Abstract
The reconstruction of gene regulatory networks (GRNs) from high-throughput experimental data has been considered one of the most important issues in systems biology research. With the development of high-throughput technology and the complexity of biological problems, we need to reconstruct GRNs that contain thousands of genes. However, when many existing algorithms are used to handle these large-scale problems, they will encounter two important issues: low accuracy and high computational cost. To overcome these difficulties, the main goal of this study is to design an effective parallel algorithm to infer large-scale GRNs based on high-performance parallel computing environments. In this study, we proposed a novel asynchronous parallel framework to improve the accuracy and lower the time complexity of large-scale GRN inference by combining splitting technology and ordinary differential equation (ODE)-based optimization. The presented algorithm uses the sparsity and modularity of GRNs to split whole large-scale GRNs into many small-scale modular subnetworks. Through the ODE-based optimization of all subnetworks in parallel and their asynchronous communications, we can easily obtain the parameters of the whole network. To test the performance of the proposed approach, we used well-known benchmark datasets from Dialogue for Reverse Engineering Assessments and Methods challenge (DREAM), experimentally determined GRN of Escherichia coli and one published dataset that contains more than 10 thousand genes to compare the proposed approach with several popular algorithms on the same high-performance computing environments in terms of both accuracy and time complexity. The numerical results demonstrate that our parallel algorithm exhibits obvious superiority in inferring large-scale GRNs.
Introduction
The reconstruction of gene regulatory networks (GRNs) from high-throughput genome-wide data can help improve our understanding of molecular regulation events and is one of the most important issues in systems biology, which explicitly characterizes regulatory processes in the cell [1, 2]. Because of the advances in high-throughput technologies, the size of the GRNs we need to understand is becoming incredibly larger than before and extremely complicated. Recently, the Dialogue for Reverse Engineering Assessments and Methods (DREAM) project provided a platform for researchers to develop new efficient computation algorithms to infer GRNs [3]. For the reverse engineering of GRNs, various state-of-the-art approaches have been developed to improve the accuracy and scalability of network inference review in [4], including correlation based estimation methods [5], model-based methods [6, 7, 8], and mutual information(MI)-based methods, such as CMI [9], MI-CMI [10], MI3 [11] and PCA-CMI [12] and IPCA-CMI [13]. Furthermore, NARROMI [14] combined regression-based optimization and information theory- based MI to achieve improved accuracy.
However, the above approaches suffer from two limitations when handling networks with large numbers of genes. One limitation is that the computation cost increases exponentially with the number of genes. The second is that the accuracy of these approaches in large-scale problems with high sparsity is not satisfactory. In recent years, there have been efforts to address these difficulties. Qin at al. applied LASSO-type regularization methods to enhance the prediction accuracy [15]. Lee at al. developed a parallelizing hybrid GA-PSO optimization method to lower the time complexity [16], but in their numerical experiments, all three datasets contain no more than 125 genes. Because the sparsity level of the large-scale GRNs is much higher than for small-scale GRNs, false positives also increase remarkably when a similar correlation cutoff is used to predict the GRNs. In this study, we used the two features of large-scale GRNs, i.e., their sparsity and modularity, to design a new parallel framework to infer GRNs. We first decomposed whole large-scale GRNs into many small modular subnetworks and used module-based optimization and asynchronous communications to identify the parameters of the GRNs. Finally, we used famous benchmark datasets from DREAM with thousands of genes to compare with several popular algorithms on the same high-performance computing environments. The results show that the proposed approach can be successfully used to infer large-scale GRNs with high accuracy, and the computation time can be greatly reduced.
Methods
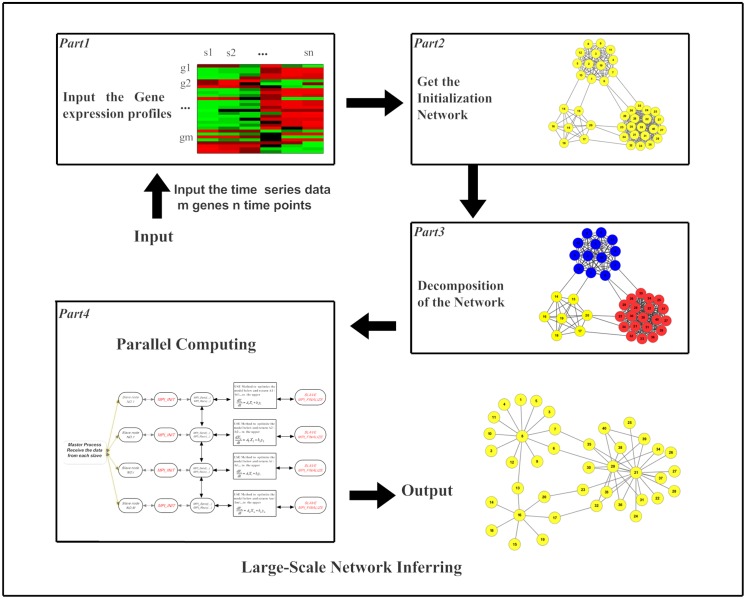
The proposed approach, namely the LSGPA, includes four parts (Fig. 1). The first part is to interpolate and normalize the gene expression data. The second is to build the initialization network based on the mutual information (MI) presented in section 2.1. In the third part, we have proposed a module-based decomposition to split the whole network into a large number of subnetworks, as detailed in section 2.2. Finally, the fourth part includes the parallel design and algorithm, which is presented in sections 2.3 and 2.4.
Fig 1. Outline of reconstructing large-scale gene networks by using the LSGPA.
2.1 Mutual information
The IFN concentration was measured by a bioassay based on the IFNα/β-mediated Mutual information (MI) is generally used as a powerful criterion for measuring the relationship between two genes X and Y in biological networks inference. MI has been widely used to construct GRNs from gene expression data [17].
For two discrete variables (genes) X and Y, the joint entropy H(X,Y) of X and Y can be denoted by
| (1) |
where p(x,y) is the joint probability of x in X and y in Y. In light of the equation displayed above, MI can be denoted as follows
| (2) |
In computational simulation, we can just use the following equivalent formula [12].
| (3) |
where C is the covariance matrix, and |C(X)| is the determinant of matrix C.
If X and Y are independent, the value will be relatively low, while a high MI value indicates that there may be a close relationship between the genes.
2.2 Module-based decomposition
In recent years, many excellent algorithms have been proposed to detect the modules of complex networks [18–23]. Most of these algorithms were only used to identify small and highly intra-connected clusters in a network, without clustering all the nodes. However, in the real biological world, GRNs often contain thousands of nodes and perform in a sparse way [24]. More recently, Glay proposed an algorithm to display and analyze large and sparse biological networks [25].
In this study, we proposed an improved approach to detect and split the modules. There are three steps.
Step1. Building the initialization networks and obtaining the network adjacency matrix
First, we construct initial networks by calculating the MI according to formula (3). By calculating the MI values between all pairs of variables, we obtain a weighted matrix A = (a ij), in which every element represents a relationship between two genes.
Second, we select a threshold “λ” to cut off edges with low value. In other words, if element a ij is greater than λ, then we set its value to 1 and let a ji = a ij = 1. Otherwise, no edges will be considered, i.e., a ji = a ij = 0. We performed simulation experiments to mine for information about how to select λ, and the detailed comparisons are displayed in S1 Table and S2 Fig. In this study, we set the MI threshold λ to 1.2.
Finally, a new network adjacency matrix can be used in clustering.
Step 2. Clustering and modularizing the large GRNs.
First, the network adjacency matrix is transformed into an interaction matrix with two columns. The first column contains source nodes and the second column contains target nodes. Second, we use Glay’s algorithm to cluster large GRNs and obtain M modules named C i (i = 1, 2,…, M).
Step 3. Using the optimization algorithm to ensure that each node in the network belongs to one of all modules.
As we all know, many algorithms have been engineered to identify small and highly intra-connected clusters in a network, without clustering all the nodes [3, 23]. In Step 1, when selecting a threshold to remove edges, certain vectors (genes) might have been "kicked out" from the whole network. To ensure the integrity of the networks to be inferred, we provide the following optimization method.
Because all the genes that were removed must be returned to the whole network, we compute the mutual information value between each "kicked out" node and the nodes in all modules. For example, if gene x was removed from the whole network, the MI value should be calculated between x and each node in all modules C i (i = 1, 2,…, M). Then, we use formula (4) to search for the index with the maximum MI value between x and C i.
| (4) |
Therefore, gene x will be inserted into module . Finally, all genes are split into modules without any isolated genes.
2.3 Mathematical Model and Optimization
Generally, a GRN consisting of N genes can be modeled by a set of ordinary differential equations (ODEs) [4, 15]. In this study, the ODE model in each cluster C i can be described as follows.
| (5) |
The first term in the right of Equation. (5) represents the internal links between the same module C i and the second term represents the external links from other modules C l(l≠i)to the module C i. x j i(t) represents the express level of gene j at time t in module C i, a j ik represents the weight value of influence from gene k to gene j in module C i and T i is the gene number in the module C i. We denoted the set that links with module C i as the Candidate_set of C i. The value b j il describes the weight value of gene l in the set to gene j, y l i(t) is the expression level of gene l at time t in set and H iare the gene number in the set . Therefore, the purpose of reconstructing the GRNs is to optimize all weight values a j ikand b j ilin Equation. (5).
First, we take the difference for the left item of the above ODE equation.
| (6) |
Thus, we can obtain a linear system.
| (7) |
For the different time points tm, (m = 1, 2,…, T), we obtain the matrix form.
| (8) |
Where
The identification of all unknown coefficients P j i for module C i (i = 1, 2,…, M) and gene j in Equation. (8) can be transformed into the following least-square problem.
| (9) |
First, we calculate the condition number of matrix H i in equation. (9). If it is relatively large, we can use the QR factorization to solve the (9) to obtain all coefficients P j i. If the condition number of matrix H i is small, the regularization method is used. Therefore, the following normal equations are obtained:
| (10) |
We use the Gauss elimination with pivoting to solve the linear systems for all modules in parallel.
2.4 Parallel Computing Algorithm
2.4.1 Parallel computational environment
The Message Passing Interface (MPI) is a standardized and portable message-passing system designed by a group of researchers from academia and industry to function on a wide variety of parallel computers. The MPI provides a method based on a message passing parallel programming environment to communicate between the service processes. Its installation is presented in S1 Text. Generally, MPI has two main modes: Peer-to-peer mode and Master/Slaves mode. In this study, we choose Master/Slaves mode, and each slave can process one or several modules.
2.4.2 Calculate the connections between modules
To achieve communication between different slaves and improve the accuracy of constructing GRNs, we need to select the edges between different modules more accurately. First, we calculate and rank all MI values of two different modules C n and C m, respectively. Because of the sparsity of large-scale biological networks, we then select those the top 5%~ 10% rankings of MI values as representatives of the connections between every two modules. In this way, all separated modules can be linked through those edges.
2.4.3 Parallel design and asynchronous communications
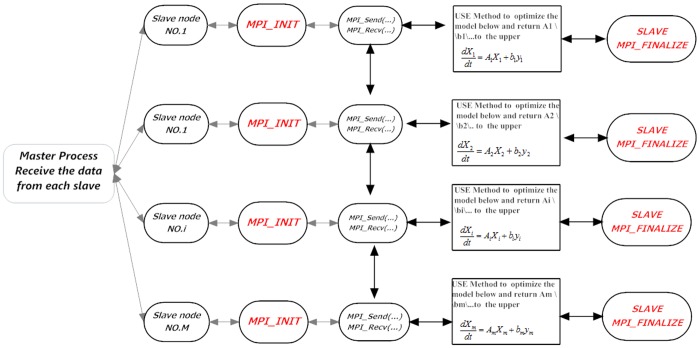
The proposed parallel framework is depicted in Fig. 2.
Fig 2. Outline of Parallel Computing.
Step 1. After finishing the splitting of network in Master, M subnetworks were sent to all Slaves.
For cases when the number of subnetworks/modules is larger than the number of computing nodes for the cluster system, we designed a special process to overcome the limitation of the number of SLAVES, which is described in S3 Text.
Step 2. For each module/Slave, we combine the ODE-based model and MLR to obtain the subnetworks and the connections among subnetworks. The algorithm is listed in Table 1.
Table 1. Algorithm 1.
| The pseudocode procedure for the parallel communications of the LSGPA. |
|---|
| for Slave i = 1 to i = M in Parrallel |
| →.Let Slave(i) MPI_Rev dataset(i) from Master, C i; |
| →.MPI_Brocast C i to all Slaves for 1 to M; |
| →.Compute I(x,y) for every pair of genes (x,y); |
| →.Give the threshold of MI values λ, if I(x,y)<λ then I(x,y) = 0, |
| else I(x,y) = 1 and edge (x,y) will be selected into C i (Candidate_set(i)); |
| →.MPI_Rev C i from Slaves1 to M |
| Compute MI values for every pair of edges (x,y j) x∈C i, y j∈C k,k = 1 to M, k≠i; |
| Rank all the MI values, selected top 5% value for every pair of edges into Candidate_set of C i. |
| →.Give the paameter threshold θ(**) |
| for j = 1 to T i |
| Solving the normal equations or using the QR factorization; |
| to obtain parameters |
| for l = 1 to T i +H i |
| if β l > θ |
| β l = 1 and edge(y k,x l) = 1; |
| else |
| β l = 1 and edge(y k,x l) = 0; |
| Endfor |
| Net(i).Matrix |
| Endfor |
| →MPI_Send Net(i).Matrix to Master; |
| Endfor |
Step 3. We send all network topologies represented by matrixes from modules/Slaves to the Master and recombine them as a whole network.
Results
All the numerical experiments were conducted on a high-performance parallel computing environment with a unified cluster system, which includes the Dawning cluster and HP cluster. The Dawning cluster has a peak speed of 19.64 TFlops and includes 93 computing nodes and two management nodes. Each node includes 2 CPUs, and each CPU includes 2 cores. The clock speed is 2.2 GHz, and the memory of each node is 128 GB. The HP cluster has a peak speed of 2.675 Tflops and includes 76 computing nodes and two management nodes. Each node includes 2 CPUs, and each CPU includes 2 cores. The clock speed is 2.2 GHz, and the memory of each node is 4 GB.
3.1 Datasets used in this study
As a well-known dataset, the DREAM5 challenge provided widely used benchmark networks with expression datasets (http://wiki.c2b2.columbia.edu/dream/index.php/D5c4) and gold standards. The E. coli dataset includes 334 TFs, 4511 target genes and 805 chips. In this study, we chose four subsets of different sizes from E. coli., i.e., including 92, 202, 1505 and 4511 genes. If the size of network is less than 4511, we simply selected genes at random. In this study, the gold-standard networks for all subsets are extracted from the overall gold-standard networks presented by DREAM5. We also selected one published dataset that contains more than 10 thousand genes [15].
3.2 Performance Evaluation
3.2.1 Result evaluation by different indexes
To evaluate the performance of LSGPA, four well-known indexes, namely true positive rate (TPR), false positive rate (FPR), positive predictive value (PPV) and accuracy (ACC) which are presented in S2 Text were calculated. To consider the overall performance of LSGPA, we also plot the receiver operating characteristic (ROC) curves. The initial networks with sizes from 92 to 4511 are depicted in S3–S6 Figs, respectively. The networks inferred by LSGPA are listed in S7–S10 Figs, and the details for those networks are shown in S2–S5 Tables, Moreover, the results for the different sizes are listed and compared in Tables 2–5, respectively.
Table 2. Comparison of four indexes on network with size 92.
| Methods | TPR | FPR | PPV | ACC |
|---|---|---|---|---|
| LSGPA | 0.7545 | 0.0443 | 0.1316 | 0.9553 |
| NARROMI | 0.7000 | 0.0500 | 0.1360 | 0.9220 |
| PCA-CMI(0-order) | 0.7222 | 0.1105 | 0.0095 | 0.8894 |
| PCA-CMI(1-order) | 0.7182 | 0.3777 | 0.0028 | 0.6225 |
| PCA-CMI(2-order) | 0.7182 | 0.3777 | 0.0028 | 0.6225 |
Remark: In all tables, the best results for the relative items are noted in bold.
Table 5. Comparison of four indexes on network with size 4511.
| Method | TPR | FPR | PPV | ACC |
|---|---|---|---|---|
| LSGPA | 0.4840 | 3.6150e-04 | 0.0863 | 0.9996 |
| NARROMI | 0.2780 | 0.0007 | 0.0650 | 0.9987 |
| PCA-CMI(0-order) | 0.3819 | 0.3238 | 0.0020 | 0.6762 |
| PCA-CMI(1-order) | non | non | non | non |
| PCA-CMI(2-order) | non | non | non | non |
Table 4. Comparison of four indexes on network with size 1505.
| Method | TPR | FPR | PPV | ACC |
|---|---|---|---|---|
| LSGPA | 0.4179 | 0.0016 | 0.0573 | 0.9983 |
| NARROMI | 0.1770 | 3.1266e-4 | 0.0139 | 0.9994 |
| PCA-CMI(0-order) | 0.4240 | 0.3633 | 2.4162e-4 | 0.6366 |
| PCA-CMI(1-order) | 0.4664 | 0.4056 | 0.0003 | 0.5943 |
| PCA-CMI(2-order) | non | non | non | non |
From Table 2, we can observe that LSGPA performed almost the best among prominent methods including NARROMI [14] and the CMI-based path consistency algorithm [12]. For PCA-CMI, we calculated three cases in the zero-order, first-order and second-order. Tables 3–5 also show that our method significantly outperforms these popular methods in terms of false positives and accuracy. In particular, with increasing network size, the first-order and second-order PCA-CMI almost cannot work. Although zero-order PCA-CMI can obtain the results, the accuracy is quite low. These results demonstrated that LSGPA is suitable for reconstructing large-scale networks.
Table 3. Comparison of four indexes on network with size 202.
| Method | TPR | FPR | PPV | ACC |
|---|---|---|---|---|
| LSGPA | 0.6000 | 7.7257e-04 | 0.0882 | 0.9992 |
| NARROMI | 0.1837 | 0.0123 | 0.0176 | 0.9867 |
| PCA-CMI(0-order) | 0.1224 | 0.3426 | 4.2502e-04 | 0.6531 |
| PCA-CMI(1-order) | 0.2653 | 0.4003 | 0.0008 | 0.7993 |
| PCA-CMI(2-order) | 0.2653 | 0.4003 | 0.0008 | 0.7993 |
Remark: In all tables, the best results for the relative items are noted in bold.
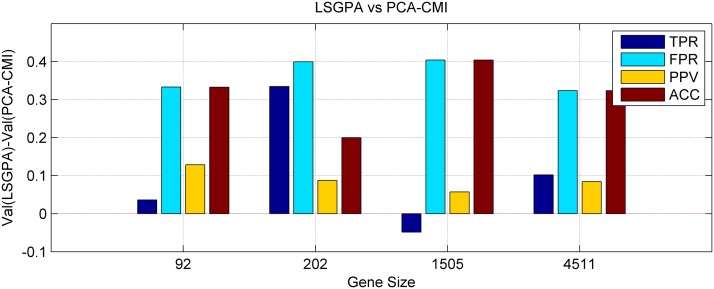
Figs. 3 and 4 depicted the difference of the LSGPA with other two methods NARROMI and PCA-CMI based on the four indexes, i.e., using the indicator value for the proposed LSGPA minus the value for other methods. We can clearly see that the vast majority of the comparison values are over the zero line, which means that the performance of our algorithm is much better than the other methods, especially in larger sets.
Fig 3. Difference between LSGPA and the two methods LSGPA and NARROMI in four indexes.
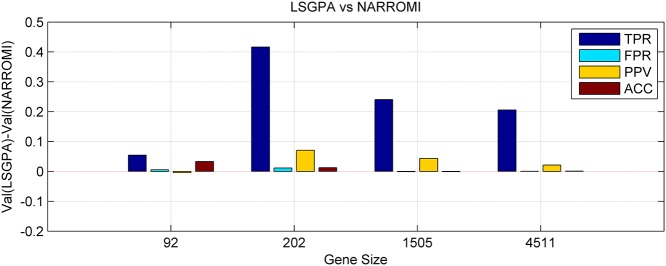
Val() in vertical axis represents one of the four indexes for different methods.
Fig 4. Difference of two methods LSGPA and PCA-CMI in four indexes.
Fig. 4 is more obvious: almost all the indexes from size 92 to 4511 are above zero except for TPR at size 1505, that is to say, LSGPA is widely superior.
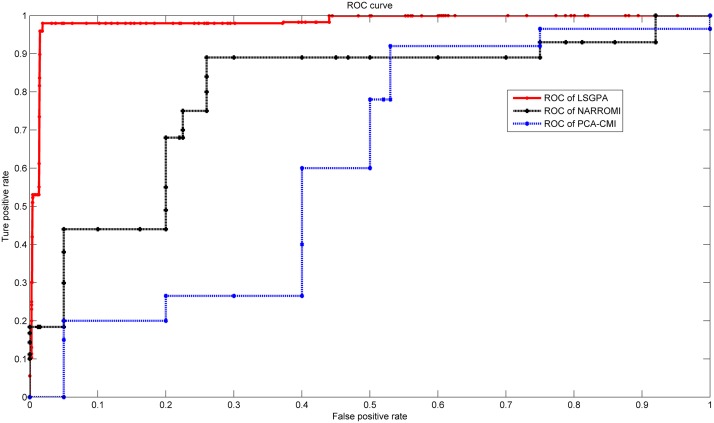
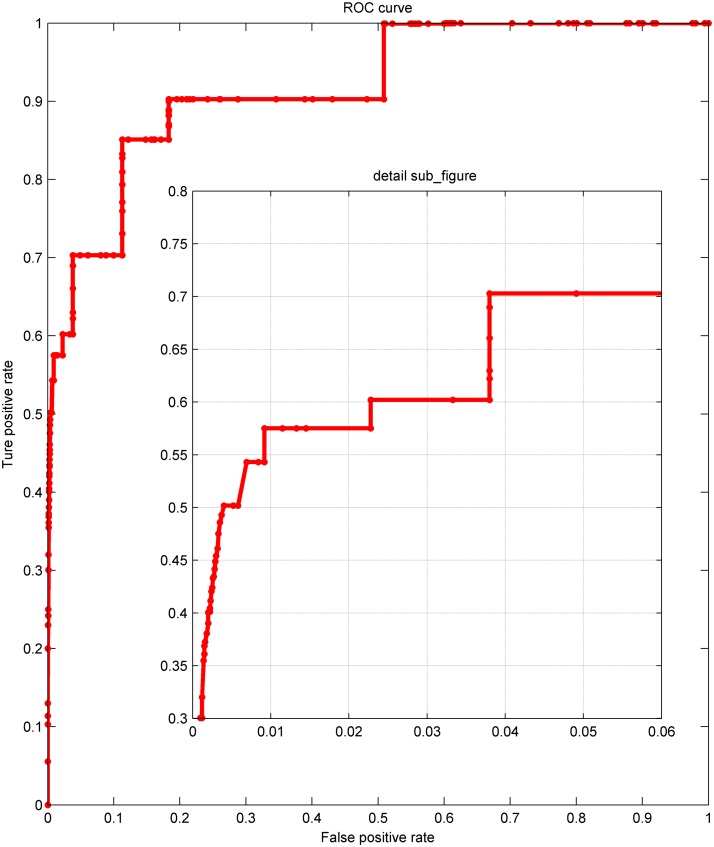
Figs. 5 and 6 displayed the overall performance of LSGPA with size 202 and 1505. these ROC curves indicate that LSGPA has reached a very high level. Because of the unacceptable runtime for calculating the ROC of NARROMI and PCA-CMI in size 1505, we just depicted the curve of LSGPA in Fig. 6.
Fig 5. ROC curves of LSGPA on DREAM5 in size 202.
Fig 6. ROC curves of LSGPA on DREAM5 in size 1505.
The subfigure shows the details.
3.2.2 The stability of the performance
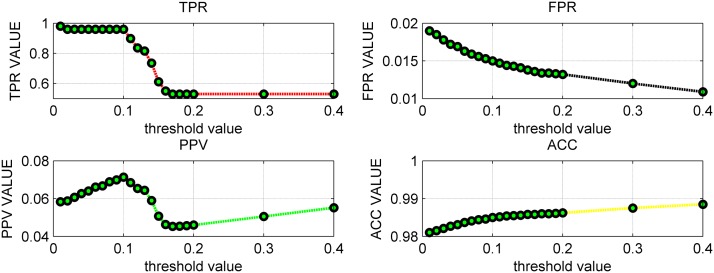
To test the stability of the performance of LSGPA, we selected different truncated threshold values θ of the identificated parameters in line (**) in the pseudocode procedure in section 2.4.3
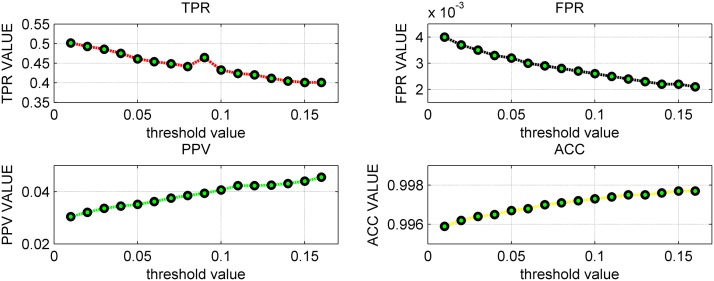
In Fig. 7 and S6 Table, the parameter threshold θ changed from 0 to 0.4 with 0.01 as the step length. Focusing on the TPR, it decreased with the increase of the threshold but stopped decreasing from 0.18 at 0.5306. FPR, PPV and ACC are all in the ideal state, for example, ACC varied from 0.9810 to 0.9885, and PPV changed between 0.0583 and 0.0552. Table 6 showed that the variances of the four indexes are all small, indicating the performance of LSGPA is not greatly influenced by the threshold value. For the size 1505, Fig. 8 (the detailed data are listed in S7 Table) and Table 7 demonstrated that all four indexes also maintained a stable level with some small fluctuations of the threshold value θ.
Fig 7. The calculated indexes for different parameter thresholds θ with size 202.
Table 6. Statistical analysis of four indexes on network with size 202.
| Indexes | TPR | FPR | PPV | ACC |
|---|---|---|---|---|
| Maximum | 0.9796 | 0.0190 | 0.0714 | 0.9885 |
| Minimum | 0.5306 | 0.0109 | 0.0453 | 0.9810 |
| Variance | 0.0382 | 4.2672e-06 | 8.1676e-05 | 3.4771e-06 |
Fig 8. The calculated indexes for different thresholds θ with size 1505.
Table 7. Statistical analysis four indexes on network with size 1505.
| Indexes | TPR | FPR | PPV | ACC |
|---|---|---|---|---|
| Maximum | 0.5018 | 0.0040 | 0.0455 | 0.9977 |
| Minimum | 0.4011 | 0.0021 | 0.0304 | 0.9959 |
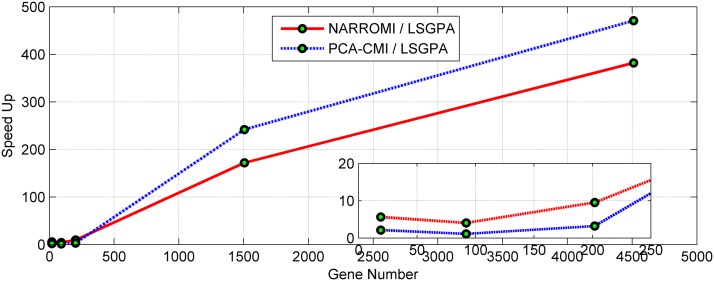
3.2.3 Comparisons of runtime and speed up
To measure the efficiency of LSGPA, the runtime and speed up of the algorithm were recorded and compared. In this section, we first compared the time consumption of LSGPA and two famous methods NARROMI and PCA-CMI by running them on the same computer environment. The method for calculating the runtime is shown in S4 Text.
Furthermore, we computed the speed up of LSGPA using the following formula, i.e., the runtime of another method over the runtime of the LGSPA in the same computing environment.
| (11) |
This definition means that larger values correspond to better performance. Compared with NARROMI and PCA-CIM, Fig. 9 shows that as the network size increases, the speed-up of the LSGPA increases exponentially. These results indicate that the proposed parallel algorithm achieved a promising speed-up and thus can be used to handle large-scale data sets effectively.
Fig 9. Speed up of the LSGPA against NARROMI and PCA-CIM for different gene numbers from E.coli.
To further prove its effectiveness, the LSGPA was also applied to construct GRNs from gene expression datasets in [15] with more than 10 thousand genes. In this dataset, there are 12488 target genes and 939 transfer factor genes, and 245 samples for each gene can be found in the expression dataset. Fig. 10 depicts the speedup of the LSGPA against Genie3 for different gene numbers in these datasets. The results further demonstrated the effectiveness of the LSGPA.
Fig 10. Speed up of the LSGPA against Genie3 for different gene numbers from datasets in [13].
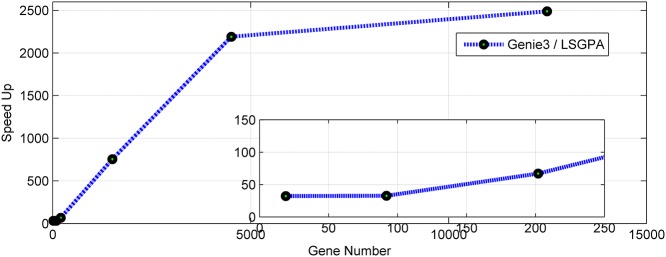
Table 8 lists the runtimes of different methods with different sizes. From size 92 to size 4511, LSGPA took less than 5 seconds, while NARROMI took over 1500 seconds, and PCA-CMI zero-order took almost 2000 seconds and also led to low accuracy. Note especially that in PCA-CMI, time cannot be counted for more than zero-order when dealing with sizes above 1505. These results indicate that the proposed algorithm is capable of handling large-scale data sets effectively.
Table 8. Runtimes for different sets with different sizes (seconds).
| Methods\Sizes | 92 | 202 | 1505 | 4511 |
|---|---|---|---|---|
| LSGPA | 1.382400 | 1.654020 | 2.354940 | 4.183680 |
| NAROMI | 5.606153 | 15.781613 | 404.341643 | 1597.859435 |
| PCA-CMI(0-order) | 1.530520 | 5.324213 | 569.249900 | 1969.614691 |
| PCA-CMI(1-order) | 56.062523 | 633.741694 | 2.6557e+005 | non |
| PCA-CMI(2-order) | 1943.272243 | 4.9291e+04 | non | non |
Discussion and Conclusion
In this study, we proposed a novel asynchronous parallel framework (namely LSGPA) to improve the accuracy and lower the time complexity of large-scale GRN inference, by combining splitting technology and ordinary differential equation (ODE)-based optimization.
Our study makes three main contributions. First, we split the whole large-scale GRNs into many small-scale modular subnetworks by using the sparsity and modularity of large-scale GRNs. Second, combining ODE-based optimization of all subnetworks in parallel and their asynchronous communications, the connected subnetworks from all modules/Slaves are sent to the Master and the large-scale networks can be easily obtained. Third, we used different performance indexes, i.e., the well-known accuracy measures, stability measure, runtime and speedup to test the performance of the proposed approach in comparison with several popular algorithms on the same high-performance computing environments. The numerical results showed that the proposed LSGPA can be used effectively to infer large-scale GRNs with high precision and the computational time can be largely reduced.
Although the proposed LSGPA was mainly used for the reconstruction of GRNs, it can also be extended to infer the undirected networks. However, because of the complexity of connections and communications between different modules/ undirected subnetworks, we need to use the optimization algorithm to search for optimal connections between different Slaves [7, 26]. The detailed description is presented in S5 Text and the results are depicted in S11 Fig. The four indexes are compared in S8 Table. For the further work, we will make the dynamical analysis based on the constructed networks [27, 28].
In summary, we established a paradigm for inferring large-scale GRNs from large-scale high-throughput data by combining a splitting technique and optimization-based asynchronous parallel communications. The novel framework of the asynchronous parallel algorithm can be applied to solve related large-scale problems presented by large-scale omics data.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors thank the anonymous reviewers for their helpful comments and suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Major Research Plan of the National Natural Science Foundation of China (No. 91230118), the National Basic Research Program of China (973 Program) (No. 2013CB911100), the Chinese National Natural Science Foundation (No. 61173060) and the Fundamental Research Funds for the Central Universities (No. 2014201020201). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A, et al. Reverse engineering of regulatory networks in human B cells. Nat Genet. 2005; 37: 382–390. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Yi M, Zou X. The linear interplay of intrinsic and extrinsic noises ensures a high accuracy of cell fate selection in budding yeast. Sci Rep. 2014; 4: 5764 10.1038/srep05764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marbach D, Prill RJ, Schaffter T, Mattiussi C, Floreano D, Stolovitzky G, et al. Revealing strengths and weaknesses of methods for gene network inference. Proc Natl Acad Sci U S A. 2010; 107: 6286–6291. 10.1073/pnas.0913357107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brunel H, Gallardo-Chacon JJ, Buil A, Vallverdu M, Soria JM, Caminal P, et al. MISS: a non-linear methodology based on mutual information for genetic association studies in both population and sib-pairs analysis. Bioinformatics. 2010; 26: 1811–1818. 10.1093/bioinformatics/btq273 [DOI] [PubMed] [Google Scholar]
- 5. Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005; 4: Article17 [DOI] [PubMed] [Google Scholar]
- 6. Chen L, Xuan J, Riggins RB, Wang Y, Hoffman EP, Clarke R, et al. Multilevel support vector regression analysis to identify condition-specific regulatory networks. Bioinformatics. 2010; 26: 1416–1422. 10.1093/bioinformatics/btq144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin S, Li Y, Pan R, Zou X. Characterizing and controlling the inflammatory network during influenza A virus infection. Sci Rep. 2014; 4: 3799 10.1038/srep03799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin S, Zou X. Construction of the influenza A virus infection- induced cell-specific inflammatory regulatory network based on mutual information and optimization.BMC Systems Biology. 2013; 7:105 10.1186/1752-0509-7-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frenzel S, Pompe B. Partial mutual information for coupling analysis of multivariate time series. Phys Rev Lett. 2007; 99: 204101 [DOI] [PubMed] [Google Scholar]
- 10. Liang KC, Wang X. Gene regulatory network reconstruction using conditional mutual information. EURASIP J Bioinform Syst Biol. 2008; 2008: 253894 10.1155/2008/253894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo W, Hankenson KD, Woolf PJ. Learning transcriptional regulatory networks from high throughput gene expression data using continuous three-way mutual information. BMC Bioinformatics. 2008; 9: 467 10.1186/1471-2105-9-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X, Zhao XM, He K, Lu L, Cao Y, Liu JD, et al. Inferring gene regulatory networks from gene expression data by path consistency algorithm based on conditional mutual information. Bioinformatics. 2012; 28: 98–104. 10.1093/bioinformatics/btr626 [DOI] [PubMed] [Google Scholar]
- 13. Aghdam R, Ganjali M, Eslahchi C. IPCA-CMI: an algorithm for inferring gene regulatory networks based on a combination of PCA-CMI and MIT score. PLoS One. 2014; 9: e92600 10.1371/journal.pone.0092600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang X, Liu K, Liu ZP, Duval B, Richer JM, Zhao XM, et al. NARROMI: a noise and redundancy reduction technique improves accuracy of gene regulatory network inference. Bioinformatics. 2013; 29: 106–113. 10.1093/bioinformatics/bts619 [DOI] [PubMed] [Google Scholar]
- 15. Qin J, Hu Y, Xu F, Yalamanchili HK, Wang J. Inferring gene regulatory networks by integrating ChIP-seq/chip and transcriptome data via LASSO-type regularization methods. Methods. 2014; 67: 294–303. 10.1016/j.ymeth.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 16. Lee WP, Hsiao YT, Hwang WC. Designing a parallel evolutionary algorithm for inferring gene networks on the cloud computing environment. BMC Syst Biol. 2014; 8: 5 10.1186/1752-0509-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altay G, Emmert-Streib F. Revealing differences in gene network inference algorithms on the network level by ensemble methods. Bioinformatics. 2010; 26: 1738–1744. 10.1093/bioinformatics/btq259 [DOI] [PubMed] [Google Scholar]
- 18. Nadakuditi RR, Newman ME. Graph spectra and the detectability of community structure in networks. Phys Rev Lett. 2012;108: 188701 [DOI] [PubMed] [Google Scholar]
- 19. Zhao XM, Wang RS, Chen L, Aihara K. Uncovering signal transduction networks from high-throughput data by integer linear programming. Nucleic Acids Res. 2008; 36: e48 10.1093/nar/gkn145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao XM, Cheung YIUM, Muang DES. Analysis of Gene Expression Data Using Rpem Algorithm in Normal Mixture Model with Dynamic Adjustment of Learning Rate. International Journal of Pattern Recognition and Artificial Intelligence. 2010; 24: 651–666. [Google Scholar]
- 21. Clauset A, Newman ME, Moore C. Finding community structure in very large networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004; 70: 066111 [DOI] [PubMed] [Google Scholar]
- 22. Radicchi F, Castellano C, Cecconi F, Loreto V, Parisi D. Defining and identifying communities in networks. Proc Natl Acad Sci U S A. 2004; 101: 2658–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, Zou X. A new method for detecting protein complexes based on the three node cliques. 2014, 10.1109/TCBB.2014.2386314 [DOI] [PubMed] [Google Scholar]
- 24. Tan J, Pan R, Qiao L, Zou X, Pan Z. Modeling and dynamical analysis of virus-triggered innate immune signaling pathways. PLoS One.2012; 7: e48114 10.1371/journal.pone.0048114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su G, Kuchinsky A, Morris JH, States DJ, Meng F. GLay: community structure analysis of biological networks. Bioinformatics. 2010;26: 3135–3137. 10.1093/bioinformatics/btq596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie W, Yu W, Zou X. Diversity-maintained differential evolution embedded with gradient-based local search, Soft Computing. 2013; 17 (8): 1511–1535. [Google Scholar]
- 27. Wang Y, Tan J, Sadre-Marandi F, Liu J, Zou X. Mathematical Modeling for Intracellular Transport and Binding of HIV-1 Gag Proteins. Mathematical Bio., 2015; 10.1016/j.mbs.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 28. Tan J, Zou X. Complex Dynamical Analysis of a Coupled Network from Innate Immune Responses. International Journal of Bifurcation and Chaos.2013; 23: 1350180 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.