Abstract
Gait decline is common among older adults and is a risk factor for adverse outcomes. Poor gait performance in dual‐task conditions, such as walking while performing a secondary cognitive interference task, is associated with increased risk of frailty, disability, and death. Yet, the functional neural substrates that support locomotion are not well established. We examined the functional connectivity associated with gait velocity in single‐ (normal pace walking) and dual‐task (walking while talking) conditions using resting‐state functional Magnetic Resonance Imaging (fMRI). We acquired 6 minutes of resting‐state fMRI data in 30 cognitively healthy older adults. Independent components analyses were performed to separate resting‐state fMRI data into group‐level statistically independent spatial components that correlated with gait velocity in single‐ and dual‐task conditions. Gait velocity in both task conditions was associated with similar functional connectivity in sensorimotor, visual, vestibular, and left fronto‐parietal cortical areas. Compared to gait velocity in the single‐task condition, the networks associated with gait velocity in the dual‐task condition were associated with greater functional connectivity in supplementary motor and prefrontal regions. Our findings show that there are partially overlapping functional networks associated with single‐ and dual‐task walking conditions. These initial findings encourage the future use of resting‐state fMRI as tool in developing a comprehensive understanding of age‐related mobility impairments. Hum Brain Mapp 36:1484–1493, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: fMRI, resting‐state, gait, dual‐task, aging
INTRODUCTION
Decline in gait performance is common among older adults, even in the absence of neurological pathology or acute clinical events. Such age‐related gait decline has been widely studied and reliably shown to increase the risk for morbidity, hospitalization, and mortality [Newman et al., 2006; Studenski et al., 2011; Verghese et al., 2006]. Age‐related gait decline also increases the risk for future cognitive decline and dementia in older adults [Marquis et al., 2002; Verghese et al., 2007b; Waite et al., 2005; Wang et al., 2006]. It is important to note that older adults are especially challenged under dual‐task gait conditions, which require walking while attending to a secondary cognitive demand [Beurskens and Bock, 2012; Holtzer et al., 2011; Li et al., 2001; Lindenberger et al., 2000]. In fact, walking while talking is conceptualized as a mobility stress test that has been shown to be a reliable predictor of falls, frailty, disability, and mortality in cognitively healthy, community‐dwelling older adults [Ayers et al., 2014; Verghese et al., 2002, 2012]. As the population of older adults increases throughout the world, developing a comprehensive understanding of these age‐related mobility impairments is an essential public health consideration.
The evidence for cognitive control, notably attention and executive functions, of gait when assessed in single‐ and dual‐task conditions in aging is robust [Holtzer et al., 2006, 2012, 2014c]. However, the underlying functional brain correlates of gait and other mobility outcomes are not well established [Holtzer et al., 2014a]. Investigations of neural activity associated with gait have been particularly challenging because traditional neuroimaging modalities cannot be applied during the act of walking. Recent functional brain imaging studies have used a variety of techniques to circumvent this limitation. Researchers have used radionuclide tracers during locomotion, and subsequently examined distribution patterns in the brain using single photon emission computerized tomography (SPECT) or positron‐emission‐tomography (PET) [Fukuyama et al., 1997; la Fougere et al., 2010; Malouin et al., 2003]. As these approaches are invasive and expensive to implement, other researchers have used functional near‐infrared spectroscopy (fNIRS), a noninvasive, low‐cost imaging modality that can map functional brain correlates during dynamic tasks such as gait [Holtzer et al., 2011; Miyai et al., 2001]. Previous studies have also explored the functional neural correlates of gait using mental imagery tasks [Blumen et al., 2014; Jahn et al., 2004; la Fougere et al., 2010; Zwergal et al., 2012]. Imaging is done while participants envision themselves walking, without actual execution. Imagery of movements has been shown to activate similar cortical and subcortical regions as the physical performance of the same movements [Anderson and Lenz, 2011; Jeannerod, 2001]. Investigation of gait through imagery creates new opportunities for the use of functional magnetic resonance imaging (fMRI) in this field.
While the majority of fMRI studies have studied the brain's response to a stimulus or task, resting‐state fMRI has emerged as an approach that does not require these conditions. Resting‐state fMRI research stems from a seminal study demonstrating that low‐frequency (0.01–0.1 Hz) blood‐oxygen‐level‐dependent (BOLD) signals were temporally correlated between regions of the primary sensory motor cortex within and across hemispheres in participants at rest [Biswal et al., 1995]. Large‐scale cortical networks corresponding to a variety of core perceptual and cognitive processes have since been widely replicated across a range of analytic approaches at both the group and individual levels in a variety of resting conditions: eyes closed, sleep, and even anesthesia [Damoiseaux et al., 2008; Erhardt et al., 2011; Fox and Raichle, 2007]. Well‐established resting‐state networks correspond strongly to functional areas identified through the use of task‐dependent paradigms, and are widely interpreted as intrinsic neural activity supporting core functional systems [Cole et al., 2010]. There are important limitations and interpretational difficulties of resting‐state fMRI because it is an indirect measure vulnerable to several confounding factors including head movements, physiological activity, and acquisition artifacts [Biswal et al., 1996; Friston et al., 1996; Glover et al., 2000]. Nevertheless, resting‐state fMRI is a potentially powerful technique to further advance our understanding of the system‐wide neural substrates underlying gait.
Our aim for this study was to identify functional neural networks associated with single‐ and dual‐task gait performance in nondemented, community‐dwelling older adults using resting‐state fMRI. Prior research from our group has demonstrated increased oxygenation levels and BOLD activity in the prefrontal cortex during WWT compared with normal walking in older adults using fNIRS [Holtzer et al., 2011] and fMRI with imagined gait [Blumen et al., in press], respectively. Based on these findings, we expect greater involvement in prefrontal regions in the dual‐task condition; however, we used a whole‐brain multivariate approach to explore this issue, as we were interested in brain function at a systems level.
METHODS
Study Population
Quantitative gait and MRI data from a convenience sample of 30 cognitively healthy older adults [M (SD) age in years = 72.50 (5.22); % female = 55.17] were used for this study from the Central Control of Mobility in Aging (CCMA) study [Holtzer et al., 2013, 2014c]. The CCMA study recruits older adults (≥65 years) residing in Yonkers, NY and aims to identify cognitive and brain predictors of mobility. General exclusion criteria included severe auditory or visual loss, recent hospitalization that affects mobility, living in a nursing home, serious chronic, or acute illness (e.g., cancer), and presence of dementia or other neurodegenerative disease. Specific MRI exclusion criteria included left‐handedness (assessed by Edinburgh Handedness Inventory [Oldfield, 1971]), claustrophobia, and surgically implanted metallic devices (e.g., pacemaker). Written informed consent was approved by the university's institutional review board.
Measures
Quantitative gait assessment
Consistent with the vast literature concerning dual‐task methodology, participants completed both the single‐ and dual‐task gait conditions. One trial was completed under each gait condition. To reduce learning effects, participants were not given practice trials or taught strategies. Task order was counterbalanced to avoid practice effects. Quantitative gait data were obtained using a 20‐foot instrumented walkway with embedded pressure sensors spanning 14 feet, allowing for 3 feet of initial acceleration and terminal deceleration (GAITRite, CIR systems, Havertown, PA). Monitoring devices were not attached to participants during the test. Each trial was one walkway in length and the software computed gait velocity (cm/s) based on the footfalls recorded. We focused on gait velocity as it is a standard quantitative performance index in gait literature and clinical practice that predicts a variety of adverse outcomes [Studenski et al., 2011; Verghese et al., 2012]. GAITRite assessments have been shown to be reliable and valid in previous research in our center and in other studies [Bilney et al., 2003; Verghese et al., 2002].
Normal pace walking (NW)
In the single‐task condition, participants were asked to walk on the instrumented walkway at their normal pace for one trial in a quiet and well‐lit room. Start and end points were clearly marked.
Walking while talking (WWT)
In the dual‐task condition, participants were asked to walk on the instrumented walkway at their normal pace while reciting alternate letters of the alphabet (skipping the letter in between) for one trial in a quiet and well‐lit room. Participants were asked to pay equal attention to their walking and talking [Verghese et al., 2007a].
We have demonstrated reliable dual‐task effects using this walking while talking paradigm in many articles using different cohorts of older adults [Brandler et al., 2012; Holtzer et al., 2014c; Li et al., 2014]. Consistent with the cognitive dual‐task literature, our findings also revealed increased attention/executive demands in the dual‐task compared to the single‐task walking condition [Holtzer et al., 2006, 2012, 2014b]. Furthermore, using fNIRS, we provided first evidence that online oxygenation levels in the prefrontal cortex were increased in walking while talking compared with walking in a single‐task condition in young and old participants [Holtzer et al., 2011]. Increasing the demands of the cognitive task (reciting letters of the alphabet compared with reciting alternative letters of the alphabet) was more strongly associated with risk of falls in older adults [Verghese et al., 2002] and changing instructions while maintaining the same cognitive and motor tasks in the dual‐task paradigm resulted in task prioritization effects [Verghese et al., 2007a]. Strong predictive validity for the walking while talking paradigm was also established in longitudinal cohort studies. Performance on this task has been shown to be a robust predictor of falls, frailty, disability, and mortality in older adults [Ayers et al., 2014; Verghese et al., 2012].
MRI data acquisition
During resting‐state MRI acquisition, participants were asked to lie still in the scanner, keep their eyes closed, and not fall asleep for 6 min of recording time [Van Dijk et al., 2010]. MRI scanning was performed with a Philips 3T Achieva Quasar TX multinuclear MRI/MRS system equipped with a Dual Quasar High Performance Gradient System, 32‐channel broadband digital RF system, Quadrature T/R Head Coil, RapidView reconstructor, Intera Achieva ScanTools Pro R2.5 Package, NetForum and ExamCards, and SENSE parallel imaging capability. All BOLD (T2*‐weighted) images were acquired with echo planar imaging using a whole brain gradient over a 240 mm field of view (FOV) on a 128 × 128 acquisition matrix, 3 mm slice thickness (no gap); TE = 30 ms, TR = 2000 ms, flip angle = 90°, and 42 trans‐axial slices per volume. A T1‐weighted whole head structural image was also acquired using axial 3D‐MP‐RAGE parameters over a 240 mm FOV and 1.0 mm isotropic resolution, TE = 4.6 ms, TR = 9.9 ms, α = 80, with SENSE factor 2.5. MRI data was obtained a few weeks to months following quantitative gait assessment (M = 96.53 days, SD = 66.20 days, range = 14–265 days). Participant health was monitored in the interim through bimonthly telephone interviews.
Image preprocessing
BOLD (T2*‐weighted) image preprocessing, using FSL (Version 4.1), FMRIB's Software Library (http://fsl.fmrib.ox.ac.uk/fsl) [Jenkinson et al., 2012; Smith et al., 2004; Woolrich et al., 2009], consisted of nonbrain removal using BET [Smith, 2002], motion correction with MCFLIRT [Jenkinson et al., 2002; Jenkinson and Smith, 2001], slice‐timing correction for interleaved acquisitions using Fourier‐space time‐series phase shifting, highpass temporal filtering using Gaussian‐weighted least‐squares straight line fitting (σ = 50 s); spatial smoothing using a Gaussian kernel with full‐width half‐maximum 8 mm, coregistration to high‐resolution T1‐weighted images, and normalization to standard space (Montreal Neurological Institute atlas, using resolutions of 4 × 4 × 4 mm) using combined affine and nonlinear registration (FSL FNIRT, with warp resolution = 10 mm).
Statistical Analysis
Independent components analysis and correlation
For each participant, smoothed normalized fMRI images were concatenated across time to form a single 4D image. The 4D images were then analyzed with FSL MELODIC Independent Component Analysis (ICA) software [Beckmann and Smith, 2004]. ICA is a data‐driven approach that separates multivariate data into statistically independent spatial components and their associated time series. When applied to resting‐state fMRI data, ICA decomposes the BOLD dataset into components representing neural signals of interest, structured noise, and random noise [Beckmann et al., 2005; Cole et al., 2010; Fox and Raichle, 2007; Greicius et al., 2004; Murphy et al., 2013]. This technique does not require a priori modeling, providing flexibility appropriate for our exploratory analysis. We used this technique to identify components that correlated with NW and WWT gait velocity in two separate analyses, and limited each analysis output to 20 components, a dimensionality used in previous resting‐state studies [Smith et al., 2009]. Criterion for statistical significance was set as P < 0.05.
Manual classification of components
Even after traditional pre‐processing steps, several confounding sources of noise may remain in resting‐state fMRI data that could compromise interpretation [Bhaganagarapu et al., 2013; Kelly et al., 2010; Power et al., 2012]. ICA accounts for the existence of noise effects by automatically isolating sources of noise within artifactual components. Identification of these components, primarily related to gross participant motion and physiological sources such as cardiac and respiratory cycles, is critical to limit spurious findings in resting‐state fMRI analyses [Murphy et al., 2013; Thomas et al., 2002]. We used an operationalized fMRI de‐noising procedure to manually classify components as representing artifacts or neural signals of interest via visual inspection. The protocol dictates that components are labeled as artifactual when the thresholded component spatial map shows 90% or more activation or deactivation in peripheral areas or in a random scattered pattern over ¼ or more of the brain without correspondence to functional‐anatomical boundaries. Components are labeled as neural signals of interest when the thresholded component spatial map shows 10% or more activation or deactivation in small to large gray matter clusters localized to nonperipheral regions of the brain. Secondary considerations include indications of noise such as high frequency activity, spikes, saw tooth pattern, and sinus coactivation. This procedure has been shown to be reliable and to improve the sensitivity of results from resting‐state fMRI data analysis [Kelly et al., 2010].
RESULTS
Characteristics of the 30 participants are presented in Table 1. A paired‐samples t‐test was conducted to evaluate the intraindividual change in gait velocity between NW and WWT conditions (also referred to as dual‐task cost in the literature [Holtzer et al., 2014c; Lindenberger et al., 2000; Yogev‐Seligmann et al., 2010]). Consistent with the literature, there was a statistically significant decrease in gait velocity from the NW condition (M = 108.83 cm/s, SD = 22.52 cm/s) to the WWT condition (M = 82.69 cm/s, SD = 25.29 cm/s), t (29) = 7.17, P < 0.001).
Table 1.
Descriptive statistics of demographic information and gait velocity (N = 30)
| M (SD) | Range | |
|---|---|---|
| Age (years) | 72.50 (5.22) | 65–87 |
| Gender (% female) | 55.17 | |
| Education (years) | 15.27 (2.99) | 12–23 |
| Global health status score | 1.37 (1.13) | 0–4 |
| RBANS total score | 95.2 (13.20) | 62–119 |
| Gait velocity NW (cm/s) | 108.83 (22.52) | 45–146 |
| Gait velocity WWT (cm/s) | 82.69 (25.29) | 35–144 |
Note. Global health status score (range 0–10) obtained from dichotomous rating (presence or absence) of diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, Parkinson's disease, chronic obstructive pulmonary disease, angina, and myocardial infarction; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; NW: normal pace walk; WWT: walking while talking
Resting‐State Networks Correlated with NW
Of the 20 components generated from the ICA correlated with NW gait velocity, 16 components were determined to be artifactual following the operationalized fMRI de‐noising procedure. The remaining four components were determined to be neural signals of interest (see Fig. 1). All anatomical and functional descriptions were classified with reference to the underlying standard‐space images in conjunction with several atlases [Lancaster et al., 1997, 2000]. These components were further identified by comparison to well‐established resting‐state networks derived from a large meta‐analysis [Smith et al., 2009; Ystad et al., 2011]. We describe each of the networks briefly below. Map 1A (“sensorimotor”) covers the premotor cortex, primary motor cortex, and supplementary motor area. This network corresponds to activations seen in bimanual motor tasks, and similar patterns have been identified in previous resting‐state studies [Beckmann et al., 2005; Smith et al., 2009]. Map 2A (“visual”) includes primary, secondary, and associative visual cortices. Strong correspondence between these areas and functionally identified visual domains has been well established in the resting‐state literature [Beckmann et al., 2005; Smith et al., 2009]. Map 3A (“vestibular”) is primarily composed of the insula, and the primary and secondary auditory cortices. This network appears to be functionally related to both auditory and vestibular paradigms. Similar patterns have been found in resting‐state networks in the past [Beckmann et al., 2005; Smith et al., 2009]. Map 4A (“left fronto‐parietal”) covers the left posterior parietal association areas, left supplementary motor cortex, left frontal eye field, and left prefrontal association cortex. This left lateralized fronto‐parietal network also includes regions of the right cerebellum. This is consistent with anatomical and functional connections, as evidenced by recent resting‐state studies that have shown cross‐lateral connectivity between regions of the cerebellum and the prefrontal and posterior‐parietal cortices [Habas et al., 2009; Krienen and Buckner, 2009; O'Reilly et al., 2010]. Fronto‐parietal networks were strongly lateralized in the resting‐state literature [Smith et al., 2009].
Figure 1.
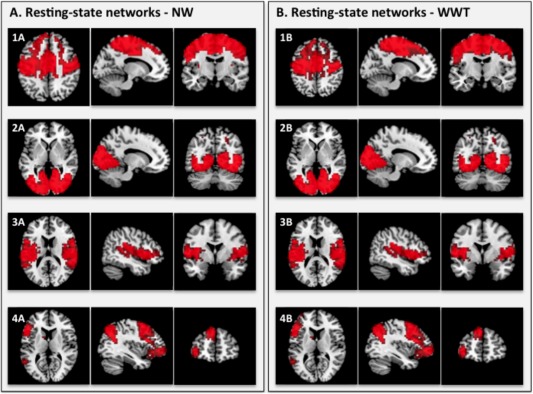
Resting‐state networks associated with NW gait velocity (A) and WWT gait velocity (B). This figure shows the three most informative axial, sagittal, and coronal slices of each resting‐state network superimposed on the Montreal Neurologic Institute (MNI) template supplied by MRIcron software. The left side of the image corresponds to the left side of the brain. All ICA spatial maps were converted to z statistic images via a normalized mixture‐model fit, and then thresholded at z = 2.30.
Resting‐State Networks Correlated with WWT
Of the 20 components generated from the ICA correlated with WWT gait velocity, 16 components were determined to represent noise, and four components were determined to be neural signals of interest using the operationalized fMRI de‐noising procedure. As with NW gait velocity, WWT gait velocity was significantly correlated with functional connectivity in well‐established sensorimotor, visual, vestibular, and left‐lateralized fronto‐parietal resting‐state networks (see Fig. 1: Maps 1B, 2B, 3B, and 4B respectively).
NW Compared with WWT
Similar resting‐state networks were correlated with NW and WWT gait velocity. The corresponding visual and auditory networks are almost identical. The sensorimotor and left fronto‐parietal networks associated with NW and WWT, however, have significant differences (Fig. 2). The sensorimotor and left fronto‐parietal networks associated with WWT include greater frontal connectivity in the supplementary motor and prefrontal areas, respectively, compared with the corresponding networks associated with NW.
Figure 2.
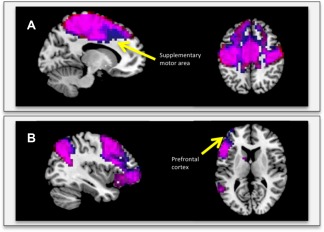
A: Overlay of the sensorimotor resting‐state networks associated with NW (red) and WWT (blue) on the same template. B: Overlay of the left fronto‐parietal resting‐state networks associated with NW (red) and WWT (blue) on the same template. The purple color represents the areas in which the networks overlap. The blue color shows where the WWT networks extend beyond the NW networks.
DISCUSSION
The present study revealed four resting‐state networks associated with NW and WWT gait velocity in healthy older adults. Our main findings were as follows: (1) Gait velocity under both walking conditions was associated with similar functional connectivity in sensorimotor, visual, vestibular, and fronto‐parietal functional networks at rest in older adults; however, (2) WWT gait velocity was associated with greater frontal functional connectivity in the sensorimotor and fronto‐parietal networks compared with NW.
Shared Neural Correlates of NW and WWT
The resting‐state networks associated with NW and WWT gait velocity in our study are consistent with findings from previous neuroimaging studies. As expected, gait in both conditions was associated with functional connectivity in the sensorimotor network. Recent studies using PET, SPECT, fNIRS, and mental imagery to investigate gait identified activations in the premotor cortex, primary motor cortex, and supplementary motor area [Blumen et al., 2014; Fukuyama et al., 1997; Godde and Voelcker‐Rehage, 2010; Jahn et al., 2004; Malouin et al., 2003; Miyai et al., 2001; Zwergal et al., 2012]. These areas have been shown to be involved in motor preparation and programming voluntary movement, both critical to locomotion [Fukuyama et al., 1997]. Gait also requires complex motor abilities related to supplementary motor area function. Patients with lesions in premotor and supplementary motor areas have difficulty in tasks such as initiating or terminating gait [Massion, 1992; Viallet et al., 1992].
Gait in both conditions was also associated with functional connectivity in the visual network, which is consistent with results from a range of neuroimaging studies of gait [Fukuyama et al., 1997; Jahn et al., 2004; la Fougere et al., 2010; Zwergal et al., 2012]. Prominent activations in the visual cortex were found across a variety of real and imagined locomotion conditions. Visual function is essential to locomotion because the processing of visual input is necessary for maintaining proper direction and adaptation to obstacles in the environment.
NW and WWT gait performance were associated with functional connectivity in the vestibular resting‐state network. Vestibular function is important in locomotion for an appropriate internal representation of the body in space. While vestibular cortical areas have been reliably associated with gait performance, the directionality of the relationship is not well established. Recent evidence suggests activity in vestibular regions during locomotion to be age‐dependent. Specifically, while younger subjects exhibited significant deactivations in vestibular cortices during motor imagery, older subjects exhibited a relatively increased activation in the same areas [Zwergal et al., 2012]. Our findings underscore the importance of further investigation into age‐related differences in neural correlates of gait performance, especially concerning vestibular functioning.
NW and WWT gait velocity were also associated with functional connectivity in the left‐lateralized frontoparietal resting‐state network. Studies of real and imagined locomotion consistently demonstrate the involvement of the frontal and frontoparietal regions during these tasks [Blumen et al., 2014; Holtzer et al., 2011; la Fougere et al., 2010; Malouin et al., 2003]. These areas sustain higher‐order cognitive processes and attentional processes, which have been shown to be integral to locomotion. Older adults with poorer executive function are at higher risk for falls [Herman et al., 2010; Holtzer et al., 2007]. An intervention study from our group showed that cognitive remediation of attention and executive functions improved gait performance during NW and WWT conditions in older adults [Verghese et al., 2010]. Our findings provide further support for the role of executive functioning and its underlying functional brain substrates in both NW and WWT gait performance.
The known lateralization of fronto‐parietal resting‐state networks is consistent with recent evidence that the left and right fronto‐parietal regions support distinct functions [Smith et al., 2009]. In particular, the left fronto‐parietal region has been associated with allocentric spatial processing and object recognition [Guerin and Miller, 2009; Iachini et al., 2009]. The left hemisphere is also associated with the cortical control of speech; however, the lack of verbal activity in the NW condition suggest that this area plays a role in locomotion that cannot be attributed solely to verbal mediation. Consistent with our results, previous studies using fMRI and mental imagery have found a preponderance of activity in the left dorsolateral prefrontal cortex during imagined gait [Jahn et al., 2004; Malouin et al., 2003]. Further investigation is necessary to explore the differential involvement of the left and right fronto‐parietal regions in gait.
Importantly, our findings are also in general agreement with prior structural MRI studies of mobility in older adults. Findings across voxel‐based morphometry, fluid attenuated inversion recovery sequences, and diffusion tensor imaging studies have emphasized the role of the primary sensorimotor, medial temporal, and prefrontal regions in gait performance [de Laat et al., 2011; Rosano et al., 2007, 2008; Srikanth et al., 2010; Sullivan et al., 2001; Van Impe et al., 2012]. While the origins and functional role of resting‐state activity remain unclear, studies suggest that functional correlations derived from resting‐state fMRI reflect direct and indirect anatomical connections to a large degree [Greicius et al., 2009; Hagmann et al., 2008; Skudlarski et al., 2008]. Therefore, the converging evidence between our results and structural studies of gait suggests additional cross validation for our findings. Presently, a number of questions remain regarding the relationship between structural and functional connection patterns, and further studies combining structural and resting‐state imaging will critical to the interpretation of present and forthcoming results.
Differences in Neural Correlates of NW and WWT
Compared to NW gait velocity, WWT gait velocity was associated with greater functional connectivity in the dorsolateral prefrontal regions of the left fronto‐parietal resting‐state network. The ability to allocate attention to competing task demands, a distinct component of executive functioning, is sub‐served in part by the prefrontal cortex [Stelzel et al., 2009; Szameitat et al., 2002] and is compromised in older adults [Davidson et al., 2006; Holtzer et al., 2004, 2005; Moscovitch and Winocur, 1995; Shimamura et al., 1990; West, 1996]. Numerous studies have demonstrated that performance on measures of executive function, assessed independently of the dual‐task paradigm, is related to gait performance in dual‐task conditions [Hall et al., 2011; Hausdorff et al., 2008; Holtzer et al., 2006, 2012; Liu‐Ambrose et al., 2009; Springer et al., 2006]. Our present findings provide converging evidence in older adults that the prefrontal cortex appears critical in supporting NW and even more so WWT, where cognitive demand is maximized.
Compared with NW gait velocity, WWT gait velocity was associated with greater functional connectivity in the supplementary motor area of the sensorimotor network. The supplementary motor area has been shown to play an important role not only in the planning and coordination of movement but also attention to movement [Johansen‐Berg and Matthews, 2002]. Increased supplementary motor area neural resources may reflect the increased attention to postural awareness and movement coordination necessary to ensure successful gait performance under challenging dual‐task conditions.
Limitations
Several limitations of this study should be considered. First, while resting‐state fMRI is a powerful approach to measure functional brain organization, it is not without its limitations. It is an indirect measure susceptible to several confounding factors that may contribute to between‐subject and between‐group differences. In addition to head movement and physiological activity, anatomic variability and atrophy are possible confounding sources particularly relevant in older adults. Second, it must be noted that due to the stochastic nature of ICA algorithms, our results may be affected by a degree of run‐to‐run variability. Although this problem is typical of ICA analyses, it must be noted that results of a single run should be interpreted with caution. We attempted to reduce this type of variability by selecting strict convergence criteria, as described previously. Lastly, the participants in this study were a relatively small sample of non‐demented, community‐dwelling older adults. The results may not generalize to older adults with significant physical and/or cognitive impairments. Future population‐based studies are needed to replicate our findings in larger, more heterogeneous populations. While methodological and interpretational limitations advise caution concerning the present results, the findings represent an exciting initial investigation into the functional neural correlates of gait.
CONCLUSION
Our results show that NW and WWT gait velocities were both associated with well‐established sensorimotor, visual, vestibular, and left fronto‐parietal resting‐state networks in older adults. Compared with gait velocity in the single‐task condition, the networks associated with gait velocity in the dual‐task condition were associated with greater functional connectivity in supplementary motor and prefrontal regions. The results suggest that WWT may require additional engagement of cognitive and motor neural regions related to attention and movement coordination. To our knowledge, this is the first study to use resting‐state fMRI to examine neural correlates of gait performance. These initial findings should encourage the future use of resting‐state fMRI as tool to examine functional neural connections underlying age‐related mobility impairments. Future avenues of research, including complementary structural and functional imaging techniques, may lead to a better understanding of the multiple neural resources underlying locomotion in older adults and eventually diagnostic or prognostic indicators and more targeted interventions.
REFERENCES
- Anderson WS, Lenz FA (2011): Review of motor and phantom‐related imagery. Neuroreport 22:939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers EI, Tow AC, Holtzer R, Verghese J (2014): Walking while talking and falls in aging. Gerontology 60:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM (2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23:137–152. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurskens R, Bock O (2012): Age‐related deficits of dual‐task walking: A review. Neural Plast 2012:131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaganagarapu K, Jackson GD, Abbott DF (2013): An automated method for identifying artifact in independent component analysis of resting‐state FMRI. Front Hum Neurosci 7:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilney B, Morris M, Webster K (2003): Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture 17:68–74. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal B, DeYoe AE, Hyde JS (1996): Reduction of physiological fluctuations in fMRI using digital filters. Magn Reson Med 35:107–113. [DOI] [PubMed] [Google Scholar]
- Blumen HM, Holtzer R, Brown LL, Gazes Y, Verghese J (2014): Behavioral and neural correlates of imagined walking and walking‐while‐talking in the elderly. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler TC, Oh‐Park M, Wang C, Holtzer R, Verghese J (2012): Walking while talking: Investigation of alternate forms. Gait Posture 35:164–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF (2010): Advances and pitfalls in the analysis and interpretation of resting‐state FMRI data. Front Syst Neurosci 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008): Reduced resting‐state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Troyer AK, Moscovitch M (2006): Frontal lobe contributions to recognition and recall: Linking basic research with clinical evaluation and remediation. J Int Neuropsychol Soc 12:210–223. [DOI] [PubMed] [Google Scholar]
- de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE (2011): Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 134:73–83. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Allen EA, Damaraju E, Calhoun VD (2011): On network derivation, classification, and visualization: A response to Habeck and Moeller. Brain Connect 1:1–19. [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346–355. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, Kimura J, Shibasaki H (1997): Brain functional activity during gait in normal subjects: A SPECT study. Neurosci Lett 228:183–186. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D (2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167. [DOI] [PubMed] [Google Scholar]
- Godde B, Voelcker‐Rehage C (2010): More automation and less cognitive control of imagined walking movements in high‐ versus low‐fit older adults. Front Aging Neurosci 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin SA, Miller MB (2009): Lateralization of the parietal old/new effect: An event‐related fMRI study comparing recognition memory for words and faces. Neuroimage 44:232–242. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009): Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CD, Echt KV, Wolf SL, Rogers WA (2011): Cognitive and motor mechanisms underlying older adults' ability to divide attention while walking. Phys Ther 91:1039–1050. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Schweiger A, Herman T, Yogev‐Seligmann G, Giladi N (2008): Dual‐task decrements in gait: Contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci 63:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM (2010): Executive control deficits as a prodrome to falls in healthy older adults: A prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci 65:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Stern Y, Rakitin BC (2004): Age‐related differences in executive control of working memory. Mem Cognit 32:1333–1345. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Stern Y, Rakitin BC (2005): Predicting age‐related dual‐task effects with individual differences on neuropsychological tests. Neuropsychology 19:18–27. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB (2006): Cognitive processes related to gait velocity: Results from the Einstein aging study. Neuropsychology 20:215–223. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J (2007): The relationship between specific cognitive functions and falls in aging. Neuropsychology 21:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011): fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66:879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J (2012): The relationship between attention and gait in aging: Facts and fallacies. Motor Control 16:64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney J, Verghese J (2013): Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults J Gerontol A Biol Sci Med Sci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM (2014a): Neuroimaging of mobility in aging: A targeted review. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney J, Verghese J (2014b): Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults. J Gerontol A Biol Sci Med Sci 69:980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J (2014c): Performance variance on walking while talking tasks: Theory, findings, and clinical implications. Age (Dordrecht, Netherlands) 36:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iachini T, Ruggiero G, Conson M, Trojano L (2009): Lateralization of egocentric and allocentric spatial processing after parietal brain lesions. Brain Cogn 69:514–520. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T (2004): Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuroimage 22:1722–1731. [DOI] [PubMed] [Google Scholar]
- Jeannerod M (2001): Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage 14:S103–S109. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): FSL. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Matthews PM (2002): Attention to movement modulates activity in sensori‐motor areas, including primary motor cortex. Exp Brain Res 142:13–24. [DOI] [PubMed] [Google Scholar]
- Kelly RE Jr., Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ (2010): Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods 189:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL (2009): Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2010): Real versus imagined locomotion: A [18F]‐FDG PET‐fMRI comparison. Neuroimage 50:1589–1598. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC (1997): Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward‐transform method. Hum Brain Mapp 5:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Verghese J, Holtzer R (2014): A comparison of two walking while talking paradigms in aging. Gait Posture 40:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KZ, Lindenberger U, Freund AM, Baltes PB (2001): Walking while memorizing: Age‐related differences in compensatory behavior. Psychol Sci 12:230–237. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB (2000): Memorizing while walking: Increase in dual‐task costs from young adulthood to old age. Psychol Aging 15:417–436. [DOI] [PubMed] [Google Scholar]
- Liu‐Ambrose T, Katarynych LA, Ashe MC, Nagamatsu LS, Hsu CL (2009): Dual‐task gait performance among community‐dwelling senior women: The role of balance confidence and executive functions. J Gerontol A Biol Sci Med Sci 64:975–982. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J (2003): Brain activations during motor imagery of locomotor‐related tasks: A PET study. Hum Brain Mapp 19:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, Camicioli R (2002): Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol 59:601–606. [DOI] [PubMed] [Google Scholar]
- Massion J (1992): Movement, posture and equilibrium: Interaction and coordination. Prog Neurobiol 38:35–56. [DOI] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K (2001): Cortical mapping of gait in humans: A near‐infrared spectroscopic topography study. Neuroimage 14:1186–1192. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G (1995): Frontal lobes, memory, and aging. Ann N Y Acad Sci 769:119–150. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA (2013): Resting‐state fMRI confounds and cleanup. Neuroimage 80:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB (2006): Association of long‐distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295:2018–2026. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen‐Berg H (2010): Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Studenski S, Newman AB (2007): A regions‐of‐interest volumetric analysis of mobility limitations in community‐dwelling older adults. J Gerontol A Biol Sci Med Sci 62:1048–1055. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB (2008): Special article: Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci 63:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR (1990): Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia 28:803–813. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G (2008): Measuring brain connectivity: Diffusion tensor imaging validates resting state temporal correlations. Neuroimage 43:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM (2006): Dual‐tasking effects on gait variability: The role of aging, falls, and executive function. Mov Disord 21:950–957. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM, Reutens DC (2010): The location of white matter lesions and gait‐‐a voxel‐based study. Ann Neurol 67:265–269. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Brandt SA, Schubert T (2009): Neural mechanisms of concurrent stimulus processing in dual tasks. Neuroimage 48:237–248. [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J (2011): Gait speed and survival in older adults. JAMA 305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A (2001): Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport 12:99–104. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Muller K, Von Cramon DY (2002): Localization of executive functions in dual‐task performance with fMRI. J Cogn Neurosci 14:1184–1199. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Harshman RA, Menon RS (2002): Noise reduction in BOLD‐based fMRI using component analysis. Neuroimage 17:1521–1537. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, Doumas M, Swinnen SP (2012): White matter fractional anisotropy predicts balance performance in older adults. Neurobiol Aging 33:1900–1912. [DOI] [PubMed] [Google Scholar]
- Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G, Lipton R (2002): Validity of divided attention tasks in predicting falls in older individuals: A preliminary study. J Am Geriatr Soc 50:1572–1576. [DOI] [PubMed] [Google Scholar]
- Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB (2006): Epidemiology of gait disorders in community‐residing older adults. J Am Geriatr Soc 54:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Holtzer R, Katz M, Xue X, Buschke H, Pahor M (2007a): Walking while talking: Effect of task prioritization in the elderly. Arch Phys Med Rehabil 88:50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X (2007b): Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 78:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Mahoney J, Ambrose AF, Wang C, Holtzer R (2010): Effect of cognitive remediation on gait in sedentary seniors. J Gerontol A Biol Sci Med Sci 65:1338–1343. [DOI] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, Wang C (2012): Mobility stress test approach to predicting frailty, disability, and mortality in high‐functioning older adults. J Am Geriatr Soc 60:1901–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallet F, Massion J, Massarino R, Khalil R (1992): Coordination between posture and movement in a bimanual load lifting task: Putative role of a medial frontal region including the supplementary motor area. Exp Brain Res 88:674–684. [DOI] [PubMed] [Google Scholar]
- Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA (2005): Gait slowing as a predictor of incident dementia: 6‐Year longitudinal data from the Sydney Older Persons Study. J Neurol Sci 229–230:89–93. [DOI] [PubMed] [Google Scholar]
- Wang L, Larson EB, Bowen JD, van Belle G (2006): Performance‐based physical function and future dementia in older people. Arch Intern Med 166:1115–1120. [DOI] [PubMed] [Google Scholar]
- West RL (1996): An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 120:272–292. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Yogev‐Seligmann G, Rotem‐Galili Y, Mirelman A, Dickstein R, Giladi N, Hausdorff JM (2010): How does explicit prioritization alter walking during dual‐task performance? Effects of age and sex on gait speed and variability. Phys Ther 90:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A (2011): Cortico‐striatal connectivity and cognition in normal aging: A combined DTI and resting state fMRI study. Neuroimage 55:24–31. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K (2012): Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol. Aging 33:1073–1084. [DOI] [PubMed] [Google Scholar]


