Abstract
Total hip arthroplasty (THA) has revolutionized the treatment of hip arthritis. A number of surgical approaches to the hip joint exist, each with unique advantages and disadvantages. The most commonly used approaches include the direct anterior, direct lateral and posterior approaches. A number of technical intricacies allow safe and efficient femoral and acetabular reconstruction when using each approach. Hip dislocation, abductor insufficiency, fracture and nerve injury are complications of THA, although their relative risk varies by approach. Numerous clinical trials have sought to elicit differences in patient-reported outcomes, complication rates and return to function among the surgical approaches. This review outlines some of the technical pearls of performing a THA through either a direct anterior, direct lateral or posterior approach. A literature review outlines the impact of surgical approach on clinical outcomes and clinically relevant complication rates.
Abstract
L’arthroplastie pour prothèse totale de la hanche (PTH) a révolutionné le traitement de l’arthrite de la hanche. Il existe plusieurs approches chirurgicales pour l’articulation de la hanche, et chacune comporte ses avantages et inconvénients propres. Les approches les plus souvent utilisées sont l’approche antérieure directe, l’approche latérale directe et les approches postérieures. Plusieurs détails techniques contribuent à une reconstruction fémorale et acétabulaire sécuritaire et efficace avec chaque approche. La dislocation de la hanche, l’insuffisance des abducteurs, la fracture et les lésions nerveuses sont les complications de la PTH, quoique leur risque relatif varie d’une approche à l’autre. Plusieurs essais cliniques ont voulu mettre en lumière les différences quant aux résultats, aux taux de complications et au rétablissement fonctionnel déclarés par les patients selon les différentes approches chirurgicales utilisées. La présente synthèse résume quelques-unes des « perles techniques » pour l’exécution de la PTH soit par approche antérieure directe, latérale directe ou postérieure. Une revue de la littérature résume l’impact de l’approche chirurgicale sur les résultats cliniques et les taux de complications cliniquement importants.
Since its inception in the 1960s, total hip arthroplasty (THA) has revolutionized the treatment of painful hip arthritis.1 More than 24 000 THA procedures are performed annually in Canada.2 Surgical approach in THA is a recent area of interest in the literature. Each approach requires a thorough understanding of anatomy to optimize femoral and acetabular visualization, minimize complications and optimize patient outcomes.
The purpose of this review is to outline the anatomy and the technical aspects of the 3 commonly used surgical approaches to the hip: the direct anterior, direct lateral and posterior approaches. We conducted an evidence-based review examining studies that compared various clinical outcomes and complication rates across the 3 approaches. Although surgeon experience and anecdotal success are important factors when choosing surgical approaches for THA, our review demonstrates many important differences among the approaches that may influence surgeon choice in the future.
Methods
We performed a comprehensive literature search using PubMed and Medline. The keywords “hip,” “arthroplasty,” and “approach” were used to identify papers examining the topic of interest. The terms “anterior,” “lateral” and “posterior” were added to our search in order to identify articles that were approach-specific. We included comparative studies published from 2000 to 2014 in our review. Study titles and abstracts were reviewed to determine level of evidence to ensure high-quality literature (i.e. meta-analyses, systematic reviews, randomized controlled trials) was included. We included articles published earlier than 2000 if they contributed to the discussion on surgical technique or the incidence of particular complications.
Direct anterior approach
Overview
The direct anterior approach to the hip was first described by Smith-Peterson in the 1940s, and was later modified by Heuter in the 1950s.3 Internationally, this approach is gaining popularity in the hip arthroplasty community.4 Advocates of this approach consider its advantages to be the muscle-sparing nature of its internervous intervals, earlier restoration of gait kinematics and low dislocation rates.5–9 The direct anterior approach can be performed with or without the use of a specialized table or fluoroscopy.10,11 Our institution favours the use of a specialized table and intraoperative fluoroscopy, which is described later in this section.
Anatomy and technical considerations
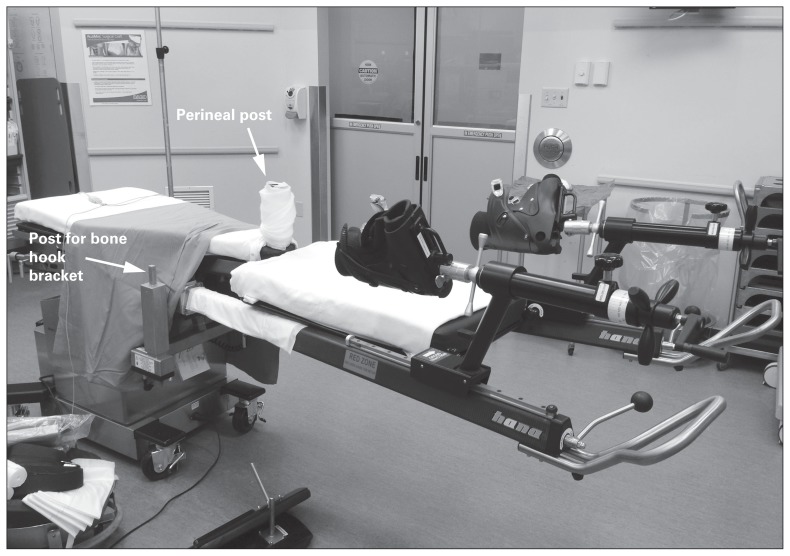
The procedure begins by positioning the patient supine on a specialized traction table (Fig. 1). Both feet are firmly secured to boots attached to lever arms that permit positioning of each lower extremity and applying traction to either limb. The perineal post located between the legs stabilizes the patient on the operating room table and provides a point of counter-traction.10
Fig. 1.
Example of the specialized table (Hana fracture table, Mizuho OSI) used during a direct anterior approach. Boots attached to lever arms allow traction and free positioning of the leg during each procedure. A perineal post provides counter-traction, and a motorized lift allows improved femoral exposure.
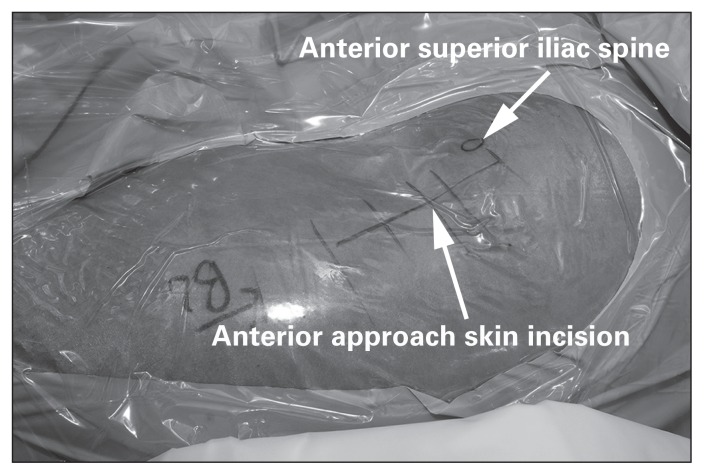
The surgical incision begins 2–4 cm lateral to the anterior superior iliac spine of the pelvis (Fig. 2). It is then carried distally and laterally for about 8–12 cm at 20° from the sagittal plane of the patient toward the lateral aspect of the patient’s ipsilateral knee. The lateral femoral cutaneous nerve (LFCN) is identified, transposed medially and protected.
Fig. 2.
The skin incision used for the direct anterior approach to the hip.
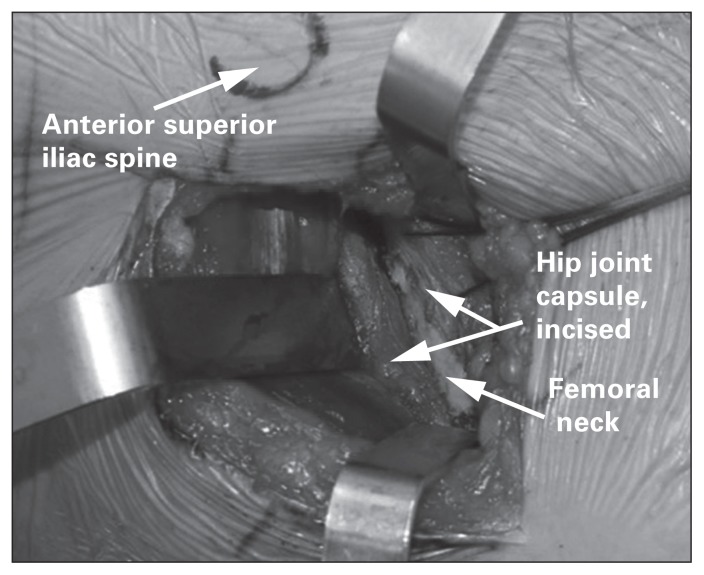
After protecting the LFCN, the fascia overlying the tensor fascia latae (TFL) is incised, and a plane is then developed between the TFL and sartorius. The surgeon will then encounter the interval between the rectus femoris and gluteus medius. A Charnley hip retractor displaces the rectus femoris medially and the gluteus medius laterally to expose the anterior joint capsule of the hip. After coagulating or suture ligating the ascending branch of the lateral femoral circumflex artery, a Mueller retractor is placed inferior to the femoral neck, and a capsulotomy is performed. The joint capsule is incised along the length of the femoral neck from the acetabulum to the intertrochanteric line (Fig. 3).
Fig. 3.
Once the hip joint capsule is exposed, a capsulotomy is performed along the long axis for the femoral neck. Heavy braided suture tags are often used to assist in retracting the joint capsule to expose the femoral neck and identify the capsule for closure.
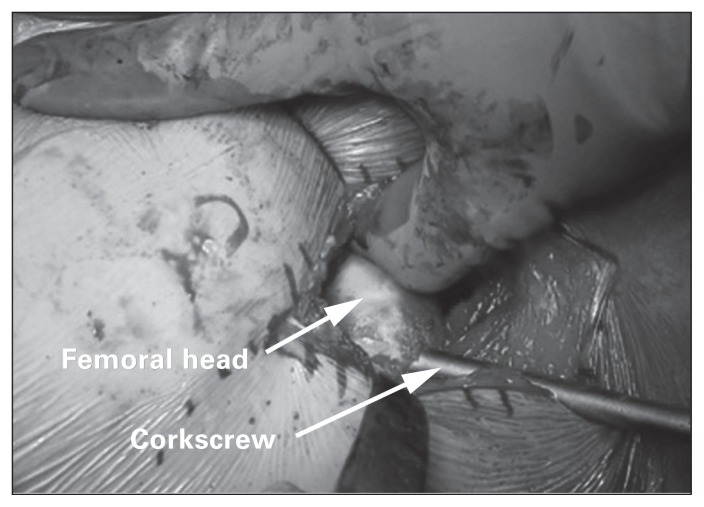
Gentle traction is then applied to the operative limb. Mueller and Hohmann retractors are placed intracapsularly around the femoral neck. A reciprocating saw is used to make a femoral neck osteotomy. The femoral head is then removed with a corkscrew (Fig. 4). The osteotomy can be repeated and the resultant napkin ring of bone removed to increase the ease of removing the femoral head.10,12
Fig. 4.
After femoral neck osteotomy, the femoral head is removed using a corkscrew. The femoral head often requires manipulation to ensure the corkscrew is positioned eccentrically in the femoral head.
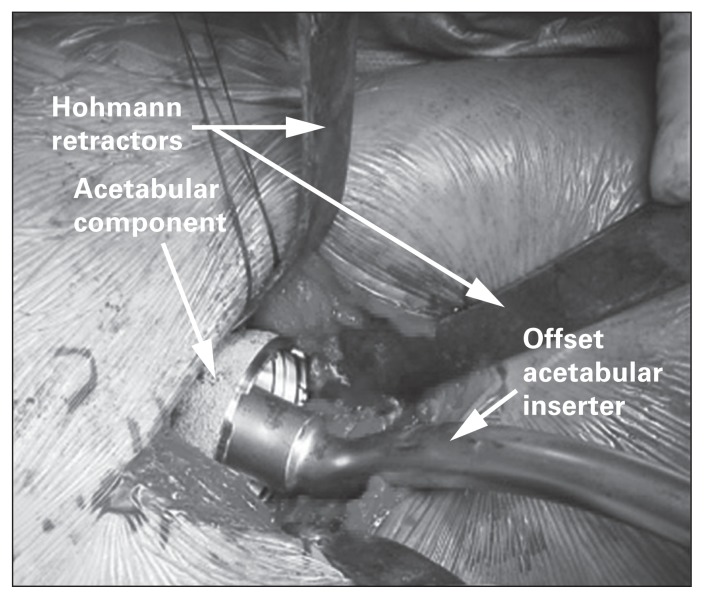
Once the femoral head is removed, traction is released and the leg is externally rotated to improve exposure for acetabular preparation. The Charnley hip retractor maintains exposure medially. Placement of the final acetabular component is facilitated by the use of an offset inserter handle to minimize soft tissue injury (Fig. 5). Intraoperative fluoroscopy is used to optimize component anteversion and inclination.
Fig. 5.
Example of retractor placement during implantation of the acetabular component. Note the use of an offset inserter handle to minimize soft tissue trauma during insertion.
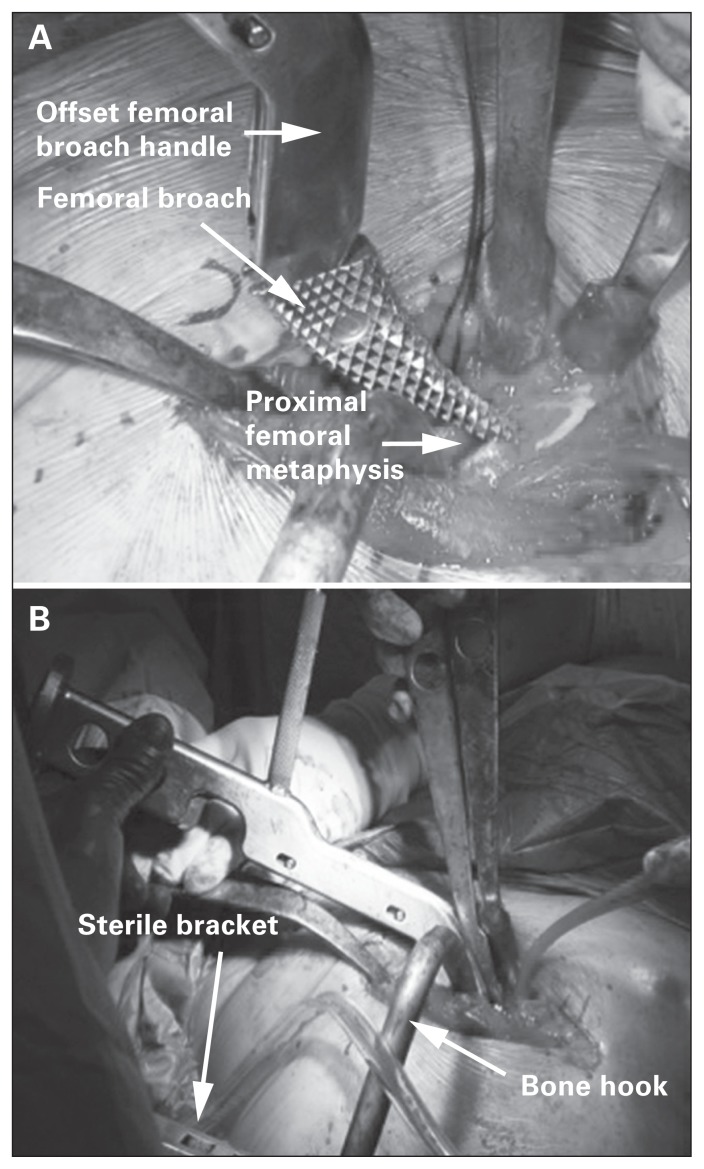
Femoral preparation can be difficult owing to limited proximal femoral exposure with this approach. The operative limb is carefully placed in a position of extension, adduction and external rotation to improve the accessibility of the proximal femur. Overly forceful external rotation can result in soft tissue injuries to the knee and ankle as well as intraoperative fracture. A specialized bone hook is then inserted around the posterior aspect of the femur just proximal to the insertion of the gluteus maximus tendon. This bone hook can be used manually to elevate the proximal femur anteriorly. In the subset of patients in whom the femur cannot be sufficiently mobilized anteriorly, sequential release of the conjoint tendon and piriformis can also improve mobilization of the femur. Rarely, a release of the anterior 1–2 cm of the origin of the TFL off the iliac wing may be required. An offset femoral broach handle eases access to the proximal femur during preparation (Fig. 6). Trialing can be combined with intraoperative fluoroscopy to assess leg length and offset. Femoral anteversion is identified based on the posterior cortex of the proximal femur or by using the femoral epicondyles as a reference point. Once the final implants are in situ and the hip is reduced, implant positioning is verified with fluoroscopy, and the stability of the construct can be assessed out of traction.10–12
Fig. 6.
(A) An offset femoral broach handle permits easier access to the proximal femur during preparation. (B) A bone hook assists with anterior displacement of the femur and can be secured in position using a sterile bracket.
Direct lateral approach
Overview
The direct lateral approach to the hip was described by Hardinge in 1982.13 Approximately 60% of Canadian orthopedic surgeons perform THAs using a direct lateral approach.14 This approach provides adequate exposure of both the proximal femur and acetabulum.12 It has the benefit of providing an extensile exposure to the femur as required. A very low dislocation rate has also been reported in clinical follow-up.15,16
Anatomy and technical considerations
The procedure begins by positioning the patient in the lateral decubitus position. The operative limb is draped freely to assist with dislocating the hip and exposing the proximal femur and acetabulum. A sterile bag is incorporated into the extremity drape to allow the surgeon to dislocate the hip and visualize the femur during preparation.
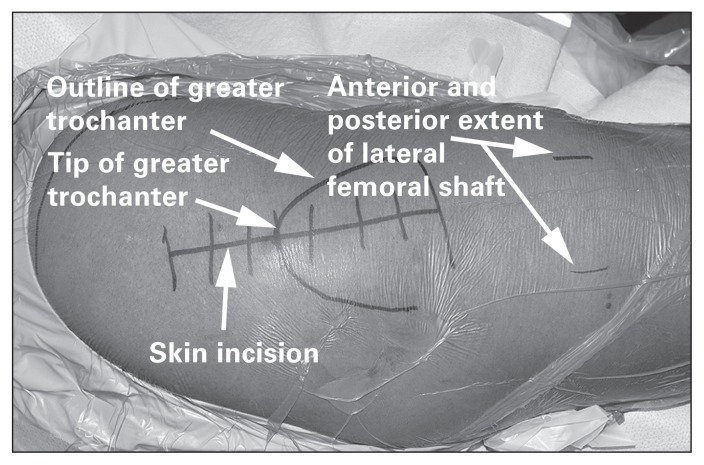
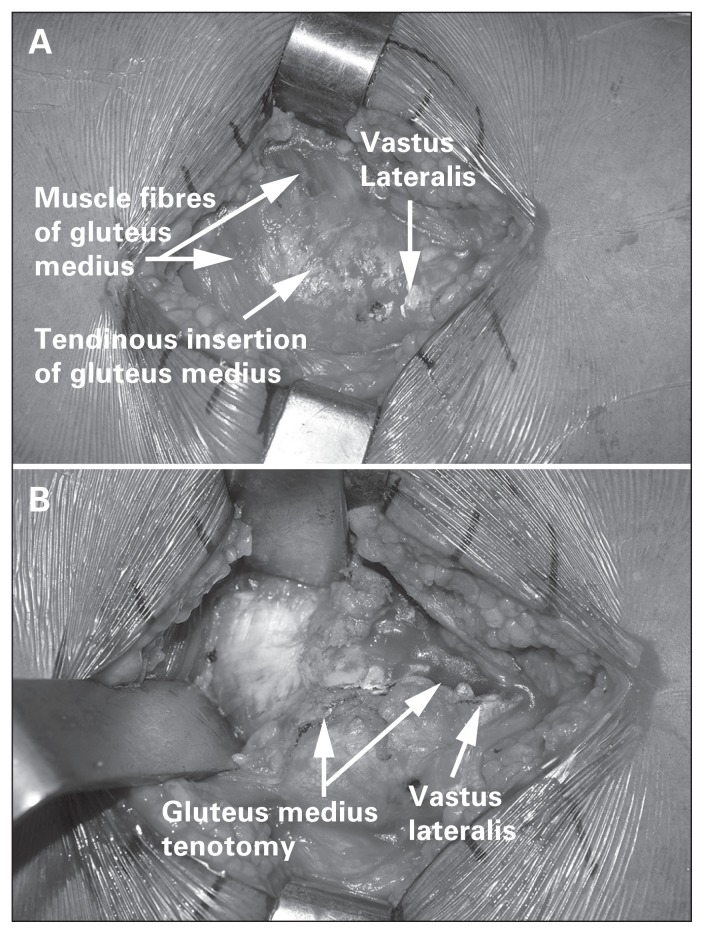
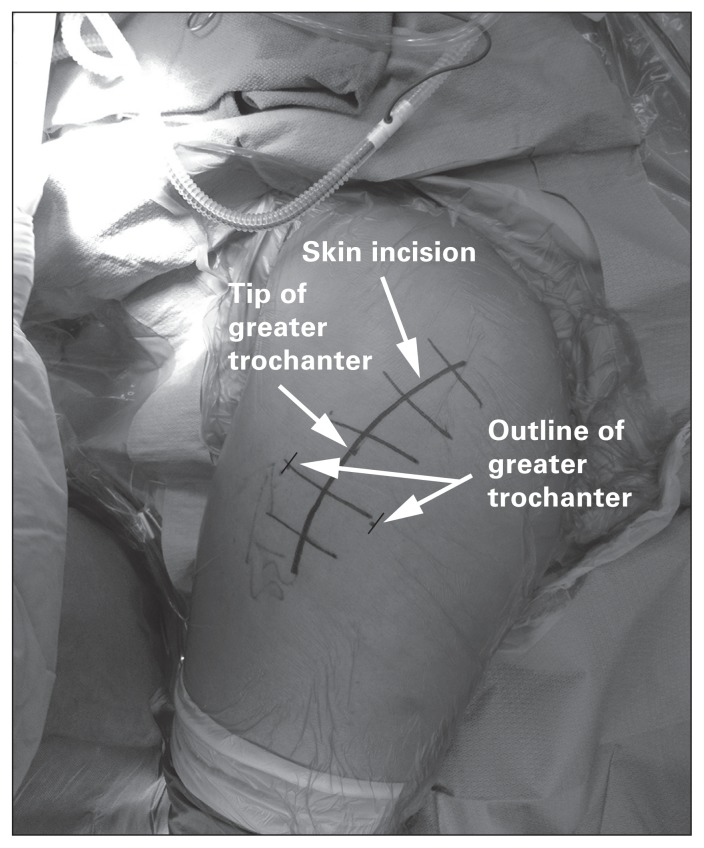
A longitudinal incision is made extending 3–5 cm proximal and about 5–8 cm distal to the tip of the greater trochanter (Fig. 7). The fascia is split at the interval between the TFL and gluteus maximus in line with the skin incision. A Charnley retractor is then used to retract the incised fascia latae. The tendon and muscle fibres of the gluteus medius are then visualized and split at the midway point between the most anterior and posterior extent of the muscle, or in a one-third anterior/two-thirds posterior fashion. The split is carried distally to the vastus ridge, leaving a cuff of gluteus medius tendon for repair following the procedure (Fig. 8). The gluteus minimus and joint capsule are split either in line with the neck of the femur or in line with the tendinous fibres of the gluteus minimus. Some surgeons perform a capsulectomy to facilitate dislocating the hip. The surgeon then dislocates the femoral head by externally rotating and flexing the hip and knee. The foot is positioned in the sterile bag anteriorly. Hohmann retractors are positioned around the femoral neck, allowing the surgeon to safely perform a femoral neck osteotomy using an oscillating saw.
Fig. 7.
The skin incision used for the direct lateral approach to the hip.
Fig. 8.
(A) The gluteus medius muscle fibres and associated tendinous insertion on the greater trochanter. (B) A tenotomy is performed through this tendinous insertion, leaving a cuff of tissue for repair during closure.
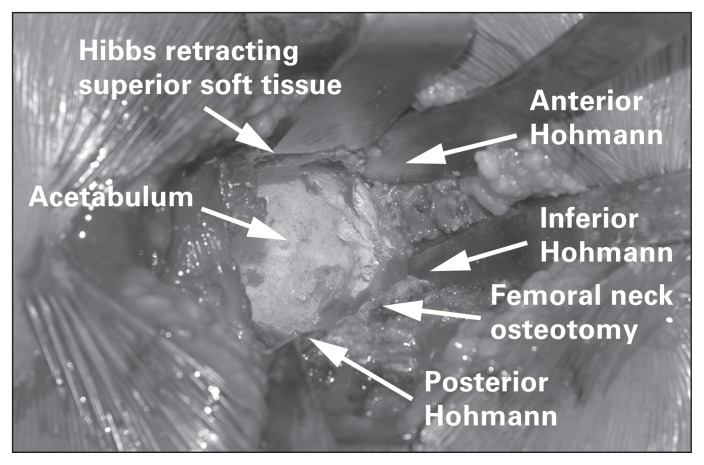
Once the femoral neck osteotomy is completed, the surgeon will have access to the acetabulum and proximal femur. The acetabulum is prepared with the leg externally rotated and the knee in extension on the table. Hohmann retractors are carefully placed anteriorly, posteriorly and inferiorly around the acetabulum to provide adequate visualization. A Hibbs retractor or additional Hohmann retractor can be used to retract superior soft tissues if visualization is impaired (Fig. 9). Soft tissue landmarks, such as the transverse acetabular ligament, reamer positioning relative to the floor and cup positioning guides, can be used to verify acetabular version and inclination.
Fig. 9.
Visualization of the acetabulum using a direct lateral approach following careful retractor placement.
When preparing the proximal femur, the hip is flexed to near 90° and externally rotated, and the foot is placed in the sterile bag anteriorly with the knee flexed. Two Hohmann retractors, 1 blunt placed posteriorly around the lateral aspect of the proximal femur and 1 sharp placed medially around the proximal femur, allow slight anterior displacement of the femur. A third Hohmann retractor is stationed posteriorly in line with the long axis of the femur to protect the abductors during femoral preparation.
Posterior approach
Overview
The posterior approach to the hip was popularized by Moore in the 1950s.12 A recent survey of surgeons from around the world suggests that the posterior approach is the most common surgical approach used internationally for THAs.4 In Canada, about 36% of arthroplasty surgeons use this approach.14 It provides adequate visualization of both the acetabulum and femur during both reconstructive procedures. The approach spares the abductor muscles during surgical exposure of the acetabulum and femur.12 It also has the benefit of providing an extensile exposure to the femur and acetabulum as required.
Anatomy and technical considerations
Similar to the direct lateral approach, for the posterior approach the patient is placed in the lateral decubitus position. Again, the involved limb is draped freely to facilitate dislocating the hip and to permit maneuverability of the limb to improve visualization throughout the procedure.
The skin incision begins 5 cm distal to the greater trochanter, centred on the femoral diaphysis. The incision continues proximal to the greater trochanter. At that point, it curves toward the posterior superior iliac spine for 6 cm. Alternatively, the incision can continue proximally in line with the femur with the hip flexed to 90° (Fig. 10).
Fig. 10.
The skin incision used for a posterior approach to the hip. A curvilinear incision or, alternatively, a straight incision with the hip flexed 90° can be used.
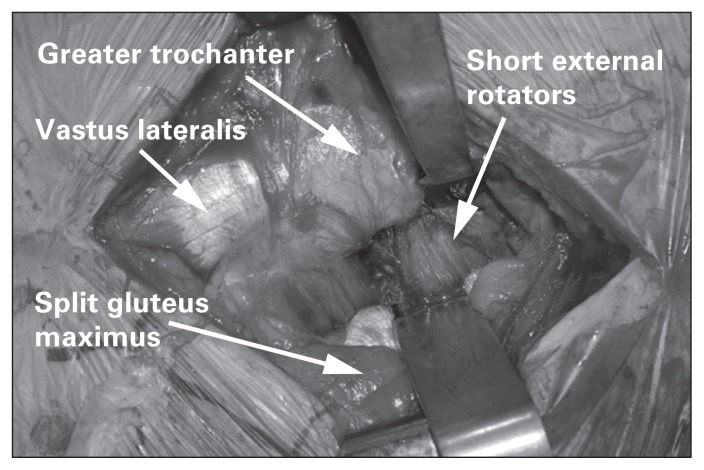
The surgeon then incises the fascia latae overlying the gluteus maximus and bluntly splits the muscle down to the short external rotators (Fig. 11). A Charnley retractor is positioned to retract the gluteus maximus. The sciatic nerve is carefully protected as it travels immediately posterior to the short external rotators. After identification of the piriformis, the short external rotators and piriformis are then tenotomized at their insertion onto the greater trochanter. They are then tagged with a braided suture for identification and repair at the end of the procedure. This will then expose the posterior joint capsule, which is incised to reveal the femoral neck and head. Alternatively, the joint capsule can be incised with the short external rotators in a single layer during tenotomy. The femoral head is then dislocated by internally rotating the hip. A femoral neck osteotomy is then performed using Hohmann retractors anteriorly and posteriorly to protect soft tissues.
Fig. 11.
Exposure of the short external rotators during a posterior approach.
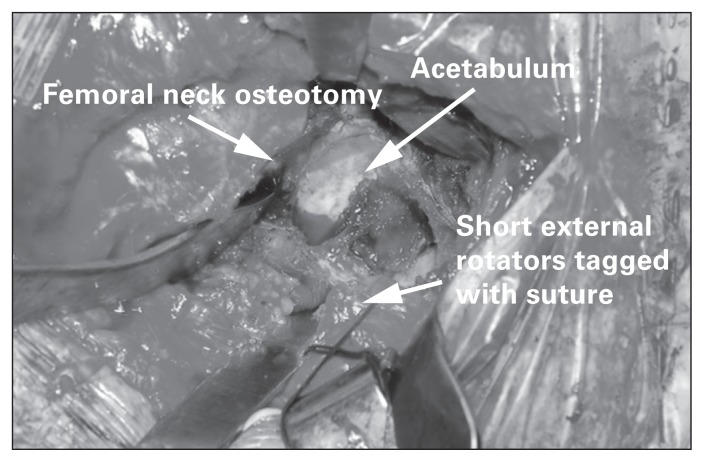
Once the osteotomized bone is removed, access is gained to the acetabulum and proximal femur. Careful placement of Hohmann retractors around the acetabulum permits adequate exposure for the reconstruction (Fig. 12). The femur is retracted anteriorly to expose the acetabulum to allow adequate restoration of acetabular anteversion. A posterior retractor or self-retaining retractor can be used to retract the posterior joint capsule to facilitate acetabular visualization. During acetabular preparation, soft tissue landmarks, such as the transverse acetabular ligament, reamer position relative to the floor and cup-positioning guides, are used to verify acetabular version and inclination.
Fig. 12.
Retractor placement and acetabular exposure using a posterior approach. The tagging suture helps retract the short external rotators, draping them over the sciatic nerve.
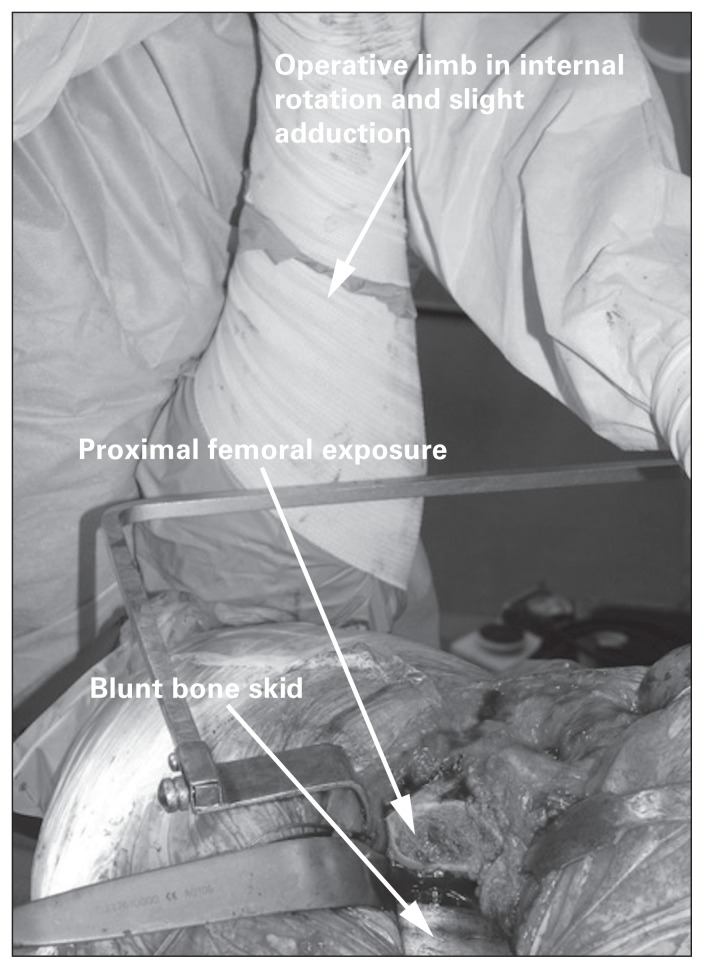
The proximal femur is exposed with the leg internally rotated, flexed and slightly adducted. This places the long axis of the tibia vertically. Blunt bone skids or Hohmann retractors can be used to elevate the femur to improve exposure (Fig. 13). Femoral preparation can then be completed in this position. Following the reconstruction, the short external rotators and posterior capsule are repaired through transosseous bone tunnels in the proximal femur or a direct repair to soft tissues.
Fig. 13.
Exposure of the proximal femur using a posterior approach. Note the position of the operative limb, held in position by a surgical assistant. Hohmann retractors or bone skids can help elevate the proximal femur during preparation.
Extensile exposures
Extensile exposures of the hip allow the surgeon to access more of the proximal femur or acetabulum in patients requiring management of complex acetabular or femoral bone defects; revision surgery; surgery for pathologic lesions of the proximal femur or acetabulum; or intraoperative complications, such as fracture. One of the disadvantages of the direct anterior approach is that exposure of the proximal femur is limited. As the direct anterior approach is part of the classic Smith–Peterson approach, acetabular exposure is adequate for THA. Access to the posterior acetabulum may require a 2-incision technique. Further proximal femoral exposure may require substantial soft tissue stripping of the vastus lateralis or a second incision using a lateral approach.17
Both the direct lateral and posterior approaches have extensile approaches. A trochanteric osteotomy or slide can improve access to the posterior column of the acetabulum using a direct lateral approach. Another option to access the posterior aspect of the acetabulum is to develop a plane posteriorly between the gluteus minimus and medius. The direct lateral approach can also be extended distally by splitting the vastus lateralis to access more of the proximal femur. Extending the exposure proximally is limited by the proximity of the superior gluteal nerve approximately 5 cm proximal to the tip of the greater trochanter. To extend the posterior approach distally along the femoral shaft, the gluteus maximus insertion can be detached.12,17
Risks and complications
Dislocation
Postoperative dislocation following THA has a deleterious effect on patient outcomes and, when required, revision surgery incurs tremendous costs to the health care system.18,19 Medicare data from more than 58 000 elective THAs in the United States suggest a dislocation rate of approximately 4%.20 However, this rate may be influenced by surgical approach at the time of the index procedure.
One of the purported benefits of the anterior and lateral approaches is lower dislocation rates than the posterior approach. A study by Sariali and colleagues21 prospectively followed 1764 patients who underwent primary THA performed through an anterior approach; patients were followed for 1 year postoperatively and had a dislocation rate (all dislocated anteriorly) of 1.5%. Another large series by Siguier and colleagues5 reported a dislocation rate of 0.96% in 1037 patients who underwent primary THA. Matta and colleagues6 reviewed 494 primary THAs performed through a direct anterior approach and reported 3 dislocations for a rate of 0.61%. The low dislocation rate has been attributed to verifying both acetabular and femoral component positioning via fluoroscopy and preserving static stabilizers, such as the posterior joint capsule.5,6
Preservation of the posterior soft tissue envelope may also explain the low dislocation rate observed with the lateral approach. A large retrospective review by Demos and colleagues22 reported 6 dislocations in 1515 patients (0.4%) undergoing a primary THA through a lateral approach. Masonis and Bourne15 performed a systematic review of the literature and determined a dislocation rate of 0.55% for 3438 THAs using the lateral approach. The definition of what constitutes a lateral approach may vary from study to study; therefore, the results of systematic reviews should be interpreted with scrutiny.
Dislocation rates for the posterior approach reported in the literature vary from 1% to 5%.16,23–26 Careful reconstruction of the capsule and short external rotators may decrease the risk of postoperative dislocation.12,16,27 Kwon and colleagues16 performed a meta-analysis to determine the rate of dislocations using a posterior approach with and without posterior soft tissue repair and found an 8 times greater relative risk of dislocation when soft tissue repair was not performed. Several repair techniques have been described for the posterior soft tissues. Examples include capsulorrhaphy of the capsule and short external rotators in 1 layer and transosseous bone tunnels in the greater trochanter.23,28
Abductor insufficiency
Abductor muscle insufficiency is a common clinical scenario following a direct lateral approach. It can cause abductor muscle weakness, a Trendelenburg gait or sign, inefficient gait mechanics and peritrochanteric pain.15,29–31 The insufficiency likely results from failure of the repaired tenotomy following a direct lateral approach, chronic degeneration of the gluteus medius tendon preoperatively, or irreparable tears at the time of THA in up to 20% of patients undergoing the procedure.32,33 The latter point, as well as technical pitfalls, such as inadequate restoration of femoral offset, may explain why some patients undergoing primary THA through a posterior or anterior approach may still exhibit abductor insufficiency postoperatively.34 Masonis and Bourne15 reviewed more than 2400 THAs involving a direct lateral approach and reported an incidence of 4%–20% for abductor insufficiency postoperatively. Careful closure of abductor tenotomy during the direct lateral approach and guided rehabilitation focusing on abductor and core strengthening in patients with preoperative abductor insufficiency can help improve patient outcomes.
Fracture
Intraoperative fractures can be a devastating complication resulting in increased duration of surgery, difficult postoperative mobilization due to weight-bearing modifications, prolonged functional recovery and poor patient outcomes. Jewett and Collis35 reviewed their experience with the direct anterior approach in 800 patients who underwent primary THA. The authors reported 19 (2.3%) intraoperative trochanteric fractures and no ankle fractures; most fractures occurred during femoral elevation with a bone hook and soft tissue avulsion. Interestingly, 15 of the intraoperative fractures occurred within the first 200 cases of the series. Matta and colleagues6 reviewed 494 direct anterior THAs and reported 7 (1.4%) intraoperative proximal femur fractures (4 fractures of the medial calcar during femoral broaching and 3 fractures of the greater trochanter during bone hook elevation). Three (0.6%) nondisplaced ankle fractures occurred when using isolated external rotation of the limb to dislocate the hip.
There is a paucity of literature examining the rate of intraoperative fracture risk with the direct lateral and posterior approaches. A retrospective review by Hendel and colleagues36 of 372 primary THAs revealed 15 intraoperative greater trochanter fractures (4.0%) using a lateral approach. Similar to the reports using the direct anterior approach, the authors suggest increased soft tissue tension and resultant avulsion during femoral preparation as the cause of the fractures.
There are some central tenets that can be applied in order to reduce the risk of intraoperative fracture. Examination of soft tissue tension before and after leg manipulation with any surgical approach can help reduce the rate of fracture. Soft tissue releases, such as the short external rotators for improved femoral exposure with a direct anterior approach, should be a part of every surgeon’s repertoire. Finally, surgeon experience with novel techniques undoubtedly plays a role in reducing the incidence of intraoperative complications.35,37,38
Nerve injury
The prevalence of nerve injuries during THA has been reported to be around 1%.39 Nerve injury can occur under several different circumstances, including direct trauma during dissection or placement of devices, such as wires or acetabular screws; retraction; thermal injury from methylmethacyrlate; compression due to hematoma; leg lengthening; and component positioning.40 Commonly injured nerves include the superior gluteal, lateral femoral cutaneous, sciatic and femoral nerves.
A superior gluteal or femoral nerve palsy is a potential complication following a direct lateral approach to the hip. The superior gluteal nerve passes between the gluteus medius and minimus muscles approximately 5 cm proximal to the greater trochanter.41 Retrospective and prospective studies suggest an incidence of 2.2%–42.5% for superior gluteal nerve injuries following reconstructive hip procedures using a direct lateral approach.41–44 This nerve palsy can lead to abductor insufficiency and poorer functional outcomes following THA; fortunately, many cases improve spontaneously. One study reported persistent electromyographic abnormalities in the gluteus medius 1 year postoperatively in 3 of 40 patients who underwent THA through a lateral approach. Interestingly, only 1 of these patients demonstrated clinical signs of abductor insufficiency (i.e., Trendelenburg sign) at latest follow-up.44
Neurapraxia of the lateral femoral cutaneous nerve can occur in 15%–80% of patients undergoing THA through a direct anterior approach45,46 owing to the nerve’s variable course around the anterior superior iliac spine and as it crosses the surgical plane at the sartorial-TFL plane more distally.6,47 Most of these neuropraxic injuries resolve without any long-term sequelae.6,8 A postoperative neuroma is a potential complication leading to increased pain, although this complication is rarely reported in the literature.46,48
The risk of sciatic nerve injury is greater during the posterior approach.49 Schmalzried and colleagues40 reviewed more than 3000 THAs and found an incidence of isolated sciatic nerve palsy of 1.3%. In most patients, sensory or motor deficits resolved spontaneously. Another study identified 14 sciatic motor nerve palsies in a cohort of more than 27 000 patients who underwent primary THA. Nine of these 14 patients had either partial or no recovery of residual motor deficits at a mean of 83 months postoperatively.49 Therefore, preserving the integrity of the nerve in order to optimize patient outcomes following THA cannot be understated.50
The femoral nerve is at risk with over-rigorous placement of soft tissue retractors over the anterior aspect of the acetabulum for all approaches. The rate of femoral nerve palsies for THA ranges from 0.1% to 2.4%.39,51 Mulliken and colleagues52 did not identify any femoral nerve injuries in 770 consecutive patients who underwent THA with a direct lateral approach. The highest reported rate of femoral nerve palsy using a direct lateral approach was that in a study by Simmons and colleagues.53 They reported 10 palsies in 440 hips, with all patients experiencing a full functional recovery 1 year postoperatively. Matta and colleagues6 reported 1 femoral nerve palsy in 494 patients. In all cases reported in the literature, the palsy was attributed to retractor placement over the anterior rim of the acetabulum.
Review of clinical outcomes
Lateral versus posterior approach
The direct lateral and posterior approaches are fundamentally similar in that they are both muscle-splitting approaches to the hip.12,13 However, as illustrated earlier, the surgical anatomy and potential complications differ between these approaches, which can influence patient outcomes.
The most important determinants of a successful THA are based on its goals of treatment: mitigation of pain, improved quality of life and restoration of function.54 Barber and colleagues55 prospectively followed for 2 years 28 patients undergoing direct posterior and 21 undergoing direct lateral THA, each performed by a single surgeon. Both groups had similar improvements on the Harris Hip Score (HHS) at the 2-year follow-up and had no observable differences in dislocations or in the incidence of a Trendelenburg gait.
A more recent prospective study56 randomly assigned 60 patients to undergo THA through either a posterior or lateral approach. The primary end point was the HHS at the 12-week follow-up. The authors also captured data from the Western Ontario and McMaster Osteoarthritis Index (WOMAC) and the Short-Form 36 (SF-36) questionnaires as well as information on complications, such as dislocations and periprosthetic fractures. Both approaches showed similar improvements across the HHS, WOMAC and SF-36 questionnaires at multiple time points up to and including 12 weeks postoperatively. The rate of dislocation and fracture did not differ significantly between the groups.
A common comparator between the posterior and lateral approach is the incidence of abductor insufficiency. Several studies have suggested the direct lateral approach has an increased incidence of abductor insufficiency following THA.15,24,30,56 The reported incidence varies from 0% to 16% for the posterior approach and from 4% to 20% for the direct lateral approach.15 However, there is tremendous heterogeneity in the methods used to diagnose abductor insufficiency in many of these studies. Many studies use subjective findings, such as the presence of Trendelenburg gait or sign or lateral trochanteric pain, which may lead to poor inter-rater reliability, to make the diagnosis. Magnetic resonance imaging (MRI) is becoming a popular method for assessing soft tissue pathology following THA.57–60 Several studies have shown that metal suppression pulsed MRI sequences can identify abductor damage in patients with symptomatic abductor tears following THA.59–61 Future prospective studies using MRI to assess soft tissue integrity postoperatively will provide a more objective measure of the incidence of abductor tears.
Anterior versus lateral approach
The direct anterior approach is increasing in popularity and is the preferred surgical approach of 10% of orthopedic surgeons performing THA.4 Reduced blood loss, earlier functional recovery, low dislocation rates and shorter stays in hospital have been attributed to the muscle-sparing properties of the anterior approach.6 The literature also suggests that minimizing muscle damage during surgery is a reason for patients to choose particular surgeons who practise muscle-sparing techniques.37 Thus, several recent studies have compared the direct anterior approach to both the direct lateral and posterior approaches.
From 2006 to 2009, Alecci and colleagues62 retrospectively reviewed peri- and intraoperative outcomes of THAs performed through either a direct lateral (n = 198) or direct anterior (n = 221) approach. The mean duration of surgery was 8 minutes longer in the direct anterior group, which was a statistically significant difference between the groups. The direct lateral group experienced increased perioperative blood loss and blood transfusions compared with the direct anterior group. Finally, length of stay in hospital was reduced significantly from 10 to 7 days when a THA was performed through an anterior approach.
A similar study by Restrepo and colleagues63 randomly assigned 100 patients to either the direct anterior or lateral approach to THA. Interestingly, the authors found no significant differences in duration of surgery, blood loss, need for blood transfusions or length of stay in hospital between the 2 groups. The authors also examined patient outcome measures. The direct anterior group outperformed the direct lateral group on the HHS, SF-36 and WOMAC questionnaires at 6 weeks postoperatively. However, these significant differences in clinical outcomes were abated when revisited at 2 years postoperatively. This study suggests that the direct anterior approach may be associated with greater early postoperative improvements in patient-reported outcomes than the direct lateral approach.
Earlier discharge from hospital may be associated with better pain mitigation after surgery. Goebel and colleagues64 retrospectively reviewed pain perception using a visual analogue scale (VAS), consumption of pain medication and length of stay in hospital in 200 patients undergoing either an anterior or lateral approach to THA. There was a significant reduction in perceived pain and consumption of pain medication in the direct anterior group during the first 24 hours postoperatively. The direct anterior group spent an average of 3 fewer days in hospital than the direct lateral group. Again, improved pain mitigation and earlier discharge were attributed to the muscle-sparing properties of the anterior approach. However, the accuracy of these data are limited by the retrospective study design and by pain assessment using a VAS and multiple different assessors.
There may be anatomic pathology that can explain the discrepancy in perceived pain between the groups. Bremer and colleagues65 obtained an MRI 1 year postoperatively in 50 patients who underwent THA through either a direct anterior or lateral approach. The authors noted significant increases in the number of abductor tears or detachments, greater trochanteric fluid collections, gluteus medius tendinosis and fatty atrophy of the abductor muscles in the direct lateral group. The abductor complex is a pain generator following the direct lateral approach and may explain differences in early pain perception between the groups.29 However, a limitation of the study by Bremer and colleagues is the absence of a clinical outcome measures assessment. They did not obtain a preoperative MRI, which could have identified patients with evidence of abductor pathology before the procedure, a common finding in patients with hip arthritis.33 Future research should compare clinical outcomes and findings on advanced imaging modalities to explain discrepancies in pain and functional outcomes.
Anterior versus posterior approach
Several studies have compared the anterior and posterior approaches, with recent literature examining the extent of muscle damage incurred by either approach. A prospective randomized trial by Barrett and colleagues66 compared 43 direct anterior and 44 direct posterior approaches to THA. The primary end point was the ability to climb stairs and walk unlimited distances, as assessed with the HHS at 6 weeks, 3 months, 6 months and 12 months postoperatively. The authors also captured intraoperative data, including total duration of surgery, and postoperative data, including length of stay in hospital. The total duration of surgery was on average 23.8 minutes longer in the direct anterior than the direct posterior group. The mean length of stay in hospital was 2.28 days for the direct anterior group and 3.02 days for the direct posterior group. At the 6-week follow-up visit, significantly more patients were walking limitlessly, were able to climb stairs normally and had a higher total HHS in the direct anterior than the direct posterior group. These differences dissipated by the 3-month mark and remained insignificant up to and including 1 year postoperatively. These results support the claim that the direct anterior approach provides earlier restoration of function after THA.
One of the purported benefits of earlier return of function is earlier discharge from hospital. Martin and colleagues67 retrospectively reviewed 41 direct anterior and 47 direct posterior approaches for THA. Length of stay in hospital was significantly shorter for the anterior than the posterior group (2.9 d v. 4.0 d). The mean duration of surgery was significantly longer with the anterior than the posterior approach (141 min v. 114 min). Both groups performed similarly on the SF-36 and WOMAC clinical outcome measures at the 6-month follow-up. This study was limited by selection bias, as the mean body mass index (BMI) was significantly higher in the posterior than the anterior group (34.1 v. 28.5). Patients with elevated BMI (> 40) were told that there was a greater risk of wound complications associated with an anterior approach and opted to undergo a posterior approach. Elevated BMI has become a relative contraindication to an anterior approach in our institution. A statistically significant difference in BMI between study cohorts is an important confounder, as obese patients require more assistance with early mobilization, thereby influencing the difference in length of stay between the groups. In the study by Martin and colleagues,67 the earlier discharge from hospital was attributed to earlier mobilization owing to the muscle-sparing properties of the anterior approach.
There is considerable interest in the degree of muscle damage sustained during surgical approaches to the hip. An interesting study by Bergin and colleagues68 compared various blood markers indicative of muscle damage in patients undergoing THA through either a direct anterior or posterior approach. This methodology has been used previously to justify the use of tissue-sparing techniques, such as laparoscopy in other surgical subspecialties.69,70 The investigators measured pre- and postoperative values of various acute phase reactant proteins, such as creatine kinase (CK), C-reactive protein (CRP), interleukin (IL)-6, tumour necrosis factor (TNF)-α and IL-1 in 57 patients undergoing THA. They found a significant increase in CK in the posterior approach group compared with the anterior approach group immediately following the procedure as well as cumulatively 2 days after THA. The other acute phase reactants did not change significantly between the groups.68 However, the duration of surgery was longer in the posterior approach than the anterior approach group (mean 118 min v. 78 min). A more prolonged period of immobilization on the operating room table could have contributed to the accumulation of additional serum CK.71 Serum CK clearance also depends on renal function,72 which was not accounted for in the study by Bergin and colleagues.68
Another study73 examined the extent of gluteus medius/minimus, TFL, rectus femoris and short external rotator muscle damage in THAs performed on 12 cadaveric hips (6 direct anterior and 6 direct posterior approaches). Minimal damage was sustained to the gluteus medius muscle with both approaches. The posterior approach caused more damage to the gluteus minimus muscle than the anterior approach (18% v. 8.5% of the mean surface area). The short external rotators were released in all posterior approach specimens and were damaged in 50% of the anterior approach specimens to improve visualization of the proximal femur. Using an anterior approach, 31% and 12% of the mean surface area of the TFL and rectus femoris muscles, respectively, was damaged. No damage to either of these muscles was sustained using a posterior approach.73 This study challenges the claim that the anterior approach is truly a muscle-sparing approach. Future studies using gait analysis could elicit the clinical effects of this muscle damage.
Conclusion
Surgical approach in THA is an area of debate among orthopedic surgeons. This review has demonstrated that the anterior, lateral and posterior approaches each have unique advantages and disadvantages. High-quality clinical comparisons among the approaches are lacking in the literature; therefore, surgeon preference is likely more a function of training and anecdotal success. The surgical approaches discussed all enable performance of a safe and clinically efficacious THA; therefore, we recommend that surgeons choose the approach with which they have the most experience and ease. Future research should elicit the long-term implications of surgical approach on clinical outcomes, restoration of function (i.e. gait analysis) and health economics.
Footnotes
Competing interests: E. Vasarhelyi is a consultant with DePuy and Smith and Nephew and has received institutional and research support from DePuy, Smith and Nephew and Stryker. No other competing interests declared.
Contributors: All authors designed the study and analyzed the data. S. Petis and E. Vasarhelyi acquired the data. S. Petis and J. Howard wrote the article, which all authors reviewed and approved for publication.
References
- 1.Charnley J. Arthroplasty of the hip. A new operation. Lancet. 1961;1:1129–32. doi: 10.1016/s0140-6736(61)92063-3. [DOI] [PubMed] [Google Scholar]
- 2.Information CIfH. Hip and knee replacements in Canada: 2012–2013 Quick Stats 2013. [accessed 2015 Feb. 12]. Available: www.cihi.ca/CIHI-ext-portal/xlsx/internet/STATS_CJRR2012-2013_EN.
- 3.Light TR, Keggi K. Anterior approach to hip arthroplasty. Clin Orthop Relat Res. 1980;152:255–60. [PubMed] [Google Scholar]
- 4.Chechik O, Khashan M, Lador R, et al. Surgical approach and prosthesis fixation in hip arthroplasty worldwide. Arch Orthop Trauma Surg. 2013;133:1595–600. doi: 10.1007/s00402-013-1828-0. [DOI] [PubMed] [Google Scholar]
- 5.Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res. 2004;426:164–73. doi: 10.1097/01.blo.0000136651.21191.9f. [DOI] [PubMed] [Google Scholar]
- 6.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115–24. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 7.Kennon RE, Keggi J, Wetmore R, et al. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am. 2003;85-A:39–48. doi: 10.2106/00004623-200300004-00005. [DOI] [PubMed] [Google Scholar]
- 8.Nakata K, Nishikawa M, Yamamoto K, et al. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty. 2009;24:698–704. doi: 10.1016/j.arth.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Mayr E, Nogler M, Benedetti M, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech (Bristol, Avon) 2009;24:812–8. doi: 10.1016/j.clinbiomech.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Horne PH, Olson S. Direct anterior approach for total hip arthroplasty using the fracture table. Curr Rev Musculoskelet Med. 2011;4:139–45. doi: 10.1007/s12178-011-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplasty. 2008;23:64–8. doi: 10.1016/j.arth.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Hoppenfeld S, DeBoer P, Buckley R. Surgical exposures in orthopaedics: the anatomic approach. Philidelphia, PA: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 13.Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br. 1982;64:17–9. doi: 10.1302/0301-620X.64B1.7068713. [DOI] [PubMed] [Google Scholar]
- 14.Burnett R. Total hip arthroplasty: techniques and results. BCMJ. 2010;52:455–64. [Google Scholar]
- 15.Masonis JL, Bourne R. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop Relat Res. 2002;405:46–53. doi: 10.1097/00003086-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Kwon MS, Kuskowski M, Mulhall K, et al. Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34–8. doi: 10.1097/01.blo.0000218746.84494.df. [DOI] [PubMed] [Google Scholar]
- 17.Masterson EL, Masri B, Duncan C. Surgical approaches in revision hip replacement. J Am Acad Orthop Surg. 1998;6:84–92. doi: 10.5435/00124635-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Vanhegan IS, Malik A, Jayakumar P, et al. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Br. 2012;94:619–23. doi: 10.1302/0301-620X.94B5.27073. [DOI] [PubMed] [Google Scholar]
- 19.Singh JA, Lewallen D. Operative diagnosis for revision total hip arthroplasty is associated with patient-reported outcomes (PROs) BMC Musculoskelet Disord. 2013;14:210. doi: 10.1186/1471-2474-14-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips CB, Barrett J, Losina E, et al. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85-A:20–6. doi: 10.2106/00004623-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23:266–72. doi: 10.1016/j.arth.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Demos HA, Rorabeck C, Bourne R, et al. Instability in primary total hip arthroplasty with the direct lateral approach. Clin Orthop Relat Res. 2001;(393):168–80. doi: 10.1097/00003086-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Chiu FY, Chen C, Chung T, et al. The effect of posterior capsulorrhaphy in primary total hip arthroplasty: a prospective randomized study. J Arthroplasty. 2000;15:194–9. doi: 10.1016/s0883-5403(00)90220-1. [DOI] [PubMed] [Google Scholar]
- 24.Jolles BM, Bogoch E. Posterior versus lateral surgical approach for total hip arthroplasty in adults with osteoarthritis. Cochrane Database Syst Rev. 2006:CD003828. doi: 10.1002/14651858.CD003828.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho KW, Whitwell G, Young S. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36mm. Arch Orthop Trauma Surg. 2012;132:1031–6. doi: 10.1007/s00402-012-1508-5. [DOI] [PubMed] [Google Scholar]
- 26.Sierra RJ, Raposo J, Trousdale R, et al. Dislocation of primary THA done through a posterolateral approach in the elderly. Clin Orthop Relat Res. 2005;441:262–7. doi: 10.1097/01.blo.0000194308.23105.f4. [DOI] [PubMed] [Google Scholar]
- 27.Pellicci PM, Potter H, Foo L, et al. MRI shows biologic restoration of posterior soft tissue repairs after THA. Clin Orthop Relat Res. 2009;467:940–5. doi: 10.1007/s11999-008-0503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop Relat Res. 1998;355:224–8. doi: 10.1097/00003086-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Lachiewicz PF. Abductor tendon tears of the hip: evaluation and management. J Am Acad Orthop Surg. 2011;19:385–91. doi: 10.5435/00124635-201107000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Iorio R, Healy W, Warren P, et al. Lateral trochanteric pain following primary total hip arthroplasty. J Arthroplasty. 2006;21:233–6. doi: 10.1016/j.arth.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Valente G, Taddei F, Jonkers I. Influence of weak hip abductor muscles on joint contact forces during normal walking: probabilistic modeling analysis. J Biomech. 2013;46:2186–93. doi: 10.1016/j.jbiomech.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 32.Miozzari HH, Dora C, Clark J, et al. Late repair of abductor avulsion after the transgluteal approach for hip arthroplasty. J Arthroplasty. 2010;25:450–7.e1. doi: 10.1016/j.arth.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Howell GE, Biggs R, Bourne R. Prevalence of abductor mechanism tears of the hips in patients with osteoarthritis. J Arthroplasty. 2001;16:121–3. doi: 10.1054/arth.2001.19158. [DOI] [PubMed] [Google Scholar]
- 34.Kiyama T, Naito M, Shinoda T, et al. Hip abductor strengths after total hip arthroplasty via the lateral and posterolateral approaches. J Arthroplasty. 2010;25:76–80. doi: 10.1016/j.arth.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Jewett BA, Collis D. High complication rate with anterior total hip arthroplasties on a fracture table. Clin Orthop Relat Res. 2011;469:503–7. doi: 10.1007/s11999-010-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendel D, Yasin M, Garti A, et al. Fracture of the great trochanter during hip replacement: a retrospective analysis of 21/372 cases. Acta Orthop Scand. 2002;73:295–7. doi: 10.1080/000164702320155284. [DOI] [PubMed] [Google Scholar]
- 37.Dosanjh S, Matta J, Bhandari M. The final straw: a qualitative study to explore patient decisions to undergo total hip arthroplasty. Arch Orthop Trauma Surg. 2009;129:719–27. doi: 10.1007/s00402-008-0671-1. [DOI] [PubMed] [Google Scholar]
- 38.Woolson ST, Pouliot M, Huddleston J. Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplasty. 2009;24:999–1005. doi: 10.1016/j.arth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Schmalzried TP, Noordin S, Amstutz H. Update on nerve palsy associated with total hip replacement. Clin Orthop Relat Res. 1997;344:188–206. [PubMed] [Google Scholar]
- 40.Schmalzried TP, Amstutz H, Dorey F. Nerve palsy associated with total hip replacement: risk factors and prognosis. J Bone Joint Surg Am. 1991;73:1074–80. [PubMed] [Google Scholar]
- 41.Khan T, Knowles D. Damage to the superior gluteal nerve during the direct lateral approach to the hip: a cadaveric study. J Arthroplasty. 2007;22:1198–200. doi: 10.1016/j.arth.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Oldenburg M, Muller R. The frequency, prognosis and significance of nerve injuries in total hip arthroplasty. Int Orthop. 1997;21:1–3. doi: 10.1007/s002640050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramesh M, O’Byrne J, McCarthy N, et al. Damage to the superior gluteal nerve after the Hardinge approach to the hip. J Bone Joint Surg Br. 1996;78:903–6. doi: 10.1302/0301-620x78b6.1289. [DOI] [PubMed] [Google Scholar]
- 44.Picado CH, Garcia F, Marques W. Damage to the superior gluteal nerve after direct lateral approach to the hip. Clin Orthop Relat Res. 2007;455:209–11. doi: 10.1097/01.blo.0000238805.87411.e8. [DOI] [PubMed] [Google Scholar]
- 45.Goulding K, Beaule P, Kim P, et al. Incidence of lateral femoral cutaneous nerve neuropraxia after anterior approach hip arthroplasty. Clin Orthop Relat Res. 2010;468:2397–404. doi: 10.1007/s11999-010-1406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhargava T, Goytia R, Jones L, et al. Lateral femoral cutaneous nerve impairment after direct anterior approach for total hip arthroplasty. Orthopedics. 2010;33:472. doi: 10.3928/01477447-20100526-05. [DOI] [PubMed] [Google Scholar]
- 47.Ropars M, Morandi X, Huten D, et al. Anatomical study of the lateral femoral cutaneous nerve with special reference to minimally invasive anterior approach for total hip replacement. Surg Radiol Anat. 2009;31:199–204. doi: 10.1007/s00276-008-0433-3. [DOI] [PubMed] [Google Scholar]
- 48.Grossman MG, Ducey S, Nadler S, et al. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336–44. doi: 10.5435/00124635-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Farrell CM, Springer B, Haidukewych G, et al. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am. 2005;87:2619–25. doi: 10.2106/JBJS.C.01564. [DOI] [PubMed] [Google Scholar]
- 50.DeHart MM, Riley L., Jr Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg. 1999;7:101–11. doi: 10.5435/00124635-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Fox AJ, Bedi A, Wanivenhaus F, et al. Femoral neuropathy following total hip arthroplasty: review and managment guidelines. Acta Orthop Belg. 2012;78:145–51. [PubMed] [Google Scholar]
- 52.Mulliken BD, Rorabeck C, Bourne R, et al. A modified direct lateral appraoch in total hip arthroplasty: a comprehensive review. J Arthroplasty. 1998;13:737–47. doi: 10.1016/s0883-5403(98)90024-9. [DOI] [PubMed] [Google Scholar]
- 53.Simmons C, Izant T, Rothman R, et al. Femoral neuropathy following total hip arthroplasty: anatomic study, case reports, and literature review. J Arthroplasty. 1991;6:S57–66. [PubMed] [Google Scholar]
- 54.Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–19. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 55.Barber TC, Roger D, Goodman S, et al. Early outcome of total hip arthroplasty using the direct lateral vs the posterior surgical approach. Orthopedics. 1996;19:873–5. doi: 10.3928/0147-7447-19961001-11. [DOI] [PubMed] [Google Scholar]
- 56.Witzleb WC, Stephan L, Krummenauer F, et al. Short-term outcome after posterior versus lateral surgical approach for total hip arthroplasty: a randomized clinical trial. Eur J Med Res. 2009;14:256–63. doi: 10.1186/2047-783X-14-6-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter HG, Nestor B, Sofka C, et al. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg Am. 2004;86-A:1947–54. doi: 10.2106/00004623-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 58.Potter HG, Foo L, Nestor B. What is the role of magnetic resonance imaging in the evaluation of total hip arthroplasty? HSS J. 2005;1:89–93. doi: 10.1007/s11420-005-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Müller M, Thotz S, Springer I, et al. Randomized controlled trial of abductor muscle damage in relation to the surgical approach for primary total hip replacement: minimally invasive anterolateral versus modified direct lateral approach. Arch Orthop Trauma Surg. 2011;131:179–89. doi: 10.1007/s00402-010-1117-0. [DOI] [PubMed] [Google Scholar]
- 60.Pfirrmann CW, Notzli H, Dora C, et al. Abductor tendons and muscles assessed at MR imaging after total hip arthroplasty in asymptomatic and symptomatic patients. Radiology. 2005;235:969–76. doi: 10.1148/radiol.2353040403. [DOI] [PubMed] [Google Scholar]
- 61.Twair A, Ryan M, O’Connell M, et al. MRI of failed total hip replacement caused by abductor muscle avulsion. AJR Am J Roentgenol. 2003;181:1547–50. doi: 10.2214/ajr.181.6.1811547. [DOI] [PubMed] [Google Scholar]
- 62.Alecci V, Valente M, Crucil M, et al. Comparison of primary total hip replacements performed with a direct anterior versus the standard lateral approach: perioperative findings. J Orthop Traumatol. 2011;12:123–9. doi: 10.1007/s10195-011-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Restrepo C, Parvizi J, Pour A, et al. Prospective randomized study of two surgical approaches for total hip arthroplasty. J Arthroplasty. 2010;25:671–9.e1. doi: 10.1016/j.arth.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Goebel S, Steinert A, Schillinger J, et al. Reduced post-operative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop. 2012;36:491–8. doi: 10.1007/s00264-011-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bremer AK, Kalberer F, Pfirrmann C, et al. Soft-tissue changes in hip abductor muscles and tendons after total hip replacement: comparison between the direct anterior and the transgluteal approaches. J Bone Joint Surg Br. 2011;93:886–9. doi: 10.1302/0301-620X.93B7.25058. [DOI] [PubMed] [Google Scholar]
- 66.Barrett WP, Turner S, Leopold J. Prospective randomized study of direct anterior vs posterolateral approach for total hip arthroplasty. J Arthroplasty. 2013;28:1634–8. doi: 10.1016/j.arth.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 67.Martin CT, Pugely A, Gao Y, et al. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28:849–54. doi: 10.1016/j.arth.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 68.Bergin PF, Doppelt J, Kephart C, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93:1392–8. doi: 10.2106/JBJS.J.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grande M, Tucci G, Adorisio O, et al. Systemic acute-phase response after laparoscopic and open cholecystectomy. Surg Endosc. 2002;16:313–6. doi: 10.1007/s00464-001-9042-5. [DOI] [PubMed] [Google Scholar]
- 70.Suter M, Martinet O, Spertini F. Reduced acute phase response after laparoscopic total extraperitoneal bilateral hernia repair compared to open repair with the Stoppa procedure. Surg Endosc. 2002;16:1214–9. doi: 10.1007/s00464-001-9164-9. [DOI] [PubMed] [Google Scholar]
- 71.Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67:272–83. [PubMed] [Google Scholar]
- 72.Lappalainen H, Tiula E, Uotila L, et al. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30:2212–5. doi: 10.1097/00003246-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Meneghini RM, Pagnano M, Trousdale R, et al. Muscle damage during MIS total hip arthroplasty: Smith-Peterson versus posterior approach. Clin Orthop Relat Res. 2006;453:293–8. doi: 10.1097/01.blo.0000238859.46615.34. [DOI] [PubMed] [Google Scholar]