Abstract
Background and Purpose
Heme oxygenase-1 (HO-1) catalyzes the rate-limiting reaction of heme breakdown, and may have both antioxidant and pro-oxidant effects. In prior studies, HO-1 overexpression protected astrocytes from heme-mediated injury in vitro. In the present study, we tested the hypothesis that selective astrocyte overexpression of HO-1 improves outcome after intracerebral hemorrhage (ICH).
Methods
Male and female transgenic mice overexpressing human HO-1 driven by the GFAP promoter (GFAP.HMOX1) and wild-type controls received striatal injections of autologous blood (25 μl). Blood-brain barrier disruption was assessed by Evans blue assay and striatal cell viability by MTT assay. Neurological deficits were quantified by digital analysis of spontaneous cage activity, adhesive removal, and elevated body swing tests.
Results
Mortality rate for wild-type mice was 34.8% and was similar for males and females; all GFAP.HMOX1 mice survived. Striatal Evans blue leakage at 24 hours was 23.4+/−3.2 ng in surviving WT mice, compared with 10.9+/−1.8 ng in transgenics. Peri-hematomal cell viability was reduced to 61±4% of contralateral at 3 days in WT mice, v. 80±4% in transgenics. Focal neurological deficits were significantly reduced in GFAP.HMOX1 mice, and spontaneous cage activity was increased.
Conclusions
Selective HO-1 overexpression in astrocytes reduces mortality, blood-brain barrier disruption, peri-hematomal cell injury, and neurological deficits in an autologous blood injection ICH model. Genetic or pharmacologic therapies that acutely increase astrocyte HO-1 may be beneficial after ICH.
Keywords: Blood-brain barrier, Heme Oxygenase, Preconditioning, Stroke
Introduction
The heme oxygenases catalyze the breakdown of heme to carbon monoxide, ferrous iron, and biliverdin, which is subsequently reduced to bilirubin. The consequences of this reaction on outcome in hemorrhagic stroke models have been quite variable to date. Mice lacking heme oxygenase-1 (HO-1), the inducible isoform, sustained less injury and inflammation than their wild-type (WT) counterparts after collagenase-induced intracerebral hemorrhage (ICH).1 Unconditional knockout of HO-2, the predominant neuronal isoform, had the opposite effect, but was somewhat protective in the blood injection ICH model.2, 3 HO inhibitors, which have less utility in mechanistic studies due to their multiple off-target effects, have also been variably protective or deleterious.4–6
These conflicting reports may be reconciled by consideration of the diverse biological activity of heme breakdown products and the selective vulnerability of neurons to iron.7 In cell culture, neuron loss after hemin or hemoglobin exposure is increased by HO-2 expression.8, 9 This effect is mitigated by protein or low molecular weight iron chelators, resulting in net neuroprotection that may be mediated by the other reaction products.3, 10, 11 Astrocytes, which are relatively resistant to acute iron toxicity due to ferritin induction,12 are protected from hemin by both HO-1 and HO-2 in the absence of exogenous chelators.13, 14 The effect of HO expression in any model may therefore depend on the cell population at greatest risk and the iron binding capacity of the cellular microenvironment.
These results suggest that nonselective targeting of HO expression or activity may not be an optimal therapeutic strategy. The efficacy of therapies that modulate HO in specific cell populations in vivo has not been defined. We have previously reported that increasing HO-1 expression in astrocytes was protective in a cell culture model of acute hemin toxicity.15 In the present study, we tested this concept in vivo, using transgenic mice expressing human HO-1 controlled by the GFAP promoter and an established ICH model.
Methods
Animals
Mice were transferred from the Schipper lab to Thomas Jefferson University where they were bred in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All protocols were approved by the local Institutional Animal Care and Use Committee. Mice expressing human HO-1 tagged with the Flag peptide in astrocytes (GFAP.HMOX1 mice, FVB background) were generated as previously described,16 and were used when 3–6 months old. Although the transgene incorporates a tetracycline-controllable regulatory system (“TET-OFF”), tetracyclines were not administered due to their effects in stroke models. Genotype was verified by PCR using DNA obtained from tail clippings and the following primers:
HMOX1 Forward: 5′-CGG CTC ATG ATG TCT AGA TTA GA -3′
Reverse: 5′-AAT TAG AAT TCT CGC GCC CCC TA -3′
tTA (tetracycline activator) Forward: 5′-CGC TGA GGA TCC ATG GAC TAC AAA GAC GAT -3′
Reverse: 5′-CGT GCA TCT AGA TCA CAT GGC ATA AAG CCC -3′
ICH Model
Mice were randomly selected from WT and GFAP.HMOX1 cohorts of similar age, and were anesthetized with 2% isoflurane in oxygen delivered via a nose mask. Temperature was monitored with a rectal probe and maintained at 37±0.5°C with a heating lamp. The scalp was incised and a burr hole was made at the following stereotactic coordinates relative to bregma: 2.5 mm lateral, 0.5 mm rostral. Blood obtained from a tail vein was loaded into a Hamilton syringe with 28 gauge needle, which was inserted 3.0 mm below the skull surface. Injection rate was 1 μl/min using a pump (Harvard Apparatus, Holliston, MA); total injected volume was 25 μl. Ten minutes later, the needle was removed, skin was sutured, and the animal was allowed to recover in a warm environment. Mice were observed for signs of distress hourly for the first 4 h and then daily.
Injury Assessment
Outcome was assessed over 7 days after ICH by researchers blinded to genotype.
Blood-Brain Barrier Permeability Assay
Mice were treated with 4 ml/kg i.p. of 2% Evans blue in sterile saline. Three hours later, they were perfused with 50 ml PBS via left ventricular injection under isoflurane anesthesia, followed by cervical dislocation. Striata were removed, and Evans blue was extracted according to the method of Uyama et al.17 Fluorescence (ex: 620 nm, em: 680 nm) of the extract solution was quantified, and striatal Evans blue content was interpolated from a standard curve.
MTT Assay
Perihematomal cell viability was quantified by MTT (methylthiazolyldiphenyl-tetrazolium bromide) assay of striatal cell suspensions.18 MTT, like the related TTC (2,3,5-triphenyltetrazolium chloride), is reduced by viable cells to a formazan dye; the product is readily extracted from cells and quantified by spectrophotometry. Tissue dissociation facilitates the delivery of an equal concentration of MTT to all cells. This approach is preferred to analysis of tetrazolium-stained tissue sections because experimental ICH does not produce a discrete infarct but rather a diffuse area of incomplete cell loss.19
Mice were euthanized by cervical dislocation after isoflurane anesthesia. Striata were removed by microdissection with Dumont forceps under a Stereomaster microscope (Fisher Scientific). Tissue was dissociated by trituration in 1 ml Hanks Balanced Salt Solution containing 27.8 mM glucose, 20.5 mM sucrose, and 4.2 mM sodium bicarbonate. The cell suspension was treated with 0.125 mg/ml MTT for 4 minutes in a 37°C water bath. Cells were then collected by centrifugation, and the formazan reaction product was extracted with 2 ml isopropanol. Absorbance (562 nm) of the solution obtained from each blood-injected striatum was normalized to that of the contralateral striatum to yield an index of cell viability. The close agreement of this method with perihematomal injury quantified by cell counts of striatal tissue sections has been established.2, 18, 20
Behavioral Outcome Measures
Functional outcome was assessed as previously described,21 via: 1) quantification of spontaneous cage activity in 1 hour videos, using Homecage Scan (CleverSys., Reston, VA USA); 2) adhesive removal test; 3) elevated body swing test.
Immunoblotting and Immunostaining
The following antibodies were used: 1) Anti-HO-1, Enzo Life Sciences, Farmingdale, NY (Cat #ADI-SPA-895), 1:4000 dilution for immunoblotting and 1:250 for immunostaining; 2) Anti-GFAP, InVitrogen (Cat #130300), 1:1000 dilution; 3) Anti-flag M2 antibody, Sigma-Aldrich (Cat #F1804) 1:250 dilution. Methods have been detailed previously.18, 20
HO-1 ELISA
Mouse and human HO-1 were quantified in injected and contralateral striata using ELISA kits marketed by Enzo Life Sciences (Cat. #ADI-960-071 and ADI-EKS-800, respectively), following the manufacturer’s instructions.
Hemoglobin assay
Blood (10 μl) collected from a tail vein was added to 5 μl of 3.5% sodium citrate to prevent clotting. Cells were lysed by passage through 3 freeze-thaw cycles. After centrifugation to remove debris, hemoglobin concentration was determined by the method of Winterbourne.22
Statistical Analyses
Mortality data were analyzed with Fisher’s exact test. Other data were analyzed with one-way ANOVA and the Bonferroni multiple comparisons test.
Results
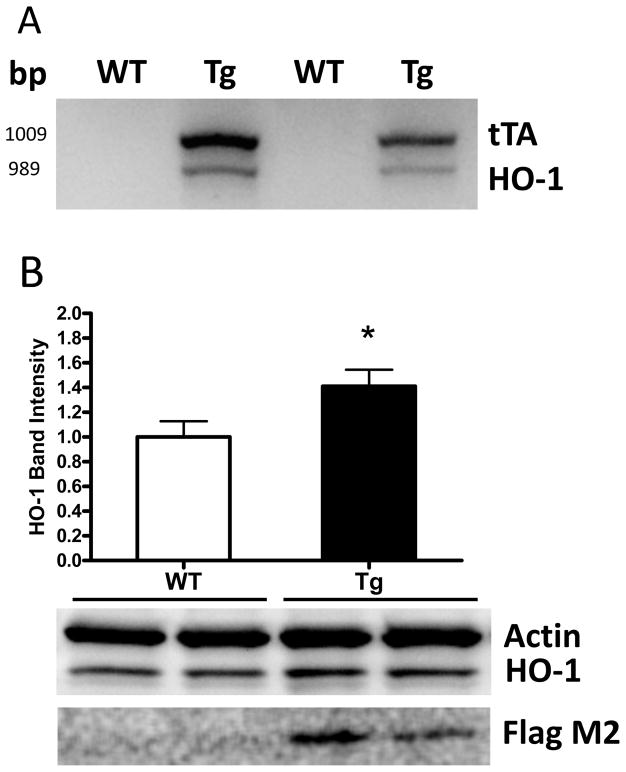
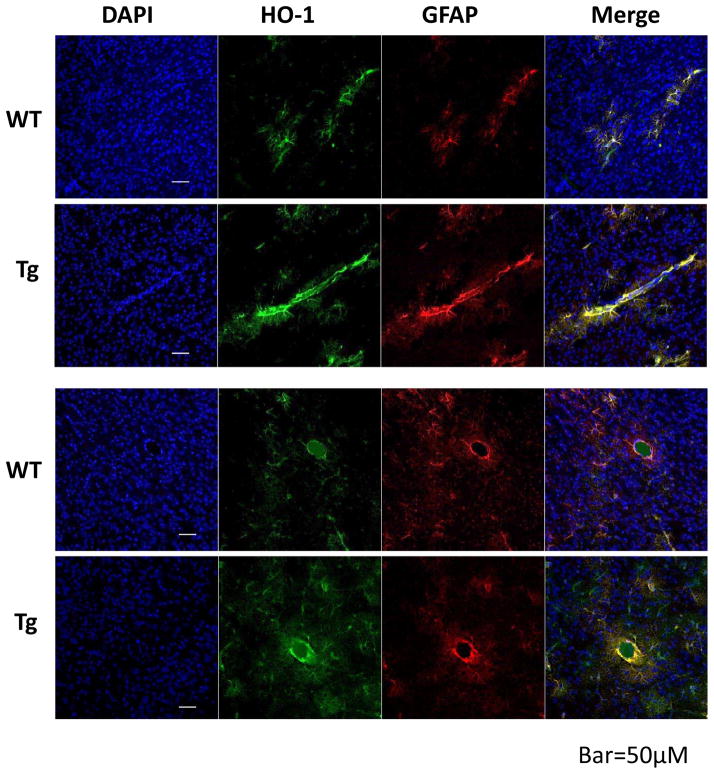
A total of 139 mice were used in these experiments (71 males and 68 females). The presence of the HMOX1 and tTA genes in transgenic mice was confirmed by genotyping (Fig 1A). Immunoblotting demonstrated a moderate increase in striatal HO-1 expression in GFAP.HMOX1 mice compared with their WT counterparts (Fig. 1B). Double immunofluorescence staining indicated that striatal HO-1 expression was predominantly localized to GFAP+ astrocytes in both wild-type and transgenic mice (Fig 2). Increased HO-1 immunoreactivity was apparent in the latter group, particularly in proximity to microvessels. Mean blood hemoglobin concentration was very similar in WT and GFAP.HMOX1 mice (7.75±0.13 mM and 7.74±0.12 mM heme, respectively).
Figure 1.
(A) PCR genotyping products demonstrating presence of Flag-HMOX1 (HO-1) and tTa genes in GFAP.HMOX1 mice. (B) Immunoblots of striatal lysates of GFAP.HMOX1 transgenic (Tg) mice and WT controls, demonstrating increased HO-1 in transgenics; expression of transgene is confirmed by immunoreactivity to Flag peptide. Bar order correlates with lane order. *P<0.05 vs. WT, 6/condition.
Figure 2.
Increased striatal HO-1 expression in GFAP.HMOX1 (Tg) mice. Sections were immunostained with antibodies to HO-1 and GFAP. Increased HO-1 expression is apparent in GFAP.HMOX1 mice, particularly in perivascular astrocytes.
Reduced mortality in GFAP.HMOX1 mice
Sixteen of forty-six WT mice died within 24 hours of blood injection (Table 1), with similar mortality rates in males and females. No deaths were observed at later time points. All transgenic mice survived until completion of the experiment. Mortality reduction was significant for both males and females.
Table 1.
Astrocyte HO-1 overexpression reduces mortality after ICH. Mortality rates in wild type (WT) and GFAP.HMOX1 mice (Tg HO-1) after striatal injection of 25 μl autologous blood. All deaths occurred within 24 hours.
| WT | Tg HO-1 | ||||
|---|---|---|---|---|---|
| Mortality | N | Mortality | N | P value | |
| Male | 30.4% | 23 | 0 | 22 | 0.0092 |
| Female | 39.1% | 23 | 0 | 21 | 0.0016 |
| Both | 34.8% | 46 | 0 | 43 | <0.0001 |
Astrocyte HO-1 overexpression reduces blood-brain barrier disruption
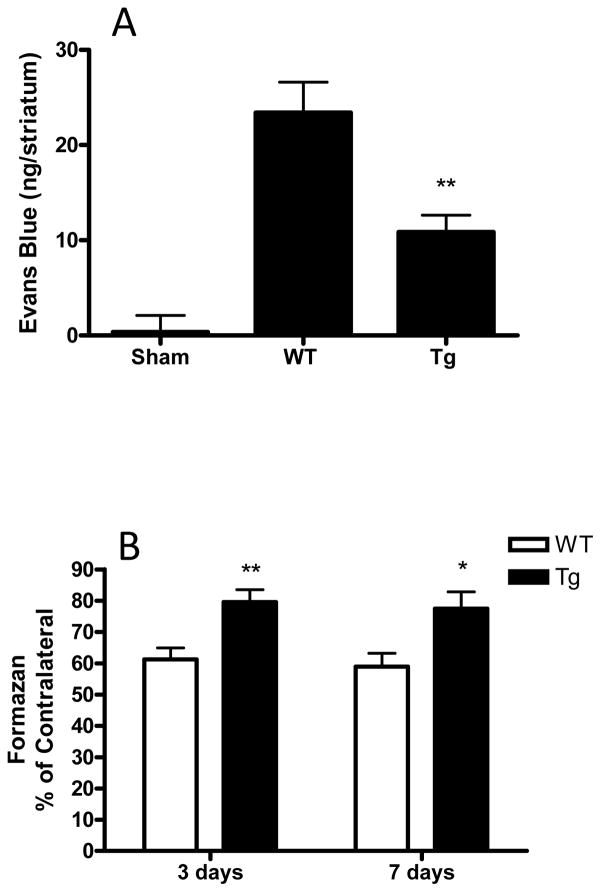
We have recently reported that systemic hemin therapy induces HO-1 overexpression in perivascular cells and reduces blood-brain barrier injury after experimental ICH.21 Since astrocyte HO-1 overexpression is particularly prominent in GFAP.HMOX1 mice adjacent to vessels (Fig 2), blood-brain barrier integrity was assessed 24 hours after striatal blood injection. Evans blue leakage into the striatal parenchyma at this time point in GFAP.HMOX1 mice was less than half of that in WT mice (Fig. 3A).
Figure 3.
Astrocyte HO-1 overexpression reduces blood-brain barrier breakdown and perihematomal cell death after ICH. (A) Evans blue leakage into the striatum was quantified 24 hours after ICH, 7–8/condition; (B) Striatal cell viability was quantified 3 and 7 days by MTT assay, 5–12/condition. *P<0.05, **P<0.01 vs. WT.
Reduced peri-hematomal cell loss in GFAP.HMOX1 mice
Striatal blood injection resulted in loss of approximately 40% of cells in the ipsilateral striata of WT mice, with minimal change between days 3 and 7 (Fig. 3B). Striatal cell viability was significantly improved in transgenic mice (p<0.05–0.01 vs. WT controls).
Effect of ICH on HO-1 expression
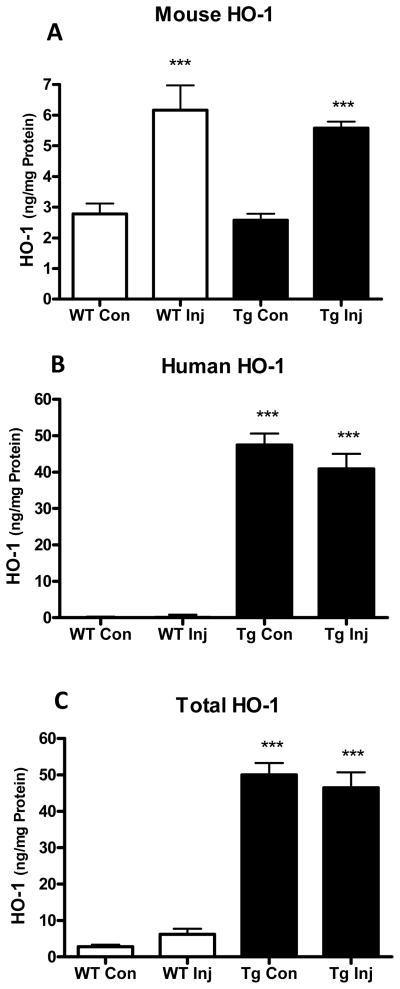
Striatal HO-1 was quantified 7 hours after blood injection using ELISA kits specific for mouse or human HO-1. Consistent with prior observations,23 native mouse HO-1 expression in injected striata was increased ~2.2-fold compared with contralateral in both WT and GFAP.HMOX1 mice (Fig. 4). Human HO-1 was expressed at a higher level in transgenics and was similar in injected and contralateral striata.
Figure 4.
Striatal HO-1 expression after ICH. Bars indicate mean mouse (A), human (B) and total (C) HO-1 in injected (Inj) and contralateral (Con) striata of WT and GFAP.HMOX1 (Tg) mice (±S.E.M., 6/condition) 7 hours after striatal blood injection. ***P < 0.001 vs. contralateral expression (A) or corresponding WT condition (B,C).
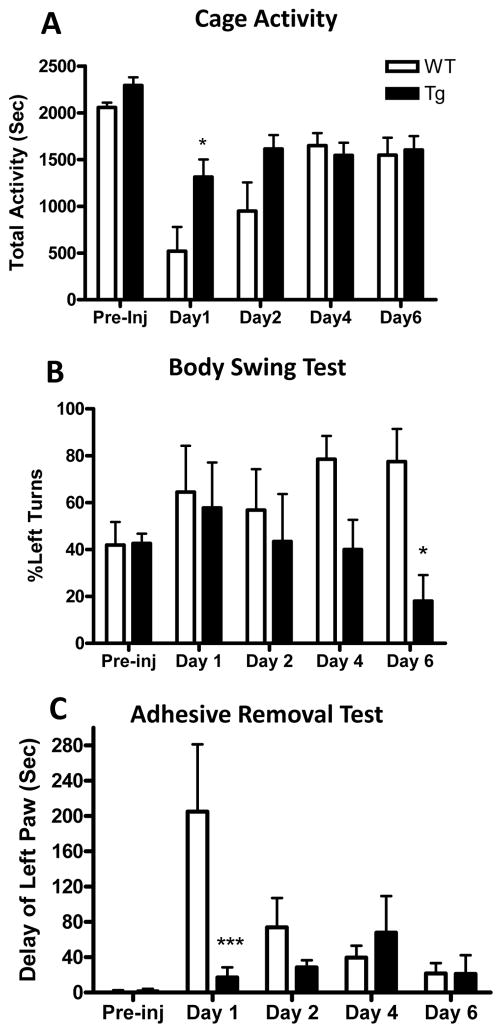
Effect of astrocyte HO-1 overexpression on behavioral outcome
Spontaneous cage activity prior to striatal blood injection was similar in WT and GFAP.HMOX1 mice. It was decreased by ~75% in WT mice 24 h after injection, compared with ~40% in transgenics (p<0.05, Fig. 5). A similar benefit at this early time point only was observed in the adhesive removal test (p<0.001); surviving WT mice rapidly regained function. A persistent deficit was observed in the elevated body swing test in WT mice, and benefit was noted in transgenics at a later time point.
Figure 5.
Astrocyte HO-1 overexpression reduces neurological deficits. Bars represent mean values (±SEM, 5–7/condition) at indicated days after striatal blood injection for: A) activity time (sum of seconds walking, feeding, vertical hanging, rearing and jumping during 1 hour recording); B) percentage left swings; C) left minus right adhesive removal times.
Discussion
This study provides the first evidence that selective over-expression of HO-1 in astrocytes is beneficial after ICH. The GFAP.HMOX1 transgene reduced mortality, an endpoint of obvious clinical relevance but an uncommon outcome measure in rodent ICH studies. Unequal mortality in WT and transgenic groups may have confounded the results of other planned outcome measures, since the most severely injured mice in only the transgenic group survived for later testing. While this bias would be predicted to diminish the observed benefit of astrocyte HO-1 overexpression, GFAP.HMOX1 mice nevertheless sustained significantly less blood-brain barrier injury, peri-hematomal cell loss, and sensorimotor deficits than surviving WT mice.
These observations are consistent with prior reports that peripheral hemin injection and ischemic preconditioning are protective in ICH models.21, 24 Both of these therapies increase CNS HO-1 expression, but also have pleiotropic effects on signaling pathways and gene expression that limit their utility as mechanistic probes. The present findings provide more specific evidence that HO-1 overexpression per se is sufficient to manifest protection. It is noteworthy that Zeynalov et al. observed that the benefit of ischemic preconditioning in a permanent middle cerebral artery occlusion model was lost in HO-1 knockout mice, consistent with an effect that also requires HO-1 expression.25
Astrocytes play a key role in the development and maintenance of the blood-brain barrier in the rodent CNS.26 Experimental evidence suggests that HO-1 expression may be essential for optimizing this function in an oxidative environment.27, 28 Alfieri et al.29 have recently reported that preconditioning stimuli increase HO-1 expression primarily in perivascular astrocytes, associated with preservation of barrier function in a rat transient middle cerebral artery occlusion model. Consistent with this observation, Evans blue leakage into the brain parenchyma was decreased by about half in GFAP.HMOX1 mice, which overexpress HO-1 prominently in perivascular astrocytes. These results add to the growing body of evidence that HO-1 improves microvascular function after a wide variety of acute injuries, including cardiac,30 liver,31 and bowel32 ischemia-reperfusion, hemorrhagic shock,33 seizures,34 and sickle cell disease.35
The protective effect of astrocyte HO-1 overexpression after ICH observed in 3–6 month old GFAP.HMOX1 mice contrasts with the pathology that develops with aging in these transgenic animals. At 48 weeks, they exhibit behavioral disturbances characterized by hyperkinesia and impaired prepulse inhibition, associated with glial iron deposition, oxidative mitochondrial injury, macroautophagy and neuritic degeneration.16, 36 The adverse effect of this transgene in older mice suggests that therapies producing a long-term increase in astrocyte HO-1 expression may be ineffective or poorly tolerated. It also highlights the need to test therapies that increase astrocyte HO-1 in older animals.
Striatal blood injection is usually a nonlethal event in rodents. In prior studies from this laboratory, mortality in Swiss-Webster or C57BL/6 × 129 mice has ranged from 0–4%, which is comparable to that reported in multiple other studies.2, 21, 37, 38 The 34% mortality rate in WT FVB mice was unexpected and highlights the prominent influence of strain on vulnerability to hemorrhagic CNS injury. FVB mice have been used infrequently in acute CNS injury models, but reported results have been largely consistent with the present observations. Compared with C57BL/6 and other strains, FVB mice sustained more hippocampal neuron loss and mortality after injection of glutamate receptor agonists.39, 40 Consistent with increased vulnerability to excitotoxic stress, they also sustained larger infarcts and increased mortality after striatal endothelin injection combined with common carotid artery occlusion and/or L-NAME injection,41 and increased lesion size after spinal cord crush injury.42 Excitotoxicity has also been implicated in early injury after ICH,43, 44 and may be exacerbated by the effects of thrombin on glutamate release45, 46 and glutamate receptor responses.47 Further investigation of the mechanisms mediating early death of FVB mice after ICH is an important topic for future investigation. The injury produced by technically-feasible blood injection volumes in this strain may accurately reflect the injury severity of clinical ICH, which also has a ~34% in-hospital mortality in the United States.48 A moderate mortality rate facilitates its use as a primary outcome measure. Although most rodent studies are not designed to quantify the effect of genetic modifications or pharmacotherapies on mortality, this hard endpoint may be more predictive of clinical efficacy than surrogate injury markers.49
HO-1 expression is increased in microglia after ICH.50 The effect of microglial HO-1 on hemorrhagic CNS injury is unknown, but prior results suggest that it may be deleterious. Wang and Doré reported that microglial activation and perihematomal free radical levels were reduced in unconditional HO-1 knockout mice, consistent with a pro-inflammatory effect.1 This was associated with reduced mean lesion volume and early neurological deficits. The specific contribution of microglial HO-1 to ICH-related injury could be determined in future experiments using transgenic mice selectively overexpressing human HO-1 in the microglial compartment.
HO-1 is readily induced in cultured astrocytes, which are then robustly protected against heme-mediated injury.51 The translational potential of upregulating astrocyte HO-1 after ICH remains undefined but initial reports suggest feasibility. Systemic therapy with the nonselective inducers sulforaphane and hemin increased HO-1 in perivascular cells including astrocytes in naïve and injured rodent brains.21, 29 This resulted in improvement in barrier function and neurobehavioral outcome, with clinically relevant time windows in TBI and ICH models.21, 52 The present results provide a rationale for the testing of genetic and pharmacologic therapies that more specifically enhance HO-1 expression or activity in astrocytes after acute hemorrhagic injuries.
Acknowledgments
Funding Sources
This study was supported by grants from the NIH to RFR. (R01NS079500) and the Canadian Institutes for Health Research to HMS. WS is a senior scientist of The Mary Katz Claman Foundation.
Footnotes
Disclosures
HMS has served as consultant to Caprion Pharmaceuticals, Molecular Biometrics Inc., Osta Biotechnologies, TEVA Neurosciences, Immunotec, and Apopharma. Other authors report no disclosures.
References
- 1.Wang J, Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J Neurosurg. 2007;106:428–435. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Zhuang H, Doré S. Heme oxygenase 2 is neuroprotective against intracerebral hemorrhage. Neurobiol Dis. 2006;22:473–476. doi: 10.1016/j.nbd.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Wagner KR, Hua Y, de Courten-Myers GM, Broderick JP, Nishimura RN, Lu SY, et al. Tin-mesoporphyrin, a potent heme oxygenase inhibitor, for treatment of intracerebral hemorrhage: in vivo and in vitro studies. Cell Mol Biol. 2000;46:597–608. [PubMed] [Google Scholar]
- 5.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- 6.Sun BL, An W, Xia ZL, Zheng CB, Li WX, Yang MF, et al. Zinc protoporphyrin aggravates cerebral ischemic injury following experimental subarachnoid hemorrhage. Clin Hemorheol Microcirc. 2006;34:241–246. [PubMed] [Google Scholar]
- 7.Kress GJ, Dineley KE, Reynolds IJ. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–5855. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad Biol Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- 9.Regan RF, Chen J, Benvenisti-Zarom L. Heme oxygenase-2 gene deletion attenuates oxidative stress in neurons exposed to extracellular hemin. BMC Neurosci. 2004;5:34. doi: 10.1186/1471-2202-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regan RF, Rogers B. Delayed treatment of hemoglobin neurotoxicity. J Neurotrauma. 2003;20:111–120. doi: 10.1089/08977150360517236. [DOI] [PubMed] [Google Scholar]
- 11.Chen-Roetling J, Chen L, Regan RF. Apotransferrin protects cortical neurons from hemoglobin toxicity. Neuropharmacology. 2011;60:423–431. doi: 10.1016/j.neuropharm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohnholt MC, Dringen R. Uptake and metabolism of iron and iron oxide nanoparticles in brain astrocytes. Biochem Soc Trans. 2013;41:1588–1592. doi: 10.1042/BST20130114. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem Biophys Res Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- 14.Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Cultured astrocytes from heme oxygenase-1 knockout mice are more vulnerable to heme-mediated oxidative injury. J Neurosci Res. 2005;82:802–810. doi: 10.1002/jnr.20681. [DOI] [PubMed] [Google Scholar]
- 15.Benvenisti-Zarom L, Regan RF. Astrocyte-specific heme oxygenase-1 hyperexpression attenuates heme-mediated oxidative injury. Neurobiol Dis. 2007;26:688–695. doi: 10.1016/j.nbd.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, Zukor H, Lin SH, Liberman A, Tavitian A, Mui J, et al. Unregulated brain iron deposition in transgenic mice over-expressing HMOX1 in the astrocytic compartment. J Neurochem. 2012;123:325–336. doi: 10.1111/j.1471-4159.2012.07914.x. [DOI] [PubMed] [Google Scholar]
- 17.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 18.Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab. 2005;25:1466–1475. doi: 10.1038/sj.jcbfm.9600143. [DOI] [PubMed] [Google Scholar]
- 19.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 20.Chen-Roetling J, Lu X, Regan KA, Regan RF. A rapid fluorescent method to quantify neuronal loss after experimental intracerebral hemorrhage. J Neurosci Methods. 2013;216:128–136. doi: 10.1016/j.jneumeth.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Chen-Roetling J, Regan RF. Systemic hemin therapy attenuates blood-brain barrier disruption after intracerebral hemorrhage. Neurobiol Dis. 2014;70C:245–251. doi: 10.1016/j.nbd.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winterbourn CC. Oxidative reactions of hemoglobin. Meth Enzymol. 1990;186:265–272. doi: 10.1016/0076-6879(90)86118-f. [DOI] [PubMed] [Google Scholar]
- 23.Matz PG, Weinstein PR, Sharp FR. Heme oxygenase-1 and heat shock protein-70 induction in glia and neurons throughout rat brain after experimental intracerebral hemorrhage. Neurosurgery. 1997;40:152–160. doi: 10.1097/00006123-199701000-00034. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Karabiyikoglu M, Hua Y, Keep RF, Xi G. Ischemic preconditioning attenuates brain edema after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3:180–187. doi: 10.1007/s12975-012-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeynalov E, Shah ZA, Li RC, Dore S. Heme oxygenase 1 is associated with ischemic preconditioning-induced protection against brain ischemia. Neurobiol Dis. 2009;35:264–269. doi: 10.1016/j.nbd.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin Y, Vreman HJ, Wong RJ, Tjoa T, Yamauchi T, Noble-Haeusslein LJ. Heme oxygenase-1 stabilizes the blood-spinal cord barrier and limits oxidative stress and white matter damage in the acutely injured murine spinal cord. J Cereb Blood Flow Metab. 2007;27:1010–1021. doi: 10.1038/sj.jcbfm.9600412. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004;21:1017–1030. doi: 10.1089/0897715041651042. [DOI] [PubMed] [Google Scholar]
- 29.Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 30.Ockaili R, Natarajan R, Salloum F, Fisher BJ, Jones D, Fowler AA, 3rd, et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–548. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, et al. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg. 2007;245:931–942. doi: 10.1097/01.sla.0000256891.45790.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallick IH, Winslet MC, Seifalian AM. Ischemic preconditioning of small bowel mitigates the late phase of reperfusion injury: heme oxygenase mediates cytoprotection. Am J Surg. 2010;199:223–231. doi: 10.1016/j.amjsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Kubulus D, Mathes A, Pradarutti S, Raddatz A, Heiser J, Pavlidis D, et al. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock. 2008;29:583–590. doi: 10.1097/SHK.0b013e318157e526. [DOI] [PubMed] [Google Scholar]
- 34.Carratu P, Pourcyrous M, Fedinec A, Leffler CW, Parfenova H. Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures. Am J Physiol Heart Circ Physiol. 2003;285:H1148–1157. doi: 10.1152/ajpheart.00091.2003. [DOI] [PubMed] [Google Scholar]
- 35.Belcher JD, Vineyard JV, Bruzzone CM, Chen C, Beckman JD, Nguyen J, et al. Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. J Mol Med. 2010;88:665–675. doi: 10.1007/s00109-010-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song W, Zukor H, Lin SH, Hascalovici J, Liberman A, Tavitian A, et al. Schizophrenia-like features in transgenic mice overexpressing human HO-1 in the astrocytic compartment. J Neurosci. 2012;32:10841–10853. doi: 10.1523/JNEUROSCI.6469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen M, Regan RF. Time course of increased heme oxygenase activity and expression after experimental intracerebral hemorrhage: correlation with oxidative injury. J Neurochem. 2007;103:2015–2021. doi: 10.1111/j.1471-4159.2007.04885.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen L, Zhang X, Chen-Roetling J, Regan RF. Increased striatal injury and behavioral deficits after intracerebral hemorrhage in hemopexin knockout mice. J Neurosurg. 2011;114:1159–1167. doi: 10.3171/2010.10.JNS10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Royle SJ, Collins FC, Rupniak HT, Barnes JC, Anderson R. Behavioural analysis and susceptibility to CNS injury of four inbred strains of mice. Brain Res. 1999;816:337–349. doi: 10.1016/s0006-8993(98)01122-6. [DOI] [PubMed] [Google Scholar]
- 40.Schauwecker PE. Modulation of cell death by mouse genotype: differential vulnerability to excitatory amino acid-induced lesions. Exp Neurol. 2002;178:219–235. doi: 10.1006/exnr.2002.8038. [DOI] [PubMed] [Google Scholar]
- 41.Horie N, Maag AL, Hamilton SA, Shichinohe H, Bliss TM, Steinberg GK. Mouse model of focal cerebral ischemia using endothelin-1. J Neurosci Methods. 2008;173:286–290. doi: 10.1016/j.jneumeth.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inman D, Guth L, Steward O. Genetic influences on secondary degeneration and wound healing following spinal cord injury in various strains of mice. J Comp Neurol. 2002;451:225–235. doi: 10.1002/cne.10340. [DOI] [PubMed] [Google Scholar]
- 43.Qureshi AI, Ali Z, Suri MF, Shuaib A, Baker G, Todd K, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–1489. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- 44.Ardizzone TD, Lu A, Wagner KR, Tang Y, Ran R, Sharp FR. Glutamate receptor blockade attenuates glucose hypermetabolism in perihematomal brain after experimental intracerebral hemorrhage in rat. Stroke. 2004;35:2587–2591. doi: 10.1161/01.STR.0000143451.14228.ff. [DOI] [PubMed] [Google Scholar]
- 45.Bell JD, Thomas TC, Lass E, Ai J, Wan H, Lifshitz J, et al. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J Neurosurg. 2014;121:1424–1431. doi: 10.3171/2014.3.JNS132130. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Dominguez M, Hernandez-Benitez R, Pena Segura C, Pasantes-Morales H. Thrombin-facilitated efflux of D-[3H]-aspartate from cultured astrocytes and neurons under hyponatremia and chemical ischemia. Neurochem Res. 2014;39:1219–1231. doi: 10.1007/s11064-014-1300-8. [DOI] [PubMed] [Google Scholar]
- 47.Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. J Neurosci. 2000;20:4582–4595. doi: 10.1523/JNEUROSCI.20-12-04582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rincon F, Mayer SA. The Epidemiology of Intracerebral Hemorrhage in the United States from 1979 to 2008. Neurocrit Care. 2013;19:95–102. doi: 10.1007/s12028-012-9793-y. [DOI] [PubMed] [Google Scholar]
- 49.Zivin JA, Lyden PD, DeGirolami U, Kochhar A, Mazzarella V, Hemenway CC, et al. Tissue plasminogen activator. Reduction of neurologic damage after experimental embolic stroke. Arch Neurol. 1988;45:387–391. doi: 10.1001/archneur.1988.00520280033012. [DOI] [PubMed] [Google Scholar]
- 50.Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radic Biol Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Regan RF, Guo YP, Kumar N. Heme oxygenase-1 induction protects murine cortical astrocytes from hemoglobin toxicity. Neurosci Lett. 2000;282:1–4. doi: 10.1016/s0304-3940(00)00817-x. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]