Abstract
Background
Although smoking-cessation interventions typically focus directly on patients, this paper conducts an economic evaluation of a novel smoking-cessation intervention focused on training physicians and/or pharmacists to use counseling techniques that would decrease smoking rates at a reasonable cost.
Purpose
To evaluate the cost-effectiveness of interventions that train physicians and/or pharmacists to counsel their patients on smoking-cessation techniques.
Methods
Using decision-analytic modeling, we compared four strategies for smoking-cessation counseling education: training only physicians, training only pharmacists, training both physicians and pharmacists (synergy strategy), and training neither physicians nor pharmacists (i.e., no specialized training, which is the usual practice). Short-term outcomes were based on results from a clinical trial conducted in 16 communities across the Houston area; long-term outcomes were calculated from epidemiological data. Short-term outcomes were measured using the cost per quit, and long-term outcomes were measured using the cost per quality-adjusted life-year (QALY). Cost data were taken from institutional sources; both costs and QALYs were discounted at 3%.
Results
Training both physicians and pharmacists added 0.09 QALY for 45-year-old men. However, for 45-year-old women, the discounted quality-adjusted life expectancy only increased by 0.01 QALY when comparing the synergy strategy to no intervention. The incremental cost-effectiveness ratio (ICER) of the synergy strategy with respect to the non-intervention strategy was US$868/QALY for 45-year-old men and US$8,953/QALY for 45-year-old women. The results were highly sensitive to the quit rates and community size.
Conclusion
Synergistic educational training for physicians and pharmacists could be a cost-effective method for smoking cessation in the community.
Keywords: medical decision making, costs and cost analysis, nicotine, smoking cessation
1. Introduction
Many smoking-cessation interventions have been successful and cost-effective. Typically, interventions focus directly on an individual patient through the use of pharmaceutical agents (e.g., bupropion (Bolin, Lindgren, & Willers, 2006) or nortriptyline (Hall, et al., 2005)), nicotine gum (Fagerstrom, 1982; Hjalmarson, 1984), and transdermal nicotine patch and nicotine nasal spray (Abelin, Buehler, Muller, Vesanen, & Imhof, 1989; Fiscella & Franks, 1996; Hurt, et al., 1994), or indirectly through physician counseling (Cromwell, Bartosch, Fiore, Hasselblad, & Baker, 1997; Cummings, Rubin, & Oster, 1989). Research on these interventions has shown that they can have significant health benefits.
Physicians are best positioned to play a crucial role in smoking cessation and prevention efforts (US PHS, 2000), and of all health care providers, pharmacists are possibly the most accessible to the public. Research shows that if trained, both physicians and pharmacists could have significant roles in helping patients quit smoking (Kottke, Brekke, Solberg, & Hughes, 1989; Richmond, Mendelsohn, & Kehoe, 1998). However, only one study (Pinget, Martin, Wasserfallen, Humair, & Cornuz, 2007) showed that such specialized training could be cost-effective.
On the basis of these previous studies, we hypothesized that an indirect physician and pharmacist training smoking-cessation intervention may also be cost-effective. The proposed study evaluates the cost-effectiveness of an intervention that trains physicians and/or pharmacists to counsel their patients on smoking-cessation techniques.
2. Methods
2.1. Intervention
Researchers at The University of Texas MD Anderson Cancer Center developed The Health Care Team Approach to Smoking Cessation: Enhanced Tobacco Outreach Education Program (eTOEP), known as the TEAM Tobacco intervention (Prokhorov, et al., 2010).
The intervention is a community-based health care provider continuing medical education (CME) training program designed to improve smoking-cessation counseling skills among physicians and pharmacists. The effectiveness of the eTOEP intervention was tested through a group-randomized trial with four treatment conditions—training both physicians and pharmacists (synergy condition), training neither physicians nor pharmacists (which is the usual practice), training only physicians, or training only pharmacists—in 16 communities around Houston, Texas.
2.2. Providers
Physicians and pharmacists (hereafter, providers) from the 16 communities were recruited to participate in the eTOEP. Each community was randomized into one of four training strategies for smokingcessation counseling. When smoking-cessation counseling training was not delivered (usual practice), an alternative duration of CME-accredited training on skin cancer prevention was delivered to counteract any potential bias or Hawthorne effect (McCarney, et al., 2007; Trudeau, 1982).
In each community, several clinicians and pharmacists were recruited for a total of 170 providers. The overarching “physicians” category included family practitioners, nurse practitioners, obstetrician/gynecologists, pediatricians, and physician’s assistants. Of 87 recruited physicians, 45 were trained for smoking-cessation counseling while 42 were trained about skin cancer prevention. Of 83 pharmacists, 45 were trained in smoking-cessation and 38 in skin-cancer prevention. The details of recruitment and retention of health care providers are presented elsewhere (Prokhorov, et al., 2010).
2.3. Participants
Participants eligible for the study were at least 18 years old, English or Spanish speaking adult smokers who consented to complete the baseline and follow-up surveys (Prokhorov, et al., 2010). The participants were surveyed four times by telephone or mail: at baseline and then 3, 6, and 12 months after entering the study. Each participant remained in the clinical trial for a 1-year period. A written informed consent was obtained from the participants during the initial contact.
Of the 888 eligible participants recruited, 240 were from a community where neither pharmacists nor physicians experienced tobacco-cessation training, 225 were from a community where only pharmacists received training, 177 were from a community where only physicians received training, and 246 were from a community where both pharmacists and physicians received training. The participants were compensated US$25 for a baseline assessment (at the time of recruitment) and for each subsequent assessment, for a total of US$100 at the end of the study.
The MD Anderson Cancer Center institutional review board approved the study protocol (BS01–129) on June 20, 2001. The study was conducted from February 2004 to May 2007.
2.4. Perspective for Economic Evaluation
A health care provider’s perspective was adopted for this economic evaluation. This perspective necessitates inclusion of direct health care costs associated with the actual delivery of the program, and the economic evaluation was conducted to determine the cost-effectiveness of implementing the intervention (Honeycutt, et al., 2006).
2.5. Decision-Analytic Model
The study constructed two decision-analytic models (Cantor, 1995) to reflect the economic costs and potential clinical benefits produced by the four smoking-cessation counseling education training strategies for the providers at two time points. Short-term outcomes (at 1 year) were evaluated in terms of cost per successful quit. Long-term outcome were modeled using the quit rates from the trial, life expectancy data for smokers and non-smokers, and other parameters from the literature, and were presented in terms of cost per quality-adjusted life-year [QALY]. According to the Health and Human Services Commission guidelines, a longer study period better reflects ongoing costs because costs stabilize over the year as more participants enroll and staffs are fully trained (Honeycutt, et al., 2006). The guidelines also recommend a time frame long enough to cover the start-up and full implementation of the program (Honeycutt, et al., 2006). Thus, this analysis uses self-reported quit rates 1 year from the baseline to determine clinical outcomes.
The economic analysis, however, incorporated a lifetime analytic horizon to capture the long-term benefits of smoking cessation. This is consistent with guidelines for cost-effectiveness analysis established by the Panel for Cost-Effectiveness in Health and Medicine (Cantor & Miller, 2009; Lipscomb, Weinstein, & Torrance, 2005).
2.6. Model Parameters
Probability data for the decision-analytic models were based on the medical literature and on data collected for this study. The 1-year quit rates from the study formed a baseline model that used costs and probabilities of quitting to estimate the cost per quit for each training strategy. The analysis uses self-reported quit rates to determine how many participants quit smoking. This is a common practice in similar community-based studies on smoking cessation interventions (Velicer, Prochaska, Rossi, & Snow, 1992; Zhu, et al., 2002). The quit rates were assessed on the basis of response to the following two survey questions at the 12-month time-point since the participant’s entry into the study:
How would you describe your smoking at this time, would you say that you have completely stopped smoking?
How would you describe your smoking at this time, would you say that you have not smoked at all since we last spoke?
Those who responded “yes” to one of the questions at the end of the one-year clinical trial period were considered quitters.
The second decision-analytic model (Sonnenberg & Beck, 1993) analyzed the long-term outcomes on the basis of data in the medical literature (Fiscella & Franks, 1996; Rogers, Hummer, Krueger, & Pampel, 2005), which enabled us to calculate the quality-adjusted life expectancy for the hypothetical cohorts. Each of the four intervention arms branches into smokers and quitters. These branches ended in simple two-state (“alive” or “dead”) Markov models that calculated life expectancies. Smokers were defined as patients who did not successfully quit smoking after the 1-year research period as discussed above. The mortality rates for smokers were based on life tables (Rogers, et al., 2005) and were adjusted by sex and age. Rogers et al categorizes mortality rates by the amount of cigarettes consumed: < 1 pack/day, 1–2 packs/day, or ≥ 2 packs/day (Rogers, et al., 2005). Accordingly, our decision-analytic model categorized hypothetical smokers using this method. Spontaneous quit rates after 1 year were assumed to be the same for all four interventions and were factored into the life expectancies from the Rogers model (Rogers, et al., 2005), as were the proportions and mortalities of former heavy, light, and very light smokers (Rogers, et al., 2005). See Table 1 for model parameters.
Table 1.
Model parameters of adult smokers participating in eTOEP (February 2004–May 2007)
| Parameters | Base case | Source | ||
|---|---|---|---|---|
| Quit rates (strategies) | Men % (95% CI) |
Women % (95% CI) |
All % (95% CI) |
Clinical trial |
| Physicians and pharmacists (both) | 17% (9% to 26%) | 9% (5% to 13%) | 11% (7% to 15%) | |
| Physicians only | 7% (0% to 14%) | 3% (0% to 6%) | 5% (1% to 8%) | |
| Pharmacists only | 9% (2% to 15%) | 8% (3% to 12%) | 8% (4% to 12%) | |
| No training (none) | 10% (3% to 17%) | 8% (4% to 12%) | 9% (5% to 12%) | |
| Prevalence of smoking intensity | Men | Women | Rogers et al., 2005 | |
| Heavy smokers (≥2 packs a day) | 13% | 6% | ||
| Light smokers (1–2 packs a day) | 56% | 47% | ||
| Very light smokers (<1 pack a day) | 31% | 47% | ||
| Prevalence of former smoking intensity | Men | Women | Rogers et al., 2005 | |
| Former heavy smokers (≥2 packs a day) | 25% | 12% | ||
| Former light smokers (1–2 packs a day) | 57% | 40% | ||
| Former very light smokers (<1 pack a day) | 18% | 48% | ||
| Life expectancy (for smokers and former smokers) | - | Rogers et al., 2005 | ||
| Health-related quality-of-life weights | - | Fiscella and Franks, 1996 | ||
| Discount rate | 3% | Gold et al., 1996 | ||
Abbreviations: eTOEP, The Health Care Team Approach to Smoking Cessation: Enhanced Tobacco Outreach Education Program; CI, confidence interval.
Sources:
Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians' smoking cessation counseling. JAMA 1996;275(16):1247–1251.
Lipscomb J, Weinstein MC, Torrance GW. Time Preference. In: Gold MR, Siegel JE, Russel LB, et al., eds. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press 2005:214–235.
Rogers RG, Hummer RA, Krueger PM, et al. Mortality attributable to cigarette smoking in the United States. Population and Development Review 2005;31(2):259 – 292
2.7. Utilities
Outcomes in the model were based on both life expectancy and quality-adjusted life expectancy, as measured in life-years and QALYs, respectively. Utility scores, representing a preference for quality of life for a particular health outcome, were derived from Fiscella and Franks (Fiscella & Franks, 1996) to adjust for quality of life in patients aged 25–69 years. The data are organized by smokers and quitters (those who have quit smoking for 15 years) and by men and women. The model assumed that the quality of life score would progressively improve in a linear fashion over the 15-year period (Fiscella & Franks, 1996). After age 70, utility values remained constant through the cohort lifetimes. The model also assumed that the quality of life did not vary depending on the amount of cigarettes consumed by current or former smokers.
2.8. Costs
Because the decision analysis was structured on a per patient basis, the costs of the intervention were allocated similarly. Costs were spread across the number of smokers who would be expected to receive each intervention and were measured alongside the clinical trial and were valued in terms of 2003 US dollars, since that was the starting year of the study and the year in which many of the initial costs were incurred.
Total costs were split into personnel, capital, supplies, and program delivery (Table 2). Following the guidelines of the US Panel on Cost-Effectiveness in Health and Medicine, the analysis did not include the research and development costs since the eTOEP program had already been created. This is because the money spent to create the program will be amortized over a long time frame and a much larger group of participants if the program is implemented in other communities. Thus, development costs will be negligible when conducting a cost-effectiveness analysis for future implementations (Luce, Manning, Siegel, & Lipscomb, 1996). The implementation costs, including project staff’s time spent implementing the program, were measured to see how effective the program would be if applied in another community. The costs included in the analysis were based on 2003 average hourly wage rates. Table 2 contains a more comprehensive list of costs and their inclusion in our analysis.
Table 2.
Summary of eTOEP implementation costs
| Cost categories | Implementation costs |
|---|---|
| Personnel | (2 years; US$) |
| Lead Investigator | 8,160 |
| Manager | 13,864 |
| Project Director | 69,000 |
| Coordinator | 96,750 |
| Trainer | 96,750 |
| Capital | |
| Note-taking materials | 231 |
| CDC smoking books | 4,899 |
| Supplies | |
| Office posters | 1,000 |
| Personalized program | 30,000 |
| Pre-training costs | |
| Advertising | 5,801 |
| Room rental | 65 |
| Office supplies | 4,034 |
| Conference services | 1,500 |
| Actual training day costs | |
| Trainers (presenters) | 22,203 |
| Education materials | 2,622 |
| Auxillary, internet-based | 19,746 |
| Maintenance costs | |
| Gifts (e.g., calendars) | 781 |
| Providers' training time costs | |
| Physicians | 1,009 |
| Pharmacists | 585 |
| Physicians and pharmacists | 1,594 |
Abbreviations: eTOEP, The Health Care Team Approach to Smoking Cessation: Enhanced Tobacco Outreach Education Program
Provider training time cost calculation
Mean hourly wage of a pharmacist, 2003 US dollars = $39.03
Mean hourly wage of a family and general practitioner, 2003 US dollars = $67.24 (Source: US Bureau of Labor statistics)
The tobacco intervention training was 3 hours long.
Overall, 16 communities participated in the trial.
Overall, there were 87 physicians and 83 pharmacists.
Therefore, let us assume that at one site there are 5 physicians and 5 pharmacists.
Therefore, the total cost incurred to provide training to physicians at one site = 5 × 3 × 67.24 = US$1,008.60
And the total cost incurred to train pharmacists at one site = 5 × 3 × 39.03 = US$585.45
The total costs incurred training physicians and pharmacists = US$1,594.05
At each site, the model assumed that a maximum of five physicians, or five pharmacists, or both (a mixed group of five) would participate in the training session. The study assumed that on average, physicians and pharmacists would see 750 unique smokers every year (Appendix). In each community the five clinicians would see 3,750 unique smokers every year. Therefore, total costs (implementation and provider training time) were allocated across 3,750 unique smokers.
2.9. Analysis
Strategies were evaluated using an incremental cost-effectiveness ratio (ICER), which determined the per unit economic and clinical value of an intervention with respect to alternative strategies. The model estimated the cost per quit, and dollars per QALY. For cost per QALY analysis, both costs and effectiveness were discounted at a rate of 3% as generally recommended by economic evaluation practice (Severens & Milne, 2004). The discount rate measures future costs and benefits in terms of net present value according to the societal preferences.
The cost-effectiveness analysis was conducted using TreeAge Pro 2013 software. The base case analysis was a 45-year-old smoker since that was the average age of participants in the clinical study (Prokhorov, et al., 2010). Sensitivity analysis determined how robust the conclusions of the base case analysis were to changes in the model parameters. One-way sensitivity analysis identified the relative effect of changes in the uncertain parameters on the ICER. With a willingness-to-pay threshold of US$50,000/QALY, two-way uncertainty analysis was conducted on the 95% confidence interval (CI) of quit rates on the non-dominated strategies (Weinstein, 2008). The two-way uncertainty analysis determines the effect of change in quit rates on the net benefits valued at the willingness-to-pay threshold.
3. Results
3.1. Provider and Participant Characteristics
The provider characteristics are presented elsewhere (Prokhorov, et al., 2010). The demographic characteristics of the participants are presented in Table 3. The average age of the participants targeted by each of the training strategies was between 43 and 47 years. The majority of the participants were white, had a high school or higher degree, were married, and were employed. There was no significant difference between demographic characteristics of the participants targeted by each of the training arms, except for race of the female participants.
Table 3.
Demographic characteristics of the participants
| Men | Women | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither N (%) |
Pharmacist only N (%) |
Physician only N (%) |
Pharmacist & physician N (%) |
P- value |
Neither N (%) |
Pharmacist only N (%) |
Physician only N (%) |
Pharmacist & physician N (%) |
P- value |
|||||||||
| Age | 0.237 | 0.735 | ||||||||||||||||
| N | 70 | 69 | 55 | 76 | 170 | 156 | 122 | 170 | ||||||||||
| Mean | 43.9 | (13.7) | 42.6 | (13.3) | 46.1 | (12.4) | 46.2 | (10.7) | 43.9 | (12.7) | 45.1 | (11.8) | 43.7 | (12.4) | 43.8 | (11.4) | ||
| Median | 44 | 43 | 46 | 47 | 45 | 47 | 45.5 | 45.5 | ||||||||||
| Race | 0.360 | <0.001 | ||||||||||||||||
| American Indian | 1 | (1.4) | 3 | (4.3) | 3 | (5.5) | 0 | (0.0) | 1 | (0.6) | 1 | (0.6) | 2 | (1.6) | 5 | (2.9) | ||
| Asian | 0 | (0.0) | 1 | (1.4) | 0 | (0.0) | 1 | (1.3) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Pacific Islanders | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 2 | (1.2) | ||
| Black | 15 | (21.4) | 11 | (15.9) | 12 | (21.8) | 18 | (23.7) | 25 | (14.7) | 49 | (31.4) | 24 | (19.7) | 33 | (19.4) | ||
| White | 47 | (67.1) | 52 | (75.4) | 34 | (61.8) | 55 | (72.4) | 140 | (82.4) | 100 | (64.1) | 81 | (66.4) | 124 | (72.9) | ||
| Hispanic | 6 | (8.7) | 2 | (2.9) | 5 | (9.1) | 2 | (2.6) | 3 | (1.8) | 6 | (3.8) | 13 | (10.7) | 5 | (2.9) | ||
| No answer | 1 | (1.4) | 0 | (0.0) | 1 | (1.8) | 0 | (0.0) | 1 | (0.6) | 0 | (0.0) | 2 | (1.6) | 1 | (0.6) | ||
| Marital status | 0.753 | 0.458 | ||||||||||||||||
| Single | 23 | (32.9) | 27 | (39.1) | 18 | (32.7) | 20 | (26.3) | 47 | (27.6) | 37 | (23.7) | 34 | (27.9) | 40 | (23.5) | ||
| Married | 31 | (44.3) | 31 | (44.9) | 25 | (45.5) | 43 | (56.6) | 67 | (39.4) | 75 | (48.1) | 56 | (45.9) | 70 | (41.2) | ||
| Separated | 4 | (5.7) | 4 | (5.8) | 6 | (10.9) | 3 | (3.9) | 11 | (6.5) | 14 | (9.0) | 7 | (5.7) | 13 | (7.6) | ||
| Divorced | 10 | (14.3) | 5 | (7.2) | 5 | (9.1) | 9 | (11.8) | 38 | (22.4) | 20 | (12.8) | 22 | (18.0) | 36 | (21.2) | ||
| Widowed | 2 | (2.9) | 2 | (2.9) | 1 | (1.8) | 1 | (1.3) | 7 | (4.1) | 10 | (6.4) | 3 | (2.5) | 11 | (6.5) | ||
| Education | 0.459 | 0.113 | ||||||||||||||||
| < 12thgrade | 11 | (15.7) | 11 | (15.9) | 16 | (29.1) | 18 | (23.7) | 23 | (13.5) | 35 | (22.4) | 33 | (27.0) | 32 | (18.8) | ||
| High school | 25 | (35.7) | 18 | (26.1) | 13 | (23.6) | 22 | (28.9) | 56 | (32.9) | 51 | (32.7) | 36 | (29.5) | 60 | (35.3) | ||
| Some college | 19 | (27.1 | 21 | (30.4) | 16 | (29.1) | 23 | (30.3) | 57 | (33.5) | 53 | (34.0) | 41 | (33.6) | 64 | (37.6) | ||
| College degree | 13 | (18.6) | 12 | (17.4) | 7 | (12.7) | 12 | (15.8) | 28 | (16.5) | 15 | (9.6) | 12 | (9.8) | 12 | (7.1) | ||
| Master’s degree | 2 | (2.9) | 6 | (8.7) | 2 | (3.6) | 1 | (1.3) | 3 | (1.8) | 2 | (1.3) | 0 | (0.0) | 2 | (1.2) | ||
| Unknown | 0 | (0.0) | 1 | (1.4) | 1 | (1.8) | 0 | (0.0) | 3 | (1.8) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| Employment | 0.185 | 0.231 | ||||||||||||||||
| Employed | 47 | 67.1 | 49 | 71.0 | 32 | 58.2 | 50 | 65.8 | 88 | 51.8 | 80 | 51.3 | 61 | 50.0 | 91 | 53.5 | ||
| Student | 1 | 1.4 | 3 | 4.3 | 0 | 0.0 | 0 | 0.0 | 8 | 4.7 | 7 | 4.5 | 5 | 4.1 | 6 | 3.5 | ||
| Retired | 11 | 15.7 | 4 | 5.8 | 10 | 18.2 | 10 | 13.2 | 18 | 10.6 | 18 | 11.5 | 12 | 9.8 | 15 | 8.8 | ||
| Keep house/take care of family | 8 | 11.4 | 6 | 8.7 | 4 | 7.3 | 6 | 7.9 | 40 | 23.5 | 44 | 28.2 | 35 | 28.7 | 48 | 28.2 | ||
| Income | 0.774 | 0.992 | ||||||||||||||||
| < $25,000 | 17 | 24.3 | 20 | 29.0 | 19 | 34.5 | 23 | 30.3 | 68 | 40.0 | 59 | 37.8 | 46 | 37.7 | 60 | 35.3 | ||
| $25,001–$55,001 | 15 | 21.4 | 10 | 14.5 | 10 | 18.2 | 16 | 21.1 | 30 | 17.6 | 37 | 23.7 | 27 | 22.1 | 42 | 24.7 | ||
| $55,001–$75,000 | 5 | 7.1 | 11 | 15.9 | 7 | 12.7 | 10 | 13.2 | 13 | 7.6 | 11 | 7.1 | 8 | 6.6 | 10 | 5.9 | ||
| $75,001–$95,000 | 3 | 4.3 | 8 | 11.6 | 4 | 7.3 | 4 | 5.3 | 5 | 2.9 | 6 | 3.8 | 4 | 3.3 | 6 | 3.5 | ||
| > $95,000 | 4 | 5.7 | 4 | 5.8 | 1 | 1.8 | 3 | 3.9 | 7 | 4.1 | 8 | 5.1 | 6 | 4.9 | 9 | 5.3 | ||
| Unknown | 26 | 37.1 | 16 | 23.2 | 14 | 25.5 | 20 | 26.3 | 47 | 27.6 | 35 | 22.4 | 31 | 25.4 | 43 | 25.3 | ||
3.2. Effectiveness Analysis
Table 4 presents the results of the effectiveness analysis in terms of survival, undiscounted QALYs, and QALYs discounted at a 3%. Training both physicians and pharmacists added 0.09 QALYs in 45-year-old men. However, in 45-year-old women, the synergy training only added 0.01 QALYs when compared to no intervention. The overall undiscounted, unadjusted survival duration was 5 years more in women than in men, which, when adjusted for quality of life and discounted, was almost an additional year.
Table 4.
Effectiveness results of eTOEPa
| Strategy | Effectiveness (unadjusted for quality of life, undiscounted) |
Effectiveness (adjusted for quality of life, undiscounted) |
Effectiveness (adjusted for quality of life, discounted at 3%) |
|---|---|---|---|
| Men | |||
| Training: none | 31.15 | 19.79 | 13.71 |
| Training: pharmacist only | 31.08 | 19.77 | 13.70 |
| Training: physician only | 31.01 | 19.74 | 13.68 |
| Training: physician and pharmacist (both) | 31.51 | 19.92 | 13.80 |
| Women | |||
| Training: none | 34.61 | 21.12 | 14.69 |
| Training: pharmacist only | 34.57 | 21.11 | 14.68 |
| Training: physician only | 34.28 | 21.02 | 14.62 |
| Training: physician and pharmacist (Both) | 34.65 | 21.13 | 14.70 |
Base-case estimates based on a 45-year-old smoker
Abbreviations: eTOEP, The Health Care Team Approach to Smoking Cessation: Enhanced Tobacco Outreach Education Program
3.3. Cost-Effectiveness Analysis
The cost per patient of the physician-only strategy and pharmacist-only strategy was $77.38 and $77.26, respectively, whereas the combined strategy cost approximately $78.39. Tables 5 and 6 present the results of the cost-effectiveness analysis in terms of cost/quit and cost/QALY analysis. Table 6 compares the no-training strategy to training both physicians and pharmacists, excluding the dominated strategies (training only physicians or training only pharmacists) from further analysis because these strategies cost more and provided fewer added life-years.
Table 5.
Cost per quit analysis of eTOEP
| Strategy | Cost/patient (US$) |
Effectiveness (men) |
Effectiveness (women) |
Effectiveness (all) |
|---|---|---|---|---|
| None | 0.00 | 10% | 8% | 9% |
| Training: pharmacists onlya | 77.27 | 9% | 8% | 8% |
| Training: physicians onlya | 77.38 | 7% | 3% | 5% |
| Training: physicians and pharmacists (both) | 78.39 | 17% | 9% | 11% |
| ICER (both compared to none) | US$1,104 | US$13,065 | US$3,105 |
The pharmacists-only and physicians-only strategies were dominated by the no intervention strategy.
Measured in terms of cost/quit
Abbreviations: eTOEP, The Health Care Team Approach to Smoking Cessation: Enhanced Tobacco Outreach Education Program; ICER, incremental cost-effectiveness ratio.
Table 6.
Incremental cost-effectiveness of the undominated strategies by sex and age at the time of intervention
| Patient age at intervention, y |
Incremental effectiveness (rounded to two decimals) |
Incremental costs per QALY saveda (US$) |
|---|---|---|
| Men | ||
| 25–29 | 0.07 | 1,198 |
| 30–34 | 0.07 | 1,100 |
| 35–39 | 0.08 | 1,016 |
| 40–44 | 0.08 | 937 |
| 45–49 | 0.09 | 868 |
| 50–54 | 0.10 | 812 |
| 55–59 | 0.10 | 776 |
| 60–64 | 0.10 | 761 |
| 65–69 | 0.10 | 762 |
| 70–74 | 0.10 | 776 |
| Women | ||
| 25–29 | 0.01 | 13,847 |
| 30–34 | 0.01 | 12,234 |
| 35–39 | 0.01 | 10,764 |
| 40–44 | 0.01 | 9,699 |
| 45–49 | 0.01 | 8,953 |
| 50–54 | 0.01 | 8,275 |
| 55–59 | 0.01 | 7,754 |
| 60–64 | 0.01 | 7,428 |
| 65–69 | 0.01 | 7,254 |
| 70–74 | 0.01 | 7,017 |
Cost-effectiveness ratios based on 2003 US dollars with quality-adjusted life-years (QALYs) saved discounted at 3%.
Abbreviations: QALY, quality-adjusted life-year.
When compared to the no training group, training both physicians and pharmacists increased the quit rate by 7% in men, but only by 1% in women. The corresponding ICER in terms of cost per quit of the combination strategy was US$1,104/quit for men, US$13,065/quit for women, and US$3,105/quit for all. Among 45-year-old women, the combination therapy saved one discounted QALY at a cost of US$8,953. The same strategy saved one discounted QALY at a cost of US$868 in 45-year-old men. With every additional year in age, the ICER decreased in both men and women.
3.4. Uncertainty Analysis
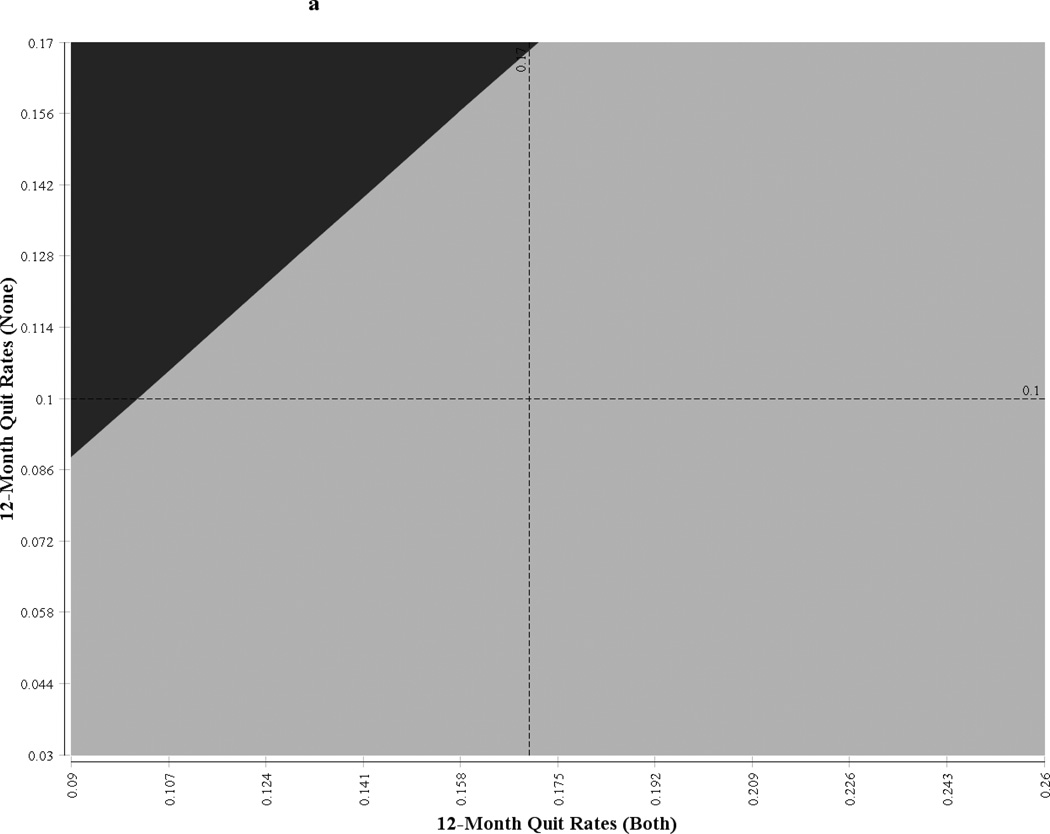
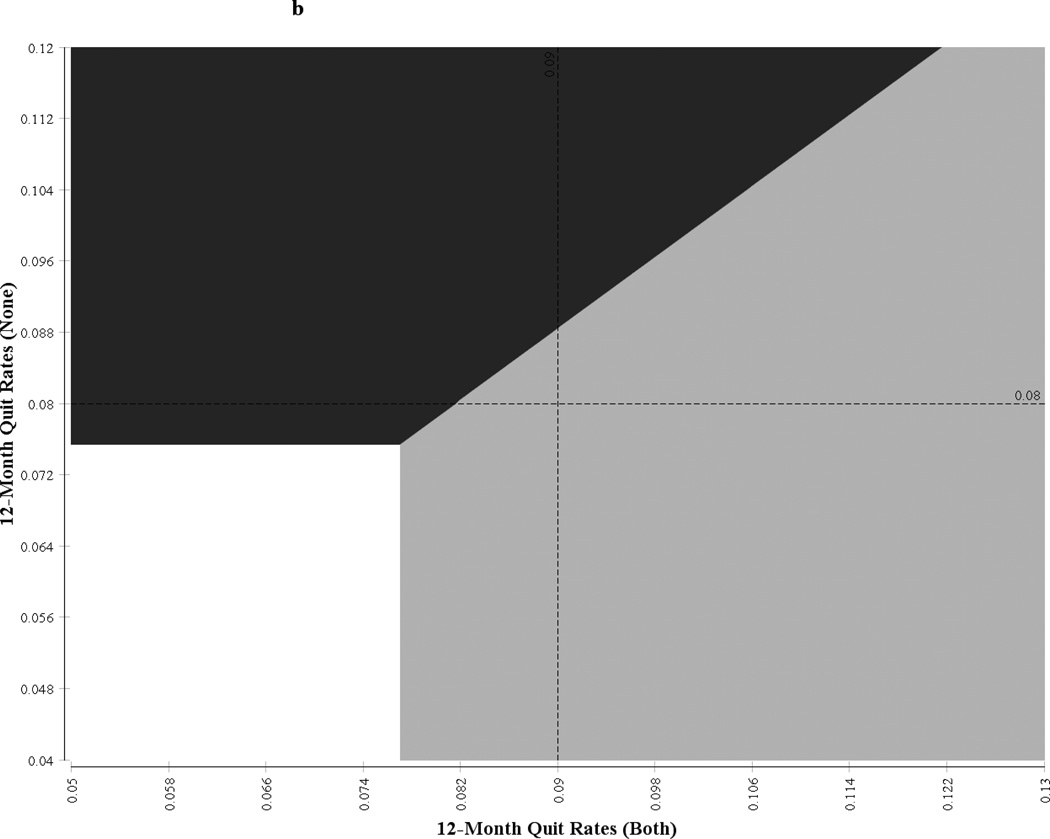
One-way sensitivity analysis showed that the ICER was sensitive to the number of patients a provider would see in a given community in 1 year and to the discount rate (see Table 7). For a community with 500 unique smokers per provider, the ICER of the synergy strategy was US$1,302/QALY for men and US$13,430/QALY for women. When the number of smokers per provider was increased to 1,000, the ICER of the intervention was reduced to US$651/QALY for men and US$6,716/QALY for women. Similarly, the ICER was highly sensitive to the change in discount rate and was moderately sensitive to the change in provider salary. The x- and y-axis of Figure 1 represent a 95% CI of the undominated strategies (synergy intervention and usual care); the quadrant is partitioned into the regions corresponding to various incremental net benefits, and the boundaries represent the points at which the strategies have the same net benefits. The points falling in a particular zone indicate the strategy that will be most cost-effective with respect to the other strategies at the willingness-to-pay threshold of US$50,000/QALY. For example if the 12-month quit rates for the combination strategy and the no-training strategy are 17.5% and 6.5%, respectively, then the combination strategy would be the most cost-effective strategy for men. Similarly, if the 12-month quit rates for the combination strategy and no-training strategy are 7% and 6%, respectively, then the pharmacist-only training strategy would be the most cost-effective strategy for women.
Table 7.
Sensitivity analysis of eTOEP
| Sensitivity analysis Base case (range) |
Incremental costs per quality-adjusted life- year saved, US$* |
|
|---|---|---|
| Men | Women | |
| Cost per hour of provider’s (physician + pharmacist) training time, base case hourly wage: physician -US$67.24; pharmacist - US$39.03 (0.5 × hourly wage – 2 × hourly wage) | (866–873) | (8,929–9,002) |
| Discount rate, base case: 3% (1%–5%) | 441–1,563 | 4,143–17,279 |
| Number of patients a physician or a pharmacist would see in a year, base case: 750 (500– 1,000) | 1,302–651 | 13,430–6,716 |
Base case estimates based on a 45-year-old smoker
Figure 1.
Two-way uncertainty analysis of the effect of change in quit rates on net benefits at willingness-to-pay threshold of US$50 000 of eTOEP
Figure 1a  Neither Neither | |
 Physician and Pharmacist Physician and Pharmacist |
|
| Figure 1b. |
 Neither Neither |
 Physician and Pharmacist Physician and Pharmacist |
|
 Pharmacist only Pharmacist only |
4. Discussion
The analysis found that the combination strategy was highly cost-effective for men and moderately cost-effective for women. The other training interventions were dominated, as they were more costly and less effective. The increase in QALYs (0.09 QALYs for men and 0.0.1 QALYs for women) in the base case show the overall effectiveness of the program spread across all participants. This does not mean an increase of 0.09 for each of the program participants. If the intervention causes approximately 1 in 10 men to quit smoking, the one quitter may gain 0.9 QALYs (about 329 additional discounted days in perfect health), but these gains have to be distributed among the other 9 participants who are still smokers. This leads to a 0.09 incremental effectiveness number. All QALY numbers should be interpreted through this framework. Thus, the higher the quit rates from the smoking cessation program, the greater the benefits and incremental QALYs gained.
Several other researchers have investigated educational smoking-cessation interventions (Cromwell, et al., 1997; Goldberg, et al., 1994; Ockene, et al., 1987; Ockene, et al., 1997; Richmond, et al., 1998; Stead, et al., 2013). The study by Pinget et al was the only one that assessed the cost-effectiveness of a physiciancentered cessation-training program (Pinget, et al., 2007). In that study, if residents received training, the ICERs were US$25 per life-year saved in men and US$35 per life-year saved in women. If physicians in private practice received training, the ICERs were US$88 per life-year saved in men and US$123 in women (currency reported in 2003 US dollars). The study was conducted in university hospitals in Switzerland and used parameters, such as hospital-specific wage rates, that are not easily generalizable. Additionally, the results were presented in terms of dollars per life-year saved rather than dollars per QALY. The present study uses a similar approach to Pinget et al with regards to the eTOEP intervention, but has a broader scope since its results may be generalized to other communities using a similar intervention program. Additionally, our study uses a dollar per QALY analysis that more accurately captures long term costs and benefits.
Other studies on smoking cessation have focused on educating patients directly. Cromwell et al compared 15 recommended smoking-cessation interventions (Cromwell, et al., 1997). The costs (measured in 1995 US dollars) per quit without nicotine replacement therapy for minimal counseling, brief counseling, and full counseling by primary care physicians were US$7,922, US$6,276, and US$2,989, respectively. The ICERs—US$2,186 cost/quit and US$1,108 cost/QALY—for the group intensive counseling intervention were less than the ICERs for the individual counseling intervention.
Our study found similar results that showed the eTOEP program to be fairly cost-effective. At a cost of $3,105 per quit, the program was effective in the short term. Moreover, among 45-year-old smokers, costs were $868/QALY and $8,953/QALY for men and women, respectively. Not only are these numbers on par with previous studies, but they also fall well under the willingness-to-pay threshold outlined above.
The combination strategy was far more cost-effective in men than in women, largely owing to the higher quit rate among men. As demonstrated in the two-way uncertainty analysis, the cost-effectiveness of the intervention depends heavily on the quit rates. Studies have shown greater challenges in helping women to quit smoking than in helping men, potentially owing to differences in confidence, although this hypothesis is controversial (Bauld, Judge, & Platt, 2007; Jarvis, Cohen, Delnevo, & Giovino, 2012). Pinget et al also observed that their intervention was more cost-effective in men than in women (Pinget, et al., 2007). This might be because in both studies more men were heavy smokers than women, which allowed men to receive more benefits in terms of QALYs saved.
The eTOEP study was subject to several limitations. Several assumptions were made regarding the number of smokers a physician or a pharmacist would see in a year. This study also does not consider relapse rates, which means that it may be overestimating the benefits of tobacco cessation. The results found here may not be generalizable to other communities in other areas, as the study was conducted in 16 communities in Texas. Another potential limitation of this study is that we did not use the biochemical validation for to determine smoking cessation. However, evidence from the literature suggests that self-reporting is actually highly accurate in low-intensive interventions that take place in settings outside of a controlled laboratory (Velicer, et al., 1992). In a community based study like this one, saliva testing is both unfeasible and unreliable. Thus, any false negatives due to misreporting should not greatly change the results of this study.
The study did not use probabilistic sensitivity analysis (PSA). The purpose of PSA is to evaluate the joint uncertainty in all model parameters, mainly costs and effectiveness. The costs associated with the interventions did not have any variation since they were point estimates calculated from the resources utilized in the clinical trial. Moreover, the major parameters, such as utilities and smoking intensity, were based on the literature and did not have variation associated with them. Therefore, we decided not to perform PSA and evaluated model uncertainty using one-way and two-way sensitivity analysis.
Since this study was conducted from the health care provider perspective, it did not account for costs that participants would face if they sought out other smoking-cessation counseling or pharmacotherapy to help them quit. Health care costs incurred owing to additional life-years gained by participants were also not considered because the topic is a controversial methodological issue that does not lie within the scope of the project.
We conclude that the eTOEP intervention yields favorable cost-effectiveness. The cost-effectiveness of the intervention depended on gender and quit rates. Implementing this program at a community level would increase the program cost marginally; however, similar success in other communities is possible.
Research highlights.
We performed an economic evaluation of smoking-cessation counseling training.
We compared training physicians or pharmacists, training both, and training none.
Outcomes were measured using cost per quit and cost per quality-adjusted life-year.
Training both physicians and pharmacists could be cost-effective.
Acknowledgments
The authors wish to thank Jill Delsigne for editorial contributions and Jennifer Gatilao for manuscript preparation.
Role of Funding Sources
Funding for this study was provided by the National Cancer Institute grant number R01-CA093969. NCI had no role in the study design, collection, analysis, or interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication. Ashish A. Deshmukh was partially supported by The Janice Davis Gordon Postdoctoral Fellowship in Colorectal Cancer Prevention and the National Institutes of Health through MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
SBC was the primary author of the manuscript. SBC, AVP, and NSL were responsible for data acquisition, study design and concept. AAD and GMN conducted statistical analyses, authored the results section of the manuscript, and contributed to the revision of the manuscript. NSL, TR, and AVP contributed to the preparation and critical revision of the manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Abelin T, Buehler A, Muller P, Vesanen K, Imhof PR. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet. 1989;1:7–10. doi: 10.1016/s0140-6736(89)91671-1. [DOI] [PubMed] [Google Scholar]
- Bauld L, Judge K, Platt S. Assessing the impact of smoking cessation services on reducing health inequalities in England: observational study. Tobacco Control. 2007;16:400–404. doi: 10.1136/tc.2007.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin K, Lindgren B, Willers S. The cost utility of bupropion in smoking cessation health programs: simulation model results for Sweden. Chest. 2006;129:651–660. doi: 10.1378/chest.129.3.651. [DOI] [PubMed] [Google Scholar]
- Cantor SB. Decision analysis: theory and application to medicine. Primary Care: Clinics in Office Practice. 1995;22:261–270. [PubMed] [Google Scholar]
- Cantor SB, Miller L. Time horizon. In: Kattan M, editor. Encyclopedia of medical decision making. Thousand Oaks, CA: SAGE Publications, Inc.; 2009. pp. 1138–1139. [Google Scholar]
- Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA. 1997;278:1759–1766. [PubMed] [Google Scholar]
- Cummings SR, Rubin SM, Oster G. The cost-effectiveness of counseling smokers to quit. JAMA. 1989;261:75–79. [PubMed] [Google Scholar]
- Fagerstrom KO. A comparison of psychological and pharmacological treatment in smoking cessation. Journal of Behavioral Medicine. 1982;5:343–351. doi: 10.1007/BF00846161. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians' smoking cessation counseling. JAMA. 1996;275:1247–1251. [PubMed] [Google Scholar]
- Goldberg DN, Hoffman AM, Farinha MF, Marder DC, Tinson-Mitchem L, Burton D, Smith EG. Physician delivery of smoking-cessation advice based on the stages-of-change model. American Journal of Preventive Medicine. 1994;10:267–274. [PubMed] [Google Scholar]
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Munoz R. Cost-effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services and Research. 2005;32:381–392. doi: 10.1007/BF02384199. [DOI] [PubMed] [Google Scholar]
- Hjalmarson AI. Effect of nicotine chewing gum in smoking cessation. A randomized, placebo-controlled, double-blind study. JAMA. 1984;252:2835–2838. [PubMed] [Google Scholar]
- Honeycutt AA, Clayton L, Khavjou O, Finkelstein EA, Prabhu M, Blitstein JL, Evans WD, Renaud JM. Guide to analyzing the cost-effectiveness of community public health prevention approaches. Research Triangle Park, NC: U.S. Department of Health and Human Services; 2006. [Google Scholar]
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, Lauger GG, Marusic Z, Neese LW, Lundberg TG. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994;271:595–600. [PubMed] [Google Scholar]
- Jarvis MJ, Cohen JE, Delnevo CD, Giovino GA. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tobacco Control. 2012 doi: 10.1136/tobaccocontrol-2011-050279. [DOI] [PubMed] [Google Scholar]
- Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians. Doctors Helping Smokers, Round I. JAMA. 1989;261:2101–2106. [PubMed] [Google Scholar]
- Lipscomb J, Weinstein MC, Torrance GW. Time Preference. In: Gold MR, Siegel JE, Russel LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 2005. pp. 214–235. [Google Scholar]
- Luce BR, Manning WG, Siegel JE, Lipscomb J. Estimating costs in cost-effectivness analysis. In: Gold MR, Siegel JE, Russel LB, Weinstein MC, editors. Cost-Effectivness in Health and Medicine. New York: Oxford University Press; 1996. p. 194. [Google Scholar]
- McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Medical Research Methodology. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene JK, Hosmer DW, Williams JW, Goldberg RJ, Ockene IS, Biliouris T, Dalen JE. The relationship of patient characteristics to physician delivery of advice to stop smoking. Journal of General Internal Medicine. 1987;2:337–340. doi: 10.1007/BF02596170. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Lindsay EA, Hymowitz N, Giffen C, Purcell T, Pomrehn P, Pechacek T. Tobacco control activities of primary-care physicians in the Community Intervention Trial for Smoking Cessation. COMMIT Research Group. Tobacco Control. 1997;6(Suppl 2):S49–S56. doi: 10.1136/tc.6.suppl_2.s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinget C, Martin E, Wasserfallen JB, Humair JP, Cornuz J. Cost-effectiveness analysis of a European primary-care physician training in smoking cessation counseling. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:451–455. doi: 10.1097/HJR.0b013e32804955a0. [DOI] [PubMed] [Google Scholar]
- Prokhorov AV, Hudmon KS, Marani S, Foxhall L, Ford KH, Luca NS, Wetter DW, Cantor SB, Vitale F, Gritz ER. Engaging physicians and pharmacists in providing smoking cessation counseling. Archives of Internal Medicine. 2010;170:1640–1646. doi: 10.1001/archinternmed.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R, Mendelsohn C, Kehoe L. Family physicians' utilization of a brief smoking cessation program following reinforcement contact after training: a randomized trial. Preventive Medicine. 1998;27:77–83. doi: 10.1006/pmed.1997.0240. [DOI] [PubMed] [Google Scholar]
- Rogers RG, Hummer RA, Krueger PM, Pampel FC. Mortality attributable to cigarette smoking in the United States. Population and Development Review. 2005;31:259–292. doi: 10.1111/j.1728-4457.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value in Health. 2004;7:397–401. doi: 10.1111/j.1524-4733.2004.74002.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau T. The Hawthorne study revisited. Hospital Topics. 1982;60:17. doi: 10.1080/00185868.1982.9954275. [DOI] [PubMed] [Google Scholar]
- US PHS. A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. JAMA. 2000;283:3244–3254. [PubMed] [Google Scholar]
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychological Bulletin. 1992;111:23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Medical Care. 2008;46:343–345. doi: 10.1097/MLR.0b013e31816a7144. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, Gutierrez-Terrell E. Evidence of real-world effectiveness of a telephone quitline for smokers. New England Journal of Medicine. 2002;347:1087–1093. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]