Abstract
There are few methodologies that yield peptides containing His residues with selective N(π), N(π)-bis-alkylated imidazole rings. We have found that, under certain conditions, on-resin Mitsunobu coupling of alcohols with peptides having a N(π)-alkylated His residue results in selective and high-yield alkylation of the imidazole N(π) nitrogen. The reaction requires the presence of a proximal phosphoric, carboxylic or sulfonic acid, and proceeds through an apparent intramolecular mechanism involving Mitsunobu intermediates. These transformations have particular application to phosphopeptides, where “charge masking” of one phosphoryl anionic charge by the cationic histidine imidazolium ion is now possible. This chemistry opens selective access to peptides containing differentially functionalized imidazolium heterocycles, which provide access to new classes of peptides and peptide mimetics.
Introduction
Derivatives of histidine (His) having alkyl groups at both the N(π)– and N(π)–positions are both unusual and useful. For example, short antimicrobial peptides that contain cationic bis-alkylated His residues have recently been synthesized.1, 2 In these cases, the His imidazole rings are identically substituted at both nitrogens. Compounds containing differentially substituted His residues are rare, and include bicyclic glycopeptide natural products, in which glycosylation occurs on the (π)-nitrogen and ring closure is through the N(π)–position.3–6 The ability to differentially functionalize the N(π)– and N(π)–positions, would significantly extend the scope and application of cationic His motifs. Here we report on-resin Mitsunobu-based reactions that readily yield peptides having bis-alkylated His residues. We provide mechanistic studies that support an intramolecular mechanism involving a proximal acidic residue and phosphorane intermediates of the Mitsunobu pathway. Importantly, the transformations are accomplished on solid-phase at the last step prior to resin cleavage. The ready access to these bifurcated His residues facilitates preparing new classes of peptides and peptide mimetics.
Results and discussion
Unexpected Mitsunobu N(π)-alkylation
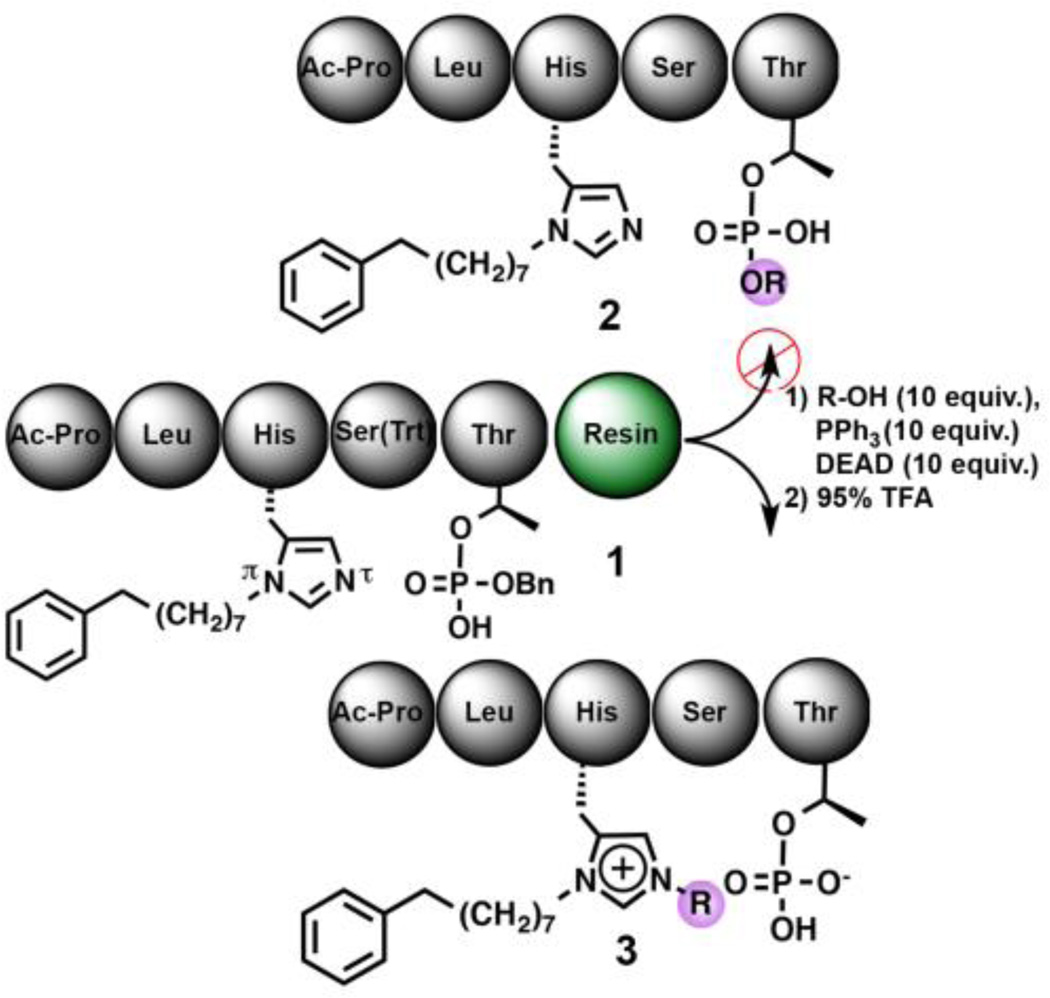
We have previously found in phosphothreonine (pThr)-containing peptides based on the polo-box domain (PBD)-binding sequence, “Ac-Pro-Leu-His-Ser-pThr-amide,” that introduction of long-chain alkylphenyl groups onto the (π)-nitrogen of the His residue can incur large increases in PBD-binding affinities. In some cases, this can represent a three-orders of magnitude enhancement.7 More recently with peptides of the form, Ac-Pro-Leu-His*-Ser-pThr-amide, where “His*” indicates the presence of an N(π)–(CH2)8Phe group, we observed that on-resin Mitsunobu coupling of alcohols with Ac-Pro-Leu-His*-Ser(Trt)-pThr(OBn)(OH)-amide resin (1), rather than giving the expected pThr phosphoryl esters 2, resulted in selective alkylation at the His N(π)-position with retention of the N(π)–(CH2)8Phe functionality (as in compound 3, Fig. 1).8 (Note that the pThr phosphoryl benzyl ester group is cleaved under the 95% TFA conditions used to cleave the peptide from the resin.) The formation of N(π), N(π) bis-alkyl-His species proved to be providential, since these peptides show little or no attenuation of PBD-binding affinity in in vitro assays, yet cellular potencies are increased. We hypothesized that the increased cellular potency may reflect greater membrane transit through intramolecular “charge masking” of an anionic pThr phosphoryl hydroxyl by the bis-alkyl-His imidazolium cation.9 Selectively alkylating the (π)-nitrogen on solid-phase provides a new and broadly accessible approach to these promising, yet underexplored molecules. In this light, we undertook work to clarify the mechanism and scope of the reaction.
Fig. 1.
Unexpected on-resin Mitsunobu N(π)-alkylation.
The presence of a proximal pThr residue is important
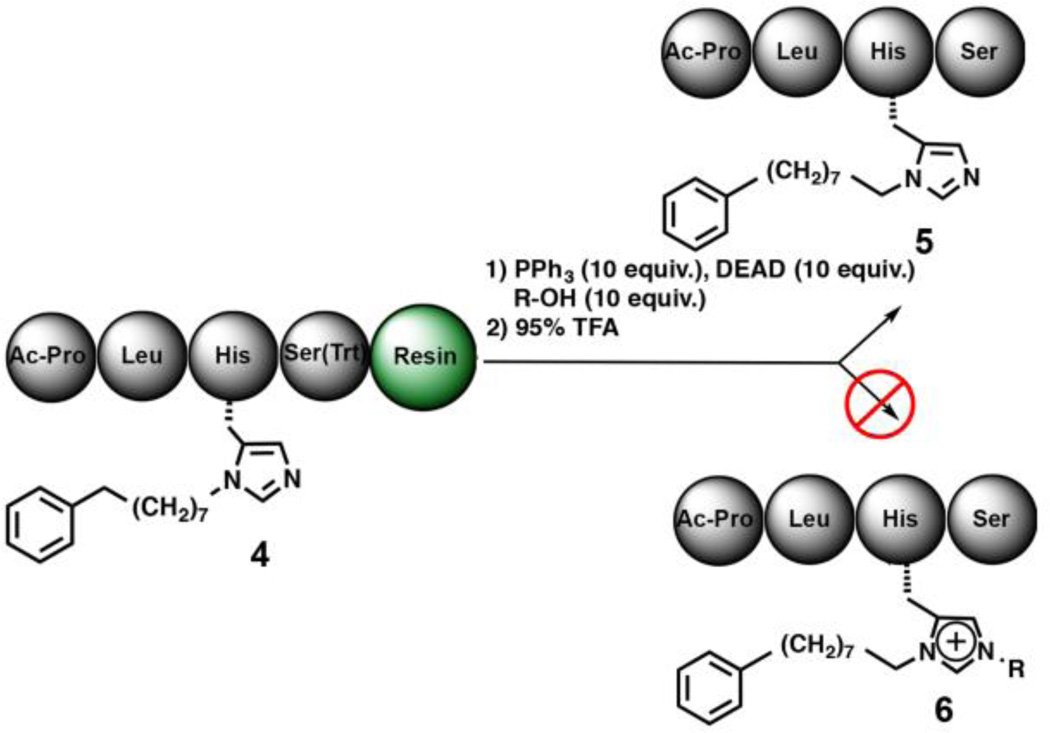
When we deleted the pThr residue from compound 1 to give Ac-Pro-Leu-His*-Ser(Trt)-amide resin (4) and performed the Mitsunobu reaction with 10 equivalents of 4-pentene-1-ol, only non-modified peptide 5 was obtained, with no detectable N(π)–alkylated product (6) (Fig. 2 and Supporting Information Table S3, Experiment 3.I). Repeating the reaction with N-Fmoc-pThr(OBn)(OH)-OH or P(O)(OMe)2(OH) (2.0 equivalents) in the reaction medium yielded predominately non-alkylated 5 with a very minor component of 6 (31:1 ratio, respectively; Supporting Information Table S3, Experiments 3.II & 3.III). Increasing the exogenous phosphoryl source in the reaction mixture to 10 equivalents, either had little [for (N-Fmoc-pThr(OBn)(OH)-OH] or modest [for (P(O)(OMe)2(OH)] effect on product ratios (Supporting Information Table S3, Experiments 3.IV and 3.V, respectively). To examine whether the pThr acidic phosphoryl hydroxyl is important for efficient N(π)–alkylation, we repeated the experiment on a peptide having the free phosphoryl hydroxyl of 1 blocked as a C6H5(CH2)8–ester and found that the ratio of N(π)- alkylated to non-alkylated product was 1:7, respectively (Supporting Information Table S3, Experiment VI; product ratios are for peptides 6-VI and 5-VI of the Table). These efforts clearly support the central role of a pendant acidic hydroxyl group in the His alkylation reactions.
Fig. 2.
Removing pThr from the peptide abrogates N(π)–alkylation.
Phosphoryl ester transfer is not involved
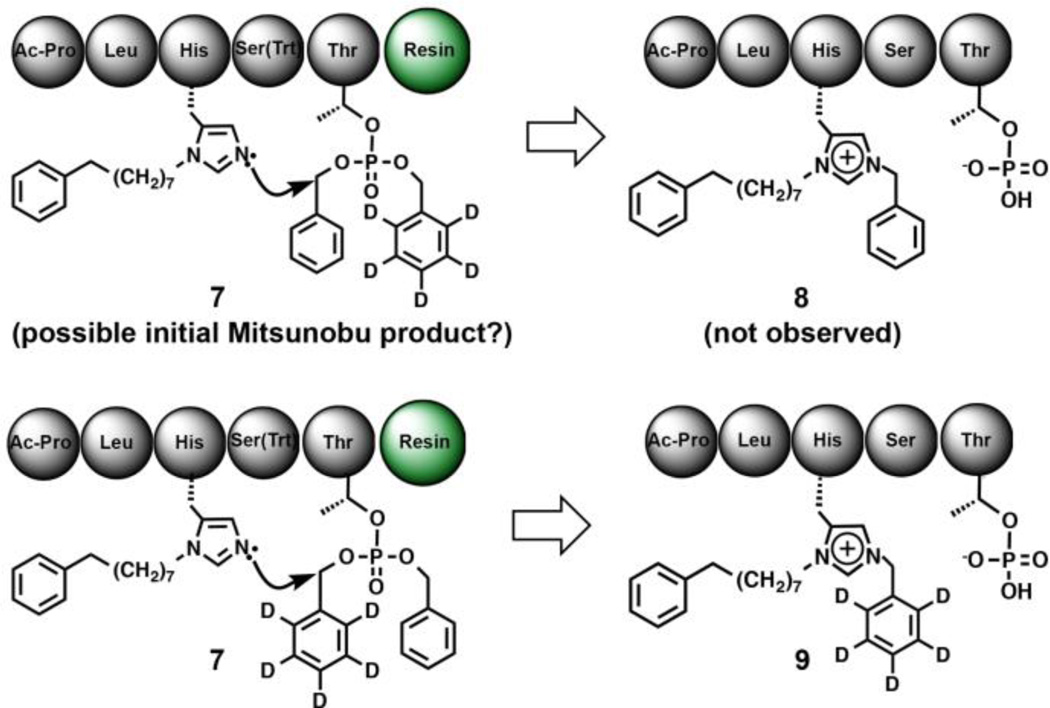
The importance of a pThr residue with an acidic hydroxyl for the N(π)–alkylation raises questions as to the role played by the acidic hydroxyl. One possibility is that phosphoryl ester transfer could be involved. Although it is possible for phosphoryl esters to alkylate imidazole nitrogens,10, 11 we had previously shown that pThr phosphoryl transfer is not involved in the Mitsunobu-based N(π)–alkylation of peptides containing a His N(π)–trityl group.12 To examine whether phosphoryl ester transfer is involved in our observed N(π)–alkylation, we performed Mitsunobu reactions on resin 1 using C6D5CH2OH as the alcohol source. Here, N(π)-alkylation involving a mixed di-ester intermediate (7) would result in transfer of both deuterated and non-deuterated benzyl groups (Fig. 3). Since non-transferred pThr phosphoryl benzyl esters would be hydrolyzed under the 95% TFA conditions used to cleave the peptide from the resin, any intramolecular ester transfer would be reflected through mass spectral analysis as a mixture of C6H5CH2– and C6D5CH2–containing products (8 and 9, respectively, Fig. 3). When we performed the experiments we observed the near exclusive presence of C6D5CH2–alkylated product [9:8 ≃ (100:1)]. This ruled out intramolecular phosphoryl ester transfer as a mechanism for the reaction.
Fig. 3.
Intramolecular phosphoryl ester transfer is not involved.
Mitsunobu His N(π)–alkylation can accommodate spatial differences between the pThr and His* residues
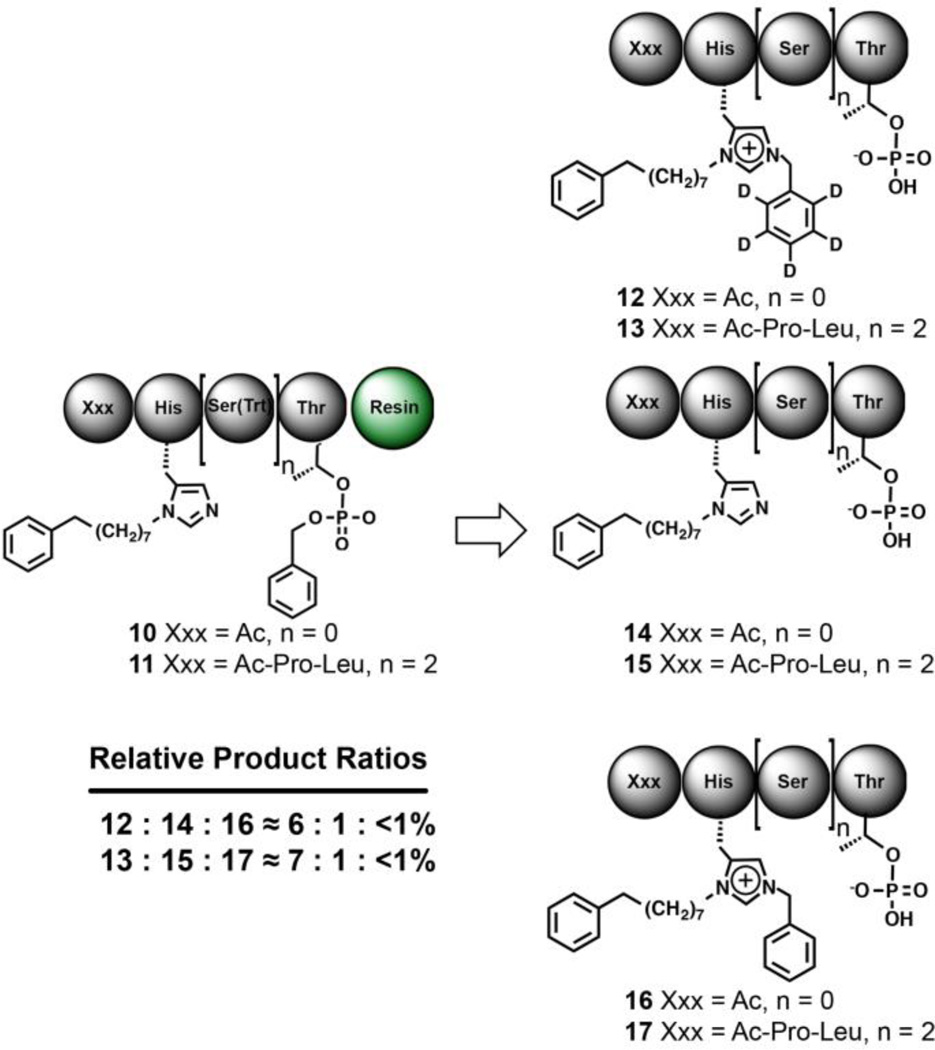
We repeated the Mitsunobu reactions using C6D5CH2OH as the alcohol source, but on a dipeptide lacking an interspaced Ser residue between the pThr and His* residues (10). We also performed the experiment on a peptide having an additional Ser residue between the His* and the pThr (11) (Fig. 4). The LC–MS analysis of the product mixtures revealed a predominant presence of the C6D5CH2–containing species 12 and 13, respectively, with a minor amount of the non-alkylated species 14 and 15, respectively. The relative ratios of alkylated to non-alkylated species were approximately 6:1 and 7:1 (Fig. 4). Less than 1% of the alkylated non-deuterated benzyl-containing products 16 and 17 were detected. This provided another support that intramolecular ester transfer does not occur significantly. It also indicates that N(π)-alkylation is compatible with differential spacing between the His* and pThr residues.
Fig. 4.
Mitsunobu His N(π)–alkylation can accommodate spatial differences between the pThr and His* residues.
Mitsunobu His N(π)–alkylation can be promoted by acidic residues other than pThr
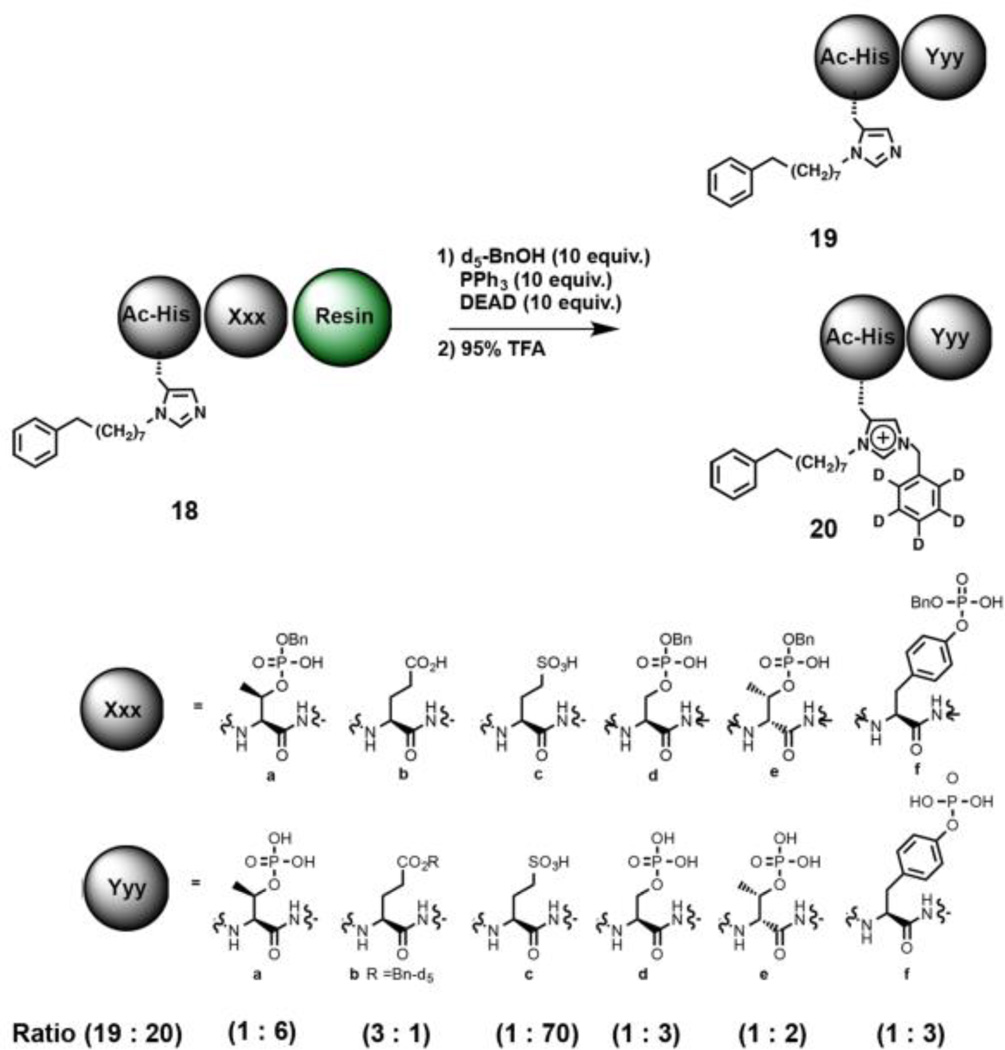
The experiments outlined above indicate that the N(π)–alkylation reactions occur by an intramolecular process involving the acidic phosphoryl hydroxyl of the pThr residue, but that alkyl transfer of a phosphoryl ester group is not involved. We wondered whether proximal acidic residues other than pThr could promote N(π)–alkylation. In order to examine this possibility, we employed dipeptide amide resins of the form “Ac-His*-Xxx” 18, where Xxx is an acidic residue. As we had done previously, we utilized C6D5CH2OH as the Mitsunobu alcohol source and measured the ratios of non-N(π)-alkylated to N(π)–alkylated products (Fig. 5, 19 and 20, respectively and Supplemental Information Table S6). We found that for the pThr-containing peptide (18a) the ratio of 19a to 20a was approximately 1:6 (Supplemental Information Table S6, Experiment 6.1). With Xxx = Glu (18b), two products were observed that contained a C6D5CH2– residue. These arose from esterification of the Glu residue with no N(π)–alkylation (19b) and N(π)–alkylation without Glu esterification (20b) (Supplemental Information Table 6, Experiment 6.2). The assignment of structure was based on matching HPLC retention times with authentic peptides synthesized by independent means. The ratio of the non-N(π)–alkylated to N(π)–alkylated products (19b to 20b) was found to be 3:1, reflecting an 18-fold decrease in N(π)–alkylation relative to pThr. When we replaced the pThr residue with sulfonic acid-containing homocysteric acid (18c), the ratio of the non-alkylated to N(π)–alkylated products (19c to 20c) was approximately 1:70 (Supporting Information Table 6, Experiment 6.3), indicating significantly enhanced N(π)–alkylation. The data show that relative to pThr (pKa ≈ 1.4), the degree of N(π)–alkylation decreased in the presence of a carboxylic acid (pKa ≈ 4.4) and significantly increased with a sulfonic acid (pKa ≈ −2.3). Importantly, these experiments also show that N(π)–alkylation can be facilitated by acidic residues other than pThr, which suggests these methods will tolerate a variety of substrates.
Fig. 5.
Mitsunobu His N(π)–alkylation can be promoted by acidic residues other than pThr.
When we replaced the pThr residue with pSer or D-pThr mono-benzyl esters (18d and 18e, respectively), we found that in both cases, the relative ratio of the N(π)–non-alkylated to N(π)–alkylated products (19d : 20d, 1:3 and 19e : 20e, 1:2, respectively) was less than with pThr (1:6 ratio). Replacing the pThr residue with pTyr (mono-benzyl phosphoryl ester) (18f) was also well-tolerated [the ratio of N(π)–non-alkylated to N(π)–alkylated peptide (19f : 20f) being 1:3].
His N(π), N(π)–bis-alkylation inhibits Mitsunobu esterification of a proximal pThr((OBn)(OH) residue
Very little product was detected that resulted from both His* N(π)-alkylation and pThr hydroxyl esterification, even though a large excess of reagent was used. We wondered whether the presence of His* N(π)-alkyl cationic residues could inhibit Mitsunobu-type phosphoryl esterification of a proximal pThr(OBn)(OH) residue. To examine this possibility, we synthesized the N-Fmoc His reagent having –(CH2)7Phe and –CH2CH2O(tBu) groups at the N(π)- and N(π)-positions, respectively (Supporting Information SI-5). We then prepared the resin-bound peptide, which contains the His residue in its bis-alkylated form (Supporting Information SI-13) and subjected the peptide to reaction with a large excess of Mitsunobu reagents and n-pent-4-en-1-ol (10 equivalents of each reagent). We found that the relative amounts of peptide having the pThr residue in its free and mono-esterified forms (Supporting Information SI-14: SI-15, respectively) was approximately 15:1. We had previously shown by performing Mitsunobu reactions on the same pentapeptide, but with the His residue bearing a single N(π)-trityl group, that efficient pThr phosphoryl esterification occurred.7, 12 The ability of N(π)-alkyl-His* to inhibit pThr phosphoryl esterification may arise by intramolecular association of the His imidazolium cation with the anionic pThr phosphoryl hydroxyl, which could present steric or electronic barriers to Mitsunobu esterification.
Potential mechanistic basis for intramolecular pThr facilitated Mitsunobu N(π)–alkylation
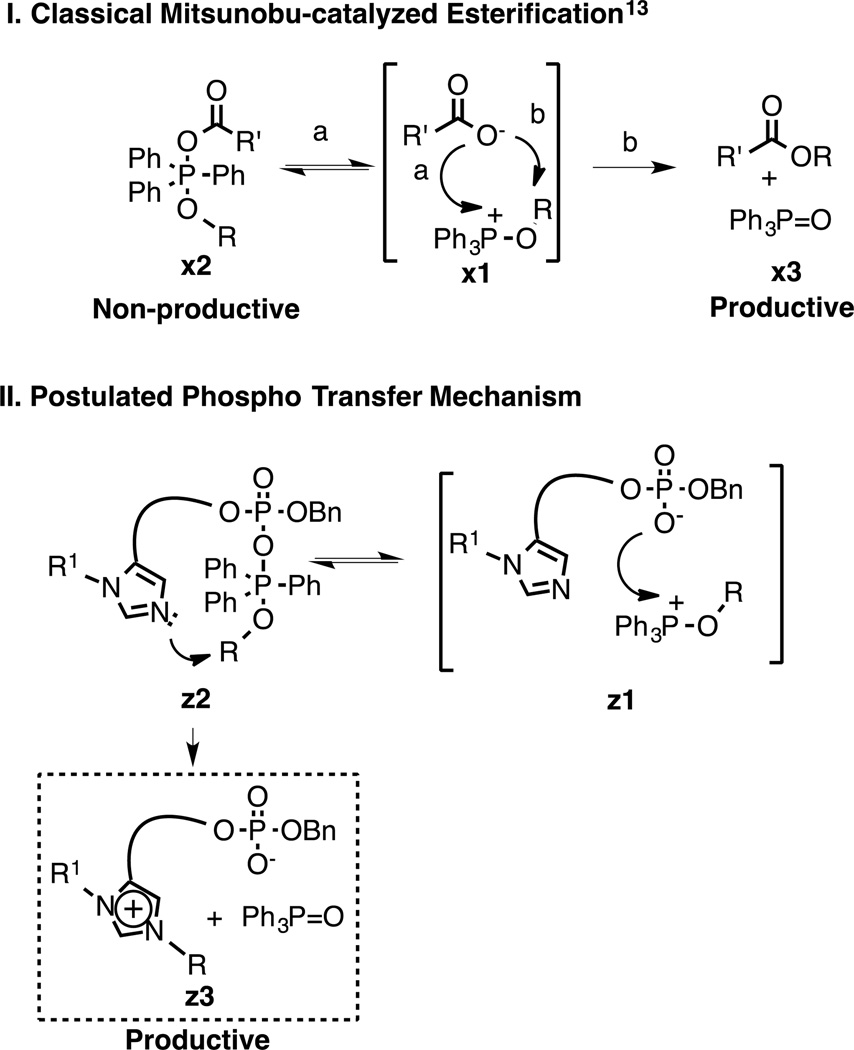
The results above provide significant insights into this unusual Mitsunobu pathway. The formation of alkoxyphosphonium carboxylate salts x1 are well established in the conventional Mitsunobu reaction on carboxylic acids (Fig. 6-I).13 Such species can proceed forward by one of two mechanisms. In one mechanism (pathway a), collapse of the salt yields species x2. However, these are non-productive intermediates that dissociate back to the original salt x1. On the other hand, nucleophilic attack of the carboxylate oxygen onto the phosphonium alkoxy group of x1 (pathway b) is productive and yields the desired ester products x3 (Fig. 6-I).14–16 Data from our current series of pThr-containing substrates suggest the formation of the intermediate salts z1 (Fig. 6-II), which are analogous to x1 in the classical Mitsunobu esterification (above), is followed by reversible collapse to the species z2. However, in contrast to the course of classical Mitsunobu transformations, this is a productive pathway. Subsequent irreversible, intramolecular attack of the imidazole N(π) moiety onto the alkoxy methylene of z2 provides the N(π)-alkylated species z3, which is observed nearly exclusively. Whether intermediate z1 proceeds by charge-driven collapse to the neutral pentavalent z2 and then onto the N(π)-alkylated product q1, would depend on the geometry of the complex and the nucleophilicity of the phosphoryloxy anion. Our data showing that the ratio of the N(π)-alkylated to non-alkylated product increases with decreasing pKa values (Fig. 6), is consistent with this latter mechanism, given that nucleophilicity generally mirrors basicity in aprotic solvents.17
Fig. 6.
Postulated mechanism for observed pThr-dependent Mitsunobu N(π)-alkylation.
Conclusion
Despite the potential utility of His residues with selective N(π), N(π)-bis-alkylated imidazole rings, the use of these agents in medicinal peptides has been limited by their synthetic intractability. The Mitsunobu-type reactions reported here provide the first general access to these compounds. Our results suggest that an intermediate derived from the collapse of an alkoxyphosphonium salt, which is generally associated with a non-productive pathway in conventional Mitsunobu reactions, may constitute the critical electrophile in route to product formation. In any event, this chemistry provides facile access to unusual bifurcated amino acids and it does so on solid-phase with N(π)–structural diversification occurring immediately prior to resin cleavage. This is particularly amenable to preparing libraries of His-functionalized peptides. The requirement of participating acidic residues to be proximal to target His* residue adds an element of sequence selectivity, and renders this methodology to be applicable to the synthesis of complex peptides and, perhaps, even proteins. Moreover, the resulting charged-masked zwiterionic phosphopeptides exhibit enhanced cellular permeability, which will facilitate addressing intracellular targets. Ready access to cationic bifurcated His residues can open the door to exploring new classes of peptides and peptide mimetics.
Supplementary Material
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, Center for Cancer Research and the National Cancer Institute, National Institutes of Health.
Footnotes
Electronic Supporting Information (ESI) available: Experimental details and NMR data for select compounds. See DOI: 10.1039/b000000x/
Notes and references
- 1.Murugan RN, Jacob B, Kim E-H, Ahn M, Sohn H, Seo J-H, Cheong C, Hyun J-K, Lee KS, Shin SY, Bang JK. Bioorg. Med. Chem. Lett. 2013;23:4633. doi: 10.1016/j.bmcl.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Ahn M, Murugan RN, Jacob B, Hyun J-K, Cheong C, Hwang E, Park H-N, Seo J-H, Srinivasrao G, Lee KS, Shin SY, Bang JK. Eur. J. Med. Chem. 2013;68:10. doi: 10.1016/j.ejmech.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Bewley CA, Faulkner DJ. J. Org. Chem. 1994;59:4849. [Google Scholar]
- 4.Matsunaga S, Fusetani N. J. Org. Chem. 1995;60:1177. [Google Scholar]
- 5.Schmidt EW, Bewley CA, Faulkner DJ. J. Org. Chem. 1998;63:1254. [Google Scholar]
- 6.Espiritu RA, Matsumori N, Murata M, Nishimura S, Kakeya H, Matsunaga S, Yoshida M. Biochemistry. 2013;52:2410. doi: 10.1021/bi4000854. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Park J-E, Qian W-J, Lim D, Graber M, Berg T, Yaffe MB, Lee KS, Burke TR., Jr Nat. Chem. Biol. 2011;7:595. doi: 10.1038/nchembio.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian W-J, Park J-E, Lim D, Lai CC, Kelley JA, Park S-Y, Lee KW, Yaffe MB, Lee KS, Burke TR. Biopolymers Pept. Sci. 2014;102:444. doi: 10.1002/bip.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allentoff AJ, Mandiyan S, Liang H, Yuryev A, Vlattas I, Duelfer T, Sytwu I-I, Wennogle LP. Cell Biochem. Biophys. 1999;31:129. doi: 10.1007/BF02738168. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlmann E, Himmler S, Giebelhaus H, Wasserscheid P. Green Chem. 2007;9:233. [Google Scholar]
- 11.Vijayaraghavan R, Surianarayanan M, Armel V, MacFarlane DR, Sridhar VP. Chem. Commun. (Cambridge U. K.) 2009 doi: 10.1039/b911568d. [DOI] [PubMed] [Google Scholar]
- 12.Qian W, Liu F, Burke TR. J. Org. Chem. 2011;76:8885. doi: 10.1021/jo201599c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Chem. Rev. 2009;109:2551. doi: 10.1021/cr800278z. [DOI] [PubMed] [Google Scholar]
- 14.Hughes DL, Reamer RA, Bergan JJ, Grabowski EJJ. J. Am. Chem. Soc. 1988;110:6487. [Google Scholar]
- 15.Camp D, Jenkins ID. J. Org. Chem. 1989;54:3049–3054. [Google Scholar]
- 16.Schenk S, Weston J, Anders E. J. Am. Chem. Soc. 2005;127:12566. doi: 10.1021/ja052362i. [DOI] [PubMed] [Google Scholar]
- 17.Uggerud E. Chem. Eur. J. 2006;12:1127. doi: 10.1002/chem.200500639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.