Abstract
OBJECTIVES
To describe the inter-individual variability in physical function responses to supervised, resistance and aerobic exercise training interventions in older adults.
PARTICIPANTS
Ninety-five older (65–79 years), overweight and obese (body mass index [BMI] ≥27 kg/m2), sedentary men and women.
INTERVENTION
Five-months of either 4 d/wk of aerobic training (AT, n=40) or 3 d/wk of resistance training (RT, n=55).
MEASUREMENTS
Physical function assessments: global measure of lower extremity function (short physical performance battery; SPPB), 400-meter walk, peak aerobic capacity (VO2peak), and knee extensor strength.
RESULTS
On average, both exercise interventions significantly improved physical function. For AT, there was a 7.9% increase in VO2peak; individual absolute increases varied from 0.4–4.3 ml/kg/min and four participants (13%) showed no change or a decrease in VO2peak. For RT, knee extensor strength improved an average of 8.1%, but individual increases varied from 1.2–63.7 Nm, and 16 participants (30%) showed no change or a decrease in strength. Majority of participants improved 400-m walk time, usual gait speed, chair rise time, and SPPB with AT, and improved usual gait speed, chair rise time, and SPPB with RT; but, there was wide variation in the magnitude of improvement. Compliance was only related to change in 400-m walk time following RT (r= −0.31; p<0.05).
CONCLUSION
Despite sufficient levels of adherence to both exercise interventions, some participants did not improve function, and the magnitude of improvement varied widely. Additional research is needed to identify factors that optimize responsiveness to exercise to maximize its functional benefits in older adults.
Keywords: aerobic training, resistance training, muscle strength, peak aerobic capacity, response variability
INTRODUCTION
Aging is universally associated with declines in physical function that lead to mobility disability and loss of independence.1–5 Regular performance of exercise that increases6–9 muscle strength and aerobic capacity enhances the ability of older adults to tolerate the functional demands of activities of daily living and presumably remain mobile.10–16 Resistance exercise training (RT) improves the ability to perform functional tasks requiring strength (e.g., chair rising, overhead lifting, lateral mobility tasks),6–9 while aerobic exercise training (AT) improves one’s ability to perform functional tasks requiring repeated muscular contraction or endurance (e.g., walking, stair climbing).17;18 Therefore, the current public health recommendation is for older adults to engage in both muscle-strengthening and aerobic physical activity for the maintenance of functional abilities and prevention of disability.13
Despite the plethora of data showing an overall benefit of both RT and AT for improving, or maintaining, functional ability in older adults, there is likely to be large inter-individual variability in functional responses to exercise. Studies in younger adults show substantial individual heterogeneity in physiological responses and benefits to standardized exercise, including maximal aerobic capacity and muscle strength.19–22 There may even be some individuals who experience a negative response to regular exercise performance for certain outcomes.23;24 In older adults, exercise training studies that show efficacy for improving functional tasks often report main effects or mean group differences without expressing the extent of inter-individual variability for these tasks. Attention to individual differences, and identification of factors that influence individual efficacy of exercise as a therapy for aging-related loss of physical function, has important clinical significance. For example, some individuals may respond more favorably to, and be more likely to engage in, one type of exercise over another. Moreover, the specific amount of exercise necessary to elicit maximal improvements in physical outcomes may differ between individuals.
Little attention is also given to identifying determinants of individual differences in response to standard exercise training. Certainly, compliance to the exercise prescription is an important factor in determining the success of the intervention for improving function.10;25;26 Therefore, the purpose of this study is to describe the extent of inter-individual variability in physical function responses to supervised, resistance and aerobic exercise training interventions in older adults. We also determined whether intervention compliance, age, gender, race, comorbidity, and baseline level of physical function were associated with this variability.
METHODS
Participants
Ninety-five, older, overweight and obese men and women completed one of two five-month exercise interventions, aerobic training (AT, n=40) or resistance training (RT, n=55). Men and women from Forsyth and surrounding counties were recruited via media advertisement and mass mailings. Participants were enrolled based on the following criteria: (a) age 65–79 years, (b) sedentary (<2×/week of structured exercise), (c) BMI=27–40 kg/m2, (d) non-smoking for the past year, (e) normal cognitive function, (f) no evidence of clinical depression, heart disease, cancer, liver or renal disease, chronic pulmonary disease, uncontrolled hypertension, physical impairment or any contraindication for exercise. After a phone screen, those eligible underwent a subsequent medical history, physical exam, and physical function assessments. Both studies were approved by the Wake Forest School of Medicine Institutional Review Board and all participants provided written, informed consent to participate. Participants were not paid to participate in the study.
Exercise Interventions
Exercise sessions were center-based and supervised by two trained exercise physiologists who provided safety oversight and monitored compliance to the exercise frequency, duration, and intensity.
Participants in the Aerobic Training (AT) study exercised four days/week on treadmills. Each session commenced with a slow-pace five-minute warm-up and concluded with a five-minute cool-down and stretching. Participants walked at an intensity of 65–70% of heart rate reserve (HRR, assessed during the peak aerobic capacity test [VO2peak]). Exercise duration progressed from 15–20 minutes at 50% HRR the 1st week to 30 minutes at 65–70% HRR by the end of the 6th week and thereafter. Treadmill speed and grade were adjusted individually to insure that participants exercised at their prescribed intensity (as monitored by heart rate). At least two heart rate readings, treadmill speed, grade, exercise duration, and the amount of energy expended were recorded each session to monitor compliance to the exercise prescription.
Participants in the Resistance Training (RT) study exercised three days/week on weight stack resistance machines. Each session commenced with a slow-pace five-minute warm-up of walking or cycling and concluded with a five-minute cool-down and stretching. Interventionists taught participants how to adjust the equipment and how to perform the exercises safely. The machines used were: 1) leg press; 2) leg extension; 3) seated leg curl; 4) seated calf; 5) incline press; 6) compound row; 7) triceps press; and 8) bicep curl. The protocol involved a gradual progression of weight and repetitions during the 1st month to allow familiarization with the equipment, minimize muscle soreness, and reduce injury potential. The maximal weight that a person could lift with correct form in a single repetition (1RM) was used to prescribe intensity. The training goal was to complete 3 sets of 10 repetitions for each exercise at 70% 1RM for that specific exercise. Participants rested one minute between sets. Resistance was increased when a participant was able to complete ≥10 reps on the 3rd set for 2 consecutive sessions. Strength testing was repeated every four weeks and training loads were adjusted to be consistent with the 70% 1RM goal. Each participant recorded the weight lifted, number of repetitions completed, and number of sets completed for each exercise.
Assessments
Baseline assessments were conducted within three weeks of the start of interventions and post-assessments were conducted 20–21 weeks after the start of intervention while participants were still exercising. Physical function assessments were performed by all participants, except for the peak aerobic capacity test, which was only conducted in the AT study and the isokinetic knee extensor strength test which was only conducted in the RT study.
Body composition (whole body fat mass, lean mass and percent body fat) was measured by dual x-ray absorptiometry (DXA; Hologic Delphi QDR, Bedford, MA).
Peak aerobic capacity (VO2peak) was determined on a motorized treadmill during a graded exercise test to exhaustion using a Ramp protocol as previously described,27 in which speed was held constant as the grade increased incrementally. Each test achieved two of the following three criteria to be considered:28;29 1) plateau in oxygen consumption with increasing workload (< 200 ml/min); 2) respiratory exchange ratio ≥1.10; and 3) maximal HR within 90% of age-predicted.
Mobility was assessed using a 400-meter walk test.28;29 Participants were instructed to complete the distance (10 laps on a flat indoor surface 20m in length) as quickly as possible. Time to complete the walk was recorded in seconds. Standardized encourage was given every lap.
Lower extremity function was assessed using the Short Physical Performance Battery (SPPB).30 The battery consists of three measures including usual gait speed over a 4m course, time to complete five repeated chair rises without use of their arms, and a standing balance test. Results from each of the three tests are scored from 0, indicating inability to perform the test, to 4 indicating highest function. Scores from the three tasks are summed for the total SPPB score, ranging from 0 (lowest function) to 12 (highest function).
Maximal knee extensor strength was measured on an isokinetic dynamometer (Biodex) at a speed of 60° per second with the participant sitting and the hips and knees flexed at 90°. Participants were asked to extend the knee and push as hard as possible against the resistance pad. Strength of the right leg recorded as peak torque in Newton-meters (Nm) was used for analyses.
Statistical Analysis
Baseline descriptive statistics were calculated and values are reported as mean ±standard deviations or as a frequency in percentage. Absolute and percent changes (post-exercise minus pre-exercise) were calculated for physical function variables. Paired t-tests were used to assess response changes to each intervention. Pearson correlation coefficient analyses and one-way ANOVA were used to determine whether inter-individual changes in physical function variables with AT or RT were related to: 1) variation in compliance (percentage of prescribed exercise sessions attended); 2) baseline values of functional variables; or 3) demographic characteristics. Analyses were performed using SPSS version 21.0 and SAS version 8.3. A P-value ≤ 0.05 was considered statistically significant.
RESULTS
Participant Characteristics at Baseline
Table 1 shows baseline demographics and physical characteristics. The mean age of all the participants was 69 years, with the majority being white and female. All participants were overweight or obese with the mean BMI > 30 kg/m2.
Table 1.
Baseline Participant Characteristics
| Aerobic Training (n=40) |
Resistance Training (n=55) |
|
|---|---|---|
| Age (yrs) | 69.0 (3.6) | 69.1 (3.4) |
| Female No. (%) | 31 (78%) | 28 (50%) |
| Whites No. (%) | 33 (83%) | 48 (87%) |
| BMI (kg/m2) | 34.1 (3.1) | 30.6 (2.4) |
| Percent body fat (%) | 44.2 (5.3) | 38.4 (6.4) |
| Fat mass (kg) | 41.8 (6.9) | 32.8 (5.8) |
| Lean mass (kg) | 52.9 (9.9) | 55.0 (12.1) |
| Waist (cm) | 104.3 (11.1) | 96.2 (9.4) |
| Hip (cm) | 117.2 (8.3) | 108.3 (6.7) |
| WHR | 0.89 (0.10) | 0.89 (0.10) |
| Systolic blood pressure (mmHg) | 133.5 (12.9) | 137.2 (22.5) |
| Diastolic blood pressure (mmHg) | 75.2 (10.8) | 77.1 (11.0) |
| Self-reported comorbidity | ||
| Hypertension | 23 (58%) | 26 (47%) |
| Diabetes* | 8 (20%) | 8 (15%) |
| Sleep apnea | 13 (33%) | 16 (29 %) |
| Arthritis | 31 (78%) | 32 (58%) |
| Chronic back pain | 15 (38%) | 10 (18%) |
| Medication use | ||
| Anti-hypertensive | 26 (65%) | 31 (56%) |
| Cholesterol-lowering | 21 (53%) | 22 (40%) |
| Glucose control | 8 (20%) | 7 (13%) |
| Thyroid | 5 (13%) | 15 (27%) |
| Anti-depressant/mood | 16 (40%) | 8 (15%) |
Values are Mean (standard deviation) or N (%)
Non-insulin-treated Diabetes
BMI-Body Mass Index
WHR-Waist to Hip Ratio
Exercise Compliance
Overall, compliance to the prescribed exercise interventions was high in both studies. Attendance to the prescribed frequency of training (AT=4 d/wk; RT=3 d/wk) averaged 86±15% for AT and 85±12% for RT. There were 31 AT participants (78%) that had attendance ≥80% (attendance range 41–100%), three had perfect attendance. With RT, 45 participants (82%) had attendance ≥80% (attendance range 30–100%), one had perfect attendance.
Changes in Physical Function with Aerobic Training and Resistance Training
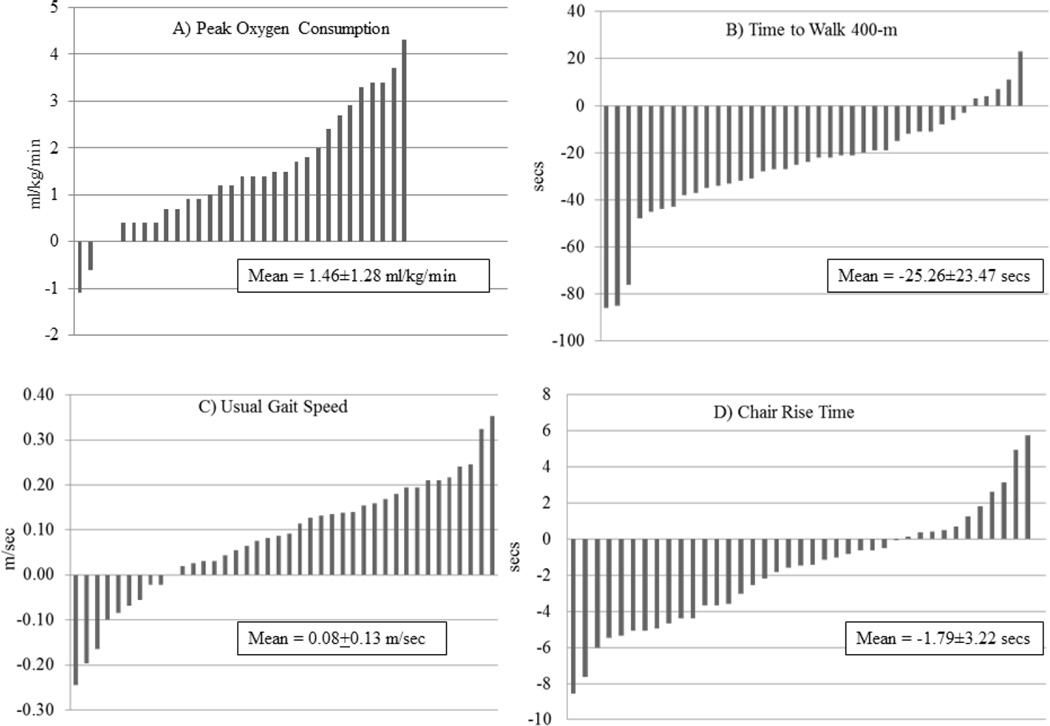
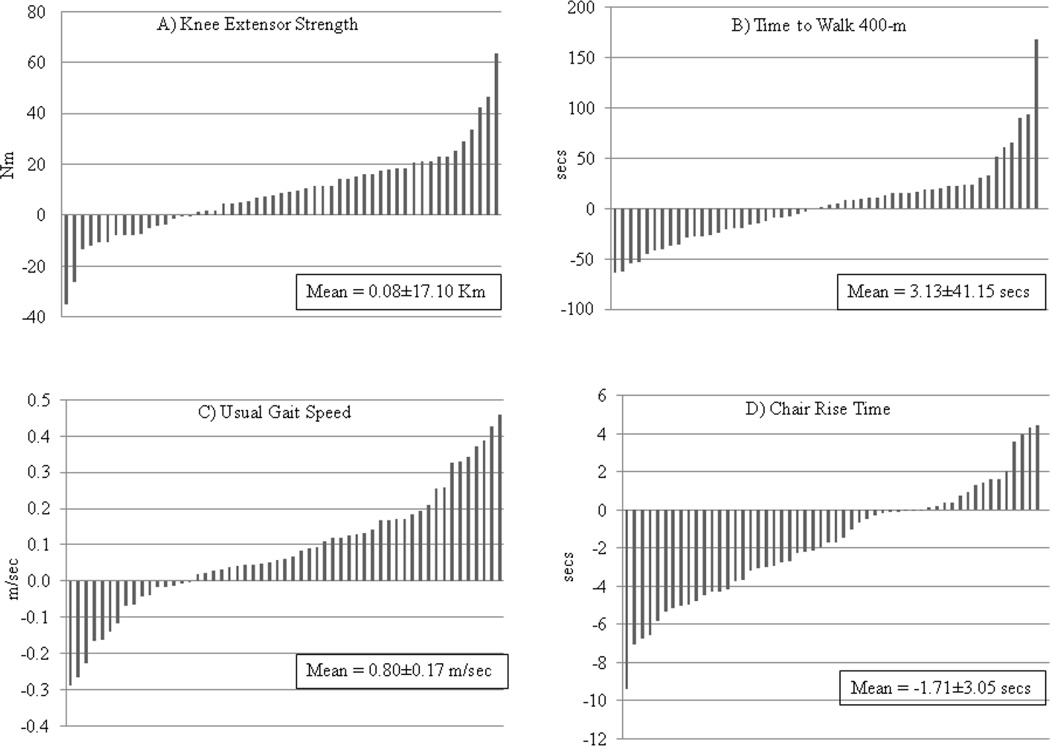
Table 2 shows the mean values for the physical function variables before and after interventions. At baseline, participants had a mean SPPB score >10, a usual gait speed >1.0 m/sec, and were able to complete the 400-meter walk in less than five and one-half minutes. On average, physical function measures significantly improved in response to AT and RT (with the exception of the 400-m walk in RT). However, there was large inter-individual heterogeneity of responses as shown in Figures 1 and 2 which depict individual absolute changes in functional variables, and Table 3 shows the range of relative changes in functional variables.
Table 2.
Physical Function Variables Before and After 5-months of Either Aerobic or Resistance Training
| Aerobic Training | Resistance Training | |||||
|---|---|---|---|---|---|---|
| Pre-ex | Post-ex | p-value | Pre-ex | Post-ex | p-value | |
| 400-meter walk time (min:sec) | 5:25 ± 1:05 | 5:00 ± 1:01 | <0.001 | 5:08 ± 0:51 | 5:10 ± 1:05 | 0.58 |
| Usual gait speed (m/sec) * | 1.02 ± 0.18 | 1.10 ± 0.19 | <0.001 | 1.15 ± 0.19 | 1.23 ± 0.21 | <0.01 |
| Chair rise time (sec) | 13.7 ± 3.7 | 12.0 ± 3.1 | <0.01 | 12.0 ± 3.3 | 10.2 ± 2.5 | <0.001 |
| SPPB score (0–12) | 10.3 ± 1.8 | 10.9 ± 1.4 | <0.01 | 10.8 ± 1.2 | 11.4 ± 1.1 | <0.01 |
| VO2peak (ml/kg/min) | 18.8 ± 3.7 | 20.3 ± 4.0 | <0.001 | Not Measured | ||
| Knee extensor strength (Nm) | Not Measured | 122 ± 44 | 130 ± 46 | <0.01 | ||
Over a 4-meter course
n’s=31–40 for aerobic training, n’s=53–55 for resistance training
Figure 1.
Variability in Physical Function Responses to Aerobic Training: A) Peak oxygen consumption; B) Time to walk 400-m; C) Usual gait speed; D) Chair rise time. Results are scaled so that the bars represent one participant and so that the results could be presented together.
Figure 2.
Variability in Physical Function Responses to Resistance Training: A) Knee extensor strength; B) Time to walk 400-m; C) Usual gait speed; D) Chair rise time. Results are scaled so that the bars represent one participant and so that the results could be presented together.
Table 3.
Physical Function Response Variability by Percent Improvement from Baseline
| Aerobic Training | |||||||
|---|---|---|---|---|---|---|---|
| Function variable | No Improvement | Improvement | |||||
| (% change as X±SD, n) | ≤ −20% | −10--19.9% | −0.1--9.9% | None | 0.1–9.9% | 10–19.9% | ≥20% |
| 400-m walk time (−7.6±6.8%, n=38) | 0 | 0 | 4 | 2 | 20 | 11 | 1 |
| Usual gait speed (7.0±12.0%, n=40) | 1 | 2 | 6 | 1 | 11 | 16 | 3 |
| Chair rise time (−10.3±22.4%, n=39) | 4 | 2 | 4 | 1 | 6 | 4 | 18 |
| SPPB score (9.4±24.6%, n=40) | 2 | 1 | 5 | 11* | 4 | 6 | 11 |
| VO2peak (7.9±6.9%, n=31) | 0 | 0 | 2 | 2 | 17 | 9 | 1 |
| Resistance Training | |||||||
|---|---|---|---|---|---|---|---|
| Function variable | No Improvement | Improvement | |||||
| (% change; X±SD) | ≤ −20% | −10–19.9% | −0.1–9.9% | None | 0.1–9.9% | 10–19.9% | ≥20% |
| 400-m walk time (1.2±13.2%, n=54) | 6 | 2 | 19 | 2 | 15 | 8 | 2 |
| Usual gait speed (5.6±12.8%, n=55) | 2 | 5 | 8 | 1 | 18 | 13 | 8 |
| Chair rise time (−10.4± 25.7%, n=54) | 4 | 6 | 5 | 3 | 7 | 7 | 22 |
| SPPB score (5.8±12.8%, n=55) | 1 | 5 | 0 | 23* | 0 | 17 | 9 |
| Knee extensor strength (8.1±15.4%, n=53) | 2 | 2 | 10 | 2 | 14 | 13 | 10 |
8 of the 11 participants showing no improvement scored a 12 (maximum score) at baseline
18 of the 23 participants showing no improvement scored a 12 (maximum score) at baseline
In response to AT, there was an overall 7.9% increase in VO2peak, with 27 of 31 (87%; 31 participants had complete data) participants experiencing an increase in peak aerobic capacity. Individual absolute increases varied from 0.4 to 4.3 ml/kg/min (Fig. 1a) and four participants showed no change or a decrease in VO2peak. Although the majority of participants improved their 400-m walk time (n=32, 84%), usual gait speed (n=30, 75%), and chair rise time (n=28, 72%) with AT, there was large variation in the magnitude of improvement and a subset of participants experienced no change or a decline in these functional variables (Fig. 1b–d; Table 3). For the SPPB, 21 of 31 (68%) participants with room for improving their score (e.g., baseline score of <12) experienced an increase in response to AT (Table 3).
In response to RT, knee extensor strength improved an average of 8.1% among participants. The majority (n=37 of 53; 70%) increased their knee extensor strength, but individual increases varied from 1.2 to 63.7 Nm, while 16 participants (30%) showed no change or a decrease in strength (Fig. 2a). Less than one-half (n=25; 46%) of participants experienced an improvement in their 400-m walk time with RT (Fig 2b; Table 3). The majority improved their usual gait speed (n=39, 71%) and chair rise time (n=36, 67%) with RT, but, again, there was wide variation in the magnitude of improvement and some participants experienced no change or a decline in usual gait speed and chair rise time (Fig. 2c–d; Table 3). Approximately 70% (n=26) of participants with room for improving their SPPB score showed an increase in response to RT (Table 3).
We also examined whether there was overlap of “non-responders” (e.g., those who did not show a change in the desired direction) across functional variables (e.g., whether the same individuals were non-responsive in multiple measures). Two of the four individuals who did not improve their VO2peak with AT were non-responsive in at least one other functional measure (usual gait speed and chair rise time). On the other hand, 15 of the 16 individuals who did not improve their knee strength with RT were non-responsive in another measure of function; four of these were non-responsive in all measures of function and seven were non-responsive in at least three other functional measures.
Correlates of Physical Function Responses to Exercise Training
Compliance to the prescribed number of exercise sessions was not associated with change or percent change in any functional variable (including change in VO2peak) in response to AT (r’s=0.05 to 0.24; p>0.15 for all). With RT, the magnitude of change in 400-m walk time was related to compliance (absolute change: r= −0.31; relative change: r= −0.32, p<0.05 for both), showing individuals with greater exercise attendance frequency had greater improvements in time to walk 400-m. However, variation in compliance was not associated with change or percent change in other functional measures (including knee extensor strength) in response to RT. Furthermore, analyses of only those individuals with >80% compliance to the prescribed exercise frequency (n=31 for AT; n=41 for RT) continued to show large variation in functional responses. For example, relative changes in VO2peak and usual gait speed with AT ranged from −5% to 23% and −15% to 39%, respectively, and relative changes in knee extensor strength and usual gait speed with RT ranged from −10% to 51% and −22% to 41%, respectively, in these subsamples with high compliance.
The magnitude of change in a physiological trait often depends on the initial level of that trait, thus we analyzed whether the baseline level of each physical function variable was associated with its change after exercise training. In response to AT, changes in 400-m walk time (r= −0.34, p<0.05), usual gait speed (r= −0.36, p<0.05), chair rise time (r= −0.62, p<0.01), and SPPB (r= −0.65, p<0.01), but not VO2peak (r= −0.23, p=0.18) were negatively correlated with initial function (e.g., greater improvement in those with worse baseline function). In response to RT, changes in usual gait speed (r= −0.28, p<0.05), chair rise time (r= −0.68, p<0.01), and SPPB (r= −0.61, p<0.01), but not 400-m walk time (r= −0.02, p=NS) or knee extensor strength (r= −0.05, p=NS) were negatively correlated with baseline function.
Next we analyzed whether absolute changes in physical function in response to AT or RT were univariately related to age, gender, adiposity (BMI, fat mass, or percent body fat), or to self-reported hypertension, sleep apnea, diabetes, back pain, arthritis, or osteoporosis. In response to AT, changes in 400-m walk correlated negatively with age (r= −0.33, p<0.05; e.g., older individuals improved more), and there was a tendency (p=0.06) for improvement in usual gait speed to be greater in individuals without (0.10±0.13 m/s) compared to with (0.00±0.12 m/sec) diabetes. In response to RT, race was associated with improvement in usual gait speed (whites: 0.10±0.16 m/sec; blacks: −0.06±0.13 m/sec, p<0.05). Also, adiposity was associated with improvements in chair rise time (BMI: r=0.41, p<0.01; fat mass: r=0.25, p=0.07) and SPPB (BMI: r= −0.33, p<0.05; fat mass: r= −0.35, p<0.05) with RT, such that greater functional improvement was seen in those with lower BMI and less fat mass at baseline.
DISCUSSION
Mean change effects of exercise interventions are well documented, whereas individual responses are reported less frequently but provide valuable insight into the individual effectiveness of exercise training. Our results showed large heterogeneity in physical function responses to well-controlled, supervised aerobic and resistance training interventions. Although the majority of participants showed some improvement, there was a wide range in their magnitude of improvement and there was a subset of “non-responders” who showed no change or a decline in function after the interventions (e.g., a range of −5% to 23% change in VO2peak in response to AT and a range of −34% to 50% increase in muscle strength with RT). These results are similar to the few studies, mostly in younger adults, that report individual adaptations in physical and functional responses to exercise, including maximal aerobic capacity and muscle strength.19–22;31–33 The wide variation in individual adaptations to exercise training may be due to differences in physiological mechanisms needed to increase strength versus aerobic capacity, or to differences in volitional effort, to the prescribed training. Fewer individuals in the AT study (50% versus 94% in the RT study) were non-responsive across multiple functional domains; in fact, four individuals did not show improvement in any functional outcome with RT. This suggests that improvements in muscle strength may be necessary to elicit improvements in gait speed and lower extremity function and has important clinical ramifications for the nearly one-third of older adults who may not experience a strength benefit from performing RT.
Other factors than age, race, gender, or compliance must play a role in responsiveness as they were not generally associated with the variation in physical function responses; only in RT was change in 400-m walk time related to compliance. In response to AT, older individuals showed greater improvements in 400-m walk time and in response to RT, blacks improved their usual walking speed more than whites. Interestingly, in response to RT, greater functional improvements were seen in those with lower BMI and less fat mass at baseline, suggesting higher adiposity may blunt the functional benefits of RT. Finally, results showed that greater improvement in functional responses was seen in participants with worse baseline function. This may be due to a regression to the mean phenomenon, or to a greater capacity for those with less function to improve with training. Overall, these findings are consistent with existing data showing that age, sex, and ethnic origin are not major predictors of exercise responsiveness, but baseline level of function may be.20;31 Moreover, there are likely multiple genetic, epigenetic, cellular, physiological and environmental factors that affect the efficacy of exercise training for improving functional and health outcomes.34–36 For example, our prior work suggests that genetic variability in the angiotensin-converting enzyme gene affects functional responses to exercise training.37 However, more research is needed to delineate these factors that affect inter-individual variability of exercise training responses.
Although we analyzed non-responsiveness as showing no change, or a decline in function, after the interventions, it may also be informative to examine results in terms of what percentage of participants showed a clinically meaningful change in the functional outcomes. A change in gait speed of 0.05 m/sec indicates a small change, and a change of 0.10 m/sec indicates a substantial change.38;39 In our study, 18% of those performing AT and 16% of those performing RT showed at least a small meaningful change (e.g., improved greater than 0.05 m/sec). Approximately 66% of participants in AT and 70% of participants in RT, showed a 1 point or greater improvement in the SPPB, which has been shown to be clinically meaningful.38;39 Walking 400 meters at least 12 seconds faster indicates a small meaningful improvement while walking at least 28 seconds faster indicates a substantial change.39 Based on these cut points, 34% of participants in AT and 15% in RT had an improvement of 12–27 secs; while 39% in AT and 22% in RT showed improvements of 28 secs or greater. On an individual basis, exercise appears to result in clinically significant functional improvements in 20–70% of participants. While showing improvements in these cut points may be clinically meaningful they lack explanation of why some individuals do not improve and miss an opportunity for early intervention when functional changes occur. Also, it may be difficult for participants who have higher baseline functioning to show a meaningful change based on these cut points. Future studies are needed to examine specific mechanisms that affect improvements in response to aerobic and resistance training, especially evaluating the role that baseline function plays in achieving desired benefits. Additional research is needed to identify non-responders based on a baseline biomarker or level of function, and/or to identify non-responders earlier in an exercise training program.
The highly controlled, supervised, and progressive exercise training protocols is a strength of this study. Another strength is that functional responses to exercise training were not confounded by the addition of a dietary component to the exercise interventions, by mixing or combining modes of exercise, or by loss of body weight during the interventions (data not shown). However, since the participants we studied were older, sedentary, and overweight/obese with several co-morbid health conditions, results may not be applicable to a younger, leaner, or more active population. It may be that exercise training responses are more variable in older populations; future research should consider whether the response heterogeneity to training is affected by age or health status. Another thing to consider is how the participant was feeling on the day of their assessments and during training. Personal attitudes, attributes, and barriers (e.g, fatigue or pain) may contribute to one’s perceived effort and intensity. Moreover, the intensity, duration, and/or total volume of exercise necessary to elicit maximal improvements in certain physical outcomes may differ between individuals.
In conclusion, understanding individual differences in functional responses to exercise in older adults is critical as some individuals may respond more favorably to, and be more likely to engage in, one type of exercise over another. Reporting the effects of exercise interventions within, as well as across, individuals is necessary to specifically tailor exercise type and dose to optimize the effectiveness of exercise as a therapy for aging-related loss of function. These results and future identification of the sources of exercise response variability are needed to develop personalized exercise prescriptions and to identify adjuvants that will enhance the benefits of exercise training in individuals who are non-responsive to a standard exercise treatment.
ACKNOWLEDGMENTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (R01HL093713) and the National Institute on Aging (R01AG020583) awarded to Dr. Nicklas, and by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30AG21332).
Sponsor’s Role: none.
Footnotes
Conflict of Interest: There were no conflicts of interest to be declared with companies or manufacturers who will benefit from the results of the present study.
Author Contributions: Nicklas, Marsh, Lyles, Brinkley: study concept and design. Chmelo, Crotts: acquisition of subjects and data. All authors: analysis and interpretation of data, preparation of manuscript.
REFERENCES
- 1.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, Penninx BW, Ferrucci L, et al. Measures of physical performance and risk for progressive and catastrophic disability: Results from the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 3.Hicks GE, Shardell M, Alley DE, et al. Absolute Strength and Loss of Strength as Predictors of Mobility Decline in Older Adults: The InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: Analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009;64:223–229. doi: 10.1093/gerona/gln022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rantakokko M, Manty M, Rantanen T. Mobility decline in old age. Exerc Sport Sci Rev. 2013;41:19–25. doi: 10.1097/JES.0b013e3182556f1e. [DOI] [PubMed] [Google Scholar]
- 6.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Latham NK, Bennett DA, Stretton CM, et al. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 8.Peterson MD, Rhea MR, Sen A, et al. Resistance exercise for muscular strength in older adults: A meta-analysis. Ageing Res Rev. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenzuela T. Efficacy of progressive resistance training interventions in older adults in nursing homes: A systematic review. J Am Med Dir Assoc. 2012;13:418–428. doi: 10.1016/j.jamda.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger WH, Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 11.Penninx BW, Messier SP, Rejeski WJ, et al. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med. 2001;161:2309–2316. doi: 10.1001/archinte.161.19.2309. [DOI] [PubMed] [Google Scholar]
- 12.Singh MA. Exercise to prevent and treat functional disability. Clin Geriatr Med. 2002;18:431-vii. doi: 10.1016/s0749-0690(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 13.Nelson ME, Rejeski WJ, Blair SN, et al. Physical Activity and Public Health in Older Adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 14.Daniels R, van RE, de WL, et al. Interventions to prevent disability in frail community-dwelling elderly: A systematic review. BMC Health Serv Res. 2008;8(278):278. doi: 10.1186/1472-6963-8-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin APM, van Uffelen JG, Riphagen I, et al. The functional effects of physical exercise training in frail older people: A systematic review. Sports Med. 2008;38:781–793. doi: 10.2165/00007256-200838090-00006. [DOI] [PubMed] [Google Scholar]
- 16.Chou CH, Hwang CL, Wu YT. Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: A meta-analysis. Arch Phys Med Rehabil. 2012;93:237–244. doi: 10.1016/j.apmr.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Pescatello LS. Physical activity, cardiometabolic health and older adults: Recent findings. Sports Med. 1999;28:315–323. doi: 10.2165/00007256-199928050-00003. [DOI] [PubMed] [Google Scholar]
- 18.Fleg JL. Aerobic exercise in the elderly: A key to successful aging. Discov Med. 2012;13:223–228. [PubMed] [Google Scholar]
- 19.Wilmore JH, Leon AS, Rao DC, et al. Genetics, response to exercise, and risk factors: the HERITAGE Family Study. World Rev Nutr Diet. 1997;81:72–83. doi: 10.1159/000059603. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc. 2001;33:S446–S451. doi: 10.1097/00005768-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 21.Hubal MJ, Gordish-Dressman H, Thompson PD, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964–972. [PubMed] [Google Scholar]
- 22.Timmons JA. Variability in training-induced skeletal muscle adaptation. J Appl Physiol (1985) 2011;110:846–853. doi: 10.1152/japplphysiol.00934.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7:e37887. doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leifer ES, Brawner CA, Fleg JL, et al. Are there negative responders to exercise training among heart failure patients? Med Sci Sports Exerc. 2014;46:219–224. doi: 10.1249/MSS.0b013e3182a44164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Gool CH, Penninx BW, Kempen GI, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis Rheum. 2005;53:24–32. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- 26.Fielding RA, Katula J, Miller ME, et al. Activity adherence and physical function in older adults with functional limitations. Med Sci Sports Exerc. 2007;39:1997–2004. doi: 10.1249/mss.0b013e318145348d. [DOI] [PubMed] [Google Scholar]
- 27.Nicklas BJ, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: A randomized, controlled trial. Am J Clin Nutr. 2009;89:1043–1052. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 29.Simonsick EM, Montgomery PS, Newman AB, et al. Measuring fitness in healthy older adults: The Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 31.Kohrt WM, Malley MT, Coggan AR, et al. Effects of gender, age, fitness level on response of VO2max to training in 60–71 yr olds. J Appl Physiol. 1991;71:2004–2011. doi: 10.1152/jappl.1991.71.5.2004. [DOI] [PubMed] [Google Scholar]
- 32.Erskine RM, Jones DA, Williams AG, et al. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol. 2010;110:1117–1125. doi: 10.1007/s00421-010-1601-9. [DOI] [PubMed] [Google Scholar]
- 33.Karavirta L, Hakkinen K, Kauhanen A, et al. Individual responses to combined endurance and strength training in older adults. Med Sci Sports Exerc. 2011;43:484–490. doi: 10.1249/MSS.0b013e3181f1bf0d. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard C, Sarzynski MA, Rice TK, et al. Genomic predictors of the maximal O(2) uptake response to standardized exercise training programs. J Appl Physiol (1985) 2011;110:1160–1170. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfarth B, Rankinen T, Hagberg JM, et al. Advances in exercise, fitness, and performance genomics in 2013. Med Sci Sports Exerc. 2014;46:851–859. doi: 10.1249/MSS.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh S, Vivar JC, Sarzynski MA, et al. Integrative pathway analysis of a genome-wide association study of (V)O(2max) response to exercise training. J Appl Physiol (1985) 2013;115:1343–1359. doi: 10.1152/japplphysiol.01487.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicklas BJ. Heterogeneity of physical function responses to exercise in older adults: Role of angiotensin-converting enzyme (ACE) gene. Perspect Psychol Sci. 2010;5:575–584. doi: 10.1177/1745691610383512. [DOI] [PubMed] [Google Scholar]
- 38.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perera S, Studenski S, Newman A, et al. Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (Health ABC Study) J Gerontol A Biol Sci Med Sci. 2014;69:1260–1268. doi: 10.1093/gerona/glu033. [DOI] [PMC free article] [PubMed] [Google Scholar]