Situ Cell Death Detection kit, TMR red) and caspase-3 activity assay kit were from Roche (Mannheim, Germany) and Beyotime Biotech (Shanghai, China), respectively. Mouse ox-LDL ELISA kits and tissue/cell total cholesterol (TC) assay kits were purchased from Bio-swamp (Shanghai, China) and Applygen (Beijing, China), respectively. Real-time PCR reagent kits and lactate dehydrogenase (LDH) assay kits were obtained from Tiangen Biological Chemistry (Beijing, China) and Jiancheng Biotech (Nanjing, China), respectively. D4F (Ac-DWFKAFYDKVAE KFKEAF-NH2) and scrambled D4F (sD4F) (Ac-DWFAKDYFKKAFVEEFAK-NH2) were synthesized from SciLight Biotechnology (Beijing, China).
Animal protocol
Seven-week-old male apoE knockout (apoE−/−) mice and C57BL/6J wild-type mice were purchased from Huafukang Bio-Technology Co. (Beijing, China). All animal study protocols were performed in accordance with the national guidelines for the care and use of animals and approved by the laboratory animals’ ethical committee of Taishan Medical University. Sixteen apoE−/− mice were fed a high-fat diet (15.8% fat and 1.25% cholesterol) for 8 weeks, and randomly distributed to receive intraperitoneal injections with either saline (model group, n = 8) or D4F (1 mg/kg per day) (D4F group, n = 8) during the final 6 weeks. Eight male C57BL/6 mice injected intraperitoneally with saline were maintained on a normal chow diet as a control group. The mice were fasted overnight at the end period of the experiment and blood samples were collected from their inner canthus for serum preparation. The mice were euthanized by cervical dislocation and hearts, including the aortic root, were perfused with ice-cold PBS, removed transversely, and fixed in 4% paraformaldehyde. The hearts were then embedded in OCT compound and frozen at −80°C. The samples were serially sectioned at 6 μm and sections were collected on glass slides for the subsequent analyses.
Cell culture
RAW264.7 macrophages were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), maintained in DMEM medium containing 2 mM glutamine, 10% FBS, and antibiotics (100 U/ml penicillin and streptomycin) in a 37°C humidified incubator containing 5% CO2 until subconfluent, and then the medium was replaced with DMEM with 1% FBS.
Isolation and oxidation of LDL
Human LDL was isolated from fresh plasma of healthy donors using sequential ultracentrifugation, and then oxidized with 10 μmol/l CuSO4 for 18 h at 37°C, as described in a previous study from our group (10).
Serum ox-LDL level measurement
Fasting blood samples from apoE−/− and C57BL/6J mice were obtained and serum ox-LDL levels were tested by ELISA assays according to the manufacturer’s guidelines.
Oil red O staining
To assess the atherosclerotic lesions, serial aortic root cryosections were stained with oil red O and counterstained with brilliant green. Atherosclerotic lesions were captured as digital images using a microscope (Olympus, Tokyo, Japan) and total mean lesion area was quantified from five sections for an animal using Image-Pro Plus software (version 6.0; Media Cybernetics, Rockville, MD).
To observe lipid droplets in RAW264.7 macrophages, cells cultured on cover glass were fixed with 4% paraformaldehyde for 20 min, washed with PBS, stained with oil red O (0.5% w/v in 60% isopropanol) for 30 min, and counterstained with hematoxylin for 5 min. The cells were viewed using an Olympus BX51 microscope (Olympus), and the lipid droplet content was analyzed using Image-Pro Plus image analysis software 6.0 and expressed as the average value of the integrated optical density (IOD).
Intracellular TC analysis
The intracellular TC concentration was measured using a tissue/cell TC assay kit according to the manufacturer’s instructions and normalized to the level of total cellular protein (10).
Immunofluorescence assay
For immunofluorescence staining of atherosclerotic lesions, serial aortic root cryosections were blocked with 5% normal donkey serum and incubated with the first primary antibodies overnight at 4°C, and then the sections were incubated with a mixture of Alexa Fluor 594-labeled donkey anti-rabbit and Alexa Fluor 488-labeled donkey anti-rat antibodies for 1 h. 4′,6-Diamidino-2-phenylindole (DAPI) was used for counterstaining. Slides were mounted with antifade reagent and viewed using a fluorescence microscope (Olympus). The mean fluorescence intensity was measured for each corresponding target protein in the macrophage-dense areas using Image-Pro Plus software (26).
To detect nuclear translocation of ATF6, the treated cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked in 10% goat serum, and then incubated with ATF6 antibody (1:200) overnight at 4°C. Following incubation with secondary antibody, the cells were incubated with SABC-Cy3 complexes and DAPI, and then observed using a laser scanning confocal microscope (Bio-Rad Radiance 2100) as described previously (11).
To measure the expression of CD36, the treated cells were rinsed with PBS, fixed with 4% paraformaldehyde, blocked with 10% goat serum, and then incubated with CD36 antibody (1:100) overnight at 4°C. Next, the cells were washed and incubated with FITC-conjugated goat anti-rabbit IgG for 30 min at room temperature. After incubation with DAPI, the cells were observed using a fluorescence microscope (Olympus), and CD36 expression was expressed as the mean fluorescence intensity per cell using Image-Pro Plus software (version 6.0, Media Cybernetics).
Cell viability and LDH assay
The viability of the treated cells was evaluated by a colorimetric assay using 3-(4,5-dimethylthiazol-2-y-l)-2,5-diphenyl-2H-tetrazolium bromide (MTT) as described previously (10) and expressed as the percentage of the optical density of treated cells relative to that of the untreated control cells (100%).
To further measure the level of cell injury, LDH activity in the media was measured using the assay kit according to the manufacturer’s instructions.
Cell apoptosis analysis
Cell apoptosis was assessed by annexin V-FITC/propidium iodide (PI) double-staining assay and TUNEL assay, respectively. In the first assay, cells of each group were harvested, washed twice with ice-cold PBS, resuspended in 500 μl binding buffer containing 5 μl annexin V-FITC and 5 μl PI, and then incubated in the dark at room temperature for 15 min. The apoptosis rates were analyzed using Cell Quest software on a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
For the second method, the treated cells on coverslips or aortic root cryosections were washed twice with PBS, fixed with 4% paraformaldehyde for 30 min, and then permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 3 min. Next, cells were incubated with TUNEL reaction mixture for 1 h at 37°C in darkness and DAPI for 5 min, respectively. After rinsing three times for 5 min in PBS, the samples were evaluated by fluorescence microscopy, and the apoptotic cells were calculated as the percentage of the number of TUNEL-positive cells to total cells.
Measurement of caspase-3 activity
Caspase-3 activity was detected with an assay kit according to the manufacturer’s instructions. Briefly, the treated cells were harvested, rinsed with PBS, resuspended in lysis buffer, and then incubated on ice for 15 min. The lysate was centrifuged at 16,000 g at 4°C for 15 min. Approximately 70 μl of reaction buffer and 10 μl of caspase-3 substrate were mixed with 20 μl lysate supernatant, and then incubated in 96-well microtiter plates at 37°C for 2 h. Caspase-3 activity was detected by an Infinite F200 microplate reader (Tecan, Switzerland) at 405 nm and described as a percentage of the control.
Western blot analysis
Cellular extracts were obtained by lysing the cells in RIPA buffer containing 1% protease inhibitors, and protein content was detected using a bicinchoninic acid assay. Equal amounts of protein were separated on SDS-PAGE by electrophoresis and then transferred onto polyvinylidene difluoride membranes. After blocking in 5% nonfat dry milk, the membranes were incubated with primary antibodies overnight at 4°C, washed with Tris-buffered saline containing 0.1% Tween-20, and then incubated with horseradish peroxidase-conjugated IgG for 1 h at room temperature. The immunoproteins were visualized by ECL detection system, and the intensities were quantified by Image-Pro Plus software (version 6.0, Media Cybernetics) and normalized to β-actin levels.
Quantitative real-time PCR
Total RNA from the treated cells was isolated with Trizol reagent (Invitrogen, Carlsbad, CA), and synthesized to the first-strand cDNA using MuLV reverse transcriptase. Primers used in this study were synthesized by Sangon Biotech (Shanghai, China) and the sequences were as follows: 5′-CCACCACACCTGAAAGCAGAA-3′ (forward primer) and 5′-GGTGCCCCCAATTTCATCT-3′ (reverse primer) for CHOP, 5′-ACATGGACCTGTTCCGCTCTA-3′ (forward primer) and 5′-TGGCTCCTTGCCATTGAAGA-3′ (reverse primer) for GRP78, 5′-CGGGGACCTGACTGACTACC-3′ (forward primer) and 5′-AGGAAGGCT GGAAGAGTGC-3′ (reverse primer) for β-actin. Quantitative real-time PCR was performed with SYBR-green PCR master mix kits on a Rotor-Gene Q real-time PCR cycler (Qiagen, Shanghai, China), analyzed using the Rotor-Gene Q software (version 1.7, Qiagen), and then relative mRNA levels were quantified by the 2–ΔΔCt method as described previously (10).
Uptake of Dil-ox-LDL
Cells were pretreated with D4F (50 mg/l), inactive control peptide sD4F (50 mg/l), or anti-CD36 mAb (2 mg/l) for 1 h, followed by treatment with or without 2 mg/l TM for 4 h, and then incubated with Dil-ox-LDL (50 mg/l) for 4 h. Cells were washed with PBS and lysed with 200 μl lysis buffer. Fluorescence intensity was detected using an Infinite F200 microplate reader (Tecan, Switzerland), and the data were normalized to the protein concentration of each sample, as reported previously (27).
The uptake of Dil-ox-LDL by RAW264.7 cells was further evaluated by fluorescence microscopy. The treated cells were washed with PBS, fixed in 4% paraformaldehyde, and counterstained with DAPI, and the mean fluorescence intensity per cell was calculated using Image-Pro Plus software (Media Cybernetics).
Statistical analysis
Results are expressed as the mean ± SEM. Statistical analysis was performed by one-way ANOVA with Student-Newman-Keuls test for multiple comparisons and Student’s t-test for comparison between two groups using the SPSS13.0 software for Windows. P values less than 0.05 were considered significant.
RESULTS
D4F attenuates serum ox-LDL level and atherosclerotic lesions in apoE−/− mice
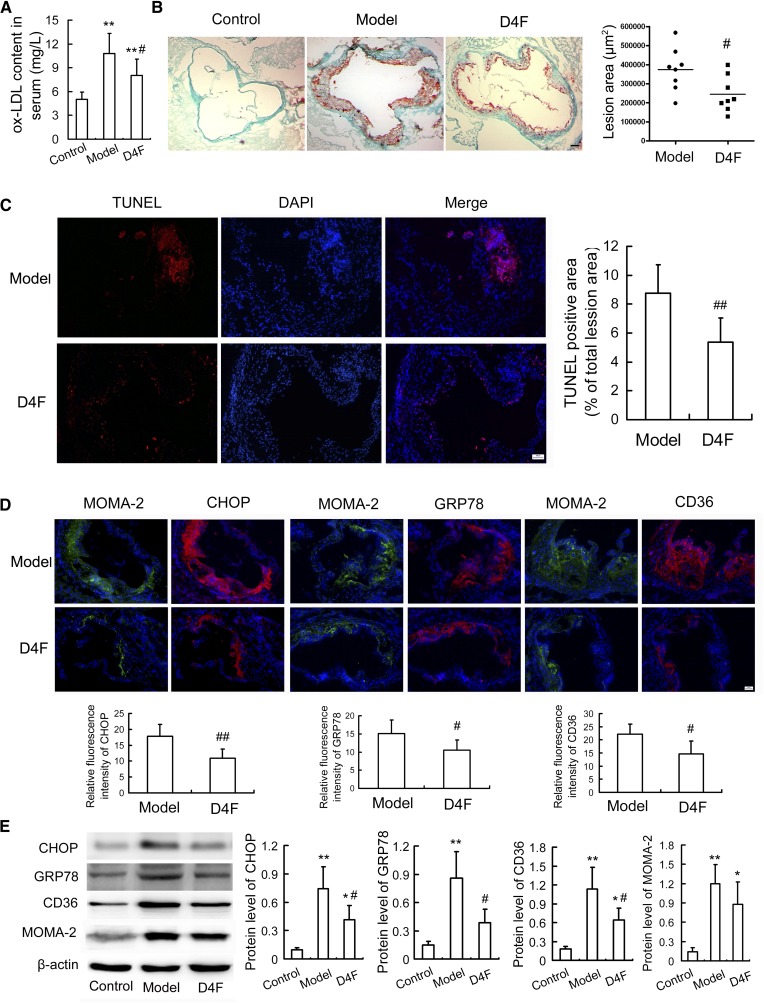
To evaluate the antiatherosclerotic function of D4F in vivo, an experimental atherosclerotic mouse model was established using apoE−/− mice following the method described previously (26). As shown in Fig. 1A, D4F administration for 6 weeks significantly reduced the serum ox-LDL level compared with the vehicle-treated model group, although there were no significant differences in body weight and serum lipids between the D4F and model groups (supplementary Fig. 1A, B). Atherosclerotic plaque formation and apoptosis in the experimental apoE−/− mice were evaluated by oil red O staining and TUNEL assay, respectively. As shown in Fig. 1B, C, D4F treatment remarkably attenuated the plaque area and cell apoptosis in the aortic roots of apoE−/− mice compared with the model group.
Fig. 1.
D4F decreases serum ox-LDL level and attenuates macrophage ER stress and apoptosis in atherosclerotic lesions. Male apoE−/− mice were fed a high-fat diet for 8 weeks, and given saline (model group) or 1 mg/kg of D4F (D4F group) per day by intraperitoneal injection during the final 6 weeks. Male C57BL/6J mice were maintained on a normal chow diet as a control group. A: Serum ox-LDL level determined by ELISA assay (n = 8). B: Atherosclerotic lesion formation stained by oil red O. Scale bar = 100 μm, n = 8. C: Cell apoptosis in atherosclerotic lesions under TUNEL staining. Representative fluorescent images and quantitative data are shown. Red, TUNEL-positive cell; blue, nuclei stained by DAPI. Scale bar = 20 μm, n = 6. D: Immunofluorescent staining with antibodies against MOMA-2, CHOP, GRP78, and CD36. Scale bar = 20 μm, n = 6. Relative fluorescence intensity for the expression of CHOP, GRP78, and CD36 (red) in the macrophage-dense areas (green) of the lesions was calculated. E: Western blot analysis of ER stress markers, CD36 and MOMA-2 in aortic arch (n = 4). Data are presented as the mean ± SEM of at least four independent experiments. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus model group.
D4F reduces the upregulation of ER stress markers and CD36 in apoE−/− mice
To elucidate whether D-4F could mitigate ER stress and CD36 upregulation in vivo, we analyzed the expression of ER stress indicators and CD36 in atherosclerotic lesions of apoE−/− mice. Immunofluorescent and immunohistochemical analysis (Fig. 1D, supplementary Fig. 2) showed a dramatic reduction in the expression of CHOP, GRP78, and CD36 in the macrophage-dense areas of aortic sinuses (shown by staining with monocyte/macrophage-specific antibody, MOMA-2) in the D4F-treated group when compared with vehicle-treated apoE−/− (model) mice. Western blot analysis of the aortic arch (Fig. 1E) also revealed the inhibitory effect of D4F treatment on the upregulation of CHOP, GRP78, and CD36. Given the important roles of the ER stress-CHOP pathway in apoptosis, these findings indicate that the reduction of CHOP and GRP78 may contribute to D4F-attenuated apoptosis in atherosclerotic lesions.
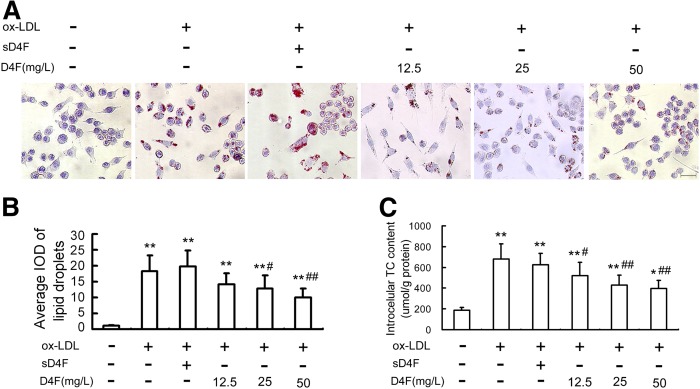
D4F attenuates ox-LDL-induced lipid accumulation in RAW264.7 cells
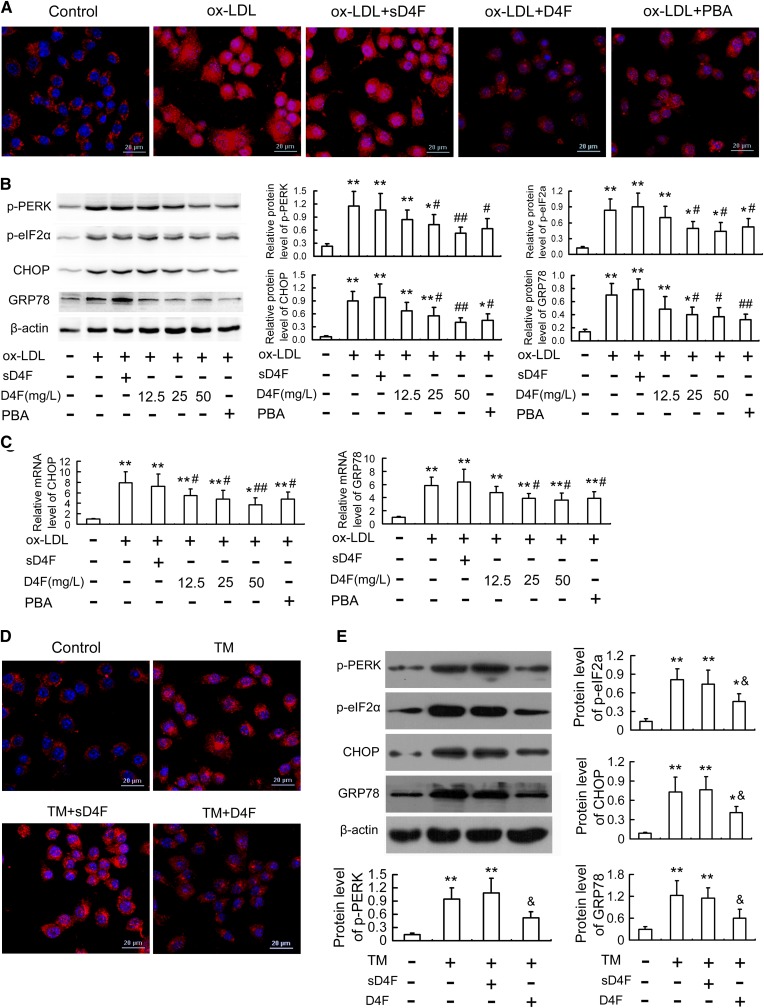
An in vitro ox-LDL-induced macrophage-derived foam cell model was established to further investigate the cytoprotective function of D4F on macrophages. The oil red O staining (Fig. 2A, B) and intracellular TC quantitative assay (Fig. 2C) revealed that pretreatment of cells with D4F (12.5, 25, and 50 mg/l) remarkably inhibited ox-LDL-induced cellular lipid accumulation in a dose-dependent manner.
Fig. 2.
D4F reduces ox-LDL-induced intracellular lipid accumulation in RAW264.7 cells. Cells were pretreated with or without D4F (12.5, 25, and 50 mg/l) or inactive control peptide sD4F (50 mg/l) for 1 h, washed, and then followed by incubation with ox-LDL (100 mg/l) for 24 h. A: The intracellular lipid droplets were stained by oil red O. Representative lipid droplet staining images are shown. Scale bar = 20 μm. B: The average IOD of lipid droplets stained with oil red O from differentiated macrophage foam cells was obtained via checking five fields in each condition. C: The intracellular TC content was detected using a tissue/cell TC assay kit. Data are expressed as the mean ± SEM of at least four independent experiments. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus ox-LDL group.
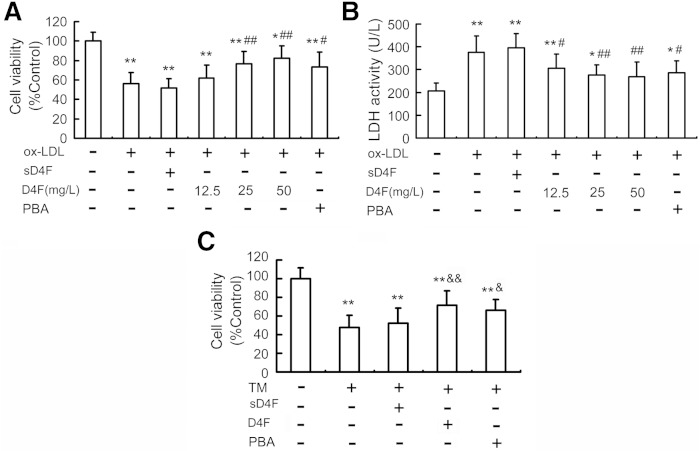
D4F suppresses cytotoxicity in RAW264.7 cells induced by ox-LDL or TM
MTT assay revealed that treatment with D4F at 12.5 up to 50 mg/l for 24 h did not remarkably affect cell viability, indicating that there is no significant cytotoxicity of D4F on RAW264.7 cells (data not shown). Treatment of cells with ox-LDL at 100 mg/l for 24 h led to about a 43.9% decrease in cell viability and a dramatic elevation in LDH leakage in cell media, which were prevented by D4F pretreatment (12.5, 25, and 50 mg/l) in a dose-dependent manner (Fig. 3A, B).
Fig. 3.
Effects of D4F on ox-LDL- or TM-induced cytotoxicity in RAW264.7 cells. RAW264.7 cells were pretreated with or without D4F (12.5, 25, and 50 mg/l), sD4F (50 mg/l), or PBA (5 mmol/l) in DMEM with 1% FBS for 1 h, washed, and then followed by incubation with ox-LDL (100 mg/l) for 24 h. Cell viability (A) and LDH activity in media (B) were measured by MTT assay and a kit, respectively. C: D4F inhibits TM-induced cell death. Cells were pretreated with D4F (50 mg/l), sD4F (50 mg/l), or PBA (5 mmol/l) for 1 h, washed, and then followed by exposure to TM (4 mg/l) for 24 h. Data are expressed as the mean ± SEM of six independent experiments. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus ox-LDL group; &P < 0.05, &&P < 0.01 versus TM group.
Next, we investigated the relative contribution of ER stress-mediated cell death to the protective effect of D4F. PBA, an ER stress inhibitor, and TM, an ER stress inducer, were used to inhibit and induce ER stress, respectively. As shown in Fig. 3A, B, PBA, like D4F, blocked the reduced cell viability and increased LDH leakage induced by ox-LDL. Conversely, TM, like ox-LDL, decreased cell viability by 52.4%, which was also prevented by D4F pretreatment (Fig. 3C). These findings indicate that D4F can reduce ER stress, which may contribute to its inhibitory effect on ox-LDL-induced cytotoxicity.
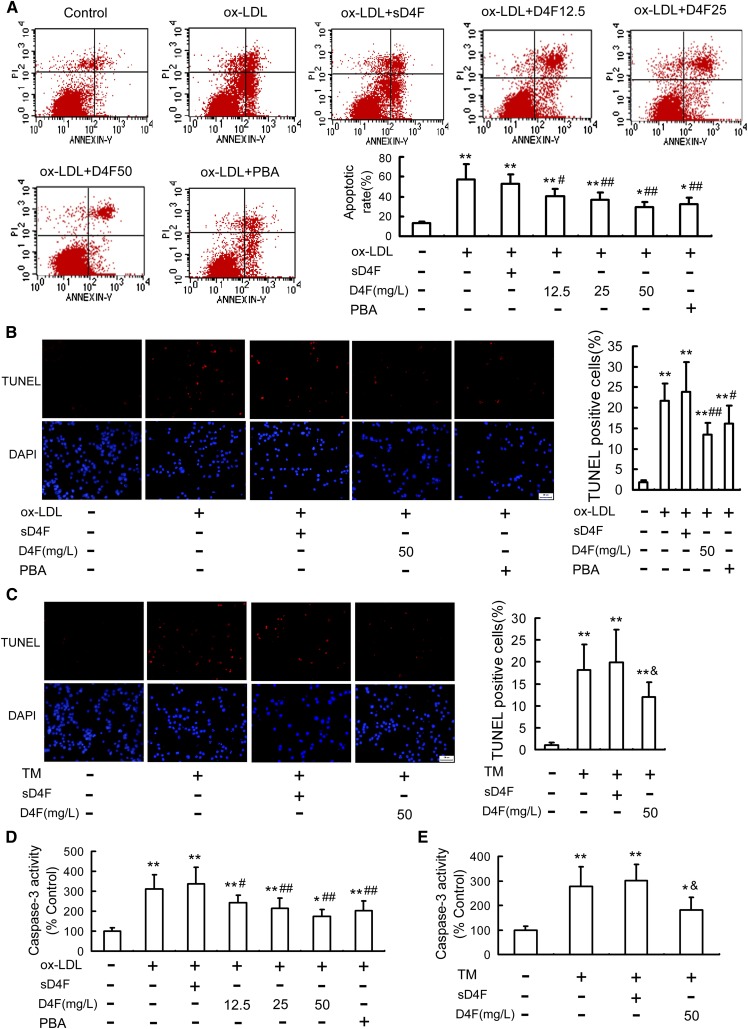
D4F inhibits apoptosis in RAW264.7 cells induced by ox-LDL or TM
To evaluate the anti-apoptotic effects of D4F, the proportion of apoptotic cells was quantified by annexin V-FITC/PI double staining and TUNEL assay. As seen in Fig. 4A, B, ox-LDL treatment for 24 h significantly increased the percentage of apoptotic cells, which were strongly attenuated by the ER stress inhibitor, PBA, and D4F pretreatment in a dose-dependent manner. In accordance with these findings, D4F pretreatment attenuated TM-induced cell apoptosis (Fig. 4C, supplementary Fig. 3). Moreover, the inhibitory effect of D4F on ox-LDL-induced macrophage apoptosis was enhanced by PBA and blocked by TM (supplementary Fig. 4A).
Fig. 4.
Effect of D4F on ox-LDL- or TM-induced apoptosis in RAW264.7 cells. A: RAW264.7 cells were pretreated with or without D4F (12.5, 25, and 50 mg/l), sD4F (50 mg/l), or PBA (5 mmol/l) in DMEM with 1% FBS for 1 h, washed, and then followed by incubation with ox-LDL (100 mg/l) for 24 h. Cell apoptosis was detected using flow cytometry and the total apoptotic cells (early and late-stage apoptosis) were represented by the right side of the panel (annexin V staining alone or together with PI). Cells were pretreated with D4F (50 mg/l), sD4F (50 mg/l), or PBA (5 mmol/l) in DMEM with 1% FBS for 1 h, washed, and then followed by incubation with ox-LDL (100 mg/l) (B) or TM (4 mg/l) (C) treatment for 24 h, and then cell apoptosis was detected by TUNEL assay. Scale bar = 20 μm. D, E: Cells were treated as described in (A) and (C), and then caspase-3 activity was determined by colorimetric assay. Data are expressed as the mean ± SEM of at least four independent experiments. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus ox-LDL group; &P<0.05 versus TM group.
The activity of caspase-3, a marker of apoptosis, was also examined to further assess the anti-apoptotic effect of D4F. As shown in Fig. 4D, E, treatment with ox-LDL or TM led to a remarkable activation of caspase-3, which was significantly inhibited by D4F and PBA. Furthermore, PBA enhanced, while TM blocked, the inhibitory effect of D4F on ox-LDL-induced caspase-3 activation (supplementary Fig. 4B). These data suggested that D4F may inhibit ox-LDL-induced macrophage apoptosis via suppressing the ER stress pathway.
D4F attenuates ER stress response in RAW264.7 cells induced by ox-LDL or TM
Given that D4F downregulated the expression of CHOP and GRP78 in atherosclerotic lesions (Fig. 1) and, like PBA, attenuated ox-LDL- and TM-induced cytotoxicity and apoptosis in RAW264.7 cells (Figs. 3, 4), we hypothesized that the suppression of the ER stress-CHOP pathway may be one of the underlying mechanisms of the anti-apoptotic effects of D4F. To confirm the hypothesis, we evaluated the changes in CHOP and its two important upstream molecules, ATF6 and PERK, in vitro. As shown in Fig. 5A–C, ox-LDL resulted in activation of the ER stress-CHOP pathway as determined by nuclear translocation of ATF6 and phosphorylation of PERK and eIF2α with concomitant upregulation of GRP 78 and CHOP, both at the protein and mRNA levels. However, pretreatment with D4F and PBA significantly inhibited the ox-LDL-induced ER stress response.
Fig. 5.
D4F inhibits ox-LDL- or TM-induced ER stress response in RAW264.7 cells. A: Cells were pretreated with D4F (50 mg/l), sD4F (50 mg/l), or PBA (5 mmol/l) in DMEM with 1% FBS for 1 h, washed, and then followed by incubation with ox-LDL (100 mg/l) for 24 h, and then immunofluorescence experiments showed ATF6 visualized by Cy3 labeling (red) and nuclei stained with DAPI (blue). Representative fluorescent images captured using a laser scanning confocal microscope are shown. Scale bar = 20 μm. B, C: Cells were treated as described in Fig. 4A, and then the protein and mRNA levels of ER stress markers were evaluated by Western blot and quantitative real-time PCR, respectively. D, E: Cells were pretreated with D4F (50 mg/l) or sD4F (50 mg/l) in the presence of TM (4 mg/l) treatment for 24 h, and then ATF6 nuclear translocation and protein levels of ER stress markers were determined by immunofluorescence experiments and Western blot, respectively. Data are expressed as the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus ox-LDL group; &P < 0.05 versus TM group.
To further demonstrate these findings, we investigated the regulatory effects of D4F on TM-induced ER stress response in RAW264.7 cells. As shown in Fig. 5D, E and supplementary Fig. 5, preincubation with D4F inhibited TM-induced activation of the ER stress-CHOP pathway as assessed by the reduced ATF6 nuclear translocation and the decreased phosphorylation of PERK and eIF2α and expression of GRP 78 and CHOP. Additionally, the inhibitory effect of D4F on ox-LDL-induced CHOP upregulation was significantly enhanced by PBA and blocked by TM (supplementary Fig. 4C).
Similarly, we observed that D4F inhibited ox-LDL-induced apoptosis and CHOP upregulation in mouse peritoneal macrophages (supplementary Fig. 6) and human THP-1-derived macrophages (supplementary Fig. 7).
D4F suppresses ox-LDL uptake and CD36 upregulation in RAW264.7 cells
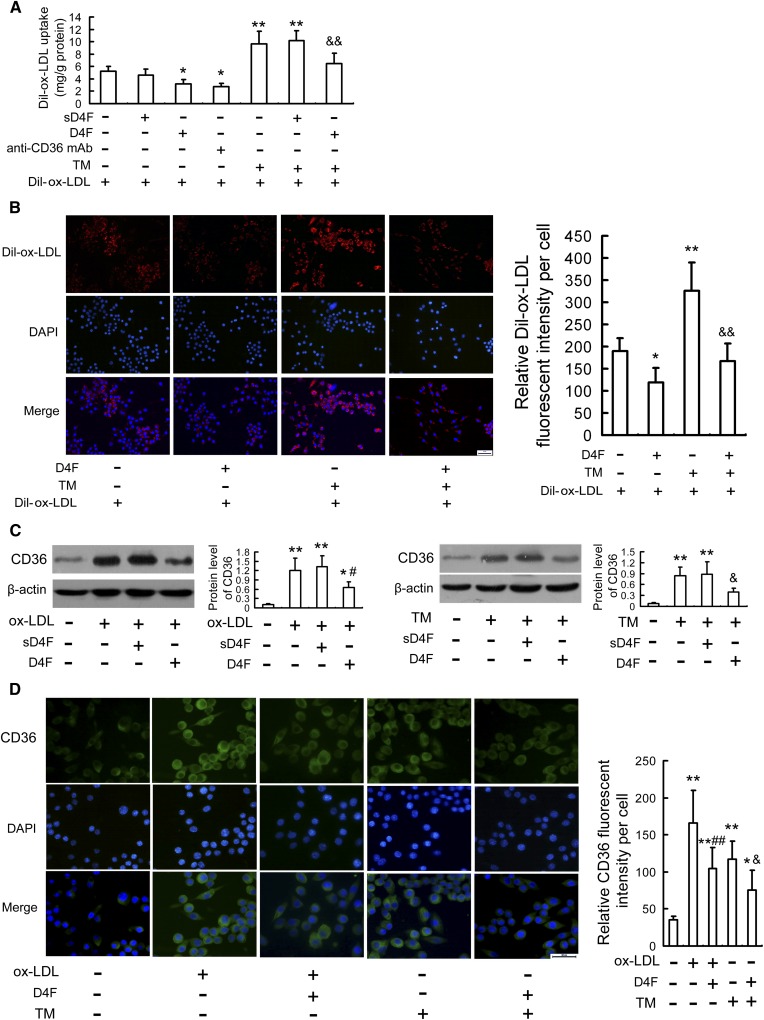
Approximately 60–70% of macrophage-derived foam cell formation is caused by scavenger receptor CD36-mediated ox-LDL uptake, which is a key factor to trigger ER stress response (28, 29), and our recent study has shown that there is a positive feedback between ER stress and CD36 expression (27). Moreover, D4F downregulated CD36 expression in atherosclerotic lesions (Fig. 1) and attenuated ox-LDL-induced lipid accumulation in RAW264.7 cells (Fig. 2). Therefore, we next explored whether the mechanism underlying the inhibitory effect of D4F on the ER stress-CHOP pathway could be through suppression of CD36 expression. As shown in Fig. 6A, B, similar to anti-CD36 mAb, D4F reduced the uptake of Dil-ox-LDL in RAW264.7 cells and also alleviated the enhancement of ox-LDL uptake induced by TM. Additionally, Western blot and immunofluorescence results (Fig. 6C, D) showed that ox-LDL- or TM-induced CD36 upregulation was remarkably attenuated by D4F pretreatment.
Fig. 6.
D4F reduces ox-LDL uptake and CD36 upregulation in RAW264.7 cells. A: Dil-ox-LDL fluorescence intensity in cells preincubated with D4F (50 mg/l), sD4F (50 mg/l), or anti-CD36 mAb (2 mg/l) in DMEM with 1% FBS for 1 h, washed, and followed by treatment with or without 2 mg/l TM for 4 h, and then incubated with Dil-ox-LDL (50 mg/l) for 4 h. B: Fluorescence microscopy shows Dil-ox-LDL uptake by RAW264.7 cells preincubated with D4F (50 mg/l) in DMEM with 1% FBS for 1 h, washed, and followed by treatment with or without 2 mg/l TM for 4 h, and then incubated with Dil-ox-LDL (50 mg/l) for 4 h. Red staining denotes Dil-ox-LDL fluorescence and the blue staining denotes nuclei visualized by DAPI (scale bar = 20 μm). C, D: Protein expression of CD36 in RAW264.7 cells pretreated with D4F (50 mg/l) or sD4F (50 mg/l) for 1 h, washed, and followed by treatment with ox-LDL (100 mg/l) or TM (2 mg/l) for 12 h were evaluated by Western blot and immunofluorescence assay, respectively. Representative fluorescent images are shown. Green staining denotes CD36 visualized by FITC labeling and the blue staining denotes nuclei visualized by DAPI (scale bar = 20 μm). Data are expressed as the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus ox-LDL group; &P < 0.05, &&P < 0.01 versus TM group.
To further confirm these findings, we knocked down the expression of CD36 by siRNA and then explored the inhibitory effects of D4F on ox-LDL-induced apoptosis and CHOP upregulation. As shown in supplementary Fig. 8, CD36 siRNA efficiently reduced the level of CD36. Similar to D4F, CD36 siRNA treatment inhibited ox-LDL-induced macrophage apoptosis, caspase-3 activation, and CHOP upregulation. However, the inhibitory effects of D4F were not enhanced when CD36 was downregulated by siRNA. These data indicate that D4F may suppress the ox-LDL uptake by macrophages through inhibition of CD36 upregulation induced by ox-LDL or TM, subsequently preventing ox-LDL-induced macrophage apoptosis.
DISCUSSION
Macrophage apoptosis has been identified as a prominent feature of advanced atherosclerotic lesions and promotes inflammatory response and enlargement of the necrotic cores, which result in plaque rupture and acute vascular events (1–5). Thus, suppression of macrophage apoptosis may be effective in combating acute atherothrombotic vascular diseases. In the present study, our results indicate for the first time that D4F alleviated the formation and apoptosis of macrophage-derived foam cells by inhibiting CD36-mediated ox-LDL uptake and subsequent activation of the ER stress-CHOP pathway, which was supported by the following observations. First, treating apoE−/− mice with D4F decreased the serum ox-LDL level and suppressed apoptosis in atherosclerotic lesions with concomitant downregulation of CD36 and inhibition of ER stress, as assessed by diminished CHOP and GRP78 expression in macrophage-dense atherosclerotic lesions. Second, D4F, like PBA (an ER stress inhibitor), inhibited macrophage-derived foam cell formation and cell injury and apoptosis induced by ox-LDL or TM (an ER stress inducer). Third, like PBA, D4F suppressed the ER stress-CHOP pathway via inhibiting ATF6 and PERK activation on the ER stress models induced by ox-LDL and TM, respectively. Fourth, the inhibitory effects of D4F on ox-LDL-induced macrophage apoptosis, caspase-3 activation, and CHOP upregulation were promoted by PBA and blocked by TM. Fifth, D4F mitigated ox-LDL uptake by macrophages and inhibited CD36 upregulation induced by ox-LDL or TM, while knockdown of CD36 expression by siRNA did not enhance the inhibitory effects of D4F on ox-LDL-induced macrophage apoptosis, caspase-3 activation, and CHOP upregulation.
It is well-known that prolonged and severe ER stress activates pro-apoptotic signals (6, 26). CHOP, a signaling mediator that plays a major role in ER stress-associated apoptosis, is upregulated in advanced lesions and plays a crucial role in macrophage apoptosis triggered by oxidative stress and a high level of intracellular cholesterol-induced prolonged ER stress (7, 8, 30). CHOP deficiency reduces macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions (9). We have shown that ox-LDL can induce macrophage apoptosis by upregulating CHOP expression during the formation of macrophage-derived foam cells (10, 11). In this study, we observed that D4F, like the ER stress inhibitor, PBA, attenuated ox-LDL- and TM (an ER stress inducer)-induced CHOP upregulation, caspase-3 activation, and macrophage apoptosis in several types of macrophages, including RAW264.7 cells, mouse peritoneal macrophages, and human THP-1-derived macrophages, and the inhibitory effects of D4F on the ox-LDL-induced macrophage changes above were enhanced by PBA and blocked by TM. Furthermore, the apoptosis and expression of CHOP were reduced in atherosclerotic lesions of D4F-treated apoE−/− mice. The translocation of ATF6 to the nucleus and phosphorylation of PERK play critical roles in the ER stress-CHOP pathway (8, 31, 32). Recently, we showed that ATF6 and PERK upregulate CHOP expression and subsequently mediate ox-LDL-induced apoptosis in macrophages (10). Here, we found that similar to PBA, D4F significantly decreased nuclear translocation of ATF6 and phosphorylation of PERK and eIF2α in ox-LDL- and TM-treated macrophages. Taken together, these findings indicate that D4F may suppress the activation of the two crucial upstream signals, nuclear translocation of ATF6 and phosphorylation of PERK, thereby inhibiting the ER stress-CHOP pathway.
How does D4F affect the upstream signals of the ER stress-CHOP pathway? Numerous experimental findings, including our own, have revealed that accumulation of intracellular cholesterol is an important inducer of ER stress and macrophage apoptosis in vivo and in vitro (33–35). CD36, a class B scavenger receptor, plays a quantitatively crucial role in ox-LDL uptake and cholesterol accumulation in macrophages, whereas suppression of CD36 expression is able to significantly decrease the ability of macrophages to accumulate ox-LDL and reduce the development of atherosclerosis, suggesting that it could be an important target for therapeutic treatment (27, 36, 37). Our recent study indicated that CD36-mediated ox-LDL uptake in macrophages triggered ER stress response (ATF6, inositol-requiring kinase/endonuclease-1, and GRP78), which, in turn, played a critical role in CD36 upregulation and then enhanced the foam cell formation by promoting uptake of ox-LDL, indicating that there may be a positive feedback loop between ER stress and CD36 expression (27). In the present work, we indeed observed that D4F reduced serum ox-LDL level and CD36 expression in atherosclerotic lesions in apoE−/− mice. In cultured macrophages, D4F significantly inhibited the upregulation of CD36 and GRP78 induced by ox-LDL or TM, and attenuated ox-LDL uptake, which was similar to anti-CD36 antibody. Furthermore, inhibition of CD36 expression alone also showed similar effects as D4F. However, we did not observe additive inhibitory effects on ox-LDL-induced macrophage apoptosis, caspase-3 activation, and CHOP upregulation when the cells were treated with both D4F and CD36 siRNA, indicating that D4F and CD36 may work in a sequential rather than parallel pathway. Thus, D4F decreases CD36-mediated ox-LDL uptake and reduces intracellular lipid accumulation, which may inhibit nuclear translocation of ATF6 and phosphorylation of PERK and the subsequent CHOP-mediated macrophage apoptosis.
A clinical trial reveals that oral administration of D4F is safe and well-tolerated, and may improve the HDL anti-inflammatory index (38). Thus, the mimetic peptide is increasingly considered as a potential effective treatment for atherosclerosis and related cardiovascular diseases. Our results indicate that D4F can alleviate macrophage-derived foam cell formation and apoptosis by inhibiting CD36-mediated ox-LDL uptake and the subsequent activation of the ER stress-CHOP pathway. However, we have to be aware of the limitations of using in vitro models. Findings obtained from cultured cells in our study may not exactly represent what happens in vivo. For example, despite the fact that Cu2+-ox-LDL is commonly used in macrophage foam cell study (39, 40), it is not the same as the ox-LDL in the circulation. Nevertheless, our findings reveal a potential novel mechanism for the antiatherogenic effects of D4F, which may provide additional support for the future therapeutic use of D4F.
Supplementary Material
Footnotes
Abbreviations:
- apoE−/−
- apoE knockout
- ATF6
- activating transcription factor 6
- CD36
- cluster of differentiation 36
- CHOP
- CCAAT/enhancer-binding protein (C/EBP) homologous protein
- DAPI
- 4′,6-diamidino-2-phenylindole
- eIF2α
- eukaryotic translation initiation factor 2α ER, endoplasmic reticulum
- GRP78
- glucose-regulated protein 78
- IOD
- integrated optical density
- LDH
- lactate dehydrogenase
- mAb
- monoclonal antibody
- MOMA-2
- monocyte/macrophage-specific antibody
- MTT
- 3-(4,5-dimethylthiazol-2-y-l)-2,5-diphenyl-2H-tetrazolium bromide
- ox-LDL
- oxidized LDL
- PBA
- 4-phenylbutyric acid
- p-eIF2α
- phospho-eukaryotic translation initiation factor 2α PERK, double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase
- PI
- propidium iodide
- p-PERK
- phospho-double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase
- sD4F
- scrambled D4F
- TC
- total cholesterol
- TM
- tunicamycin
- TUNEL
- transferase-mediated dUTP nick end-labeling
This research was supported by the Taishan Scholars Foundation of the Shandong Province (zd056, zd057) and the National Natural Science Foundation of China (81370381, 81202949). The authors declare no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of eight figures.
REFERENCES
- 1.Moore K. J., Tabas I. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell. 145: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I. 2010. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabas I. 2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25: 2255–2264. [DOI] [PubMed] [Google Scholar]
- 4.Littlewood T. D., Bennett M. R. 2003. Apoptotic cell death in atherosclerosis. Curr. Opin. Lipidol. 14: 469–475. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari R. L., Singh V., Barthwal M. K. 2008. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 28: 483–544. [DOI] [PubMed] [Google Scholar]
- 6.Scull C. M., Tabas I. 2011. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31: 2792–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X., Wang Y., Zhao W., Zhou H., Yang W., Guan X. 2014. Toll-like receptor 7 promotes the apoptosis of THP-1-derived macrophages through the CHOP-dependent pathway. Int. J. Mol. Med. 34: 886–893. [DOI] [PubMed] [Google Scholar]
- 8.Tsukano H., Gotoh T., Endo M., Miyata K., Tazume H., Kadomatsu T., Yano M., Iwawaki T., Kohno K., Araki K., et al. 2010. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 30: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 9.Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. 2009. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metab. 9: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao S., Zong C., Zhang Y., Sang H., Yang M., Jiao P., Fang Y., Yang N., Song G., Qin S. 2013. Activating transcription factor 6 mediates oxidized LDL-induced cholesterol accumulation and apoptosis in macrophages by up-regulating CHOP expression. J. Atheroscler. Thromb. 20: 94–107. [DOI] [PubMed] [Google Scholar]
- 11.Yao S., Sang H., Song G., Yang N., Liu Q., Zhang Y., Jiao P., Zong C., Qin S. 2012. Quercetin protects macrophages from oxidized low-density lipoprotein-induced apoptosis by inhibiting the endoplasmic reticulum stress-C/EBP homologous protein pathway. Exp. Biol. Med. (Maywood). 237: 822–831. [DOI] [PubMed] [Google Scholar]
- 12.Cao S. S., Kaufman R. J. 2013. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin. Ther. Targets. 17: 437–448. [DOI] [PubMed] [Google Scholar]
- 13.Minamino T., Komuro I., Kitakaze M. 2010. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 107: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 14.Nayyar G., Mishra V. K., Handattu S. P., Palgunachari M. N., Shin R., McPherson D. T., Deivanayagam C. C., Garber D. W., Segrest J. P., Anantharamaiah G. M. 2012. Sidedness of interfacial arginine residues and anti-atherogenicity of apolipoprotein A-I mimetic peptides. J. Lipid Res. 53: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anantharamaiah G. M., Mishra V. K., Garber D. W., Datta G., Handattu S. P., Palgunachari M. N., Chaddha M., Navab M., Reddy S. T., Segrest J. P., et al. 2007. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J. Lipid Res. 48: 1915–1923. [DOI] [PubMed] [Google Scholar]
- 16.Kruger A. L., Peterson S., Turkseven S., Kaminski P. M., Zhang F. F., Quan S., Wolin M. S., Abraham N. G. 2005. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 111: 3126–3134. [DOI] [PubMed] [Google Scholar]
- 17.Leman L. J., Maryanoff B. E., Ghadiri M. R. 2014. Molecules that mimic apolipoprotein A-I: potential agents for treating atherosclerosis. J. Med. Chem. 57: 2169–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgantini C., Imaizumi S., Grijalva V., Navab M., Fogelman A. M., Reddy S. T. 2010. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 59: 3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. 2002. Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 105: 290–292. [DOI] [PubMed] [Google Scholar]
- 20.Ou J., Wang J., Xu H., Ou Z., Sorci-Thomas M. G., Jones D. W., Signorino P., Densmore J. C., Kaul S., Oldham K. T., et al. 2005. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ. Res. 97: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Q., Zhao S. P., Li F. 2010. D-4F, an apolipoprotein A-I mimetic peptide, promotes cholesterol efflux from macrophages via ATP-binding cassette transporter A1. Tohoku J. Exp. Med. 220: 223–228. [DOI] [PubMed] [Google Scholar]
- 22.Troutt J. S., Alborn W. E., Mosior M. K., Dai J., Murphy A. T., Beyer T. P., Zhang Y., Cao G., Konrad R. J. 2008. An apolipoprotein A-I mimetic dose-dependently increases the formation of prebeta1 HDL in human plasma. J. Lipid Res. 49: 581–587. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z., Qun J., Cao C., Wang J., Li W., Wu Y., Du L., Zhao P., Gong K. 2012. Apolipoprotein A-I mimetic peptide D-4F promotes human endothelial progenitor cell proliferation, migration, adhesion though eNOS/NO pathway. Mol. Biol. Rep. 39: 4445–4454. [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Yao S., Wang S., Jiao P., Song G., Yu Y., Zhu P., Qin S. 2014. D-4F, an apolipoprotein A-I mimetic peptide, protects human umbilical vein endothelial cells from oxidized low-density lipoprotein-induced injury by preventing the downregulation of pigment epithelium-derived factor expression. J. Cardiovasc. Pharmacol. 63: 553–561. [DOI] [PubMed] [Google Scholar]
- 25.de Souza J. A., Vindis C., Nègre-Salvayre A., Rye K. A., Couturier M., Therond P., Chantepie S., Salvayre R., Chapman M. J., Kontush A. 2010. Small, dense HDL 3 particles attenuate apoptosis in endothelial cells: pivotal role of apolipoprotein A-I. J. Cell. Mol. Med. 14: 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao S., Miao C., Tian H., Sang H., Yang N., Jiao P., Han J., Zong C., Qin S. 2014. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J. Biol. Chem. 289: 4032–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahaman S. O., Lennon D. J., Febbraio M., Podrez E. A., Hazen S. L., Silverstein R. L. 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seimon T. A., Nadolski M. J., Liao X., Magallon J., Nguyen M., Feric N. T., Koschinsky M. L., Harkewicz R., Witztum J. L., Tsimikas S., et al. 2010. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12: 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., et al. 2007. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 116: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 31.Adachi Y., Yamamoto K., Okada T., Yoshida H., Harada A., Mori K. 2008. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Funct. 33: 75–89. [DOI] [PubMed] [Google Scholar]
- 32.Bromati C. R., Lellis-Santos C., Yamanaka T. S., Nogueira T. C., Leonelli M., Caperuto L. C., Gorjão R., Leite A. R., Anhê G. F., Bordin S. 2011. UPR induces transient burst of apoptosis in islets of early lactating rats through reduced AKT phosphorylation via ATF4/CHOP stimulation of TRB3 expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300: R92–R100. [DOI] [PubMed] [Google Scholar]
- 33.Devries-Seimon T., Li Y., Yao P. M., Stone E., Wang Y., Davis R. J., Flavell R., Tabas I. 2005. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., et al. 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 35.Yao S., Yang N., Song G., Sang H., Tian H., Miao C., Zhang Y., Qin S. 2012. Minimally modified low-density lipoprotein induces macrophage endoplasmic reticulum stress via toll-like receptor 4. Biochim. Biophys. Acta. 1821: 954–963. [DOI] [PubMed] [Google Scholar]
- 36.Kuchibhotla S., Vanegas D., Kennedy D. J., Guy E., Nimako G., Morton R. E., Febbraio M. 2008. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 78: 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Febbraio M., Podrez E. A., Smith J. D., Hajjar D. P., Hazen S. L., Hoff H. F., Sharma K., Silverstein R. L. 2000. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. 2008. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari R. L., Singh V., Singh A., Rana M., Verma A., Kothari N., Kohli M., Bogra J., Dikshit M., Barthwal M. K. 2014. PKCδ-IRAK1 axis regulates oxidized LDL-induced IL-1β production in monocytes. J. Lipid Res. 55: 1226–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boullier A., Li Y., Quehenberger O., Palinski W., Tabas I., Witztum J. L., Miller Y. I. 2006. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler. Thromb. Vasc. Biol. 26: 1169–1176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.