Summary
Objective
The matricellular protein NOV/CCN3, is implicated in osteoarthritis (OA) and targeted mutation of NOV in mice (Novdel3) leads to joint abnormalities. This investigation tested whether NOV is required for joint homeostasis and if its disruption causes joint degeneration.
Method
NOV expression in the adult mouse joint was characterized by immunohistochemistry. A detailed comparison of the joints of Novdel3−/− and Novdel3+/+ (wild-type) males and females at 2, 6 and 12 months of age was determined by X-ray, histology and immunohistochemistry.
Results
NOV protein was found in specific cells in articular cartilage, meniscus, synovium and ligament attachment sites in adult knees. Novdel3−/− males exhibited severe OA-like pathology at 12 months (OARSI score 5.0 ± 0.5, P < 0.001), affecting all tissues of the joint: erosion of the articular cartilage, meniscal enlargement, osteophytic outgrowths, ligament degeneration and expansion of fibrocartilage. Subchondral sclerosis and changes in extracellular matrix composition consistent with OA, were also seen. The density of articular cartilage cells in Novdel3+/+ knee joints is maintained at a constant level from 2 to 12 months of age whereas this is not the case in Novdel3−/− mice. Compared with age and sex-matched Novdel3+/+ mice, a significant increase in articular cartilage density was seen in Novdel3−/− males at 2 months, whereas a significant decrease was seen at 6 and 12 months in both Novdel3−/− males and females.
Conclusion
NOV is required for the maintenance of articular cartilage and for joint homeostasis, with disruption of NOV in ageing Novdel3−/− male mice causing OA-like disease.
Keywords: NOV/CCN3, Joint homeostasis, Osteoarthritis, Targeted mouse mutant
Introduction
An impaired ability to maintain homeostasis of the joint is associated with tissue degeneration, yet the mechanisms that regulate this process are largely unknown. Failure to maintain healthy articular cartilage cells is a common feature of ageing and an important underlying cause of osteoarthritis (OA)1. The association of OA with impaired joint homeostasis suggests common regulators in the pathways mediating these processes.
NOV (CCN3) is a member of the CCN family of matricellular proteins which have important roles in development, wound healing, angiogenesis and disease. In skeletal development and disease CTGF (CCN2) is required for coordinating chondrogenesis and angiogenesis2, WISP1 (CCN4) is upregulated in OA3 and mutations in WISP3 (CCN6) cause progressive pseudorheumatoid dysplasia4, while NOV (CCN3) has roles in skeletal development5, regulation of osteogenesis6,7, bone regeneration8 and bone cancer9. All CCN proteins share a common modular structure: an insulin-like growth factor binding protein (IGFBP) domain, a von Willibrand factor type C (WVC) domain, a thrombospondin type 1 repeat (TSP1) and a carboxy-terminal (CT) domain containing a cysteine knot (absent in CCN5). By specific interactions with these domains, CCN proteins modulate multiple signalling pathways including BMPs, Wnt, TGFs, Notch and integrins to regulate cell proliferation, survival, adhesion, migration and differentiation10.
Recent work has implicated NOV in OA. In experimentally induced OA in mice elevated NOV expression is seen in gene array studies using RNA isolated from arthritic joints11. Similarly, in human OA NOV is upregulated12. This may reflect a role for NOV in OA pathology or alternatively, that NOV is induced in response to joint damage and acts to mediate repair. Indeed, studies comparing OA susceptible and non-susceptible rat strains have identified NOV as a potential gene conferring protection from OA13.
Our preliminary characterization of mice homozygous for the targeted mutation Novdel3 revealed joint abnormalities in addition to other mild skeletal phenotypes5. In this paper, we have performed a detailed investigation of the role of NOV in the adult joint. We have characterized the expression pattern of NOV in the adult knee joint, and show that homeostasis of articular cartilage cells is compromised in the Novdel3−/− mutants, and that 12 month Novdel3−/− males exhibit joint degeneration with an OA-like pathology.
Materials and methods
Experimental animals
Male and female Novdel3−/− mice carrying a targeted mutation in Nov5 and wild type littermates (Novdel3+/+) were maintained on a 129Sv background. Breeding and analysis of mice were approved by the Cardiff University's Ethical Committee and performed in accordance with the Animal [Scientific Procedures] Act 1986 under project licences PPL 30/2672, PPL 30/2600. Mice housed together on a 12-h light–dark cycle with a temperature range of 21C ± 2, with free access to tap water and standard chow.
X-ray imaging and skeletal preparation
Novdel3+/+ and Novdel3−/− male and female adult mice were fixed by perfusion with 4% paraformaldehyde (PFA) in PBS at 2, 6 and 12 months (n = 6 for each sex and genotype at each time point). The hind limbs were dissected and imaged by X-ray (KODAK In-Vivo Imaging System FX Pro) prior to decalcification in 10% EDTA pH 7. 7 μm coronal paraffin sections were prepared semi-serially.
Histological assessment and OARSI score
Comparable sections from each animal, stained using haematoxylin and eosin (H&E), toludine blue and safranin O were assessed for histopathological changes (by KR blinded to genotype), using the OARSI scheme14 (see Supplementary Methods), assigning a score between 0 and 6 to all four quadrants averaged over three sections (n = 6 of each sex and genotype at each time point). OA severity is expressed as the summed score across the entire joint. Collagen birefringence in the tibial plateau (two sections, n = 4 for each sex and genotype at each time point) was imaged by polarized light microscopy (Polarisation equipped Zeiss Photomicroscope).
Analysis of articular cartilage cell density and thickness
Articular cartilage cell density was determined for each of the four quadrants of the joint from four H&E sections per animal (n = 4 for each sex and genotype at each time point) (KR blinded to genotype). Cell number and area of articular and calcified cartilage were measured within a box of 0.32 × 0.23 mm placed on load-bearing regions of articular cartilage. Cartilage thickness, measured at three equidistant points across the cartilage on both the medial and lateral side of the tibia, was averaged across a minimum of three sections (n = 4 for each sex and genotype at each time point).
Immunohistochemistry
Anti-NOV rabbit polyclonal antibody Novpep5170 (Pepceuticals Ltd), against peptide CPQNNEAFLQDLELKTSRGEI, was used to analyse expression in 6 month Novdel3+/+ and Novdel3−/− males and females (n = 3 for each genotype and sex). Cell proliferation and apoptosis were analysed at 2, 6 and 12 months using a rabbit polyclonal to PCNA (Abcam) and PARP p85 (Promega), (n = 4 for each genotype, sex and stage). The percentage positive cells was determined for each of four quadrants, averaged over two slides by KR blinded to genotype. Collagen I and Collagen X expression was assayed using mouse monoclonals anti-ColI (1/1000, Sigma) and anti-ColX (1/10, gift of Klaus von der Mark) (n = 4 for each genotype, sex and stage). Immunohistochemistry details in Supplementary Methods.
Statistical analysis
The effects of genotype, sex and age on measurements of articular cartilage histological (OARSI) score, thickness and cell density, and chondroctye proliferation/apoptosis were tested using three way ANOVA (SPSS) and included analysis of both simple and main effects. Normality was checked using the Shapiro–Wilk test and homogeneity of variances was verified using Levene's test (SPSS).
Results
NOV is expressed in the adult knee joint
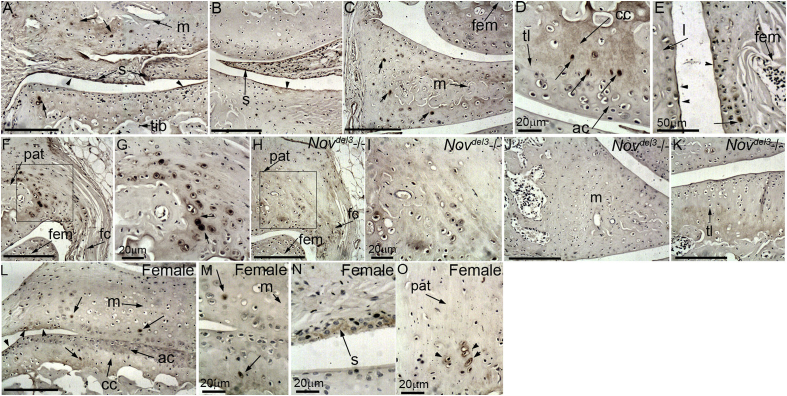
NOV was detected by immunohistochemistry in multiple tissues of male and female knee joints: the synovial intima, a membrane lining all the non-articular structures in the joint [Fig. 1(A), (B), (N)]; the superficial layer of the articular cartilage, particularly at the extremities of the joint e.g., near the cruciate ligament [Fig. 1(A), (B) arrowhead, L]; a subset of cells in the calcified cartilage [Fig. 1(A), (D) (arrow), L]; the outmost layer of cells in the meniscus [Fig. 1(A)] and a subset of cells in the core [Fig. 1(A), (C) (arrow), M]; the synovial lining over the femur and in a thin layer lining the inner surface of the adjacent collateral ligament [Fig. 1(E)]; the patella, in large round cells at the fibrocartilage attachment sites of the capsular ligaments [Fig. 1(F), (G), (O)]. No signal was detected in Novdel3−/− mice [Fig. 1(H)–(K)].
Fig. 1.
Immunohistochemical characterization of NOV expression in 6 month males (A–K) and females (L–O). NOV is expressed in the most superficial layer of the articular cartilage at margins of the joint and meniscus (A, B, arrowhead), the synovial intima (A, B) and a subset of cells in the calcified layer (A, D, arrow) and meniscus (B). NOV is also present in the synovial lining of the femur and lining the collateral ligaments (E), and in the attachment site of the fibrous capsule (fc) to the patella (F, G). No signal was detected in Novdel3−/− fibrous capsule (H, I), meniscus (J) or articular cartilage (K). In females NOV is also expressed in the superficial layer (L, arrowhead), the synovial intima (N), a subset of cells in the calcified layer and meniscus (L, M) and the patella (O). Scale bars are 100 μm unless indicated. tib; tibia, m; meniscus, s; synovium, fem; femur, ac; articular cartilage, tl; tideline, cc; calcified cartilage, l; collateral ligament, pat; patella.
Novdel3−/− mice exhibit joint defects with features characteristic of OA
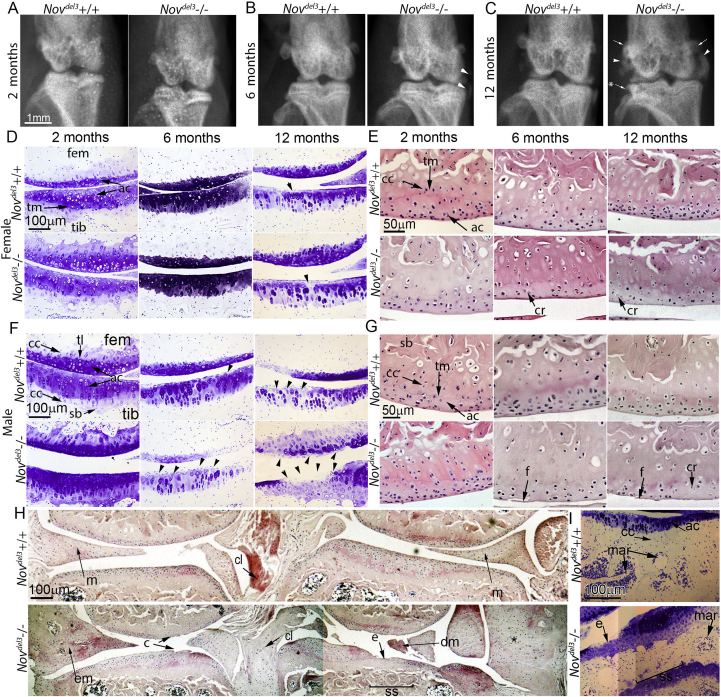
To determine the effect of NOV expression on the gross anatomy of the knee joint, Novdel3−/− males and females were analysed by X-ray at 2, 6 and 12 months of age and compared with age and sex matched Novdel3+/+ littermates (n = 6 for each group) [Fig. 2(A)–(C)]. While the structure of the female joint at all time points appeared overtly normal (not shown), the Novdel3−/− males displayed a number of pathological changes compared with Novdel3+/+ males [Fig. 2(B), (C)]. The menisci of 12 month Novdel3−/− males (4/6) were abnormally large with regions of calcification adjacent to the knee [Fig. 2(C) arrowhead], as previously noted in Ref. 5. While normal at 2 months [Fig. 2(A)], early stages of meniscal enlargement could already be seen at 6 months (1/6) [Fig. 2(B) arrowhead]. At 12 months, osteophytes were seen, particularly on the tibia [Fig. 2(C)] and the femoral epicondyle also appeared enlarged [Fig. 2(C)] (4/6).
Fig. 2.
X-ray and histological analysis of Novdel3−/− and Novdel3+/+ knee joints. A–C: X-rays of 2, 6 and 12 month male Novdel3−/− joints revealed abnormal bone (B, C, arrowhead), osteophytes (C,*), enlarged epicondyles (C, arrow), whereas male Novdel3+/+ joints were normal. Knee joints of both Novdel3−/− and Novdel3+/+ females were normal (data not shown). D–G: Histological analysis of 2, 6 and 12-month-old Novdel3−/− and Novdel3+/+ females (D, E) and males (F, G) by staining with toluidine blue (D, F) and H and E (E, G). Normal toluidine blue staining of Novdel3−/− females compared with controls (D), but H and E staining showed decreased articular cartilage cell densities at 2, 6 and 12 months (E) with cell remnants (cr) present at 6 and 12 months (E) (see inserts). Compared to age and sex matched controls, Novdel3−/− male joints had reduced toluidine blue staining (F), depleted articular cartilage cell density and articular damage at 6 and 12 months (G) with fibrillation (f) at 6 and 12 months. H: H and E staining of Novdel3−/− and Novdel3+/+ males at 12 months showing significant joint pathology in the mutant with erosion (e), clefts (c), enlarged (em) or damaged (dm) meniscus (m), cruciate ligament (cl) degeneration and fibrocartilage expansion (*) in the joint margins and subchondral sclerosis (ss). I: 12 month males showed distinct subchondral sclerosis (ss) with loss of marrow cavities (mar). tib; tibia, fem; femur, ac; articular cartilage, cc; calcified cartilage, tm; tidemark. Scale bar 1 mm (A–C), 100 μm (D, F, H) and 50 μm (E, G).
Histological analysis was performed by toluidine blue staining of cartilage extracellular matrix (ECM) and H&E staining of coronal sections of the knees isolated from Novdel3−/− and Novdel3+/+ males and females at 2, 6 months and 12 months of age (n = 6 for each group) (Fig. 2). Novdel3−/− female mice were comparable to Novdel3+/+ controls at 2, 6 and 12 months, with both showing slight reductions in toluidine blue staining at 12 months [Fig. 2(D) arrowhead]. The number of articular cartilage cells at 6 and 12 months appeared reduced compared to Novdel3+/+ females, with evidence of cell remnants near the articular surface [Fig. 2(E)]. In contrast, the joints of Novdel3−/− males were clearly abnormal [Fig. 2(F)–(H)]. Toluidine blue staining of cartilage ECM was increased in 2 month Novdel3−/− males compared with Novdel3+/+ controls [Fig. 2(F)], consistent with the increased numbers of articular cartilage cells also seen [Fig. 2(G)]. However, at 6 and 12 months toluidine blue staining was reduced in Novdel3−/− males compared to Novdel3+/+ controls [Fig. 2(F)], consistent with the depletion of articular cartilage cells also observed [Fig. 2(G)]. Six-month-old Novdel3−/− males also showed distinct fibrillation of the articular surface [Fig. 2(F), (G)] and by 12 months the male joint was badly compromised [Fig. 2(F), (H)]. Severe pathological changes included loss of ECM staining [Fig. 2(F)], cracks in the articular surface, loss of chondrocytes and loss of the articular surface, with exposure of bone and damage to the meniscus [Fig. 2(H)]. Enlarged menisci with peripheral marrow cavities were present in 12 month Novdel3−/− males [Fig. 2(H)]. Abnormal fibrocartilage-like tissue was also found around the knee joint in spaces normally occupied by synovium or collateral ligaments. Subchondral sclerosis was evident at 12 months in Novdel3−/− males, but not in Novdel3+/+ males [Fig. 2(I)] or in females of either genotype (data not shown). In summary, the pathological changes observed in the Novdel3−/− males were consistent with an OA-like phenotype.
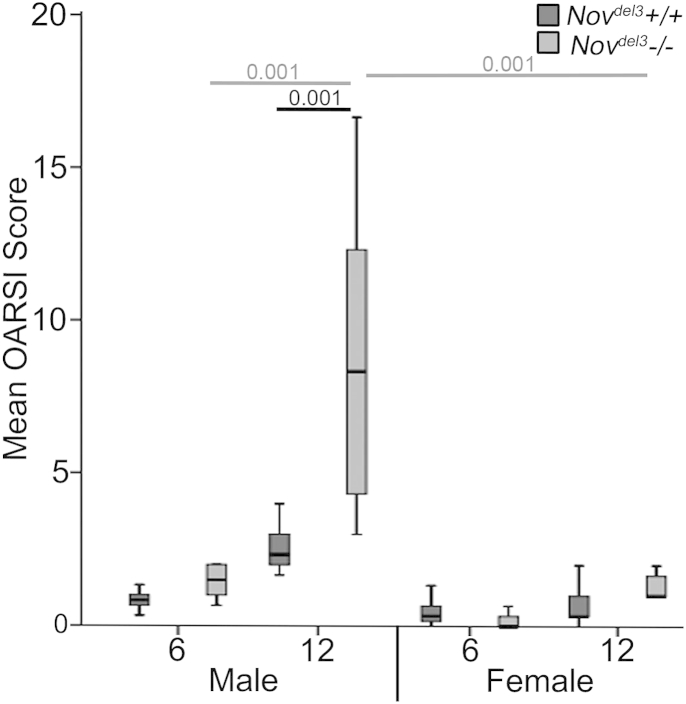
To determine the severity of OA in the Novdel3−/− mice, the OARSI grading scheme14 was used. No pathological changes were apparent at 2 months in Novdel3−/− or Novdel3+/+ males or females (OARSI score of 0, data not shown). No significant changes in the OARSI scores were observed between Novdel3−/− mice and sex-matched controls at 6 months. However, at 12 months Novdel3−/− males, but not females, showed significantly higher OARSI scores (Fig. 3; OARSI score 5.0 ± 0.5, P < 0.001), indicating severe OA-like joint pathology. The OARSI score of the 12 month Novdel3−/− males was also significantly higher than those of the 6 month Novdel3−/− males (P < 0.001) and 12 month Novdel3−/− females (P < 0.001). No significant differences were seen between the Novdel3+/+ males and females.
Fig. 3.
Mean OARSI score of the knee joints of Novdel3−/− and Novdel3+/+ males and females at 6 and 12 months (n = 6 of each sex and genotype at each time point), (±95% CI), P values indicated.
Abnormal differentiation in multiple joint tissues in male Novdel3−/− mice
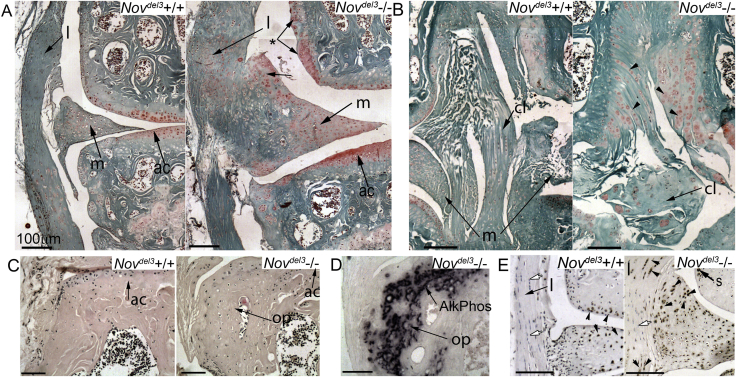
Safranin O and toluidine blue staining revealed that proteoglycans characteristic of cartilage were present in multiple ectopic sites in the joints of 12 month Novdel3−/− males, indicating inappropriate cartilage differentiation in these tissues, but not in Novdel3−/− females and Novdel3+/+ control mice of either sex. Increased safranin O staining was found at multiple sites in the enlarged menisci of Novdel3−/− males, particularly at the periphery [Fig. 4(A)], in the synovial intima lining the femur [Fig. 4(A),*], in the collateral ligament and throughout the femoral notch [Fig. 4(B)], an area normally occupied by the cruciate ligaments. In the ligaments, proteoglycan changes ranged from abnormal safranin O staining near the insertion sites to complete degeneration of the cruciate ligament and its replacement with extensive safranin O stained fibre-like structures [Fig. 4(B)], possibly indicating cartilage metaplasia in the ligament. Osteophytes, expressing the osteoblast marker alkaline phosphatase, were also found in Novdel3−/− male mice at 12 months [Fig. 4(C), (D)] and in males at 6 months, but not in Novdel3+/+ males or in females of either genotype (data not shown). Abnormal clusters of proliferating cells were present in the meniscus of Novdel3−/− males at 12 months but not in Novdel3+/+ male littermates, based on immunohistochemistry with an anti-PCNA antibody [Fig. 4(E)]. Increased numbers of PCNA positive cells were also identified in the synovial intima over the femur and in the collateral ligaments [Fig. 4(E)] and cruciate ligament (not shown) of Novdel3−/− males. This suggests that NOV mutation is associated with ectopic cell proliferation in joint tissues with an OA-like pathology.
Fig. 4.
Histological changes at 12 months in knee joints of Novdel3−/− males compared with Novdel3+/+ males. A, B: safranin O staining. Increased staining seen in the meniscus (m), articular cartilage (ac), collateral ligament (l), cruciate ligament (cl) and synovium (*) (A, B). Note expansion of fibrocartilage stained with safanin O in the collateral ligament (l) (A) and cruciate ligament (cl) (arrowheads) (B). C: H and E staining of joint margins showing the presence of osteophytic outgrowths (op) in Novdel3−/− males (C), but not in Novdel3+/+ control. D: Alkaline phosphatase staining of adjacent section shown in C. Positive staining of the osteophytes with the osteoblast marker alakaline phosphatase (AlkPhos) (D). E: Immunohistochemistry with anti-PCNA antibody, counterstained with haematoxlyin. Proliferating cells expressing PCNA (black arrowhead) were found in ligaments (l), synovium (s) and in the groups of cells in the margins of the meniscus in Novdel3−/− males. Negative cells indicated by white arrow. Scale bar 100 μm.
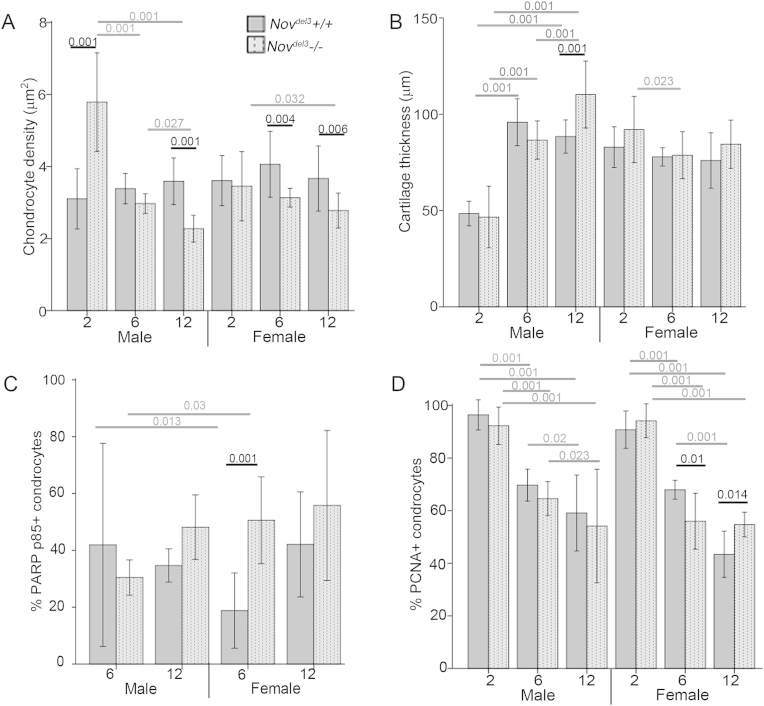
Articular cartilage cell density and thickness is altered in Novdel3−/− mice
Initial observations of H&E stained sections indicated differences in the density of articular cartilage cells in Novdel3−/− mice [Fig. 2(E), (G)]. To quantify this, the mean density of articular chondrocytes was determined for the medial and lateral compartments of the femur and tibia at each time point and the overall mean used for analysis (n = 4 mice of each genotype and sex at each time point). Articular cartilage cell density is maintained at a constant level in Novdel3+/+ mice over the timescale 2–12 months [Fig. 5(A)]. In contrast, this is not seen in Novdel3−/− mice. Compared to aged matched Novdel3+/+ males, a significant increase in articular cartilage cell density was found in Novdel3−/−males at 2 months (P < 0.001) while a significant decrease was seen at 12 months (P < 0.001) [Fig. 5(A)]. Articular cartilage cell densities were also significantly reduced in Novdel3−/− females at 6 months (P = 0.004) and 12 months (P = 0.006) compared with age matched Novdel3+/+ females [Fig. 5(A)].
Fig. 5.
Histological analysis of articular chondrocytes in Novdel3−/− and Novdel3+/+ knee joints at 2, 6 and 12 months. A: Chondrocyte density measurements (n = 4 at each age, genotype and sex). B: Cartilage thickness (n = 4 at each age, genotype and sex). C: Percentage of cells positive for immunohistochemical staining with anti-PARP p85 antibody apoptotic marker (n = 4 at each age, genotype and sex). D: Percentage of cells positive for immunohistochemical staining with anti-PCNA antibody proliferation marker (n = 4 at each age, genotype and sex). No PARP p85 positive cells were detected at 2 months (data not shown). Graphs depict mean (±95% CI), P values indicated.
The mean thickness of the articular cartilage was also measured, in order to determine whether this might account for the alterations in cell density [Fig. 5(B)]. In 12-month-old Novdel3−/− males, a significant increase in articular cartilage thickness was observed (P < 0.001). However, compared to sex and aged matched controls, no significant differences were observed in Novdel3−/− females at any timepoint and in Novdel3−/− males at 2 and 6 months, indicating that other factors contributed to the early cell density changes seen in these mice.
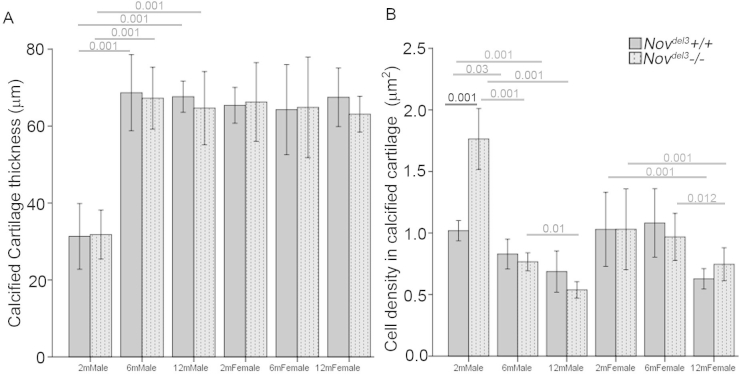
In contrast to the articular cartilage, the calcified cartilage was much less severely affected, with no significant changes to the overall thickness, comparing Novdel3−/− and Novdel3+/+ age and sex-matched mice at 2, 6 and 12 months [Supplementary Fig. 1(A)]. Interestingly, at 2 months calcified cartilage was significantly thinner in males of both genotypes than in females and older males (P < 0.001). Similarly, no significant changes in chondrocyte cell density were seen in the calcified cartilage of Novdel3−/− and Novdel3+/+ sex matched mice at 6 and 12 months [Supplementary Fig. 1(B)], although a significant increase was noted in 2 month Novdel3−/−males (P < 0.001). Thus, the calcified cartilage appears less severely affected by Nov mutation than is the articular cartilage.
Altered cell proliferation and cell death in Novdel3−/− mice
To determine whether articular cartilage cell density changes might reflect altered apoptosis or cell proliferation, immunohistochemistry for the apoptosis marker PARP p85 [Fig. 5(C)] and the proliferation marker PCNA [Fig. 5(D)] was performed. A significant increase in the percentage of PARP p85 and significant decrease in the percentage of PCNA positive cells were observed in 6-month-old females (P < 0.001 and P = 0.01, respectively), indicating that increased apoptosis and decreased proliferation contribute to the decrease in articular cartilage cell density of Novdel3−/− females at this time point [Fig. 5(C), (D)]. However, this was not observed in 12-month-old Novdel3−/− females where no significant difference was seen in the percentage of apoptotic cells and a significant increase (P < 0.014) in proliferation was apparent compared to control mice, despite a decrease in articular cartilage cell density. No significant differences were seen in the percentage of PCNA or PARP p85 positive cells in 2, 6 and 12 month Novdel3−/− males compared to Novdel3+/+ males, indicating that altered rates of cell proliferation or apoptosis could not account for the cell density differences observed in these mice [Fig. 5(C), (D)].
Abnormal ECM in Novdel3−/− mice characteristic of OA
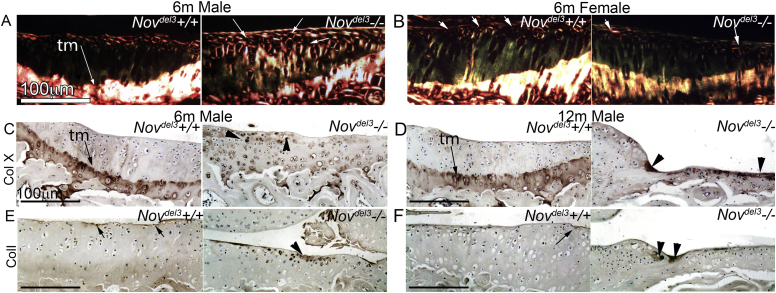
Specific changes to the distribution of ECM molecules in articular cartilage are indicative of OA, including altered collagen fibre structure15 and aberrant expression of collagen I and collagen X15. We determined the structure of collagen in articular cartilage of 6 month Novdel3−/− and Novdel3+/+ mice by picrosirus red staining and polarised light microscopy (n = 4 for each sex and genotype). Brighter and strikingly thicker collagen fibril structure was seen in Novdel3−/− males compared with Novdel3+/+ males [Fig. 6(A)]. In contrast, no obvious structural changes were apparent in Novdel3−/− females compared with controls [Fig. 6(B)].
Fig. 6.
Collagen content and distribution in articular cartilage of knee joints of Novdel3−/− mice and Novdel3+/+ mice. A, B: Collagen birefringence, captured from picrosirus Red stained sections of 6 month males (A) and females (B) imaged under polarized light. Thicker collagen fibres seen in Novdel3−/− males compared with Novdel3+/+ males (A, white arrows) but not in Novdel3−/− females (B). C–F: Immunohistochemistry with anti-collagen X (C, D) and anti-collagen I antibodies (E, F) of Novdel3−/− and Novdel3+/+ male articular cartilage at 6 (D, E) and 12 months (D, F). In Novdel3+/+, collagen X is adjacent to the tidemark (tm) (A, B, arrows) and collagen I is in the superficial articular cartilage (C, D, arrows). Aberrant expression of collagen I and collagen X in Novdel3−/− males at 6 and 12 months is indicated by arrowheads (C–F). Scale bar 100 μm.
Collagen X is a marker of hypertrophy in chondrocytes and is normally restricted in the joint to the thin layer of calcified cartilage below the tidemark. However in OA, collagen X is expressed in cells above the tidemark16,17, consistent with abnormal differentiation of articular cartilage cells down the endochondral pathway. Immunohistochemistry using an anti-collagen X antibody gave the expected pattern of expression in knee joints in Novdel3+/+ males and females at 6 and 12 months [Fig. 6(C), (D); data not shown]. Novdel3−/− females at 6 and 12 months also expressed collagen X as normal (data not shown). In contrast, in joints of Novdel3−/− males, the normal expression of collagen X at the tidemark was disrupted [Fig. 6(C), (D)] and was also present near areas of obvious cartilage damage [Fig. 6(C), (D)], consistent with an OA-like pathology.
Collagen I is normally expressed in the superficial layer of the articular cartilage and its expression increases in areas of damage17–19. As expected, Novdel3+/+ males at 6 and 12 months showed normal collagen I localization [Fig. 6(E), (F)]. In contrast, 6 and 12 month Novdel3−/− males exhibited stronger collagen I staining in the articular cartilage surface layer and in areas of visible cartilage damage [Fig. 6(F)].
Discussion
This is the first detailed characterization of the joint phenotype in Novdel3 mutant mice and identifies NOV as a new regulator of joint homeostasis with a role in OA. Homeostasis of articular cartilage is crucial to joint health. Here we show that the density of articular cartilage cells is normally maintained at a constant level in adult 129Sv mice between 2 and 12 months of age, whereas, this homeostatic process is compromised in Novdel3−/− mutants. Novdel3−/− females exhibited depletion of articular cartilage cell density compared to age matched Novdel3+/+ females at 6 and 12 months; at 6 months this was due, at least in part, to decreased proliferation and increased apoptosis. In contrast, altered proliferation and apoptosis could not account for the large variation seen in articular cartilage cell densities in Novdel3−/− males where, compared with age-matched Novdel3+/+ males, a significant depletion in cell density was seen at 12 months, whereas a dramatic increase was observed at 2 months. This suggests that differences in the density of articular chondrocytes in Novdel3−/− males have a different underlying origin, possibly reflecting changes in development or in behaviour of articular cartilage cells or their progenitor populations, as discussed below. Thus, normal NOV function is essential for maintenance of articular cartilage cell density in adult mice in the first year of life.
Multiple lines of evidence indicate loss of normal NOV function in Novdel3−/− males leads to OA-like joint pathology. Moderate pathological changes are present in 6 month Novdel3−/− males and, by 12 months, severe joint pathology is seen, reflected in a high OARSI score. Subchondral bone is less severely affected with subchondral sclerosis, but little effect on the calcified cartilage. Collagen X positive cells near damaged articular surfaces indicate aberrant differentiation of articular chondrocytes, also seen in OA16. In Novdel3−/− mice, clusters of cells in the margins of the meniscus and in the synovium express the proliferation marker PCNA, consistent with the expansion of these tissues. Severe disruption of the knee joint is apparent at 12 months in Novdel3−/− males with osteophytes, degenerate ligaments, enlarged menisci and abnormal proteoglycan content. The worst cruciate ligament degeneration is associated with the presence of the largest menisci and most osteophytes. Abnormal ligaments and menisci would significantly alter the distribution of load across the joint, leading to changes in gene expression in the mechanically sensitive cartilage and ligaments. Abnormal loading of the joint is a key factor in the progression and severity of OA20–22 and is likely to be a contributing factor in the early onset OA-like pathology seen in the Novdel3−/− mice. Moreover, joint laxity has also been observed in Novdel3−/− mice, correlating with the expression of NOV in mechano-responsive ligament attachment sites and tendons5. The meniscus, important in correct load transmission and abnormal in Novdel3−/− males, is also a site of NOV expression. Indeed, NOV has been shown to be regulated by mechanical stress23. The expression of NOV in a subset of cells in joint tissues may reflect several parameters including differentiation, cell cycle status or mechanical load. Thus, the aberrant behaviour of joint tissues in Novdel3−/− mice may result directly from NOV mutation, rather than being solely a consequence of abnormal joint structure and biomechanics. Whilst joint defects have not previously been reported in another NOV mouse mutant7, this may reflect differences in the mutation or strain in the proclivity of mice to develop OA-like pathology.
The ECM of the articular cartilage in Novdel3−/− male mice was also significantly different at 6 months, with decreased levels of proteoglycans and increased collagen fibre thickness compared to age and sex-matched littermates. Consistent with this, NOV has been reported to promote sulphated proteoglycan synthesis in chondrocytes isolated from developing rat epiphysis24. It would be of interest to determine whether ECM proteins that regulate collagen fibril size and diameter, such as decorin and collagen IX are altered in the Novdel3−/−mice. NOV has been shown to bind to fibrillin-1, and, is over expression in Tsk mice is associated with increased fibrillin-1 expression and repression of microfibril assembly25. Given that fibrillin-1 has recently been implicated in OA26, it would also be of interest to determine whether fibrillin-1 staining in the mutant is affected.
The observation that male Novdel3−/− mice are more affected than age-matched females is consistent with other mouse models of OA27. The higher incidence of spontaneous OA in male mice has been linked directly to the action of male hormones in exacerbating OA pathology27 and the protective role of female hormones28, suggesting that there may be a regulatory network involving sex hormones that influences OA severity. The androgen receptor has recently been shown to inhibit the Nov promoter via epigenetic silencing29. The increased susceptibility of Novdel3−/−males to OA cannot be explained through the androgen inhibition of the Nov promoter, but it may suggest that in mice NOV is part of a wider regulatory network involving sex hormones that leads directly or indirectly to increased OA susceptibility.
NOV has previously been linked to OA through its increased expression demonstrated in micro-array studies in mouse11 and in man12 suggesting a role for NOV in OA processes. We believe that these results can be reconciled with ours by the hypothesis that NOV is induced in damaged tissue in OA and plays a role in healing. Indeed, a role for NOV in tissue regeneration has been reported in the skin30 and tooth31. Articular cartilage is normally very limited in its ability to heal and it appears that this is further compromised in Novdel3−/− mice. Cartilage homeostasis and repair is thought to involve expansion and differentiation of progenitor cell populations; putative stem/progenitor cells are located in the synovium32 and in the superficial layer of the articular cartilage33,34 and a population of stem/progenitor cells has been shown to migrate from below the tidemark in late stage OA35. These cells are capable of differentiating and some have been shown respond to damage and to attempt healing33. Expression of NOV in the articular cartilage superficial layer and synovium suggests that it might influence the behaviour of the progenitor cells located there. Indeed, a role for NOV in regulating precursor cells has been shown in other systems31,36,37. It will be of interest to determine whether the alterations in articular chondrocyte density seen in Novdel3−/− mice is due to abnormal progenitor cell function; abnormal stem cell activation in young mice, could result in subsequent stem cell depletion, thereby contributing to the decreased articular cartilage cell density in older mice. NOV is also reported to direct articular chondrocyte differentiation and inhibit endochondral differentiation38,39. Our data is consistent with this: inappropriate collagen X staining is seen in Novdel3−/− males at 6 months, prior to serious OA-like pathology.
The Novdel3−/− mutant is a new mouse model for OA which crucially affects multiple tissues of the joint and arises during ageing, with many features seen in human OA. Importantly, unlike many other animal models of OA, disease onset is not dependent on surgical intervention to destabilize the medial meniscus or damage the ligaments40–42, allowing the involvement of these tissues in OA progression to be studied. The Novdel3 mutation thus provides a new model to study joint ageing and degeneration in a known genetic background, potentially allowing better understanding of the early changes induced in OA throughout the entire structure of the joint.
Author contributions
KAR co-designed, performed and analysed the experiments, and co-wrote manuscript. CAB obtained funding, co-designed and supervised project and co-wrote manuscript.
Role of the funding source
This work was funded by a project grant to CAB from the charity Arthritis Research UK (ref. 19283).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to acknowledge excellent technical assistance from Dr Mari Nowell, Bridget Allen and Luke Bradshaw and we are grateful to colleagues for helpful discussions and comments on the manuscript.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Fig. 1.
Histological analysis of calcified cartilage in Novdel3−/− and Novdel3+/+ knee joints at 2, 6 and 12 months. A: Calcified chondrocyte density measurements (n = 4 at each age, genotype and sex). B: Calcified cartilage thickness (n = 4 at each age, genotype and sex). Graphs depict mean (±95% CI), P values indicated.
References
- 1.Goldring M.B., Goldring S.R. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 2.Ivkovic S., Yoon B.S., Popoff S.N., Safadi F.F., Libuda D.E., Stephenson R.C. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130(12):2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blom A.B., Brockbank S.M., van Lent P.L., van Beuningen H.M., Geurts J., Takahashi N. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60(2):501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 4.Hurvitz J.R., Suwairi W.M., Van Hul W., El-Shanti H., Superti-Furga A., Roudier J. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 1999;23(1):94–98. doi: 10.1038/12699. [DOI] [PubMed] [Google Scholar]
- 5.Heath E., Tahri D., Andermarcher E., Schofield P., Fleming S., Boulter C.A. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8(1):18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rydziel S., Stadmeyer L., Zanotti S., Durant D., Smerdel-Ramoya A., Canalis E. Nephroblastoma overexpressed (Nov) inhibits osteoblastogenesis and causes osteopenia. J Biol Chem. 2007;282(27):19762–19772. doi: 10.1074/jbc.M700212200. [DOI] [PubMed] [Google Scholar]
- 7.Canalis E., Smerdel-Ramoya A., Durant D., Economides A.N., Beamer W.G., Zanotti S. Nephroblastoma overexpressed (Nov) inactivation sensitizes osteoblasts to bone morphogenetic protein-2, but nov is dispensable for skeletal homeostasis. Endocrinology. 2010;151(1):221–233. doi: 10.1210/en.2009-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita Y., Sakamoto K., Tamamura Y., Shibata Y., Minamizato T., Kihara T. CCN3 protein participates in bone regeneration as an inhibitory factor. J Biol Chem. 2013;288(27):19973–19985. doi: 10.1074/jbc.M113.454652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouellet V., Siegel P.M. CCN3 modulates bone turnover and is a novel regulator of skeletal metastasis. J Cell Commun Signal. 2012;6(2):73–85. doi: 10.1007/s12079-012-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C.-C., Lau L.F. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41(4):771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng J., Ma X., Ma D., Xu C. Microarray analysis of differential gene expression in temporomandibular joint condylar cartilage after experimentally induced osteoarthritis. Osteoarthritis Cartilage. 2005;13(12):1115–1125. doi: 10.1016/j.joca.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson C., Dehne T., Lindahl A., Brittberg M., Pruss A., Sittinger M. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):581–592. doi: 10.1016/j.joca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins E., Brenner M., Laragione T., Gulko P.S. Synovial expression of Th17-related and cancer-associated genes is regulated by the arthritis severity locus Cia10. Genes Immun. 2012;13(3):221–231. doi: 10.1038/gene.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasson S.S., Chambers M.G., Van Den Berg W.B., Little C.B. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi: 10.1016/j.joca.2010.05.025. (null) [DOI] [PubMed] [Google Scholar]
- 15.Hyttinen M.M., Arokoski J.P., Parkkinen J.J., Lammi M.J., Lapveteläinen T., Mauranen K. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarthritis Cartilage. 2001;9(8):694–701. doi: 10.1053/joca.2001.0466. [DOI] [PubMed] [Google Scholar]
- 16.Walker G.D., Fischer M., Gannon J., Thompson R.C., Oegema T.R. Expression of type-X collagen in osteoarthritis. J Orthop Res. 1995;13(1):4–12. doi: 10.1002/jor.1100130104. [DOI] [PubMed] [Google Scholar]
- 17.Gay S., Müller P.K., Lemmen C., Remberger K., Matzen K., Kühn K. Immunohistological study on collagen in cartilage-bone metamorphosis and degenerative osteoarthrosis. Klin Wochenschr. 1976;54(20):969–976. doi: 10.1007/BF01468947. [DOI] [PubMed] [Google Scholar]
- 18.Ronzière M.-C., Ricard-Blum S., Tiollier J., Hartmann D.J., Garrone R., Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta Protein Struct Mol Enzymol. 1990;1038(2):222–230. doi: 10.1016/0167-4838(90)90209-x. [DOI] [PubMed] [Google Scholar]
- 19.Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973;181(4101):751–752. doi: 10.1126/science.181.4101.751. (80-. ) [DOI] [PubMed] [Google Scholar]
- 20.Little C., Ghosh P., Rose R. The effect of strenuous versus moderate exercise on the metabolism of proteoglycans in articular cartilage from different weight-bearing regions of the equine third carpal bone. Osteoarthritis Cartilage. 1997;5(3):161–172. doi: 10.1016/s1063-4584(97)80011-8. [DOI] [PubMed] [Google Scholar]
- 21.Appleton C.T.G., McErlain D.D., Pitelka V., Schwartz N., Bernier S.M., Henry J.L. Forced mobilization accelerates pathogenesis: characterization of a preclinical surgical model of osteoarthritis. Arthritis Res Ther. 2007;9(1):R13. doi: 10.1186/ar2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei L., Hjerpe A., Brismar B.H., Svensson O. Effect of load on articular cartilage matrix and the development of guinea-pig osteoarthritis. Osteoarthritis Cartilage. 2001;9(5):447–453. doi: 10.1053/joca.2000.0411. [DOI] [PubMed] [Google Scholar]
- 23.Schild C., Trueb B. Three members of the connective tissue growth factor family CCN are differentially regulated by mechanical stress. Biochim Biophys Acta. 2004;1691(1):33–40. doi: 10.1016/j.bbamcr.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Janune D., Kubota S., Lazar N., Perbal B., Iida S., Takigawa M. CCN3-mediated promotion of sulfated proteoglycan synthesis in rat chondrocytes from developing joint heads. J Cell Commun Signal. 2011;5(3):167–171. doi: 10.1007/s12079-011-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaire R., Farina G., Bayle J., Dimarzio M., Pendergrass S.A., Milano A. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J Invest Dermatol. 2010;130(6):1514–1523. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanayake W., Jones H., Orriss I., Arnett T., Pitsillides A., Denton C. Fibrillin-1 expression, which regulates of TGF-B bioavailability, is modified during osteoarthritis and mutations lead to osteoarthritis. Osteoarthritis Cartilage. 2014;22:S141. [Google Scholar]
- 27.Ma H.-L., Blanchet T.J., Peluso D., Hopkins B., Morris E.A., Glasson S.S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15(6):695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Kinney R.C., Schwartz Z., Week K., Lotz M.K., Boyan B.D. Human articular chondrocytes exhibit sexual dimorphism in their responses to 17beta-estradiol. Osteoarthritis Cartilage. 2005;13(4):330–337. doi: 10.1016/j.joca.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu L., Runkle C., Jin H.-J., Yu J., Li J., Yang X. CCN3/NOV gene expression in human prostate cancer is directly suppressed by the androgen receptor. Oncogene. 2014;33(4):504–513. doi: 10.1038/onc.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C.G., Chen C.-C., Leu S.-J., Grzeszkiewicz T.M., Lau L.F. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280(9):8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., He H., Wu X., Hu J., Tan Y. Promotion of dentin regeneration via CCN3 modulation on Notch and BMP signaling pathways. Biomaterials. 2014;35(9):2720–2729. doi: 10.1016/j.biomaterials.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Kurth T.B., Dell'accio F., Crouch V., Augello A., Sharpe P.T., De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63(5):1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 33.Seol D., McCabe D.J., Choe H., Zheng H., Yu Y., Jang K. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64(11):3626–3637. doi: 10.1002/art.34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowthwaite G.P., Bishop J.C., Redman S.N., Khan I.M., Rooney P., Evans D.J.R. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 35.Koelling S., Kruegel J., Irmer M., Path J.R., Sadowski B., Miro X. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4(4):324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Gupta R., Hong D., Iborra F., Sarno S., Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316(5824):590–593. doi: 10.1126/science.1136031. (80-. ) [DOI] [PubMed] [Google Scholar]
- 37.Minamizato T., Sakamoto K., Liu T., Kokubo H., Katsube K., Perbal B. CCN3/NOV inhibits BMP-2-induced osteoblast differentiation by interacting with BMP and Notch signaling pathways. Biochem Biophys Res Commun. 2007;354(2):567–573. doi: 10.1016/j.bbrc.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Janune D., Kubota S., Nishida T., Kawaki H., Perbal B., Iida S. Novel effects of CCN3 that may direct the differentiation of chondrocytes. FEBS Lett. 2011;585(19):3033–3040. doi: 10.1016/j.febslet.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Kawaki H., Kubota S., Suzuki A., Lazar N., Yamada T., Matsumura T. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Min Res Off J Am Soc Bone Min Res. 2008;23(11):1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 41.McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Jt Surg Br. 1977;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- 42.Kamekura S., Hoshi K., Shimoaka T., Chung U., Chikuda H., Yamada T. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.