Abstract
Gold nanoparticles (AuNPs) are promising vehicles for cancer immunotherapy, with demonstrated efficacy in immune delivery and innate cell stimulation. Nevertheless, their potential has yet to be assessed in the in vivo application of peptide cancer vaccines. In this study, we hypothesized that the immune distribution and adjuvant qualities of AuNPs could be leveraged to facilitate delivery of the ovalbumin (OVA) peptide antigen and the CpG adjuvant and enhance their therapeutic effect in a B16-OVA tumor model. AuNP delivery of OVA (AuNP-OVA) and of CpG (AuNP-CpG) enhanced the efficacy of both agents and induced strong antigen-specific responses. In addition, we found that AuNP-OVA delivery alone, without CpG, was sufficient to promote significant antigen-specific responses, leading to subsequent anti-tumor activity and prolonged survival in both prophylactic and therapeutic in vivo tumor models. This enhanced therapeutic efficacy was likely due to the adjuvant effect of peptide coated AuNPs, as they induced inflammatory cytokine release when cultured with bone marrow dendritic cells. Overall, we demonstrate that AuNP mediated OVA peptide delivery can produce significant therapeutic benefit without the need of adjuvant, indicating that AuNPs are effective peptide vaccine carriers with the potential to permit the use of lower and safer adjuvant doses during vaccination.
Keywords: Gold nanoparticles, Immunotherapy, Biodistribution, Immune system, Cancer
1. Introduction
Nanotechnology can promote the efficacy of various facets of cancer immunotherapy, from adoptive T cell therapy to vaccine delivery.[1–4] Nanotechnology is particularly beneficial for peptide vaccination, an avenue that has shown clinical promise but important limitations. Peptides present a number of advantages for treatment, including safety, stability, defined epitopes, and efficacy at promoting T cell stimulation.[5,6] However, peptide vaccines have shown low clinical effect in trials because they can be quickly degraded in the body, producing only transient responses.[6] Nanotechnology can protect peptides from degradation and facilitate delivery to the immune system, but conventional carriers such as liposomes are of limited benefit due to large sizes that are inappropriate for lymphatic drainage and cell uptake.[7,8] Gold nanoparticles (AuNPs), however, are particularly well-suited for this application, for they can be tuned in size to optimize delivery to the immune system, can be functionalized with the relevant molecules for immune modulation,[9] and have been shown to produce adjuvant effects in vaccination.[2] By leveraging these characteristics, AuNPs can be used to both overcome the delivery limitations of peptides and to promote their therapeutic benefit.
The biodistribution of nanoparticles has been well characterized, with the majority of nanoparticle doses accumulating in the spleen and liver.[10–14] Recent studies, however, have shown that this distribution could be applied in immunotherapy. Reddy and colleagues, for example, assessed the lymphatic uptake of poly(propylene sulfide) nanoparticles of varying sizes and determined that they accumulated in about 50% of lymph node dendritic cells, with particles in the 20-45 nm range showing the highest retention.[8] Similarly, our group has found that 50 nm AuNPs accumulate in dendritic cells, B cells, and MDSCs of the spleen after intravenous injection.[15] Although further study is needed to fully characterize AuNP distribution within the immune system, various groups have begun exploring the application of AuNPs in immunotherapy, particularly in the delivery of antigens and adjuvants. For instance, Niikura and colleagues tested AuNPs of varying sizes and shapes for vaccination against West Nile virus and demonstrated that 40 nm spheres were optimal for antibody production and inflammatory response. The group observed that AuNPs have a direct adjuvant effect, evidenced by the release of inflammatory cytokines in cultured dendritic cells.[16] Lee et al., in turn, used 7 nm AuNPs to deliver red fluorescent protein (RFP) and demonstrated that these constructs can inhibit tumor growth in an RFP-B16F10 tumor model.[17] Tao and colleagues utilized gold nanoclusters for antigen and adjuvant delivery, demonstrating enhanced efficacy in stimulating antigen presenting cells in vitro, as well as enhanced antibody production in vivo.[18] Similarly, Ahn et al. developed antigen-carrying AuNPs capable of inducing an inflammatory response in vitro and an anti-tumor response in vivo without the need of an external adjuvant.[19] Finally, our group has previously developed AuNPs capable of carrying self and non-self peptides that induce an antigen specific response in vitro.[20]
Numerous groups have also explored methods by which to optimize AuNP mediated delivery of CpG, a synthetic oligodeoxynucleotide that binds to toll-like receptor 9 (TLR9) in the endosomes of antigen presenting cells, resulting in an inflammatory response.[21–23] AuNP delivery facilitates uptake into endosomes, thereby enhancing the stimulatory activity of CpG and producing a strong immune response with anti-tumor activity.[21,22] Interestingly, peptide coated AuNPs can also induce stimulation of innate immune cells, and the mechanisms behind this effect are under study. Bastus et al., for instance, observed that AuNPs homogenously coated with peptides caused macrophages to produce pro-inflammatory cytokines, and they posit that this effect is due to the ordered and repetitive coating of the peptide.[24] Yang et al. also assessed the inflammatory response to peptide coated AuNPs and found that cytokine release is mainly dependent on the amino acid composition of the peptide, with aromatic amino acids causing the strongest stimulatory effect.[25]
Cumulatively, AuNPs have been shown to facilitate delivery to the immune system, to promote the therapeutic effect of antigens and adjuvants, and to have an adjuvant effect on their own. Therefore, we hypothesized that one could leverage the immune distribution and adjuvant effect of AuNPs to develop AuNP-peptide vaccines that could produce systemic immune responses against tumors. To that end, we used AuNPs coated with the ovalbumin peptide antigen (AuNP-OVA) alone or in combination with AuNPs coated with the CpG adjuvant (AuNP-CpG) for cancer immunotherapy. AuNP-OVA treatments induced a significantly stronger antigen specific immune response than delivery of free OVA and did not require the addition of adjuvant. Furthermore, this response resulted in significant tumor inhibition in both a prophylactic and therapeutic setting. This anti-tumor effect in turn led to significant survival extension, demonstrating that AuNPs can be an effective carrier of peptide cancer antigens and adjuvants for cancer treatment. In addition, delivery with AuNPs may reduce the need for high doses of adjuvants, thereby reducing possible toxicities such as spleen enlargement and systemic cytokine release.[26,27]
2. Results and Discussion
2. 1 AuNP-OVA and AuNP-CpG promote antigen specific immune responses
AuNP-OVA and AuNP-CpG particles were prepared as previously described.[20,21] Briefly, 30 nm AuNPs were first coated with carboxyl-terminated polyethylene glycol (PEG), then conjugated with OVA peptide using EDC/Sulfo-NHS chemistry to bind the amine groups of the peptide to the activated carboxyl groups of the PEG.[20] The AuNP-CpG particles were prepared by coating 30 nm AuNPs with CpG1826 via an Au-thiol dative bond.[21] A spectral shift in the UV-Vis absorbance spectrum of the particles was indicative of successful conjugation (Supplemental Figure 1). Both AuNP-OVA and AuNP-CpG particles remained under 100 nm in diameter (Supplementary Table 1), the size range that is optimal for lymphatic drainage and immune uptake.[7,8,28]
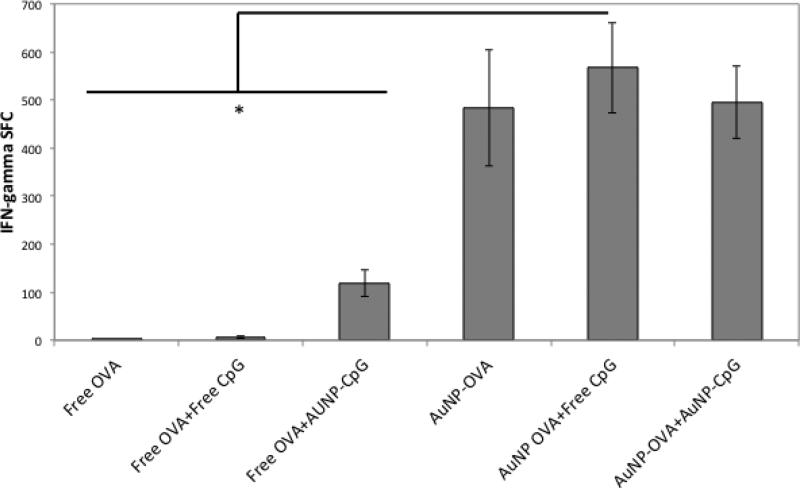
To analyze the antigen-specific response induced by treatment, the particles were injected subcutaneously in both flanks of mice for a total dose of 2×1011 AuNP OVA and 1012 AuNP-CpG, or the equivalent of at most 50 μg OVA and approximately 4.7 μg CpG. A booster injection was given 10 days later, and the mice were euthanized after an additional 7 days and the spleens were harvested for Enzyme Linked ImmunoSpot (ELISPOT) analysis. The conditions tested were: Free OVA, Free OVA+ Free CpG, Free OVA+ AuNP-CpG, AuNP-OVA, AuNP-OVA+ Free CpG, AuNP OVA+ AuNP-CpG (Figure 1). The injection of free OVA with or without free CpG induced no OVA-specific immune response, while the application of Free OVA+ AuNP-CpG caused a measureable response by ELISPOT. Therefore, AuNP mediated delivery of CpG enhanced the adjuvant’s immune stimulation, and this enhancement consequently promoted vaccination with free OVA. The strongest responses were observed in the AuNP-OVA conditions (p<0.02), with approximately 1.5% of CD8+ T cells specific for OVA (Supplementary Figure 2). This strong response illustrates that AuNPs enhance antigen specific activity, likely due to facilitated delivery and through an adjuvant effect of AuNPs. In fact, when cultured in vitro with bone marrow derived dendritic cells (BMDCs), AuNP-OVA induced significantly higher release of the inflammatory cytokine IL-6 than free OVA or unconjugated, PEGylated AuNPs (Supplementary Figure 3a). AuNP-CpG particles also caused IL-6 release, as expected. Interestingly, AuNP-PEG particles also had an inflammatory effect, but to a lesser extent than AuNP-OVA and AuNP-CpG particles. This effect may be mediated by the carboxyl groups on the nanoparticle surface, as this surface modification has been previously shown to induce inflammatory cytokine release.[29] When cultured with the J774.A1 monocyte and macrophage cell line, however, the AuNP-PEG and AuNP-OVA treatments showed no stimulatory effect, whereas the AuNP-CpG particles did (Supplementary Figure 3b). This finding suggests that the AuNP-OVA adjuvant effect may be specific to dendritic cells.
Figure 1.
IFN-gamma producing splenocytes in ELISPOT assay after treatment with various conditions. Mice were injected subcutaneously on both flanks on day 0 with a total dose of 2×1011 AuNP-OVA and 1012 AuNP-CpG, or the equivalent of at most 50 μg OVA and approximately 4.7 μg CpG. The dose was repeated on day 10, and the spleens were harvested on day 17. *, p<0.02.
The AuNP-OVA inflammatory response is consistent with previous work describing the adjuvant effect of AuNPs coated with proteins or with peptides. Niikura and colleagues found that spherical AuNPs in the 40 nm range coated with West Nile Virus Envelope protein induced the highest release of TNFα and IL-6 in bone marrow dendritic cells when compared to particles of different shapes and sizes.[16] As aforementioned, Bastus et al. attributed macrophage pro-inflammatory response against peptide-coated AuNPs to the repetitive coating on the particle surface,[24] while Yang and colleagues concluded that the presence of aromatic amino acids in peptide coated AuNPs induced inflammation.[25] In this study, the main contribution to the cytokine production may stem from the foreign OVA antigen or from the presence of the aromatic amino acid phenylalanine in the peptide, but further work is needed to determine the role of the core particle, the peptide structure, and the choice of antigen in inflammatory responses.
2.2 AuNP treatment promotes immunity against tumor challenge
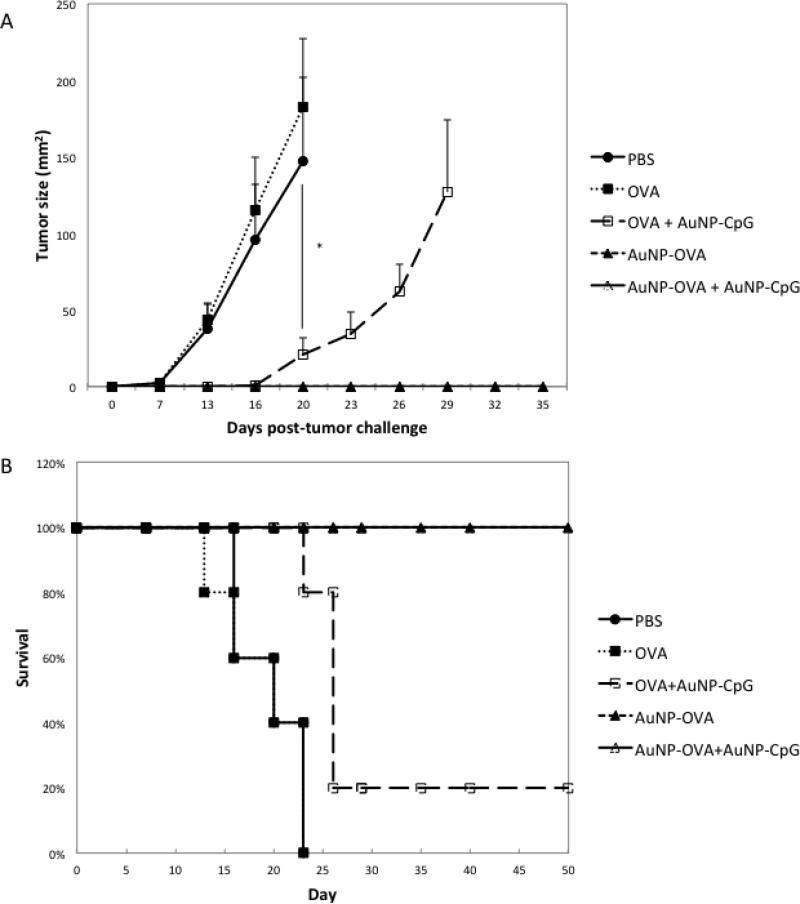
To assess whether the antigen-specific response then translated to an anti-tumor effect, we applied the nanoparticles in a tumor challenge model. The treatments were again given at the same doses 10 days apart, followed by tumor challenge 7 days later with 105 B16-OVA cells subcutaneously (Figure 2a). Tumors grew in PBS treated mice (n=5) and mice treated with free OVA (n=5), consistent with the lack of antigen specific response observed in Figure 1. Mice treated with free OVA+ AuNP-CpG (n=5) displayed a significant delay in tumor growth (p<0.02) starting on day 13, but the tumors eventually grew. Nevertheless, the addition of AuNP-CpG enhanced the vaccination and significantly prolonged survival when compared to Free OVA alone (p=0.0082). Mice treated with AuNP-OVA (n=5) and AuNP-OVA+ AuNP-CpG (n=5) showed no tumor growth at all in any of the mice, indicating that the antigen specific response provided protection against tumor growth. These anti-tumor effects ultimately resulted in significantly prolonged survival (p<0.0001), with 100% of the AuNP-OVA and AuNP-OVA+ AuNP-CpG mice surviving throughout the 50 day duration of the study (Figure 2b).
Figure 2.
A) Tumor growth following challenge with B16-OVA on mice treated with various conditions. Mice were injected subcutaneously on both flanks on day 0 with a total dose of 2×1011 AuNP-OVA and 1012 AuNP-CpG, or the equivalent of at most 50 μg OVA and approximately 4.7 μg CpG. The dose was repeated on day 10, and the mice were then challenged with 105 B16-OVA cells subcutaneously on day 17. B) Survival following challenge with B16-OVA. N=5 for all conditions. *, p<0.02.
2.3 AuNP treatment inhibits tumor growth in established tumor models
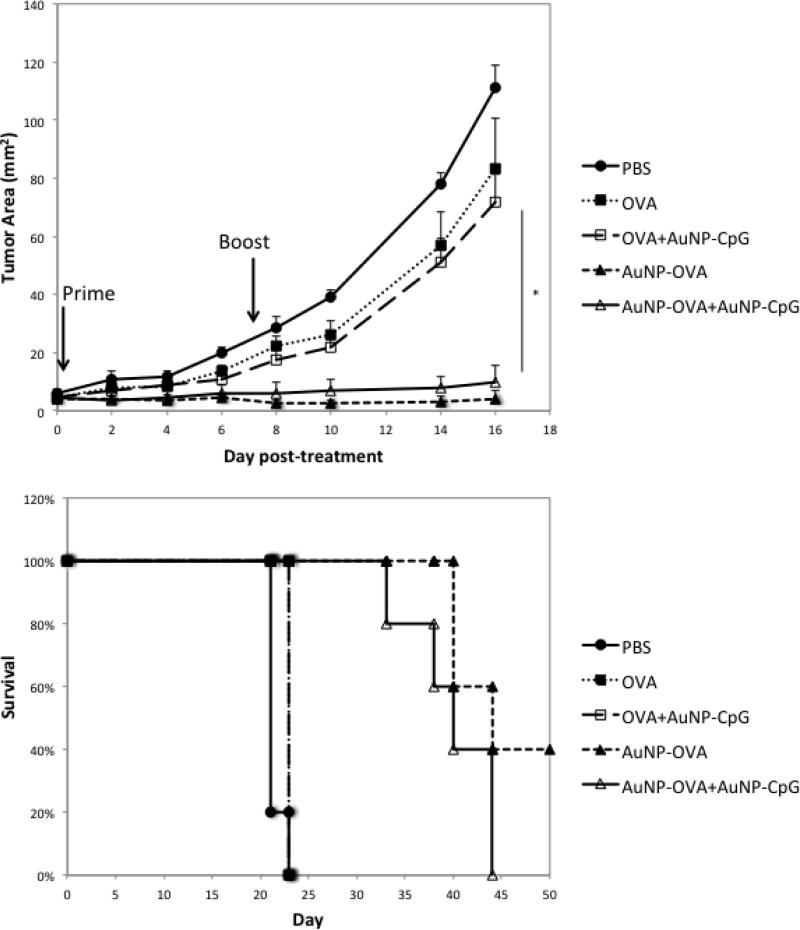
Next, we assessed the benefit of these treatments in a therapeutic setting. Here, mice were implanted with 5×105 B16-OVA cells, and the tumors were allowed to grow for 5 days to a size of 5 mm2 prior to treatment (Figure 3). The mice were then given the same doses, with a priming injection on day 5 and a booster injection on day 12 after tumor implantation. In this model, the free OVA and free OVA+AuNP-CpG condition had no effect on tumor growth when compared to PBS treated mice. Yet, the conditions with AuNP-OVA showed a significant inhibition of tumor growth (p<0.02), which also resulted in significantly prolonged survival when compared to PBS, Free OVA, and Free OVA+AuNP-OVA (p<0.003). In addition, 40% of mice in the AuNP-OVA condition showed no palpable tumor by the end of the 50-day study. Interestingly, the addition of AuNP-CpG had no effect when combined with free OVA or AuNP-OVA. Perhaps the tumor was too advanced to be affected by CpG alone or by the weak immune response produced by injections of free OVA+ AuNP-CpG. Furthermore, the addition of AuNP-CpG to AuNP-OVA may not add benefit because the antigen and adjuvant are on separate particles. Immune stimulation of antigen and adjuvant is indeed more potent when the two agents are co-localized,[30,31] and the use of separate particles may not result in uptake by the same cell populations.
Figure 3.
A) Tumor growth in an established B16-OVA 5 mm2 tumor model following treatment with various conditions. Mice were implanted with 5×105 B16-OVA tumor cells subcutaneously, and the tumors were allowed to grow for 5 days to a size of about 5 mm2. The first treatment dose was then administered on both flanks subcutaneously, for a total dose of 2×1011 AuNP-OVA and 1012 AuNP-CpG, or the equivalent of at most 50 μg OVA and approximately 4.7 μg Cpg. The dose was repeated 7 days. B) Survival following treatment. N=5 for all conditions. *, p<0.02.
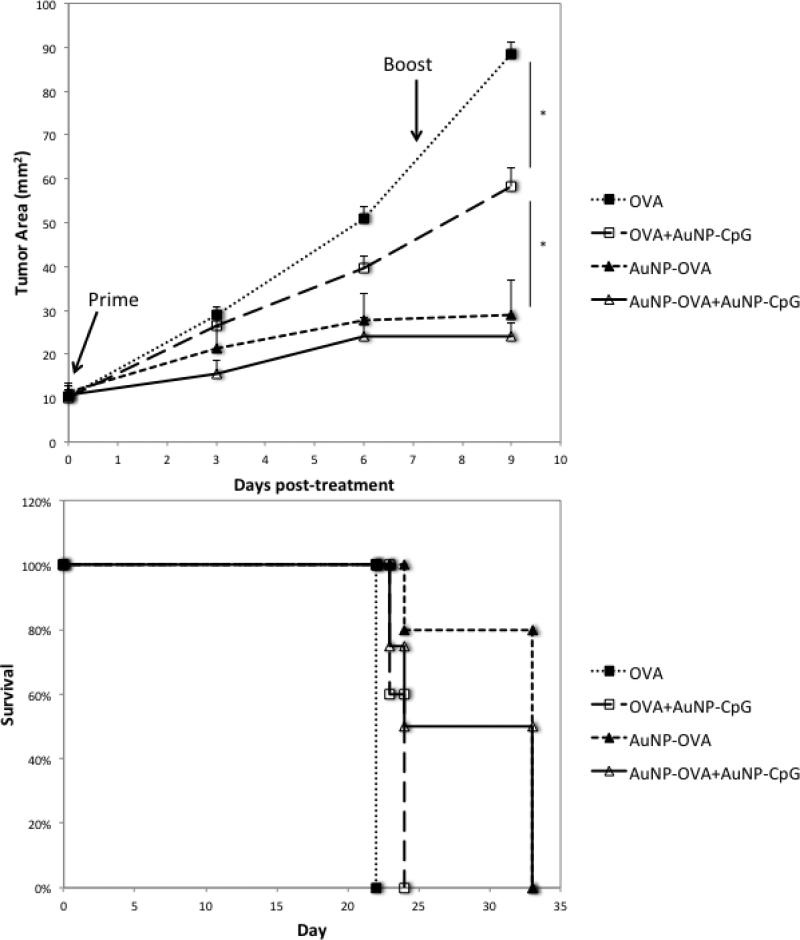
Finally, we explored the effect of these treatments on a larger tumor, as advanced tumors are more immunosuppressive and more difficult to treat (Figure 4).[32–34] In this case, we allowed the B16-OVA tumors to grow for 11 days, reaching an average size of 10 mm2, or double the size used in the previous experiment. In this case, the free OVA condition was again ineffective, but the free OVA+ AuNP-CpG condition caused a significant inhibition of tumor growth (p<0.03) and a modest, significant increase in survival (p=0.0027). This finding was unexpected and may be a result of the tumor stage. More advanced tumors have higher infiltration of MDSCs, and the application of CpG at this stage may be inhibiting the MDSC suppressive activity.[35,36] In agreement with previous experiments, the AuNP-OVA conditions caused the most significant inhibition in tumor growth (p<0.03), leading to significantly enhanced survival when compared to free OVA (p<0.005).
Figure 4.
A) Tumor growth in an established B16-OVA 10 mm2 tumor model following treatment with various conditions. Mice were implanted with 5×105 B16-OVA tumor cells subcutaneously, and the tumors were allowed to grow for 11 days to a size of about 10 mm2. As with the small tumor, the first treatment dose was then injected on both flanks subcutaneously, for a total dose of 2×1011 AuNP-OVA and 1012 AuNP-CpG, or the equivalent of at most 50 μg OVA and approximately 4.7 μgμg CpG. The dose was repeated 7 days later. B) Survival following treatment. N=5 for all conditions. *, p<0.03.
Once again, the AuNP-OVA treatment was strong enough to promote an anti-tumor effect without the inclusion of adjuvant. The efficacy of AuNP-OVA alone is particularly striking given that similar studies of nanoparticle-delivered OVA require the presence of an adjuvant for anti-tumor activity. Bourquin et al., for example, delivered OVA in gelatin nanoparticles, but the immunization was only effective against tumor challenge when the particles were complexed with 100 μg CpG.[26] Similarly, in a study by de Titta and colleagues, OVA delivery with small polymeric particles required co-delivery with CpG in order to induce immune protection against tumor challenge.[37] Finally, with liposomal delivery, van Broekhoven et al. observed that particles delivering OVA needed to be encapsulated with danger signals such as LPS or interferon to provide anti-tumor immunity.[38] Thus, the efficacy of AuNP-OVA alone shown here suggests the potential for AuNPs to deliver peptide antigens with reduced adjuvant doses. In fact, our findings are consistent with those of Lee et al., who also observed that AuNP delivery of red fluorescent protein (RFP) antigen alone, without CpG, induced protection against tumor challenge. In a therapeutic model AuNP-RFP delivery was again effective on its own, but in this case the addition of CpG led to a modest improvement in tumor inhibition.[17] In their work, Niikura and colleagues delivered West Nile virus envelope protein without adjuvants and observed significant inflammatory response and antibody production, consistent with previous observations of the inflammatory impact of AuNPs.[16][39–41] in vitro Finally, Ahn et al. observed antigen specific responses and an anti-tumor response in vivo with delivery of OVA coated AuNPs alone, without the need for adjuvant, consistent with the findings here.[19] The results discussed here indicate that AuNPs can significantly promote vaccination with the OVA antigen, but future studies need to assess their efficacy in delivery of less immunogenic, endogenous antigens. In addition, more research is needed to elucidate the mechanisms behind the stimulatory effect of AuNPs, as they may be mediated by a variety of factors such as shape, size, hydrophobicity, and coating.[29,41,42] Further understanding may then allow researchers to leverage these characteristics for improved vaccine delivery. Here, we expanded upon the findings that AuNPs can serve as adjuvants and can promote inflammation when coated with peptides to demonstrate that AuNP delivery of antigen alone causes anti-cancer activity without co-delivery of danger signals. The ability to induce an anti-tumor immune response without adjuvants positions AuNPs as promising immunotherapy carriers, with the potential to limit toxicities normally associated with high adjuvant doses.
3. Conclusion
AuNP mediated delivery of OVA and CpG induced systemic antigen-specific immune responses that resulted in anti-tumor effect in a prophylactic model and in small and large established tumor models. The addition of AuNP-CpG was beneficial when combined with delivery of free OVA, indicating that AuNP delivered adjuvant can boost the efficacy of free antigen to a higher extent than free adjuvant. However, the addition of AuNP-CpG had no effect when administered with AuNP-OVA in vivo, likely because the effect of AuNP-OVA was strong enough on its own and because the different particle treatments did not distribute to the same cellular populations. In fact, AuNP-OVA demonstrated higher inflammatory effects in vitro than PEGylated particles and free OVA. Cumulatively, the findings indicate that AuNPOVA alone can induce a strong, therapeutic response because of the adjuvant effect of peptide coated AuNPs. AuNPs therefore can serve as effective carriers for peptide vaccines, promoting their anti-tumor effect and potentially reducing the need for high doses of other adjuvants. Further studies will focus on the delivery of other tumor antigens and on the design of particles capable of delivering antigen and adjuvant simultaneously.
4. Experimental Section
Cell culture
B16-OVA cells were kindly provided by Dr. Xiao-Tong Song (Baylor College of Medicine).[43] They were maintained at 37 °C and 5% CO2 and cultured with Dulbecco's Modified Eagle Medium (DMEM) (ATCC), supplemented with 0.5 mg/ml geneticin (Invitrogen), 2 mM Glutamax (Invitrogen), and 10% FBS (ATCC). The J774.A1 macrophage line (ATCC) was maintained in DMEM with 10% FBS and 1% penicillin/streptomycin.
AuNP-OVA conjugation
AuNP-OVA particles were prepared as previously described.[20] Briefly, 10 ml of 30 nm gold colloid (Ted Pella, Inc.) at 2×1011 AuNPs/ml was incubated with 0.5 mM carboxyl-terminated 5,000 MW PEG overnight. The solution was then raised to 10 mM sodium phosphate, 0.1 M sodium chloride, and 0.1% tween 20 w/v% and incubated overnight. Next, the particles were spun and pelleted at 7,500 g for 20 minutes and re-suspended in 2-(N - morpholino)ethanesulfonic acid (MES) buffer (Thermo Scientific). 4.5 mg of EDC and 6.4 mg Sulfo-NHS (Thermo Scientific) were added to the solution and incubated for 15 minutes. After incubation, the solution was washed two times at 7,500 g for 20 minutes, and the pellet was re-suspended in 10 ml PBS. Then, the solution was incubated with 50 μg/ml of the SIINFEKL OVA peptide (Genemed Synthesis) for 1 hour. The reaction was terminated with 10 mM NH2OH and incubated for 1 hour.
Finally, the solution was washed three times at 7,500 g for 20 minutes, and the particles were re-suspended in PBS and stored at 4 °C until use.
AuNP-CpG conjugation
AuNP-CpG particles were prepared as previously published.[21] CpG 1826 sequences with the modifications previously described were purchased from Integrated DNA Technologies. The sequence is: 5’-HS-C6-TTTTTTTTTTT-(CH2CH2O)3-TCCATGACGTTCCTGACGTT-3’. The thiol modification was reduced by incubating for 1 hour in 100 mM dithiothreitol (Thermo Scientific) in HPCE buffer solution, pH 8.5 (Fluka Analytics). Next, the solution was eluted through NAP-5 columns (GE Healthcare) in HPCE buffer at pH 6.5. After elution, 10 ml of 30 nm gold colloid was incubated with 0.5 μM uncapped CpG overnight. The solution was then raised to 10 mM sodium phosphate, 0.1 M sodium chloride, and 0.1% tween 20 w/v% and incubated overnight. The particles were then washed three times at 7,500 g for 20 minutes, re-suspended PBS, and stored at 4°C until use.
Particle characterization.
UV-VIS absorbance was measured using a Cary 60 UV-Vis (Agilent Technologies), and the particle size was measured using a 90-Plus Particle Size Analyzer (Brookhaven).
Enzyme Linked Immunospot (ELISPOT) Animal studies
Albino C57BL/6J mice were purchased from Jackson Laboratories and kept in the pathogen free mouse facility at Rice University. All studies were approved and done in accordance with the Institutional Animal Care and Use Committee at Rice University. Particle treatments were given subcutaneously at both flanks for a total dose of 2×1011 AuNP-OVA particles and 1012 AuNP-CpG particles. Free OVA was injected at a total dose of 50 μg, and free CpG was injected at a dose of 4.7 μg. As the OVA peptide does not absorb in the 260-280 nm range, we could not use UV-Vis absorbance to measure the OVA content found on the particles, as we did with the AuNP-CpG particles. We thus compared AuNP-OVA injections with the total amount of OVA added during the conjugation process, which was 50 μg per 2×1011 AuNP particles. AuNP-OVA in mice, we compared that treatment Therefore, as we injected 2x1011 with 50 μg of free OVA. This is a conservative estimate, as not all 50μg of OVA will successfully be conjugated onto the surface of the PEGylated AuNPs.
In ELISPOT studies, mice were injected with a priming dose, followed by a booster dose 10 days later. After 7 additional days, the mice were euthanized, and the spleen was harvested for the ELISPOT assay, as previously described by Bear et al.[3] Spleens were passed through a 70 μ m cell strainer (BD Falcon), and red blood cells were removed using red blood cell lysis buffer (Sigma). 3×105 splenocytes were cultured in 96 well plates pre-coated with anti-Interferon γ antibodies. The spot forming cells )SFC) were quantified at ZellNet.
Dextramer staining
The harvested splenocytes were also stained with anti-CD8 PerCP-Cy5.5 antibodies (Biolegend) and SIINFEKL Dextramer PE (Immundex) and analyzed using a LSR2 flow cytometer and FACSDiva Software (BD).
Inflammatory cytokine release studies
BMDCs were harvested from the tibia and femur of mice. Red blood cells were lysed and the cells were grown for 48 hours at 37°C and 5% CO2 in media supplemented with IL-4 and GM-CSF. Cells were cultured with 50 μg OVA, 2×1011 AuNP-PEG, 2×1011 AuNP-OVA, or 2×1011 AuNP-CpG. After overnight incubation, the supernatant was collected and analyzed using an IL-6 ELISA kit (eBioscience). The experiment was repeated in the J774.A1 monocyte/macrophage cell line.
Tumor studies
In challenge studies, the mice were treated as described, and then challenged with 1×105 B16-OVA cells. Tumor area was measured by digital caliper and mice were euthanized once the tumor area exceeded 1 cm2.
In therapeutic studies, mice were implanted with 5×105 B16-OVA cells, and the tumors were allowed to grow for 5 days to a size of ~5 mm2 in the small tumor model and for 11 days to ~10 mm2 in the large tumor model. Then the mice were treated with the previously described doses 7 days apart. Again, the tumor size was measured using digital calipers, and mice were euthanized once tumors exceeded an area of 1 cm2
Statistical analysis
Significance was set at α=0.05, and multiple comparisons were done using Tukey’s HSD. Survival analysis was done using the log-rank test, accounting for multiple comparisons with the Bonferroni correction. All analysis was done in JMP Pro Software.
Supplementary Material
Acknowledgements
This project was supported by the National Institutes of Health R01CA172836. J Almeida is also funded by the Keck Center of the Gulf Coast Consortia, on the Nanobiology Interdisciplinary Graduate Training Program, National Institute of Biomedical Imaging and Bioengineering (NIBIB) T32EB009379, as well as the National Science Foundation Graduate Research Fellowship #0940902 and the Howard Hughes Medical Institute Med into Grad fellowship.
References
- 1.Moon JJ, Huang B, Irvine DJ. Adv. Mater. 2012;24:3724. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida JPM, Figueroa ER, Drezek RA. Nanomedicine-Nanotechnology Biol. Med. 2014;10:503. doi: 10.1016/j.nano.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bear AS, Kennedy LC, Young JK, Perna SK, Mattos Almeida JP, Lin AY, Eckels PC, Drezek RA, Foster AE. PLoS One. 2013;8:e69073. doi: 10.1371/journal.pone.0069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE, Drezek RA. Nanoscale Res. Lett. 2011;6:X1. doi: 10.1186/1556-276X-6-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang XQ, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. J. Immunol. 1995;154:3961. [PubMed] [Google Scholar]
- 6.Slingluff CL., Jr. Cancer J. 2011;17:343. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy ST, Swartz MA, Hubbell JA. Trends Immunol. 2006;27:573. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Reddy ST, Rehor A, Schmoekel HG, Hubbell JA, Swartz MA. J. Control. Release. 2006;112:26. doi: 10.1016/j.jconrel.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Han G, Ghosh P, Rotello VM. Nanomedicine. 2007;2:113. doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- 10.Almeida JPM, Chen AL, Foster A, Drezek R. Nanomedicine. 2011;6:815. doi: 10.2217/nnm.11.79. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian SK, Jittiwat J, Manikandan J, Ong CN, Yu LE, Ong WY. Biomaterials. 2010;31:2034. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 12.Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schäffler M, Takenaka S, Möller W, Schmid G, Simon U, Kreyling WG. Eur. J. Pharm. Biopharm. 2011;77:407. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkilany AM, Murphy CJ. J. Nanoparticle Res. 2010;12:2313. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khlebtsov N, Dykman L. Chem. Soc. Rev. 2011;40:1647. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 15.Almeida JPM, Lin AY, Langsner RJ, Eckels P, Foster AE, Drezek RA. Small. 2013;10:812. doi: 10.1002/smll.201301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, Ijiro K, Sawa H. ACS Nano. 2013;7:3926. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 17.Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, Lee JH, Lee TS, Im SH, Jon S. Angew. Chemie - Int. Ed. 2012;51:8800. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 18.Tao Y, Ju E, Li Z, Ren J, Qu X. Adv. Funct. Mater. 2014;24:1004. [Google Scholar]
- 19.Ahn S, Lee I-H, Kang S, Kim D, Choi M, Saw PE, Shin E-C, Jon S. Adv. Healthc. Mater. 2014;3:1194. doi: 10.1002/adhm.201300597. [DOI] [PubMed] [Google Scholar]
- 20.Lin AY, Lunsford J, Bear AS, Young JK, Eckels P, Luo L, Foster AE, Drezek RA. Nanoscale Res. Lett. 2013;8:1. doi: 10.1186/1556-276X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin AY, Mattos Almeida JP, Bear A, Liu N, Luo L, Foster AE, Drezek RA. PLoS One. 2013;8:e63550. doi: 10.1371/journal.pone.0063550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei M, Chen N, Li J, Yin M, Liang L, He Y, Song H, Fan C, Huang Q. Angew. Chemie - Int. Ed. 2012;51:1202. doi: 10.1002/anie.201105187. [DOI] [PubMed] [Google Scholar]
- 23.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. Expert Rev. Vaccines. 2011;10:499. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastus NG, Sanchez-Tillo E, Pujals S, Farrera C, Lopez C, Giralt E, Celada A, Lloberas J, Puntes V. ACS Nano. 2009;3:1335. doi: 10.1021/nn8008273. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Zhou Y, Fung S-Y, Wu L, Tsai K, Tan R, Turvey SE, Machuca T, de Perrot M, Waddell TK, Liu M. Part. Part. Syst. Charact. 2013;30:1039. [Google Scholar]
- 26.Bourquin C, Anz D, Zwiorek K, Lanz AL, Fuchs S, Weigel S, Wurzenberger C, Von Der Borch P, Golic M, Moder S, Winter G, Coester C, Endres S. J. Immunol. 2008;181:2990. doi: 10.4049/jimmunol.181.5.2990. [DOI] [PubMed] [Google Scholar]
- 27.Kwong B, Liu H, Irvine DJ. Biomaterials. 2011;32:5134. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 29.Bartneck M, Keul HA, Singh S, Czaja K, Bornemann J, Bockstaller M, Moeller M, Zwadlo-Klarwasser G, Groll J. ACS Nano. 2010;4:3073. doi: 10.1021/nn100262h. [DOI] [PubMed] [Google Scholar]
- 30.Nierkens S, den Brok MH, Sutmuller RPM, Grauer OM, Bennink E, Morgan ME, Figdor CG, Ruers TJM, Adema GJ. Cancer Res. 2008;68:6859. doi: 10.1158/0008-5472.CAN-07-6023. [DOI] [PubMed] [Google Scholar]
- 31.Fischer NO, Rasley A, Corzett M, Hwang MH, Hoeprich PD, Blanchette CD. J. Am. Chem. Soc. 2013;135:2044. doi: 10.1021/ja3063293. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Nat. Rev. Immunol. 2009;9:162. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. J. Immunol. 2008;181:5791. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Int. Immunopharmacol. 2011;11:814. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Shirota Y, Shirota H, Klinman DM. J. Immunol. 2012;188:1592. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoglmeier C, Bauer H, Nörenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. Clin. Cancer Res. 2011;17:1765. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 37.de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19902. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Cancer Res. 2004;64:4357. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 39.Yen HJ, Hsu SH, Tsai CL. Small. 2009;5:1553. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- 40.Kim EY, Schulz R, Swantek P, Kunstman K, Malim MH, Wolinsky SM. Gene Ther. 2012;19:347. doi: 10.1038/gt.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. J. Am. Chem. Soc. 2012;134:3965. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartneck M, Ritz T, Keul HA, Wambach M, Bornemann J, Gbureck U, Ehling J, Lammers T, Heymann F, Gassler N, Lüdde T, Trautwein C, Groll J, Tacke F. ACS Nano. 2012;6:8767. doi: 10.1021/nn302502u. [DOI] [PubMed] [Google Scholar]
- 43.Song XT, Kabler KE, Shen L, Rollins L, Huang XF, Chen SY. Nat. Med. 2008;14:258. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.