Abstract
Poor executive function (EF; pre-frontal cognitive control processes governing goal-directed behavior) and elevated hedonic hunger (i.e., preoccupation with palatable foods in the absence of physiological hunger) are theoretical risk and maintenance factors for binge eating (BE) distinct from general obesity. Recent theoretical models posit that dysregulated behavior such as BE may result from a combination of elevated appetitive drive (e.g., hedonic hunger) and decreased EF (e.g., inhibitory control and delayed discounting). The present study sought to test this model in distinguishing BE from general obesity by examining the independent and interactive associations of EF and hedonic hunger with BE group status (i.e., odds of categorization in BE group versus non-BE group). Treatment-seeking overweight and obese women with BE (n = 31) and without BE (OW group; n = 43) were assessed on measures of hedonic hunger and EF (inhibitory control and delay discounting). Elevated hedonic hunger increased the likelihood of categorization in the BE group, regardless of EF. When hedonic hunger was low, poor EF increased the likelihood of categorization in the BE group. Results indicate that the interplay of increased appetitive drives and decreased cognitive function may distinguish BE from overweight/obesity. Future longitudinal investigations of the combinatory effect of hedonic hunger and EF in increasing risk for developing BE are warranted, and may inform future treatment development to target these factors.
Keywords: binge eating, hedonic hunger, executive function, delayed discounting, inhibitory control
INTRODUCTION AND AIMS
Binge eating (BE) is defined as consuming large amounts of food within a discrete time period, accompanied by a sense of loss of control (LOC). BE is a key symptom of binge eating disorder (BED), and is linked to serious psychological and physical co-morbidity (Latner, Hildebrandt, Rosewall, Chisholm, & Hayashi, 2007) and impaired social functioning (Rieger, Wilfley, Stein, Marino, & Crow, 2005; Robinson et al., 2006). A majority of those with BED are overweight or obese (Hudson, Hiripi, Pope Jr, & Kessler, 2007); however, individuals with BED demonstrate increased functional impairment compared to non-binge eating overweight and obese peers with similar BMIs (Wilfley, Wilson, & Agras, 2003).
Although the current diagnostic criteria for BED require that an individual's binge episodes consist of an “objectively large” amount of food, mounting evidence indicates that the presence of LOC is the primary indicator of BE severity and associated psychosocial impairment. Indeed, empirical research has shown that the presence and severity of LOC eating (Colles, Dixon, & O'Brien, 2008), and frequency of LOC eating episodes (Latner et al., 2007; Picot & Lilenfeld, 2003), are closely related to eating disorder psychopathology, even when the amount consumed in a given episode fails to reach an objectively large size. Individuals with BED or sub-threshold BED appear to be similar in terms of psychosocial and quality of life impairment, weight outcomes, and psychological distress (Mond, Latner, Hay, Owen, & Rodgers, 2010). Thus, for the purposes of the current study, we refer to BE pathology as recurrent episodes LOC eating, including objective and subjective binge sizes.
Despite the fact that BE and BED are much more common among those who are overweight and obese, most overweight and obese individuals do not endorse BE pathology (Hsu et al., 2002). Thus, research has begun to examine variables involved in the development and maintenance of BE as distinct from those associated with being overweight or obese. Deficits in cognitive function have been investigated as possible risk and maintenance factors for BE—particularly deficits in executive function (EF), which represent higher-order control processes that govern goal-directed behavior (Van den Eynde et al., 2011). A large body of evidence has linked EF deficits with obesity in the absence of BE (Smith, Hay, Campbell, & Trollor, 2011); however, research has also suggested that BE may present with distinct and more pronounced cognitive deficits (Duchesne et al., 2010; Manasse et al., 2014). Deficits along two dimensions of EF show compelling theoretical and empirical associations with BED: 1) inhibitory control, manifested as reduced inhibition of prepotent responses (Duchesne et al., 2010; Manasse et al., 2014; Svaldi, Naumann, Trentowska, & Schmitz, 2014), and 2) monetary delay discounting, or the preference for immediate, smaller reward over delayed, larger reward (Manasse et al., in press; Manwaring, Green, Myerson, Strube, & Wilfley, 2011). Although preliminary studies have implicated deficits in these EF domains in BED, the evidence remains mixed (Van den Eynde et al., 2011). For example, three (Duchesne et al., 2010; Mobbs, Iglesias, Golay, & Van der Linden, 2011; Svaldi et al., 2014) of six total studies have detected inhibitory control deficits in individuals with binge eating compared to weight-matched controls. Similarly, one study found no differences in delay discounting between obese BED and non-BED subjects, (Davis, Patte, Curtis, & Reid, 2010), although another reported steeper discounting (i.e., choosing smaller, short-term rewards in preference to larger, long-term rewards) in obese BED versus non-BED subjects (Manasse et al., in press). Although inhibitory control and delay discounting reflect dimensions of impulsivity broadly, research suggests that that inhibitory control measured via tasks such as the Stroop or stop-signal paradigms are classified as measures of “impulsive inhibition” (i.e., late-stage inhibition of a prepotent response), whereas delayed discounting may be classified as a measure of “impulsive decision-making” (i.e., deliberate choice of a smaller short-term over a larger, long-term reward) (Reynolds, Ortengren, Richards, & de Wit, 2006). Thus, these distinct constructs that theoretically underlie impulsive behavior each warrant investigation as variables that play a role in the maintenance of BE.
However, one possible reason for inconsistent results across studies is a failure to consider potential moderating variables. In fact, an influential theory of self-control posits that poor EF interacts with increased appetitive drive to predict dysregulated behavior, such as BE or alcohol use (Hofmann, Friese, & Strack, 2009; Hofmann, Rauch, & Gawronski, 2007). According to this theory, EF processes are necessary to override persistent and difficult-to-control urges or impulses. Thus, when appetitive desire is high, a well-functioning EF system may be essential to prevent dysregulated behavior from occurring; however, if appetitive desire is low, only minimal EF capabilities may be necessary to regulate behavior (e.g., overeating) (Nederkoorn, Houben, Hofmann, Roefs, & Jansen, 2010; Rollins, Dearing, & Epstein, 2010).
One common conceptualization of increased appetitive desire for food is “hedonic hunger,” which refers to a preoccupation with highly palatable food when not physically hungry (Lowe & Butryn, 2007). Overweight individuals with BE may be distinguished from overweight counterparts without BE by a combination of elevated hedonic hunger and reduced EF, and emerging evidence shows that this combination predicts palatable food intake in overweight and obese samples without BE when they are energy replete (Appelhans et al., 2011). One study found that hedonic hunger, as measured by the Power of Food Scale (Cappelleri et al., 2009; Lowe et al., 2009), is positively related to BE frequency in those with anorexia nervosa or bulimia nervosa (Witt & Lowe, 2014), but no studies have directly compared hedonic hunger between obese samples with and without BED. Additionally, no studies have tested the interacting effect of hedonic hunger and executive dysfunction on the presence of BE, over and above overweight and obesity.
As such, the current study sought to test Hofmann's model of self-control in predicting the presence of BE in an overweight and obese sample. First, we hypothesized that elevated hedonic hunger, poorer inhibitory control and increased delayed discounting would each be independently associated with BE status. We additionally hypothesized that hedonic hunger would moderate the association of executive dysfunction (specifically, inhibitory control and delayed discounting) with membership in BE and non-BE groups. Specifically, we hypothesized that EF deficits would be most strongly associated with the presence of BE at the highest levels of hedonic hunger.
METHODS
Participants and procedure
The current study included overweight and obese (BMI = 26-50 kg/m2) females who endorsed BE in the preceding three months (BE group) and a group of overweight and obese women without any past or present BE (OW group). Participants were seeking treatment for weight loss and/or BE. All participants provided informed consent.
Participants in the OW group (n = 43) met the following criteria: a) no LOC eating episodes in the past 3 months and b) no current or past history of BE or eating disorder (e.g., anorexia nervosa, bulimia nervosa, BED). Participants in the BE group (BE; n = 31) must have endorsed an average of at least one subjective or objective binge episode per week over the past three months (12 total binge episodes over the past 3 months), and must not have met criteria for bulimia nervosa. We chose to include those with subjectively large binge episodes (i.e., subthreshold BED) given evidence that neurocognitive factors (Manasse et al., 2014) and functional impairment associated with binge eating is most associated with presence of LOC, rather than size of binge episodes (Latner et al., 2007; Mond et al., 2006).
Recruitment took place over the course of one year (June 2013 - May 2014). A neuropsychological battery and BE screening were included as part of a baseline assessment prior to entry in either intervention. A licensed clinical psychologist supervised all neuropsychological assessments. Order of administration of tasks was randomly generated for each participant to control for order effects. Participants received free treatment from either of the trials and also received $50 for completion of the assessments. The study protocol was approved by Drexel University's Institutional Review Board.
Measures
Binge Eating
The Eating Disorders Examination (EDE) version 16D is the gold-standard semi-structured interview for assessing for BE (Grilo, Masheb, Lozano-Blanco, & Barry, 2004; Wilfley, Schwartz, Spurrell, & Fairburn, 1997). The Overeating section (“Questions for Identifying Bulimic Episodes and Other Episodes of Overeating”) was administered to all participants to examine for presence of LOC eating and BE. The EDE has high inter-rater reliability and test-retest reliability (Rizvi, Peterson, Crow, & Agras, 2000) and good internal consistency (Cooper, Cooper, & Fairburn, 1989).
IQ
Wechsler Test of Adult Reading (WTAR) (Wechsler, 2001): The WTAR is a single-word oral reading test used to estimate verbal intelligence; scores were converted to Full Scale IQ estimates. The WTAR has strong correlations (.70–.80) with WAIS-III FSIQ scores for a wide age range of WTAR scores (Wechsler, 2001).
Inhibitory Control
The Delis Kaplan Executive Functioning System Color-Word Interference Task (D-KEFS) (Delis, Kaplan, & Kramer, 2001): Color-Word Interference is a modified Stroop task assessing response inhibition in the presence of distractors. This modified Stroop task contained four trials: 1) Participants were presented with blocks of color and were told to name the colors; 2) Participants were told to read words; 3) Color names were written in dissonant color ink, and participants were told to name the ink color; and 4) Same instructions, except if a word is in a box, participants were to read the word. Inhibitory control was operationalized as the raw total number of errors committed on the task across trials.
Delayed Discounting
The Delayed Discounting Task (Robles & Vargas, 2007) is a widely-used computerized monetary delayed discounting task. Participants are asked to choose between a smaller amount of hypothetical money that they would receive sooner and a larger amount of money they would receive later. The “indifference point” is the point at which the participant chooses the more immediate, smaller amount over the delayed, larger amount. Area-under-the-curve (AUC) was calculated from indifference points across trials (Myerson, Green, & Warusawitharana, 2001).
Hedonic Hunger
The Power of Food Scale (PFS) (Lowe et al., 2009) is a self-report measure which assesses the extent to which highly palatable foods influence a person's food-related thoughts and feelings when not physically hungry. The PFS has adequate internal and test-retest reliability and convergent and discriminant validity (Lowe et al., 2009). The PFS has been used in several previous investigations of hedonic hunger and its association with dysregulation of food intake—both among obese populations (Appelhans et al., 2011; Cappelleri et al., 2009; Schultes, Ernst, Wilms, Thurnheer, & Hallschmid, 2010) and those with bulimic-spectrum eating pathology (Witt & Lowe, 2014).
Statistical Analysis
Two separate logistic regressions (dependent variable: categorization in either OW or BE group) were completed to examine main and interaction effects of hedonic hunger with both inhibitory control and delayed discounting on BE status. Age was included as a covariate to control for differences between groups. We conducted two separate logistic regression models, consistent with testing Hoffman's model (i.e., the interaction between hedonic drive and executive control in predicting dysregulated behavior) for two distinct facets of impulsivity. For both models, the covariates, EF variable (inhibitory control or delayed discounting) and PFS were entered in Step 1 of the regression. In Step 2, the interaction term was added to examine the additive effect of the interaction term to the model. Statistical Package for the Social Sciences (SPSS) v. 20.0 (IBM, 2013) was used to analyze data.
RESULTS
Descriptive statistics
Sample demographics and clinical characteristics are presented in Table 1. As expected, the BE group endorsed significantly more eating pathology as measured by the EDE than the OW group. In our sample, the association between delayed discounting and inhibitory control was small (r = .10, p = .44), indicating separable constructs, consistent with existing literature.
Table 1.
Sample descriptive and clinical characteristics by group
| BE Group (n=31) | OW Group (n=43) | t | p | Cohen's d | |
|---|---|---|---|---|---|
| Age (yrs) | 45.06 (14.86) | 51.09 (8.26) | 2.04 | < .05* | .50 |
| Objective binge episodesa | 10.97 (9.32) | -- | -- | -- | -- |
| Subjective binge episodesa | 5.74 (11.39) | -- | -- | -- | -- |
| Body Mass Index (kg/m2) | 36.84 (7.97) | 37.85 (6.27) | .02 | .61 | .14 |
| IQ | 111.74 (12.31) | 112.63 (10.52) | .33 | .54 | .08 |
| BDI-II | 17.94 (10.17) | 7.58 (6.78) | 5.26 | < .01** | 1.20 |
| EDE Restraint | 1.76 (1.34) | 1.45 (1.22) | .90 | .37 | .24 |
| EDE Eating Concern | 2.52 (1.35) | 1.04 (.97) | 4.65 | < .01** | 1.26 |
| EDE Shape Concern | 4.06 (1.48) | 3.54 (1.20) | 1.42 | .16 | .39 |
| EDE Weight Concern | 3.80 (1.17) | 3.04 (.82) | 2.79 | .01* | .75 |
| EDE Global Score | 3.07 (1.07) | 2.27 (.75) | 3.1 | < .01** | .87 |
p < .05
p <.01
in the past 28 days
Outcome analyses
Both of the overall regression models with the interaction terms were statistically significant (p < .01), and Hosmer and Lemeshow's goodness-of-fit tests were statistically non-significant (ps > .05), indicating appropriate model fit. There were strong associations of hedonic hunger (Wald χ2 = 10.38, p < .01; OR = 5.71) and delayed discounting (Wald χ2 = 4.88, p = .03; OR = .33) with the presence of BE (because a higher DDT score indicates less monetary discounting, the odds ratio of < 1 indicates that steeper discounting is associated with membership in the BE group). However, the association of inhibitory control with BE status was small (Wald χ2 = 2.17, p = .14; OR = 1.62). The interaction between hedonic hunger and inhibitory control was statistically significant (Wald χ2 = 4.03, p < .05, OR = .42) and the interaction between hedonic hunger and delayed discounting trended towards statistical significance (Wald χ2 = 3.58, p = .06, OR = 12.10).
To test for simple effects, we conducted four separate logistic regression models to detect main effects of inhibitory control and delayed discounting at high and low levels of hedonic hunger (See Figures 1 and 2). For these analyses, we divided hedonic hunger into “higher” and “lower” levels using a median split. At higher levels of hedonic hunger, the effect of delayed discounting was small and statistically non-significant (Wald χ2 = .78, p = .38, OR = .45). At lower levels of hedonic hunger, the effect of delayed discounting borderline trended towards significance, with a large effect size (Wald χ2 = 2.69, p = .10, OR = .13). At higher levels of hedonic hunger, inhibitory control had a small and statistically non-significant negative relation with the likelihood of categorization in the BE group (Wald χ2 = 2.21, p = .14, OR = .23) At lower levels of hedonic hunger, worse inhibitory control was significantly associated with increased likelihood of categorization in the BE group (Wald χ2 = 4.45, p = .04, OR = 2.55). Figures 1 and 2 present the mean predicted probabilities of categorization in the BE group with dichotomized PFS and EF variables.
Figure 1.
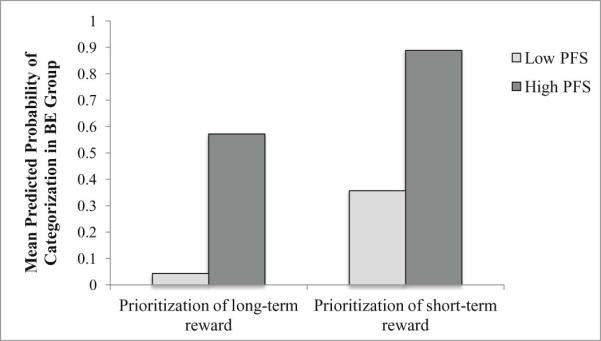
The moderating effect of hedonic hunger on the ability of delayed discounting to predict the presence of binge eating.
PFS = Power of Food Scale (hedonic hunger)
The figure provides a representation of the mean predicted probabilities of categorization in the BE group (within a logistic regression model conducted with dichotomized variables) in order to simplify graphical presentation.
Figure 2.
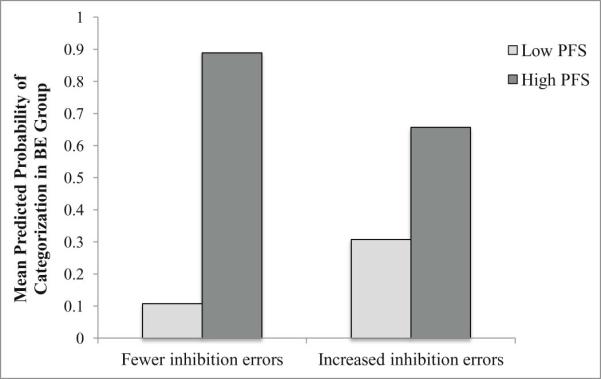
The moderating effect of hedonic hunger on the ability of inhibitory control to predict the presence of binge eating.
PFS = Power of Food Scale (hedonic hunger)
The figure provides a representation of the mean predicted probabilities of categorization in the BE group (within a logistic regression model conducted with dichotomized variables) in order to simplify graphical presentation.
DISCUSSION
This study examined the associations of hedonic hunger and two measures of EF (inhibitory control and delay discounting) with BE status among a sample of overweight and obese women. Results demonstrated a strong positive association between hedonic hunger and the probability of being categorized in the BE group. When hedonic hunger was high, likelihood of categorization in the BE group was substantially increased, regardless of EF. Steeper monetary discounting and worse inhibitory control were associated with increased probability of being categorized in the BE group, but only among those with lower levels of hedonic hunger. However, the overall interaction between delayed discounting and hedonic hunger in association with BE status was significant only at trend level, as was the simple effect of delayed discounting in those with lower levels of hedonic hunger, so these results should be interpreted with caution.
Results are partially consistent with those which would be predicted by Hofmann's model, and highlight the importance of both hedonic drives to eat, and executive abilities to regulate and override such drives, in predicting BE status. Overweight and obese individuals who experience strong appetitive motivation to consume highly palatable foods are more likely to endorse BE, regardless of their executive abilities. This finding is consistent with a previous observation that hedonic hunger distinguishes those with BE pathology from their weight-matched, non-BE peers (Witt & Lowe, 2014). Convergent neuroimaging evidence suggests that although overweight and obese individuals appear to exhibit increased response to food stimuli in reward-related brain region, individuals with BE may display even greater activation in reward-related brain regions in response to the presence of highly palatable foods when compared to weight-matched peers (Schienle, Schäfer, Hermann, & Vaitl, 2009). Differential reward response may explain elevations in appetitive desire for consuming palatable foods in the absence of physiological hunger, above and beyond elevated appetitive desire in those with obesity in the absence of BE. It is possible that increased food reward sensitivity and elevated hedonic hunger contribute to a drive to overconsume highly palatable foods when available. These elevated drives may promote feelings of LOC associated with BE.
Although hedonic hunger showed the strongest association with membership in the BE group, results from this study also highlight the potential role of EF in the maintenance of BE, particularly when hedonic hunger is low. The trend-level interaction between delayed discounting and hedonic hunger in association with the presence of BE indicates that these two constructs should continue to be examined as potential combinatorial risk and/or maintenance factors for BE. A tendency to prioritize immediate reward (e.g., the pleasant taste of a binge food) may further exacerbate a susceptibility to the hedonically-rewarding properties of food, leading to LOC eating in the presence of such rewarding stimuli. Even among those who are less sensitive to the rewarding properties of palatable foods, the immediate reward of consuming a palatable food (or distracting from a negative affective state) may be more compelling than the delayed satisfaction associated with abstaining from binge eating and reducing shame and guilt overall in the long term. However, it must be noted that both the overall interaction and simple effect of delayed discounting in those with low hedonic hunger were significant only at trend level. It is possible that while delayed discounting and hedonic hunger may each be important independent predictors of BE, the interaction between the two variables may not be as robust as each variable's independent effect.
The significant interaction between inhibitory control and hedonic hunger indicated that, for those with lower hedonic hunger, more inhibitory control (commission) errors increased likelihood of categorization in the BE group. This result indicates that inhibitory control may play a role in the maintenance of binge eating, especially when hedonic hunger is not elevated. Results indicated that when hedonic hunger is high, risk for binge eating is significantly elevated, regardless of inhibitory control capacity. Interestingly, previous literature has implicated inhibitory control deficits in both general overeating and obesity (Batterink, Yokum, & Stice, 2010; Loeber et al., 2011; Nederkoorn, Smulders, Havermans, Roefs, & Jansen, 2006). Other results suggest that inhibitory control deficits that distinguish individuals with BED from their weight-matched counterparts are food-specific (Hege et al., 2014; Svaldi et al., 2014), and that no differences exist on general measures of inhibition (Wu et al., 2013), although findings remained mixed (Van den Eynde et al., 2011). We did not detect a main effect of inhibitory control in association with BE status in our sample. Mixed results regarding inhibitory control deficits in those with BE may be attributable to use of different measures of inhibitory control across studies. These conflicting results also highlight the importance of examining potential moderating variables (such as hedonic hunger) which may explain how inhibitory control (general and food-specific) deficits play differential roles in risk for obesity versus BE. Given the robust effect size of the inhibitory control-hedonic hunger interaction, it appears that impulsive inhibition, rather than impulsive decision-making as measured by the DDT, may be especially important in its relation with hedonic drive to predict BE behavior. Future research should aim to distinguish between and examine the role of these distinct constructs in the maintenance of BE.
Interpretation of the present results should be made in the context of important limitations. First, the sample sizes were relatively small, and the delayed discounting-hedonic hunger interaction only trended towards statistical significance. Simple effects analyses were also especially underpowered, as they were conducted with subsets (higher and lower hedonic hunger) of the sample. Thus, although they provide a promising direction for future research, our findings should be interpreted cautiously. Secondly, our sample consisted of treatment-seeking women, limiting our ability to generalize to males and non-treatment-seeking individuals. Additionally, while the variables examined were concurrent predictors of BE status, the design was cross-sectional. Thus, no temporal relations can be inferred. Future research should examine hedonic hunger, inhibitory control, and delay discounting as prospective predictors, differentiating between risk for onset of overweight/obesity with and without BE. Additionally, we utilized a control sample completely absent of LOC eating, and a BE sample consisting of a mixed sample of those who met full criteria for BED and those who did not, limiting our ability to generalize results to full threshold BED groups and overweight groups that may have LOC pathology but do not meet BED frequency criteria. Future research should aim to include and differentiate full, sub-threshold, and control groups. Finally, neuroimaging research should investigate the underlying neural substrates of executive deficits in BE, as well as how they might interact with hedonic reward to promote overconsumption during binge episodes.
With replication, these findings could have important implications for the treatment of overweight and obese individuals with BE. For example, existing interventions could be adapted to address the intense cravings for highly palatable foods. Acceptance-based behavioral interventions may be indicated, due to preliminary evidence that strategies to accept, and gain psychological distance from, intense food cravings are effective for individuals with elevated hedonic hunger (Baer, Fischer, & Huss, 2005; Forman, Hoffman, Juarascio, Butryn, & Herbert, 2013; Forman et al., 2007). However, no studies have targeted hedonic hunger directly in the treatment of BE.
Results from the current pilot study indicate that further examination of the combinatorial associations of hedonic hunger and poor EF with BE is warranted; future research will benefit from replication in order to provide directions for treatment development that could be tailored to specific risk and maintenance factors for BE.
Highlights.
We examined the role of executive function and hedonic hunger in predicting binge eating status
Treatment-seeking overweight and obese women (with and without binge eating) were assessed
Delayed discounting and hedonic hunger were independently associated with binge eating status
Executive function also interacted with hedonic hunger to predict binge eating status
Acknowledgements
This study was funded by a grant from the National Institute of Diabetes & Digestive & Kidney Diseases (R01DK095069) awarded to Dr. Forman, and two grants from the American Psychological Association of Graduate Students and Psi Chi, respectively, to Ms. Manasse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity. 2011;19(11):2175–2182. doi: 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA, Fischer S, Huss DB. Mindfulness and acceptance in the treatment of disordered eating. Journal of rational-emotive and cognitive-behavior therapy. 2005;23(4):281–300. [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri JC, Bushmakin AG, Gerber RA, Leidy NK, Sexton CC, Karlsson J, Lowe MR. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: development and measurement properties. International Journal of Obesity. 2009;33(8):913–922. doi: 10.1038/ijo.2009.107. [DOI] [PubMed] [Google Scholar]
- Colles SL, Dixon JB, O'Brien PE. Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity. 2008;16(3):608–614. doi: 10.1038/oby.2007.99. [DOI] [PubMed] [Google Scholar]
- Cooper Z, Cooper PJ, Fairburn CG. The validity of the eating disorder examination and its subscales. Br J Psychiatry. 1989;154:807–812. doi: 10.1192/bjp.154.6.807. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system (D-KEFS): Psychological Corporation. 2001.
- Duchesne M, Mattos P, Appolinário JC, de Freitas SR, Coutinho G, Santos C, Coutinho W. Assessment of executive functions in obese individuals with binge eating disorder. Rev Bras Psiquiatr. 2010;32(4):381–388. doi: 10.1590/s1516-44462010000400011. [DOI] [PubMed] [Google Scholar]
- Forman EM, Hoffman KL, Juarascio AS, Butryn ML, Herbert JD. Comparison of acceptance-based and standard cognitive-based coping strategies for craving sweets in overweight and obese women. Eating behaviors. 2013;14(1):64–68. doi: 10.1016/j.eatbeh.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Forman EM, Hoffman KL, McGrath KB, Herbert JD, Brandsma LL, Lowe MR. A comparison of acceptance-and control-based strategies for coping with food cravings: An analog study. Behaviour research and therapy. 2007;45(10):2372–2386. doi: 10.1016/j.brat.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. Int J Eat Disord. 2004;35(1):80–85. doi: 10.1002/eat.10238. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- Hege M, Stingl K, Kullmann S, Schag K, Giel K, Zipfel S, Preissl H. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. International Journal of Obesity. 2014 doi: 10.1038/ijo.2014.99. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science. 2009;4(2):162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Rauch W, Gawronski B. And deplete us not into temptation: Automatic attitudes, dietary restraint, and self-regulatory resources as determinants of eating behavior. Journal of Experimental Social Psychology. 2007;43(3):497–504. [Google Scholar]
- Hsu LK, Mulliken B, McDonagh B, Krupa DS, Rand W, Fairburn CG, Shikora S. Binge eating disorder in extreme obesity. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002;26(10):1398–1403. doi: 10.1038/sj.ijo.0802081. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM . SPSS Statistics for Macintosh, Version 22.0. IBM Corp.; Armonk, NY: 2013. [Google Scholar]
- Latner JD, Hildebrandt T, Rosewall JK, Chisholm AM, Hayashi K. Loss of control over eating reflects eating disturbances and general psychopathology. Behaviour research and therapy. 2007;45(9):2203–2211. doi: 10.1016/j.brat.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, Kiefer F. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. International Journal of Obesity. 2011;36(10):1334–1339. doi: 10.1038/ijo.2011.184. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiology & behavior. 2007;91(4):432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Wallaert M. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53(1):114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Manasse SM, Forman EM, Ruocco AC, Butryn ML, Juarascio AS, Fitzpatrick KK. Do executive functioning deficits underpin binge eating disorder? An examination of overweight women with and without binge eating pathology. International Journal of Eating Disorders. doi: 10.1002/eat.22383. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, Juarascio AS, Forman EM, Berner LA, Butryn ML, Ruocco AC. Executive Functioning in Overweight Individuals with and without Loss - of - Control Eating. European Eating Disorders Review. 2014;22(5):373–377. doi: 10.1002/erv.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE. Discounting of various types of rewards by women with and without binge eating disorder: Evidence for general rather than specific differences. The Psychological Record. 2011;61(4):561. doi: 10.1007/bf03395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;57(1):263–271. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Mond J, Hay P, Rodgers B, Owen C, Crosby R, Mitchell J. Use of extreme weight control behaviors with and without binge eating in a community sample: Implications for the classification of bulimic - type eating disorders. International Journal of Eating Disorders. 2006;39(4):294–302. doi: 10.1002/eat.20265. [DOI] [PubMed] [Google Scholar]
- Mond J, Latner J, Hay P, Owen C, Rodgers B. Objective and subjective bulimic episodes in the classification of bulimic-type eating disorders: another nail in the coffin of a problematic distinction. Behaviour research and therapy. 2010;48(7):661–669. doi: 10.1016/j.brat.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the experimental analysis of behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Houben K, Hofmann W, Roefs A, Jansen A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychology. 2010;29(4):389. doi: 10.1037/a0019921. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Havermans RC, Roefs A, Jansen A. Impulsivity in obese women. Appetite. 2006;47(2):253–256. doi: 10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Picot AK, Lilenfeld LR. The relationship among binge severity, personality psychopathology, and body mass index. International Journal of Eating Disorders. 2003;34(1):98–107. doi: 10.1002/eat.10173. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and individual differences. 2006;40(2):305–315. [Google Scholar]
- Rieger E, Wilfley DE, Stein RI, Marino V, Crow SJ. A comparison of quality of life in obese individuals with and without binge eating disorder. International Journal of Eating Disorders. 2005;37(3):234–240. doi: 10.1002/eat.20101. [DOI] [PubMed] [Google Scholar]
- Rizvi SL, Peterson CB, Crow SJ, Agras WS. Test-retest reliability of the eating disorder examination. Int J Eat Disord. 2000;28(3):311–316. doi: 10.1002/1098-108x(200011)28:3<311::aid-eat8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Robinson S, Perkins S, Bauer S, Hammond N, Treasure J, Schmidt U. Aftercare intervention through text messaging in the treatment of bulimia nervosa—Feasibility pilot. International Journal of Eating Disorders. 2006;39(8):633–638. doi: 10.1002/eat.20272. [DOI] [PubMed] [Google Scholar]
- Robles E, Vargas PA. Functional parameters of delay discounting assessment tasks: order of presentation. Behavioural Processes. 2007;75(2):237–241. doi: 10.1016/j.beproc.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. 2010;55(3):420–425. doi: 10.1016/j.appet.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biological psychiatry. 2009;65(8):654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. The American journal of clinical nutrition. 2010;92(2):277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- Smith E, Hay P, Campbell L, Trollor J. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obesity Reviews. 2011;12(9):740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Naumann E, Trentowska M, Schmitz F. General and food - specific inhibitory deficits in binge eating disorder. International Journal of Eating Disorders. 2014 doi: 10.1002/eat.22260. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Guillaume S, Broadbent H, Stahl D, Campbell I, Schmidt U, Tchanturia K. Neurocognition in bulimic eating disorders: a systematic review. Acta Psychiatrica Scandinavica. 2011;124(2):120–140. doi: 10.1111/j.1600-0447.2011.01701.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- Wilfley DE, Schwartz MB, Spurrell EB, Fairburn CG. Assessing the specific psychopathology of binge eating disorder patients: interview or self-report? Behav Res Ther. 1997;35(12):1151–1159. [PubMed] [Google Scholar]
- Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. International Journal of Eating Disorders. 2003;34(S1):S96–S106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]
- Witt AA, Lowe MR. Hedonic hunger and binge eating among women with eating disorders. International Journal of Eating Disorders. 2014;47(3):273–280. doi: 10.1002/eat.22171. [DOI] [PubMed] [Google Scholar]
- Wu M, Giel KE, Skunde M, Schag K, Rudofsky G, Zwaan M, Friederich HC. Inhibitory control and decision making under risk in bulimia nervosa and binge - eating disorder. International Journal of Eating Disorders. 2013;46(7):721–728. doi: 10.1002/eat.22143. [DOI] [PubMed] [Google Scholar]


