Abstract
Sulfatides are found in brain as components of myelin, oligodendrocytes, and neurons but are also present in various visceral tissues. Metachromatic leukodystrophy (MLD) is an inherited lysosomal storage disorder caused by a deficiency of arylsulfatase A, leading to severe white matter disease due to the accumulation of sulfatides and lysosulfatides. To study the physiological role of sulfatides, accessible and sensitive quantitative methods are required. We developed a sensitive LC/MS/MS method to quantify total sulfatide and lysosulfatide content as well as individual molecular species in urine and plasma from MLD patients and plasma and tissues from an MLD mouse model. Our results demonstrate that the method can quantify a wide range of sulfatide concentrations and can be used to quantify total sulfatide content and levels of individual molecular species of sulfatides in tissues, cells, and body fluids. Even though plasma sulfatides and lysosulfatides would seem attractive candidate biomarkers that could possibly correlate with the severity of MLD and be of use to monitor the effects of therapeutic intervention, our results indicate that it is unlikely that the determination of these storage products in plasma will be useful in this respect.
Keywords: metachromatic leukodystrophy, biomarker, lipidomics
Sulfatides are anionic sulfoglycolipids mainly found in the outer leaflet of the plasma membranes of cells (1, 2). Sulfatides are particularly abundant in the brain, where they constitute an important component of myelin. Oligodendrocytes are responsible for the synthesis of sulfatides and the maintenance of sulfatide levels in myelin (1). However, recent studies in mice indicate that their presence is not restricted to oligodendrocytes and Schwann cells; they are also present in neurons and astrocytes (3). Sulfatides are also components of extraneural tissues, and high concentrations are found in selected organs, including kidney, colon, pancreas, and gall bladder. Increased concentrations of sulfatides have been reported in renal cell carcinoma, adenocarcinoma of colon and lung, and ovarian cancer (2, 4). Sulfatides have been proposed to play a role as ion barriers to osmotic stress and as counterions of ammonium in the renal adaptation to chronic metabolic acidosis (5, 6)
Sulfatides are synthesized from their precursor galactosylceramide in the Golgi apparatus by 3-O-sulfation of the galactose residue by the enzyme 3′-phosphoadenosine-5′-phosphosulfate: cerebroside sulfotransferase (CST) (EC2.8.2.11). Galactosylceramide is synthesized in the endoplasmic reticulum from ceramides and UDP-galactose by the enzyme UDP-galactose:ceramide galactosyltransferase (CGT) (EC 2.4.1.45) and is then transported to the Golgi apparatus prior to sulfation to sulfatides. Sulfatides consist of many molecular species, with structures differing in acyl chain length and hydroxylation and sphingoid base. These sulfatide molecular species are cell and tissue specific, which may be explained by the cell- and tissue-specific expression of ceramide synthases and fatty acid 2-hydroxylase (1, 7). It is difficult to assign specific functions to the different molecular species of sulfatides, but sulfatides with C16:0 fatty acids were reported to be involved in the regulation of insulin secretion in rat pancreatic β-cells (8), and elevated levels of C18:0 sulfatides have been implicated as a cause of audiogenic seizers in mice overexpressing CGT (9). There is also heterogeneity of the sphingoid base composition of sulfatides. Most of the sulfatides contain 4-sphingenine (sphingosine, d18:1), but the presence of sphingadienine (d18:2) has been reported in mammalian brain (10).
Degradation of sulfatides takes place in the lysosomes and is catalyzed by the lysosomal enzyme arylsulfatase A (ASA) (EC 3.1.6.8). An accessory protein saposin B, a so-called “sphingolipid activator protein,” is required for the lysosomal degradation of sulfatides by ASA. A deficiency of ASA or of saposin B leads to intralysosomal storage of sulfatides and is the cause of the lysosomal storage disease metachromatic leukodystrophy (MLD) (11). Lysosulfatide, the deacylated form of sulfatides, also accumulates in tissues of patients with MLD (12). The accumulation of sulfatides in kidney leads to an increased excretion of sulfatides in urine. Determination of sulfatides in urine is a convenient diagnostic tool to confirm MLD in patients with arylsulfatase A deficiency or saposin B deficiency (13–15). In addition to mutation analysis of the ASA gene, it is also used to confirm a pseudodeficiency of arylsulfatase A. Severe demyelination is an important feature of MLD in humans (11). A mouse model of arylsulfatase A deficiency was created by targeted disruption of the ASA gene (16). This model was extensively characterized and exhibits many features of human MLD, including storage of sulfatides and lysosulfatide (3, 17). However, in contrast to the human disease, this mouse model of MLD does not show demyelination (3, 16). Demyelination could be induced in ASA knockout mice overexpressing CGT, presumably by increasing the substrate load in the lysosomes that lack the ability to degrade sulfatides (18).
Several methods have been used to quantify sulfatides, including TLC (19), HPLC (14, 20), and, more recently, MS (4, 13, 15, 21–25). Here we describe a LC/MS/MS method to quantify sulfatides and their molecular species over a wide range of concentrations in normal and diseased tissues, plasma, and urine. In addition, we describe a sensitive and facile LC/MS/MS method for the quantification of lysosulfatides in the same tissues.
EXPERIMENTAL PROCEDURES
Materials
LC/MS–grade methanol, water, and HPLC-grade chloroform were purchased from Biosolve; ammonium formate LC/MS grade from Sigma-Aldrich; ammonium hydroxide and ammonia solution (25%) from Merck; and silica Gel (SiOH) for SPE columns from Bakerbond.
Lipid standards
Lysosulfatide, N-acetyl-sulfatide, and N-octadecanoyl-D3-sulfatide were obtained from Matreya LLC. Bovine brain sulfatides were from Sigma-Aldrich.
Animals
Twenty-month-old wild-type (+/+) C57BL/6J and ASA knockout (−/−) mice carrying the human ASA-C96S transgene to make them immune-tolerant to human arylsulfatase A were used in this study (26).
Patient and control samples
Urine and plasma samples were obtained from patients with MLD during diagnostic work-up or follow-up. Informed consent was obtained from all patients or their guardians. Approval of the institutional medical ethical committee was not required because of the observational nature of the study. The diagnosis of MLD had been confirmed by demonstrating a deficiency of arylsulfatase A in leukocytes and mutations in the ASA gene. Sulfatide concentrations of anonymous control samples (male and female subjects) from individuals 0–65 years of age without any known metabolic disease were measured to establish a reference range. Creatinine in urine was measured by the Jaffe reaction on the Cobas P800 (Roche Diagnostics).
Extraction of sulfatides from human urine
Sulfatides were extracted from urine by a modification of the method of Folch (27). Briefly, to 0.5 ml of urine in a 5 ml glass tube, 25µl of internal standard N-Octadecanoyl-D3-sulfatide (10 nmol/ml in chloroform-methanol, 1:2, v/v) was added (final concentration: 0.5 nmol/ml urine), and the solution was stirred briefly. Then, 2 ml chloroform-methanol (2:1) was added. The solution was stirred briefly and centrifuged for 5 min at 3,220 g. The lower phase was transferred to a clean glass tube. The upper phase was re-extracted by addition of 1.3 ml chloroform and pooled with the first lower phase. Recovery of the sulfatides in the combined lower phases was >95%. The combined lower phases were dried under a gentle stream of nitrogen at 35°C, and the residue was dissolved in 500 µl chloroform:methanol (98:2). This total lipid extract was subsequently fractionated on Bakerbond Silica Gel (SiOH) SPE columns using vacuum-assisted flow (1 ml, 100 mg per column). The columns were conditioned by consecutive washes with 2 ml methanol, 2 ml chloroform-methanol (1:1, v/v), and 2 ml chloroform. Samples were loaded onto the column, and the sample tube was rinsed with 200 µl chloroform-methanol (98:2, v/v), which was also loaded onto the column. Lipids were fractionated by a modified procedure (28) by eluting the column with 2 ml of chloroform-methanol (98:2, v/v, fraction 1) and 1 ml acetone-methanol (90:10, v/v) (fraction 2). The recovery of sulfatides in fraction 2 with this method is >90%. The phospholipids that remain bound to the column can be eluted with 100% methanol (fraction 3). The fractionation on SPE serves as a clean-up step of the urinary extract to reduce matrix effects and to reduce contamination of the ultra-high pressure liquid chromatography (UPLC)-column and the mass spectrometer.
Preparation of tissue homogenates
Tissue homogenates (brain, liver, and kidney) were prepared in water (one part tissue, three parts water v/v). The tissues were cut into small pieces with scissors prior to sonication (3 × 5 s) on melting ice. After vortexing (1 min), the homogenate was sonicated again (2 × 5 s). The homogenates from brain and kidney from ASA knockout mice and brain from wild-type mice were diluted 10-fold in water prior to extraction.
Extraction of sulfatides from plasma and tissue homogenates
For extraction, 50 µl of plasma, 25 µl of brain, 25 µl of kidney, and 100 µl of liver homogenates were used for extraction. N-octadecanoyl-D3-sulfatide was added as the internal standard at 4 nmol/ml for mouse plasma; 2 nmol/ml for human plasma; 200 nmol/ml for the brain homogenates; 200 nmol/ml and 120 nmol/ml for the kidney homogenates of ASA knockout and wild-type mice, respectively; and 5 nmol/ml and 3 nmol/ml for liver homogenates from ASA knockout and wild-type mice, respectively. Plasma and tissues were extracted by a modification of the method of Folch (27) as described here for a 50 µl sample. The sample was pipetted into a 5 ml glass tube, 1 ml methanol was added, and the solution was stirred briefly. Subsequently, 1 ml of chloroform was added and stirred for 1 min. The sample was incubated 30 min at room temperature and stirred occasionally and then centrifuged 10 min at 3,220 g to remove protein. The supernatant was transferred to a glass tube to which 1 ml chloroform and 0.75 ml water were added, mixed, and centrifuged for 2 min at 3,220 g. The lower phase was transferred to a glass tube, and the upper phase was re-extracted with 1.8 ml of chloroform, which was pooled with the first lower phase. The combined lower phases were dried, redissolved, and fractioned by SPE as described above for the urine samples. Fractionation has a strong influence on the signal intensity of all molecular species of sulfatides in plasma, which increased by a factor of 2 to 3 as a result of diminished ion suppression. An example is given in supplementary Table 4, where we discuss the precursor spectra of m/z 96.9 in plasma of a MLD patient with and without fractionation of the lipid extract on SPE. In addition, the removal of phospholipids decreases the contribution precursors of m/z 96.9 due to HPO3− ions. This leads to an increase in the reliability of the annotation of the sulfatide species.
Extraction of lysosulfatide from plasma and tissue
Lysosulfatide was extracted from plasma and mouse tissue homogenates (same sample volumes as described for sulfatide) by a modification of the method of Bligh and Dyer (29). Briefly, a 50 µl sample was pipetted in an Eppendorf tube, internal standard (N-acetyl-sulfatide, 0.1 nmol/ml in methanol) (30) was added, and the solution was mixed briefly. Subsequently, 25 µl water, 300 µl methanol, and 150 µl chloroform were added, with 1 min of mixing after each addition. The samples were left for 30 min at room temperature. After centrifugation for 10 min at 15,700 g to spin down precipitated protein, the supernatants were transferred to Eppendorf tubes, and 150 µl chloroform and 225 µl water were added. After mixing, the tubes were centrifuged for 2 min at 15,700 g to separate the phases. From the upper phase, 400 µl was transferred to a 2 ml tube. The lower phase was re-extracted by the addition of 300 µl methanol and 300 µl water, which was pooled with the first upper phase. The combined upper phases were dried at 35°C under a nitrogen stream. For tissues, at this point a further sample clean-up may be obtained by dissolving the sample in 800 µl butanol saturated water and extracting the solution with 800 µl of water-saturated butanol. The samples are centrifuged for 5 min at 15,700 g, and the upper butanol phase is removed. The lysosulfatides and the internal standard are quantitatively recovered in the butanol phase. The residue was dissolved in 100 µl methanol, stirred for 1 min, and sonicated for 30 s in a bath sonifier. The samples were centrifuged for 10 min at 15,700 g in the Eppendorf centrifuge to precipitate any insoluble material. The recoveries of lysosulfatide and N-acetylsulfatide were >95%.
Sample preparation for mass spectrometry
For the sulfatides, Fraction 2 from the SPE column was dried under a nitrogen stream at 35°C and dissolved in 100 µl methanol prior to LC/MS/MS analysis. For lysosulfatide, dried upper phases were redissolved in 100 µl methanol, vortexed for 1 min, and sonicated for 30 s in a bath sonifier. The samples were centrifuged for 10 min at 15,700 g to precipitate any insoluble material prior to LC/MS/MS analysis. For direct infusion measurements, dried upper or lower phases were redissolved in 500 µl 1% ammonium hydroxide in chloroform-methanol (1:2, v/v) and centrifuged for 1 min at 15,700 g to remove insoluble material.
Instrumentation and data analysis
An Acquity UPLC (Waters) coupled to a tandem quadrupole mass spectrometer (Waters) was used for all analyses. Data were analyzed with Masslynx Software (Waters), for the identification and quantification of sulfatides in direct infusion experiments we used the Lipid Mass Spectrum Analysis (LIMSA) program (31).
Measurement of sulfatides and lysosulfatides by MS/MS
The instrument settings for the detection of sulfatides and lysosulfatides were optimized by measuring pure standards (lysosulfatide, N-acetyl-sulfatide, and N-octadecanoyl-D3-sulfatide) by direct infusion into the mass spectrometer. Optimal conditions for collision-induced dissociation were established for transitions m/z 888.7 > 96.9 and 890.7 > 96.9 for sulfatides and m/z 540.4 > 96.9 for lysosulfatide and are given in supplementary Tables 1 and 2. Major molecular species of sulfatides were identified by precursor ion scanning of 96.9 of direct infusion of extracted urine from MLD patients (not shown) or mouse brain and kidney (supplementary Fig. 1). Precursor masses were annotated with LIMSA (31). These m/z values were used to set up transitions to monitor during LC/MS/MS experiments (supplementary Table 3).
Separation of sulfatides by reversed-phase UPLC
Sulfatides were separated on a BEH C18 reversed-phase column (2.1 × 50 mm, particle size 1.7 µm; Waters) by applying a gradient from methanol-water (37:63 v/v), with 5 mM ammonium formate (Eluent A) to methanol and 5 mM ammonium formate (Eluent B) at 0.350 ml/min. During the 11.5 min program, a linear gradient was applied from 0% to 100% eluent B in 2.5 min and kept at 100% B until 10 min before going back to 0% B at 11 min. The eluent was diverted to waste between 0.00 and 2.30 min and after 11.05 min to keep the mass spectrometer free of contaminants. Mass spectrometric data were collected between 2.30 and 11.00 min. The retention times (RTs) of the individual molecular species are shown in supplementary Table 3. Sulfatides elute in order of decreasing polarity (i.e., molecular species were separated according to chain length of the fatty acids, with molecular species with the shortest chain length eluting first). For each carbon chain length of the fatty acid, the molecular species of the sulfatides eluted in the order: monounsaturated fatty acids containing molecular species, then hydroxy fatty acid containing molecular species, and finally the saturated fatty acid containing molecular species.
Separation of lysosulfatides by reversed phase UPLC
Lysosulfatides were separated using the same column and mobile phases as for the sulfatides. A linear gradient was applied during a 5.50 min program from 0% to 100% B in 2.50 min and kept at 100% B until 4.00 min before going back to 0% B at 5.00 min. The flow rate was 0.250 ml/min. The eluent was diverted to waste between 0.00 and 2.30 min and after 4.05 min to keep the source free of contaminants. Data were collected between 2.30 and 4.05 min. The RTs of the lysosulfatides and the internal standard are shown in supplementary Table 3.
RESULTS
Quantification of total sulfatides in human urine and plasma
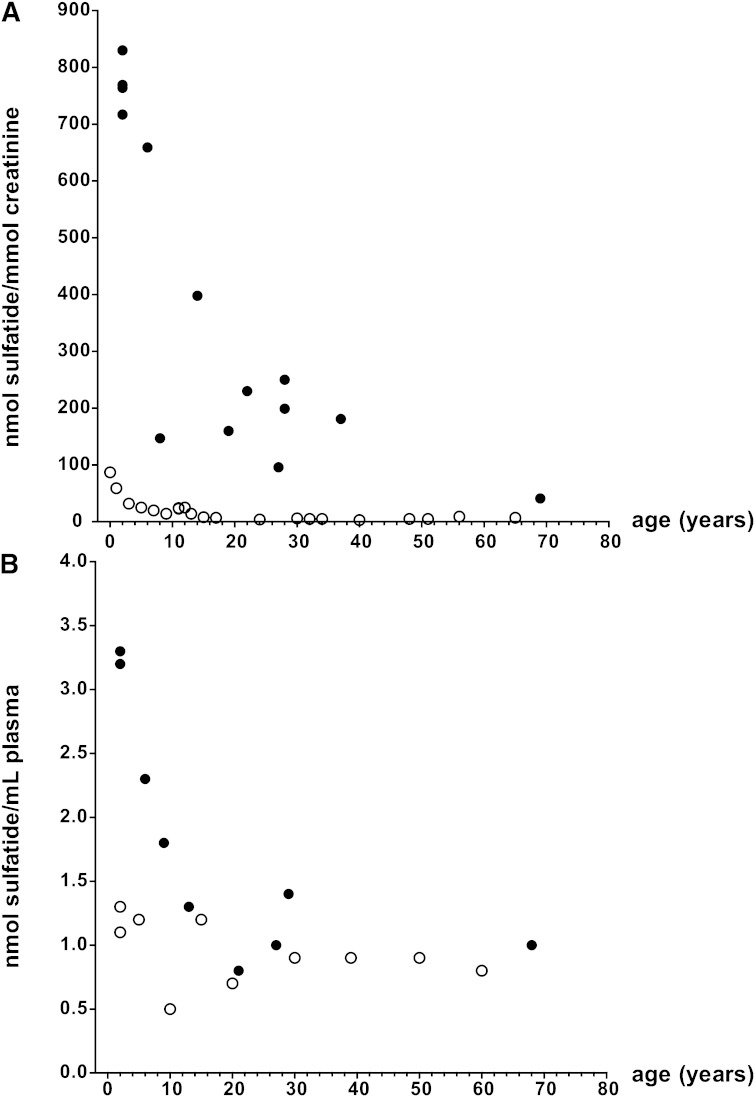
Control urine was spiked with sulfatides from bovine brain (concentrations of 0, 0.1, 0.2, 0.4, 1, 2, and 5 µM), internal standard was added, and samples were extracted. The ratio, the sum of the area from transitions of sulfatides over the area from the transition of N-octadecanoyl-D3-sulfatide, was plotted against the concentration of sulfatides spiked in the samples. A linear response was obtained over the entire concentration range (y = 2.518x; r2 = 0.995). With this calibration curve, the concentrations of sulfatides (expressed as nmol sulfatide/mmol creatinine) of four patients with the late infantile form of MLD, six patients with the juvenile form, and eight patients with the adult form of MLD (11) were determined and compared with the sulfatide concentration in control urines of individuals of 0–65 years of age. MLD patients show approximately 10-fold increased concentrations of sulfatides (Fig. 1A). The limit of quantification is 10 pmol/ml (S/N ratio, >10). The within-run coefficient of variation (five duplicate determinations of the same sample) was 5.1%, and the between-run coefficient of variation (10 consecutive measurements of the same sample) was 13.5% (average value: 779 nmol sulfatides/mmol creatinine; SD: 105; n = 10).
Fig. 1.
A: Urinary sulfatide content in MLD patients. Sulfatide concentration is shown for MLD patients (black circles) and age-matched controls (open circles) as a function of age. B: Plasma sulfatide content in MLD patients. Sulfatide concentration is shown for MLD patients (black circles) and age-matched controls (open circles) as a function of age.
Human control plasma was spiked with sulfatides from bovine brain (concentrations of 0, 0.5, 1.0, 2.0, and 5 µM). A linear response was obtained over the entire concentration range (y = 0.7665; r2 = 0.997) after subtraction of sulfatides from the blank plasma. .
In contrast to urine, no consistent increases in human MLD plasma sulfatides were observed as compared with controls (Fig. 1B). The range of sulfatide concentrations in human control plasma was 0.5–1.3 µM (n = 10). The concentrations of sulfatide of early-onset patients (two late infantile and two juvenile patients) were above the control range, whereas that of the five late onset patients fell within the control range. The range of sulfatide concentrations in plasma of all MLD patients tested was 0.8–3.3 µM (n = 9). The limit of quantification is 8 pmol/ml (S/N ratio, >10).
Quantification of total sulfatides in murine tissues and plasma
Tissue and plasma sulfatides were quantified based on a single point calibration using the sum of the area from 10 major molecular species relative to that of the internal standard N-octadecanoyl-D3-sulfatide. Table 1 shows the results of sulfatide analysis of selected tissues and plasma from two normal 20 month old wild-type mice (+/+, C57BL/6J) and ASA knockout mice (−/− carrying the human ASA-C96S) (26). Strongly increased levels of sulfatides were found in brain (∼2.6-fold), kidney (∼84-fold), and liver (∼19-fold) from the ASA knockout mice. These results show the ability of the method to quantify sulfatides over a wide range of concentrations in tissue (at least 7,000-fold difference in concentration of control liver vs. ASA knockout brain). The levels of sulfatides in the ASA knockout mice were increased 3-fold in plasma.
TABLE 1.
Sulfatides in mouse tissues and plasma
| Genotype | Brain | Kidney | Liver | Plasma |
| nmol/mg protein | nmol/mg protein | nmol/mg protein | pmol/ml | |
| ASA−/− | 205 | 209 | 0.84 | 780 |
| ASA−/− | 203 | 246 | 0.30 | 724 |
| WT | 76 | 3.5 | 0.02 | 247 |
| WT | 83 | 1.9 | 0.04 | 193 |
ASA, arylsulfatase A; WT, wild type.
Measurement of different molecular sulfatide species in fluids and tissues from human and murine origin
The use of LC/MS/MS allows quantification of individual molecular species of sulfatides. Table 2 compares the percentage composition of the molecular species of sulfatides in brain, kidney, and liver from control mice (C57BL/6J) and ASA knockout mice (−/−). A characteristic set of 10 major sulfatide species was found for each organ, mainly consisting of long- and very-long-chain saturated, mono-unsaturated, and hydroxyl fatty acids. Although no single species was unique for a tissue, the relative composition is tissue specific. An exception is C20:0-OH sulfatide (m/z 850.7), which was found to be a major species unique for kidney (15.5% in ASA knockout vs. 9.5% in C57BL/6J control mice). The transition m/z 850.7 > 96.9 was not routinely included in the set of transitions monitored for the quantification of total sulfatides in tissues (Table 2), leading to a slight underestimation of the sulfatide content in kidney. Only small quantitative differences in the percentage composition are observed between the molecular species in tissues from control mice and ASA knockout mice (−/−). An exception was the C16:0 species of sulfatides, which were much higher in plasma from ASA knockout mice (−/−) compared with wild-type plasma. Table 3 shows the percentage composition of the 13 major molecular species of sulfatide in human plasma and urine. The C16:0, C16:0-OH, and C24:1 molecular species were higher in MLD plasma compared with human control plasma, whereas hydroxy fatty acids containing sulfatides were generally lower in MLD plasma. In addition to the molecular species shown in Table 3, we observed in human plasma and urine a sulfatide species with transition 892.7 > 96.9 and a RT of 4.80 min. This species could not be identified with certainty but fits best with a C23:0-OH sulfatide species. The percentage of this sulfatide species was 5.7% in control plasma, 4.0% in MLD plasma, 9.8% in control urine, and 7.3% in MLD urine.
TABLE 2.
Sulfatide compositions in mouse tissue and plasma
| Molecular Species | Brain | Kidney | Liver | Plasma | ||||
| ASA−/−−/− | WT | ASA−/−−/− | WT | ASA−/−−/− | WT | ASA−/−−/− | WT | |
| % | % | % | % | % | % | % | % | |
| C16:0 | 0.3 | 0.1 | 4.8 | 3.4 | 5.8 | 1.0 | 20.1 | 5.0 |
| C18:0 | 5.2 | 1.3 | 0.5 | 0.4 | 1.8 | 1.1 | 3.3 | 3.5 |
| C20:0 | 2.3 | 0.9 | 3.2 | 2.8 | 3.9 | 1.7 | 1.9 | 1.9 |
| C22:0 | 4.1 | 3.3 | 8.9 | 10.9 | 14.3 | 6.7 | 4.5 | 8.0 |
| C22:0-OH | 8.4 | 11.2 | 35.6 | 32.0 | 10.5 | 13.2 | 14.4 | 19.0 |
| C24:1 | 42.6 | 44.6 | 7.0 | 11.0 | 24.8 | 41.8 | 31.4 | 33.0 |
| C24:0 | 16.0 | 11.2 | 11.1 | 14.5 | 18.0 | 11.7 | 8.8 | 8.7 |
| C24:1-OH | 7.2 | 14.0 | 2.5 | 2.9 | 7.9 | 10.0 | 5.0 | 10.1 |
| C24:0-OH | 13.4 | 12.9 | 26.5 | 22.2 | 12.9 | 12.6 | 10.8 | 11.9 |
| C26:1 | 0.8 | 0.6 | 0.1 | 0.2 | 0.3 | 0.5 | 0.0 | 0.0 |
ASA, arylsulfatase A; WT, wild type. Data are the average composition from ASA−/− (n = 2) or WT (n = 2) mice.
TABLE 3.
Sulfatides composition human plasma and urine
| Molecular Species | Plasma | Urine | ||
| MLD | CTRL | MLD | CTRL | |
| % | % | % | % | |
| C16:0 | 16.7 | 15.5 | 3.9 | 2.8 |
| C16:0-OH | 30.6 | 20.9 | 2.3 | 1.8 |
| C18:0 | 1.9 | 1.0 | 0.9 | 1.1 |
| C18:0-OH | 1.1 | 1.0 | 1.2 | 0.3 |
| C20:0 | 1.1 | 1.1 | 3.3 | 2.3 |
| C20:0-OH | 1.4 | 1.5 | 3.0 | 1.4 |
| C22:1 | nd | nd | nd | nd |
| C22:0 | 5.4 | 7.7 | 18.8 | 20.6 |
| C22:0-OH | 5.9 | 9.0 | 12.4 | 11.5 |
| C24:0 | 4.9 | 5.7 | 14.5 | 19.9 |
| C24:1 | 15.4 | 13.5 | 5.9 | 5.4 |
| C24:1-OH | 8.8 | 13.3 | 11.3 | 5.0 |
| C24:0-OH | 6.4 | 10.1 | 22.3 | 26.8 |
| C26:1 | 0.6 | 0.3 | 0.2 | 1.1 |
CTRL, control; MLD, metachromatic leukodystrophy; nd, not determined. Data are the average sulfatide composition in plasma or urine from MLD patients (n = 9 or n = 5) and control subjects (n = 10 or n = 5).
Quantification of lysosulfatide in mouse tissues and plasma and human plasma
Mouse tissue and plasma lysosulfatide were quantified based on a single point calibration relative to the area of the internal standard N-acetyl-sulfatide added (Table 4). The method has sufficient sensitivity to reliably quantify lysosulfatide in normal mouse brain. The lysosulfatide levels in brain of the ASA knockout mice are 76 times higher than in the C57BL/6J control mice. Strongly increased levels of lysosulfatide were also observed in kidney and liver of ASA knockout mice, but, although lysosulfatide could be detected in the normal control kidney and liver, their levels were below the limit of quantification. In addition to lysosulfatide with a sphingosine base (4-sphingenine, d18:1, RT 3.29 min, m/z 540.4), we consistently observed a lysosulfatide with a molecular mass 2 m/z smaller and a RT of 3.10 min. This suggests the presence of a lysosulfatide with a sphingadienine (d18:2) base. This d18:2 species was present at 20% of the d18:1 lysosulfatide signal intensity in brain and kidney and in >30% in liver of ASA knockout mice. Lysosulfatide could not be reliably quantified in plasma from normal and ASA knockout mice or in plasma from MLD patients and healthy volunteers because its concentration was below the limit of quantification.
TABLE 4.
Lyso-sulfatides in mouse tissues
| Genotype | Brain | Kidney | Liver | |||
| Lysosulfatide | Lyso-ene-sulfatide | Lysosulfatide | Lyso-ene- sulfatide | Lysosulfatide | Lyso-ene-sulfatide | |
| pmol/mg protein | ||||||
| ASA−/− | 228 | 46 | 32 | 6 | 1.06 | 0.68 |
| ASA−/− | 248 | 48 | 13 | 3 | 0.64 | 0.22 |
| WT | 2.9 | nd | tracea | nd | trace | nd |
| WT | 3.3 | nd | trace | nd | trace | nd |
ASA, arylsulfatase A; nd, not determined; WT, wild type.
Discernable signal but below limit of quantitation.
DISCUSSION
We developed an LC/MS/MS method for identification and quantitation of sulfatides and lysosulfatides in different biological materials. For sulfatide determination, we used fractionation on SPE prior to analysis by LC/MS/MS rather than direct analysis of the total lipid extract. This fractionation serves three purposes: i) clean-up of urine samples and removal of excess neutral lipids and phospholipids especially from plasma samples in order to protect the analytical column and the mass spectrometer against contamination, ii) removal of phospholipids (specifically phosphoinositides) that may interfere with the precursor ion scan of precursors of m/z 96.9 (4, 7), and iii) reduction of matrix effects and ion suppression in samples containing small amounts of sulfatides in the presence of large amounts of neutral and phopholipids like plasma. Overall, fractionation on SPE led to better performance on LC/MS/MS and allowed for a much more sensitive detection of sulfatides in tissues, cells (not shown), and body fluids that contain low amounts of sulfatides. This enables measurement of sulfatides in different biological matrices over a wide range of concentrations. MLD mice models and human patients can easily be distinguished from controls. In contrast to recently published methods on the quantification of sulfatides in dried blood spots and urine (24, 25) from MLD patients and normal controls, our method could easily quantify sulfatides in normal plasma and urine with a signal to noise ratio of >10 for all peaks quantified. Because of its sensitivity, only 0.5 ml of a representative urine sample suffices for the analysis of MLD and normal urines instead of the large volumes or even 24 h urine collections, which are needed when traditional methods like TLC or HPLC are used (14, 19, 32).
An additional advantage of the fractionation on SPE is that separate analyses of ceramides and neutral lipids in fraction 1, sulfatides and neutral glycolipids in fraction 2, and phospholipids in fraction 3 in tissue samples are possible. The chromatographic separation allows fast analysis of sulfatides species separated according to hydrophobicity, which facilitates the study of changes in the tissue content of individual molecular species of sulfatides in conditions like insulin resistance and diabetes, cancer and metastasis, and multiple sclerosis (4, 8, 33, 34).
A consistent, although rather small, difference between sulfatide levels in plasma from age-matched wild-type (+/+) C57BL/6J and ASA knockout mice (−/−) was found. In contrast, there was overlap between MLD patients and healthy volunteers. Late infantile and juvenile patients show increased levels of plasma sulfatides, whereas adolescent and adult patients do not. This finding suggests that the determination of sulfatides in plasma has insufficient sensitivity to reliably discriminate all MLD patients from healthy individuals, especially in later-onset patients, and may have limited potential as a biomarker to monitor the efficacy of treatment. Plasma samples from human MLD patients show relatively high levels of C16:0 and C16:0-OH sulfatides. A similar observation for the C16:0 species was recently reported for sulfatides in blood spots from MLD patients (24, 25)
In addition to the sulfatide determination, we describe the determination of lysosulfatide, the deacylated form of the primary storage product sulfatide, by LC/MS/MS for the first time. Previous methods used primarily HPLC methods for detection and quantification of lysosulfatide (12, 17). In addition to sensitivity and specificity, our method has the advantage of a simple sample clean-up procedure compared with previously described methods. This is due to the quantitative partitioning to the polar upper phase in the Bligh and Dyer extraction, whereas most other lipids partition to the apolar lower phase. Lysosulfatide binds to the reversed phase column because of its amphiphilic nature, whereas most of the polar compounds present in the extract do not bind and are diverted to waste. The presence of lysosulfatide has been shown in normal tissues, especially in the brain. Our method easily detects lysosulfatide in small tissue samples of wild-type (+/+) C57BL/6J mice brain. Trace amounts were found in normal kidney and liver. The content of lysosulfatide in 20 month old ASA knockout (−/−) mice was greatly increased in brain and kidney, but increased levels were also found in liver, an organ not functionally affected in MLD. Our finding of a lysosulfatide species with an m/z of 538.4 suggests that this may originate from sulfatide species with a sphingadienine (d18:2) base previously shown to be present in galactosylceramides and sulfatides from mouse brain (10). Prompted by the presence of globotriaosylsphingosine in plasma from Fabry mice and human Fabry patients (35, 36) and glucosylsphingosine in plasma from Gaucher mice and human Gaucher patients (37, 38), we searched for the presence of lysosulfatide in plasma from the wild-type (+/+) C57BL/6J and ASA knockout (−/−) mice. Although the presence of lysosulfatide was detected in trace amounts in plasma from normal and ASA knockout mice as well as normal human plasma and plasma from MLD patients, it could not be reliably quantified. This suggests that, in contrast to Fabry and Gaucher disease, where globotriaosylsphingosine and glucosylsphingosine are promising biomarkers, in MLD lysosulfatide may not be a useful biomarker to monitor the progress of the disease and the effects of therapy. This may be explained by the lesser involvement of visceral organs in the storage process in metachromatic leukodystrophy as compared with Gaucher and Fabry disease. These visceral organs, especially the tissue macrophages, may be an important source of plasma lysosphingolipids (35, 37).
Supplementary Material
Acknowledgments
The authors thank Dr. V. Gieselmann (University of Bonn, Germany) for providing the mouse tissues, the referring physicians for providing human plasma and urine samples from patients with metachromatic leukodystrophy, Drs. P. Haimi and P. Somerharju (University of Helsinki, Finland) for making LIMSA available, and Dr. J.M.F.G. Aerts (Academic Medical Center, Amsterdam, The Netherlands) for stimulating discussions.
Footnotes
Abbreviations:
- ASA
- arylsulfatase A
- CGT
- UDP-galactose:ceramide galactosyltransferase
- CST
- cerebroside sulfotransferase
- LIMSA
- Lipid Mass Spectrum Analysis
- MLD
- metachromatic leukodystrophy
- RT
- retention time
- SPE
- solid phase extraction
- UPLC
- ultra-high pressure liquid chromatography
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and four tables.
REFERENCES
- 1.Eckhardt M. 2008. The role and metabolism of sulfatide in the nervous system. Mol. Neurobiol. 37: 93–103. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T., Suzuki T. 2012. Role of sulfatide in normal and pathological cells and tissues. J. Lipid Res. 53: 1437–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittke D., Hartmann D., Gieselmann V., Lullmann-Rauch R. 2004. Lysosomal sulfatide storage in the brain of arylsulfatase A-deficient mice: cellular alterations and topographic distribution. Acta Neuropathol. 108: 261–271. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Chen Y., Momin A., Shaner R., Wang E., Bowen N. J., Matyunina L. V., Walker L. D., McDonald J. F., Sullards M. C., et al. 2010. Elevation of sulfatides in ovarian cancer: an integrated transcriptomic and lipidomic analysis including tissue-imaging mass spectrometry. Mol. Cancer. 9: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niimura Y., Nagai K. 2008. Metabolic responses of sulfatide and related glycolipids in Madin-Darby canine kidney (MDCK) cells under osmotic stresses. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 149: 161–167. [DOI] [PubMed] [Google Scholar]
- 6.Stettner P., Bourgeois S., Marsching C., Traykova-Brauch M., Porubsky S., Nordstrom V., Hopf C., Kosters R., Sandhoff R., Wiegandt H., et al. Sulfatides are required for renal adaptation to chronic metabolic acidosis. Proc. Natl. Acad. Sci. U S A. 110: 9998–10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka I. 1997. Chemistry and functional distribution of sulfoglycolipids. Prog. Lipid Res. 36: 245–319. [DOI] [PubMed] [Google Scholar]
- 8.Buschard K., Blomqvist M., Mansson J. E., Fredman P., Juhl K., Gromada J. 2006. C16:0 sulfatide inhibits insulin secretion in rat beta-cells by reducing the sensitivity of KATP channels to ATP inhibition. Diabetes. 55: 2826–2834. [DOI] [PubMed] [Google Scholar]
- 9.van Zyl R., Gieselmann V., Eckhardt M. 2010. Elevated sulfatide levels in neurons cause lethal audiogenic seizures in mice. J. Neurochem. 112: 282–295. [DOI] [PubMed] [Google Scholar]
- 10.Colsch B., Afonso C., Popa I., Portoukalian J., Fournier F., Tabet J. C., Baumann N. 2004. Characterization of the ceramide moieties of sphingoglycolipids from mouse brain by ESI-MS/MS: identification of ceramides containing sphingadienine. J. Lipid Res. 45: 281–286. [DOI] [PubMed] [Google Scholar]
- 11.Von Figura K. G. V., Jaeken J. 2001. Metachromatic leukodystrophy. In The metabolic and molecular basis of inherited disease. C. R. Scriver, A. L. Beaudet, D. Valle, and W. S. Sly, editors. McGraw Hill, New York. 3695–3724. [Google Scholar]
- 12.Toda K., Kobayashi T., Goto I., Kurokawa T., Ogomori K. 1989. Accumulation of lysosulfatide (sulfogalactosylsphingosine) in tissues of a boy with metachromatic leukodystrophy. Biochem. Biophys. Res. Commun. 159: 605–611. [DOI] [PubMed] [Google Scholar]
- 13.Cui Y., Colsch B., Afonso C., Baumann N., Tabet J. C., Mallet J. M., Zhang Y. 2008. Synthetic sulfogalactosylceramide (sulfatide) and its use for the mass spectrometric quantitative urinary determination in metachromatic leukodystrophies. Glycoconj. J. 25: 147–155. [DOI] [PubMed] [Google Scholar]
- 14.Natowicz M. R., Prence E. M., Chaturvedi P., Newburg D. S. 1996. Urine sulfatides and the diagnosis of metachromatic leukodystrophy. Clin. Chem. 42: 232–238. [PubMed] [Google Scholar]
- 15.Whitfield P. D., Sharp P. C., Johnson D. W., Nelson P., Meikle P. J. 2001. Characterization of urinary sulfatides in metachromatic leukodystrophy using electrospray ionization-tandem mass spectrometry. Mol. Genet. Metab. 73: 30–37. [DOI] [PubMed] [Google Scholar]
- 16.Hess B., Saftig P., Hartmann D., Coenen R., Lullmann-Rauch R., Goebel H. H., Evers M., von Figura K., D’Hooge R., Nagels G., et al. 1996. Phenotype of arylsulfatase A-deficient mice: relationship to human metachromatic leukodystrophy. Proc. Natl. Acad. Sci. USA. 93: 14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blomqvist M., Gieselmann V., Mansson J. E. 2011. Accumulation of lysosulfatide in the brain of arylsulfatase A-deficient mice. Lipids Health Dis. 10: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramakrishnan H., Hedayati K. K., Lullmann-Rauch R., Wessig C., Fewou S. N., Maier H., Goebel H. H., Gieselmann V., Eckhardt M. 2007. Increasing sulfatide synthesis in myelin-forming cells of arylsulfatase A-deficient mice causes demyelination and neurological symptoms reminiscent of human metachromatic leukodystrophy. J. Neurosci. 27: 9482–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berná L., Asfaw B., Conzelmann E., Cerny B., Ledvinova J. 1999. Determination of urinary sulfatides and other lipids by combination of reversed-phase and thin-layer chromatographies. Anal. Biochem. 269: 304–311. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka G., Kishimoto Y. 1979. Simultaneous determination of picomole levels of gluco- and galactocerebroside, monogalactosyl diglyceride, and sulfatide by high performance liquid chromatography. Biochim. Biophys. Acta. 572: 423–431. [DOI] [PubMed] [Google Scholar]
- 21.Tan M. A., Fuller M., Zabidi-Hussin Z. A., Hopwood J. J., Meikle P. J. 2010. Biochemical profiling to predict disease severity in metachromatic leukodystrophy. Mol. Genet. Metab. 99: 142–148. [DOI] [PubMed] [Google Scholar]
- 22.Hsu F. F., Bohrer A., Turk J. 1998. Electrospray ionization tandem mass spectrometric analysis of sulfatide. Determination of fragmentation patterns and characterization of molecular species expressed in brain and in pancreatic islets. Biochim. Biophys. Acta. 1392: 202–216. [DOI] [PubMed] [Google Scholar]
- 23.Kuchař L., Asfaw B., Poupetova H., Honzikova J., Turecek F., Ledvinova J. 2013. Direct tandem mass spectrometric profiling of sulfatides in dry urinary samples for screening of metachromatic leukodystrophy. 425: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han M., Jun S. H., Song S. H., Park H. D., Park K. U., Song J. 2014. Ultra-performance liquid chromatography/tandem mass spectrometry for determination of sulfatides in dried blood spots from patients with metachromatic leukodystrophy. Rapid Commun. Mass Spectrom. 28: 587–594. [DOI] [PubMed] [Google Scholar]
- 25.Barcenas M., Suhr T. R., Scott C. R., Turecek F., Gelb M. H. 2014. Quantification of sulfatides in dried blood and urine spots from metachromatic leukodystrophy patients by liquid chromatography/electrospray tandem mass spectrometry. Clin. Chim. Acta 433: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzner U., Matthes F., Herbst E., Lullmann-Rauch R., Callaerts-Vegh Z., D’Hooge R., Weigelt C., Eistrup C., Fogh J., Gieselmann V. 2007. Induction of tolerance to human arylsulfatase A in a mouse model of metachromatic leukodystrophy. Molecular medicine (Cambridge, Mass) 13: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 28.Vance D. E., Sweeley C. C. 1967. Quantitative determination of the neutral glycosyl ceramides in human blood. J. Lipid Res. 8: 621–630. [PubMed] [Google Scholar]
- 29.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama E., Hara A., Uemura K. 1999. A quantitative analysis of serum sulfatide by matrix-assisted laser desorption ionization time-of-flight mass spectrometry with delayed ion extraction. Anal. Biochem. 274: 90–97. [DOI] [PubMed] [Google Scholar]
- 31.Haimi P., Chaithanya K., Kainu V., Hermansson M., Somerharju P. 2009. Instrument-independent software tools for the analysis of MS-MS and LC-MS lipidomics data. Methods in molecular biology (Clifton, N.J) 580: 285–294. [DOI] [PubMed] [Google Scholar]
- 32.Nonaka G., Kishimoto Y. 1979. Levels of cerebrosides, sulfatides, and galactosyl diglycerides in different regions of rat brain. Change during maturation and distribution in subcellular fractions of gray and white matter of sheep brain. Biochim. Biophys. Acta. 572: 432–441. [DOI] [PubMed] [Google Scholar]
- 33.Morichika H., Hamanaka Y., Tai T., Ishizuka I. 1996. Sulfatides as a predictive factor of lymph node metastasis in patients with colorectal adenocarcinoma. Cancer. 78: 43–47. [DOI] [PubMed] [Google Scholar]
- 34.Marbois B. N., Faull K. F., Fluharty A. L., Raval-Fernandes S., Rome L. H. 2000. Analysis of sulfatide from rat cerebellum and multiple sclerosis white matter by negative ion electrospray mass spectrometry. Biochim. Biophys. Acta. 1484: 59–70. [DOI] [PubMed] [Google Scholar]
- 35.Aerts J. M., Groener J. E., Kuiper S., Donker-Koopman W. E., Strijland A., Ottenhoff R., van Roomen C., Mirzaian M., Wijburg F. A., Linthorst G. E., et al. 2008. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA. 105: 2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold H., Mirzaian M., Dekker N., Joao Ferraz M., Lugtenburg J., Codee J. D., van der Marel G. A., Overkleeft H. S., Linthorst G. E., Groener J. E., et al. 2013. Quantification of globotriaosylsphingosine in plasma and urine of fabry patients by stable isotope ultraperformance liquid chromatography-tandem mass spectrometry. Clin. Chem. 59: 547–556. [DOI] [PubMed] [Google Scholar]
- 37.Mistry P. K., Liu J., Yang M., Nottoli T., McGrath J., Jain D., Zhang K., Keutzer J., Chuang W. L., Mehal W. Z., et al. 2010. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. USA. 107: 19473–19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dekker N., van Dussen L., Hollak C. E., Overkleeft H., Scheij S., Ghauharali K., van Breemen M. J., Ferraz M. J., Groener J. E., Maas M., et al. 2011. Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 118: e118–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.