Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a ubiquitous environmental toxicant found in consumer products that causes ovarian toxicity. Antral follicles are the functional ovarian units and must undergo growth, survival from atresia, and proper regulation of steroidogenesis to ovulate and produce hormones. Previous studies have determined that DEHP inhibits antral follicle growth and decreases estradiol levels in vitro; however, the mechanism by which DEHP elicits these effects is unknown. The present study tested the hypothesis that DEHP directly alters regulators of the cell cycle, apoptosis, and steroidogenesis to inhibit antral follicle functionality. Antral follicles from adult CD-1 mice were cultured with vehicle control or DEHP (1-100μg/ml) for 24-96 hr to establish the temporal effects of DEHP on the follicle. Following 24-96 hr of culture, antral follicles were subjected to gene expression analysis, and media were subjected to measurements of hormone levels. DEHP increased the mRNA levels of cyclin D2, cyclin dependent kinase 4, cyclin E1, cyclin A2, and cyclin B1 and decreased the levels of cyclin-dependent kinase inhibitor 1A prior to growth inhibition. Additionally, DEHP increased the mRNA levels of BCL2-associated agonist of cell death, BCL2-associated X protein, BCL2-related ovarian killer protein, B-cell leukemia/lymphoma 2, and Bcl2-like 10, leading to an increase in atresia. Further, DEHP decreased the levels of progesterone, androstenedione, and testosterone prior to the decrease in estradiol levels, with decreased mRNA levels of side-chain cleavage, 17α-hydorxylase-17,20-desmolase, 17β-hydroxysteroid dehydrogenase, and aromatase. Collectively, DEHP directly alters antral follicle functionality by inhibiting growth, inducing atresia, and inhibiting steroidogenesis.
Keywords: di(2-ethylhexyl) phthalate, ovary, antral follicle, atresia, steroidogenesis
INTRODUCTION
Di(2-ethylhexyl) phthalate (DEHP) is the most commonly used phthalate ester, and it is predominantly used in the manufacturing of a wide range of polyvinyl chloride consumer, medical, and building products to impart flexibility [1, 2]. Because of its incorporation in numerous commonly used consumer products, DEHP is produced in vast quantities. Domestic production of dioctyl phthalates, a subgroup of phthalate esters in which DEHP is classified, exceeds 300 million pounds annually [2]. Humans are exposed to DEHP on a daily basis via oral ingestion, inhalation, and dermal contact [1]. This is because DEHP is non-covalently bound to the plastic, allowing the chemical to frequently leach out into the environment and in the products that humans consume on a daily basis [1]. In fact, it is estimated that the range of daily human exposure to DEHP is between 3-30 μg/kg/day [3, 4]. Continuous, daily exposure to DEHP is a major concern because DEHP and its metabolite mono(2-ethylhexyl) phthalate (MEHP) have been identified in human blood samples [5, 6], urine samples [1, 5-9], amniotic fluid samples [10-12], cord blood samples from newborns [13, 14], breast milk samples [6], and in ovarian follicular fluid samples tested [15], indicating the ability of these chemicals to reach the ovary.
Important for public health, DEHP is a known endocrine disrupting chemical and reproductive toxicant [1, 2]. In women, chronic occupational exposure to phthalates is associated with an increased risk of miscarriage and a decreased rate of pregnancy [1]. In laboratory animals, DEHP causes pregnancy complications, including reduced implantations, increased resorptions, and decreased fetal weights of offspring [16, 17].
The mechanisms by which DEHP disrupts these endocrine and reproductive events remain unknown, but interestingly, antral follicles from the ovary are critical regulators of these processes. Follicles are the functional units of the ovary, and the antral follicle is the most mature follicle type. Antral follicles are the major sources of sex steroid hormone production in the female and are the only follicle type capable of ovulation [18]. Normal antral follicle function requires follicle growth, survival from atresia, and appropriate regulation of steroidogenesis [18].
Antral follicle growth is predominantly regulated by proliferation of granulosa cells and theca cells, which are somatic cells located in the follicle [18]. Proliferation of these cells, like most mammalian cells, is regulated by cyclins, cyclin dependent kinases, and cyclin dependent kinase inhibitors [19-21]. Although some antral follicles are rescued from atresia and ovulate in a female’s reproductive lifespan, the vast majority of follicles (99%) are lost via atretic demise [18, 22]. The regulation of follicular atresia involves a balance of pro- and anti-apoptotic factors that signal through the B cell leukemia/lymphoma 2 (BCL2) signaling pathway promoting caspase-induced apoptosis [23-28]. Antral follicle production of sex steroid hormones, a process termed ovarian steroidogenesis, is also essential for reproductive and non-reproductive health [29-46]. Steroidogenesis is the enzymatic conversion of cholesterol to 17β-estradiol and other necessary sex steroid hormones.
Interestingly, recent work also suggests that DEHP targets the ovary and adversely affects antral follicle functionality. Specifically, in vivo studies using oral exposure to DEHP have shown that DEHP decreases serum estradiol levels, decreases aromatase levels, decreases antral follicle size, causes anovulation, and disrupts estrous cyclicity [47-51]. Further, studies using ovarian granulosa cell cultures and minced ovary cultures show that DEHP also decreases estradiol and aromatase levels [52, 53]. Our group has also reported that DEHP inhibits antral follicle growth and decreases estradiol and aromatase levels in a whole antral follicle culture system following 96 hrs of culture [54]. However, few studies have investigated the effects of DEHP on the precursor hormones and enzymes upstream of estradiol and aromatase. Further, the direct effect of DEHP on antral follicle atresia is unclear. Additionally, the mechanisms by which DEHP disrupts antral follicle growth, health, and steroidogenesis remain unknown.
The present study was designed to conduct a time-course study using an antral follicle culture system to investigate the initial and time specific DEHP-induced defects in antral follicle growth, atresia, and steroidogenesis upstream of the previously reported effects on estradiol. Specifically, we tested the hypothesis that DEHP directly alters regulators of the cell cycle, apoptosis, and steroidogenic pathway to inhibit antral follicle growth, induce atresia, and inhibit steroid production. To test this hypothesis, antral follicles were cultured with vehicle or DEHP for 24, 48, 72, and 96 hrs. Following culture, antral follicles were collected for histological evaluation of atresia and the measurements of mRNA levels of regulators of the cell cycle (Ccnd2, Cdk4, Ccne1, Ccna2, Ccnb1, and Cdkn1a), apoptosis (Bad, Bax, Bok, Bcl2, Bcl2l10, Casp8, and Casp3), and the enzymes responsible for generating estradiol (Star, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b1, and Cyp19a1). Further, media were collected for the measurements of antral follicle produced progesterone, DHEA, androstenedione, testosterone, estrone, and estradiol.
MATERIALS AND METHODS
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of DEHP were prepared using dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) as the vehicle in various concentrations (1.33, 13.3, and 133 mg/ml). This allowed for an equal volume of each stock to be added to the culture wells to control for vehicle concentration. Final concentrations of DEHP in culture were 1, 10, and 100 μg/ml, which is approximately equivalent to 2.77, 27.7, and 277 μM respectively.
The concentrations of DEHP were chosen based their ability to cause inhibition of antral follicle growth, decreased estradiol production from antral follicles, and decreased antral follicle Cyp19a1 expression [54, 55]. These concentrations of DEHP also have been determined to be clinically relevant in reproductive and non-reproductive cell and tissue cultures [56-58]. Additionally, the selected concentrations of DEHP are environmentally relevant. Plasma concentrations of DEHP in healthy women have been reported to be 0.18 μg/ml, and peritoneal fluid concentrations of DEHP in these women were reported to be 0.46 μg/ml, which are close to the lowest concentration in this study [59]. The no-observed-adverse-effect level (NOAEL) for DEHP is 5.8 mg/kg/day which equates to 14.3 μM [2]. In addition, patients undergoing consistent medical care have markedly higher levels of DEHP than healthy people due to the extensive use of DEHP in medical care products [60, 61]. In intensive neonatal care units, patients receiving blood transfusions have plasma levels of DEHP at 11.1 μg/ml [62]. Further, the lowest-observed-adverse-effect level (LOAEL) of DEHP is 140 mg/kg/day, which equates to 344.6 μM [2]. Each of the selected concentrations of DEHP falls below the LOAEL concentration.
Animals
Cycling, adult CD-1 female mice (34-37 days of age) were obtained from Charles River Laboratories (Wilmington, MA). The mice were housed in groups of 4 in the College of Veterinary Medicine Animal Facility at the University of Illinois at Urbana-Champaign and were allowed to acclimate to the facility prior to experimentation. The mice were housed in a controlled animal room environment (temperature at 22 ± 1 °C and 12-hour light–dark cycles) and were provided food and water ad libitum. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
In vitro antral follicle culture
Unprimed, female CD-1 mice were euthanized and their ovaries were aseptically removed for antral follicle isolation. Antral follicles were isolated from the ovary based on relative size (250-400 μm) and were cleaned of interstitial tissue using watchmaker’s forceps [63, 64]. At least 2-3 mice were used in each experiment, in which we could obtain approximately 20-30 antral follicles per mouse. Each treatment group contained 10-16 follicles. Isolated antral follicles were randomly and individually plated in wells of a 96-well culture plate containing unsupplemented α-minimal essential medium (α-MEM, Life Technologies, Grand Island, NY) prior to treatment.
Treatment groups included DMSO (vehicle control) and DEHP (1, 10, and 100 μg/ml) and were prepared in supplemented α-MEM. Supplemented α-MEM contained 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium, Sigma-Aldrich, St. Louis, MO), 100 U/ml penicillin (Sigma-Aldrich, St. Louis, MO), 100 mg/ml streptomycin (Sigma-Aldrich, St. Louis, MO), 5 IU/ml human recombinant follicle-stimulating hormone (FSH; Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA) as described previously [63, 64]. An equal volume of chemical (0.75 μl/ml of media) was added for each dose to control for the amount of vehicle in each preparation (final concentration of DMSO=0.075%). Each follicle was cultured in 150 μl of medium for 24–96 hr in an incubator at 37°C supplying 5% CO2. Following each 24 hr culture window, media were collected for measurements of the levels of sex steroid hormones and follicles were collected, snap-frozen, and stored at −80°C for gene expression analysis. Further, follicles were collected following 96 hr of culture and processed for histological analysis of atresia. A time-course was conducted to observe when the effects of DEHP on antral follicle growth, atresia, and steroidogenesis begin and to determine which enzymes and/or hormones cause the inhibition of growth and reduction of estradiol production from antral follicles following 96 hr as reported previously [54].
Histological analysis of antral follicle atresia
Following 96 hr of culture, antral follicles were collected and processed for histological evaluation of atresia as described previously (n=10-16 follicles/3 separate experiments) [65, 66]. Briefly, each section of the follicle was observed for the presence of apoptotic bodies, which is a morphological sign of apoptosis. Based on the abundance of apoptotic bodies, the follicles were scored on a 1-4 scale. A rating of 1 indicated a healthy follicle without apoptotic bodies, a rating of 2 indicated the presence of apoptotic bodies encompassing 1-10% of the follicle section, a rating of 3 indicated the presence of apoptotic bodies encompassing 11-30% of the follicle section, and a rating of 4 indicated the presence of apoptotic bodies encompassing >30% of the follicle section. Atresia ratings were reported based on the average of all ratings throughout the follicle sections.
Analysis of gene expression
After 24, 48, 72, and 96 hr of culture, antral follicles were snap frozen in liquid nitrogen, and stored at −80°C for quantitative real-time polymerase chain reaction (qPCR) analysis (n=10-16 follicles/3-9 separate experiments). We elected to focus on mRNA gene expression analysis based on the limited amount of sample that can be retrieved from antral follicles. Total RNA (200 ng) was extracted from the follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol, and was then reverse transcribed to complementary DNA (cDNA) using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s protocol. Each cDNA sample was diluted 1:4 using nuclease-free water prior to qPCR analysis. Analysis of qPCR was conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA) and accompanying CFX Manager Software according to the manufacturer’s protocol. All qPCR reactions were done in triplicate using 1 μl cDNA, forward and reverse primers (5 pmol) for Ccnd2, Cdk4, Ccne1, Ccna2, Ccnb1, Cdkn1a, Bad, Bax, Bok, Bcl2, Bcl2l10, Casp8, Casp3, Star, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b1, Cyp19a1, Cyp1b1, or Actb, in addition to a SsoFastEvaGreen Supermix for a final reaction volume of 10 μl. Specific qPCR primers (Integrated DNA Technologies, Inc., Coralville, IA) for the genes of interest as well as the reference gene, beta-actin (Actb), which did not differ across treatment groups, can be found in the supplemental data. The genes tested were chosen because they are regulators of cell cycle (Ccnd2, Cdk4, Ccne1, Ccna2, Ccnb1, and Cdkn1a) [19, 20, 67, 68], apoptosis (Bad, Bax, Bok, Bcl2, Bcl2l10, Casp8, and Casp3) [23-26, 69], and ovarian steroidogenesis (Star, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b1, and Cyp19a1) [70-73].
The CFX96 machine quantifies the amount of PCR product generated by measuring SsoFastEvaGreen dye (Bio-Rad Laboratories, Inc., Hercules, CA) that fluoresces when bound to double-stranded DNA. The qPCR program consisted of an enzyme activation step (95°C for 1 min), an amplification and quantification program (40 cycles of 95°C for 10 sec, 60°C for 10 sec, single fluorescence reading), a step of 72°C for 5 min, a melt curve (65°C–95°C heating 0.5°C per sec with continuous fluorescence readings), and a final step at 72°C for 5 min as per the manufacturer’s protocol. Expression data were generated using the mathematical standard comparative (ΔΔCt) method. The ΔCt was calculated by subtracting the Actb Ct value from the gene of interest Ct value. The ΔΔCt was calculated as the difference between the ΔCt between the treatment groups and the DMSO groups. The relative fold-change of expression was then equaled to 2(−ΔΔCt) for each sample.
Analysis of sex steroid hormone levels
After 24, 48, 72, and 96 hr of culture, media were subjected to enzyme-linked immunosorbent assays (ELISAs) for the measurements of the levels of dehydroepiandrosterone (DHEA), progesterone, androstenedione, testosterone, estrone, and 17β-estradiol. ELISA kits were purchased from Diagnostics Research Group (DRG, Mountainside, NJ) (n=10-16 wells of pooled media containing follicles/3-9 separate experiments). The analytical sensitivity of each kit was 0.044 μg/ml for DHEA, 0.1 ng/mL for progesterone, 0.019 ng/mL for androstenedione, 0.083 ng/mL for testosterone, 6.3 pg/mL for estrone, and 9.71 pg/mL for estradiol. The assays were performed following the manufacturer’s protocol. All samples were run in duplicate and all intra- and inter-assay coefficients of variability were less than 10%. Some samples were diluted to match the dynamic range of each ELISA kit. Mean values for each sample were used in this analysis. These steroid hormones were chosen because of their necessity for normal female reproductive function and endocrinology. Further, these hormones serve as precursors for estradiol production, and estradiol production has been shown to be reduced in response to DEHP treatment [54].
Statistical analysis
All data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± standard error of the means (SEM), and at least three separate experiments were conducted for each treatment group prior to data analysis. Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance (ANOVA) followed by Tukey post-hoc comparison. Multiple comparisons between non-normally distributed experimental groups were made using the Kruskal Wallis test when appropriate. Statistical significance was assigned at p ≤ 0.05.
RESULTS
Effect of DEHP on antral follicle growth and the mRNA levels of key regulators of the cell cycle
As previously reported, DEHP exposure for 72 and 96 hr inhibits antral follicle growth in vitro [54, 55]. To confirm this, antral follicles were cultured with vehicle control (DMSO) or DEHP (1-100μg/ml) for 96 hr, and follicle diameters were measured on a perpendicular axis every 24 hr. Similar to the previous reports, DEHP exposure for 72 hr inhibited antral follicle growth by roughly 10% at the 10 and 100μg/ml doses when compared to the vehicle control group (data not shown). Also comparable to the previous studies, DEHP exposure for 96 hr inhibited antral follicle growth by roughly 15% at all selected doses of DEHP when compared to the vehicle control group (data not shown).
Because DEHP has been shown to inhibit antral follicle growth in vitro beginning at 72 hr, we investigated the initial effects and potential mechanisms by which DEHP inhibits antral follicle growth. Specifically, we subjected antral follicles to qPCR following 24, 48, and 72 hr of culture to investigate the direct effects of DEHP on the mRNA levels of key cyclins, cyclin dependent kinases, and cyclin dependent kinase inhibitors, which are heavily involved in follicular cell proliferation and ultimately antral follicle growth [18-20]. Cyclins (Ccnd2, Ccne1, Ccna2, and Ccnb1) and cyclin dependent kinases (Cdk4) promote progression through the cell cycle, whereas cyclin dependent kinase inhibitors (Cdkn1a, also known as p21) promote cell cycle arrest.
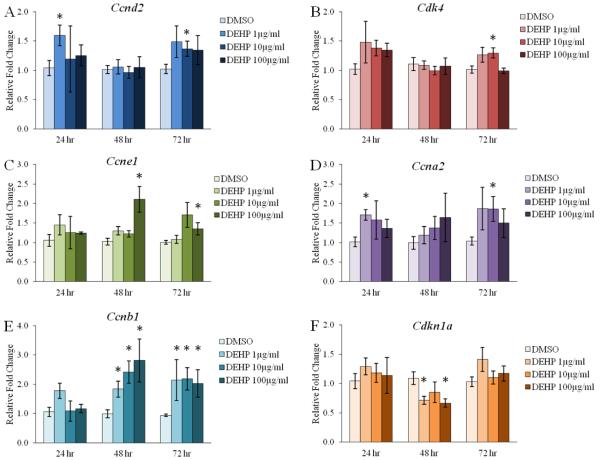
DEHP exposure for 24 hr significantly increased the mRNA levels of Ccnd2 at the 1μg/ml dose when compared to the vehicle control group (Fig. 1A, n=3, p≤0.05). Similarly, DEHP exposure for 72 hr significantly increased the mRNA levels of Ccnd2 at the 10μg/ml dose when compared to the vehicle control group (Fig. 1A, n=5-9, p≤0.05). DEHP exposure for 72 hr also increased the mRNA levels of Cdk4 at the 10μg/ml dose when compared to the vehicle control group (Fig. 1B, n=5-9, p≤0.05). Further, DEHP exposure for 48 and 72 hr increased the mRNA levels of Ccne1 at the 100μg/ml dose when compared to the vehicle control group (Fig. 1C, n=5-9, p≤0.05). The mRNA levels of Ccna2 were significantly increased following 24 hr at the 1μg/ml dose and following 72 hr at the 10μg/ml dose when compared to the vehicle control group (Fig. 1D, n=3-9, p≤0.05). Strikingly, DEHP exposure for 48 and 72 hr significantly increased the mRNA levels of Ccnb1 at all selected doses when compared to the vehicle control group (Fig. 1E, n=5-9, p≤0.05). The mRNA levels of the inhibitor Cdkn1a were significantly decreased by the 1 and 100μg/ml doses following 48 hr when compared to the vehicle control group (Fig. 1F, n=5-8, p≤0.05).
Figure 1. Effect of DEHP on the antral follicle mRNA expression of key regulators of the cell cycle.
Antral follicles were isolated from adult CD-1 mice and were cultured with vehicle (DMSO) or DEHP (1-100μg/ml) for 24-72 hr. Following each 24 hr time-point, antral follicles were pooled per treatment group and were subjected to qPCR for the measurements of Ccnd2 (panel A), Cdk4 (panel B), Ccne1 (panel C), Ccna2 (panel D), Ccnb1 (panel E), and Cdkn1a (panel F). All values were normalized to Actb. Graph represents means ± SEM from 3-9 separate experiments, with 10-16 follicles/treatment group in each experiment. Asterisks (*) represent significant difference from vehicle control (p ≤ 0.05).
Effect of DEHP on antral follicle atresia
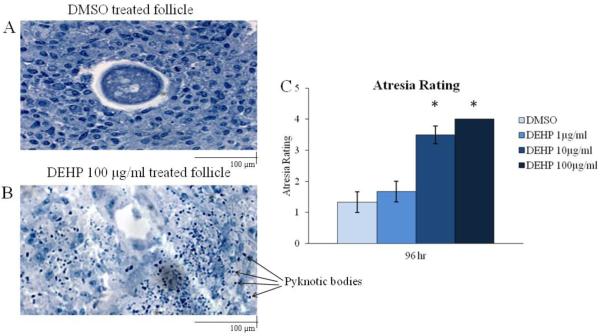
An important aspect of antral follicle health and functionality is survival from atresia, which is an apoptotic process. Although we have previously determined that DEHP exposure inhibits antral follicle growth and decreases antral follicle-produced estradiol levels [54, 55], the direct effects of DEHP on antral follicle atresia were unknown. Thus, we cultured antral follicles with vehicle control (DMSO) or DEHP (1-100μg/ml) for 96 hr and processed the follicles for histological evaluation of atresia. Atresia was rated by the presence of apoptotic bodies. A representative image of a DMSO treated follicle is displayed in Fig. 2A, whereas a representative image of a DEHP 100μg/ml treated follicle is exhibited in Fig. 2B. Qualitatively, it appears that DEHP treatment increased the presence and abundance of apoptotic bodies within the follicular unit when compared to the vehicle control treated follicles. When quantified, DEHP exposure for 96 hr significantly increased the number of apoptotic bodies, represented by a higher atresia rating, at the 10 and 100μg/ml doses when compared to the vehicle control group (Fig. 2C, n=3, p≤0.05).
Figure 2. Effect of DEHP on antral follicle atresia.
Antral follicles were isolated from adult CD-1 mice and were cultured with vehicle (DMSO) or DEHP (1-100μg/ml) for 96 hr. Following 96 hr, antral follicles were processed for histological evaluation of atresia. A representative image of a DMSO treated follicle is found in panel A. A representative image of a DEHP 100 μg/ml is found in panel B. Atresia ratings were assigned based on the presence of apoptotic bodies. A rating of 1 indicated a healthy follicle without apoptotic bodies, a rating of 2 indicated the presence of apoptotic bodies encompassing 1-10% of the follicle section, a rating of 3 indicated the presence of apoptotic bodies encompassing 11-30% of the follicle section, and a rating of 4 indicated the presence of apoptotic bodies encompassing >30% of the follicle section. Graph represents means ± SEM from 3 separate experiments, with 10-16 follicles/treatment group in each experiment. Asterisks (*) represent significant difference from vehicle control (p ≤ 0.05).
Effect of DEHP on the antral follicle mRNA levels of key regulators of apoptosis
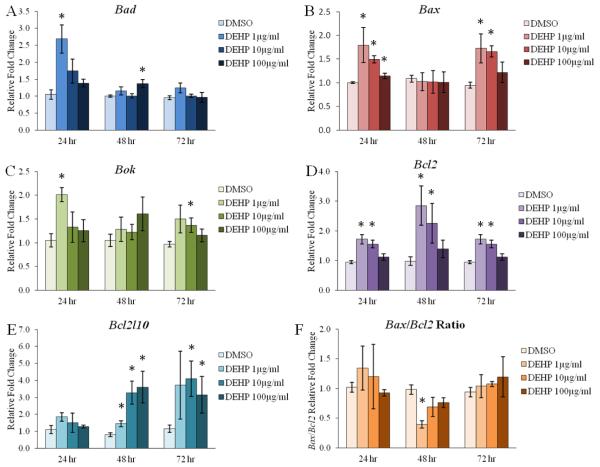
Although atresia is a natural occurrence in antral follicles, an increase in atresia can have negative impacts on ovarian and reproductive health [18, 22]. Because we observed an increase in antral follicle atresia following 96 hr of DEHP exposure, we investigated the initial effects and potential mechanisms by which DEHP induces atresia, or apoptosis, in the antral follicle. Specifically, antral follicles were cultured with vehicle control (DMSO) or DEHP (1-100μg/ml) for 24-72 hr. Following 24, 28, and 72 hr of culture, we subjected antral follicles to qPCR to investigate the direct effects of DEHP on the mRNA levels of factors that drive apoptosis to promote atresia (Bad, Bax, and Bok) and factors that inhibit apoptosis to promote follicle survival (Bcl2 and Bcl2l10). DEHP exposure significantly increased the mRNA levels of pro-apoptotic Bad following 24 hr at the 1μg/ml dose and following 48 hr at the 100μg/ml dose when compared to the vehicle control group (Fig. 3A, n=3-8, p≤0.05). DEHP exposure also significantly increased the mRNA levels of pro-apoptotic Bax following 24 hr at all selected doses of DEHP and following 72 hr at the 1 and 10μg/ml doses when compared to the vehicle control group (Fig. 3B, n=3-9, p≤0.05). The mRNA levels of pro-apoptotic Bok were significantly increased following 24 hr at the 1μg/ml dose and following 72 hr at the 10μg/ml dose when compared to the vehicle control group (Fig. 3C, n=3-9, p≤0.05). Interestingly, DEHP exposure for 24, 48, and 72 hr significantly increased the mRNA levels of anti-apoptotic Bcl2 at the 1 and 10μg/ml doses when compared to the vehicle control group (Fig. 3D, n=3-9, p≤0.05). Similarly, the mRNA levels of anti-apoptotic Bcl2l10 were significantly increased following 48 hr at all selected doses of DEHP and following 72 hr at the 10 and 100μg/ml doses when compared to the vehicle control group (Fig. 3E, n=5-9, p≤0.05).
Figure 3. Effect of DEHP on the antral follicle mRNA expression of key regulators of apoptosis.
Antral follicles were isolated from adult CD-1 mice and were cultured with vehicle (DMSO) or DEHP (1-100μg/ml) for 24-72 hr. Following each 24 hr time-point, antral follicles were pooled per treatment group and were subjected to qPCR for the measurements of Bad (panel A), Bax (panel B), Bok (panel C), Bcl2 (panel D), Bcl2l10 (panel E). All values were normalized to Actb. Further, ratios of Bax values/Bcl2 values were calculated (panel F). Graph represents means ± SEM from 3-9 separate experiments, with 10-16 follicles/treatment group in each experiment. Asterisks (*) represent significant difference from vehicle control (p ≤ 0.05).
Apoptosis and cell survival rely on the balance of pro- and anti-apoptotic factors, and often the Bax/Bcl2 ratio is used to determine susceptibility to apoptosis [74, 75]. Following qPCR analysis, we calculated the Bax/Bcl2 ratio at 24, 48, and 72 hr of culture. Interestingly, DEHP exposure significantly decreased the Bax/Bcl2 ratio following 48 hr at the 1μg/ml dose when compared to the vehicle control group (Fig. 3F, n=5-8, p≤0.05).
Effect of DEHP on the antral follicle mRNA levels of downstream apoptosis factors
The present study indicates that DEHP exposure induced atresia in cultured mouse antral follicles; however, DEHP exposure also increased the mRNA levels of both pro- and anti-apoptotic BCL2 regulators of apoptosis. In addition, the Bax/Bcl2 ratio is relatively unchanged in response to DEHP exposure. In order to confirm the histological presence of atresia in the antral follicles, we measured the levels of key downstream facilitators of apoptosis. Specifically, we subjected antral follicles to qPCR following 24, 48, and 72 hr of culture to measure the mRNA levels of Casp8 and Casp3. These caspases are known executors of apoptosis that initiate the disassembly and degradation of the cell in response to pro-apoptotic BCL2 signaling [27, 28]. DEHP exposure significantly increased the mRNA levels of Casp8 following 72 hr at the 10 and 100μg/ml doses when compared to the vehicle control group (Fig. 4A, n=5-9, p≤0.05). Interestingly, DEHP exposure significantly increased the mRNA levels of Casp3 following 24 hr at the 1 and 100μg/ml doses when compared to the vehicle control group, but this effect was not observed following 48 hr of exposure (Fig. 4B, n=3-9, p≤0.05). However, following 72 hr of exposure, DEHP significantly increased the mRNA levels of Casp3 at all selected doses of DEHP when compared to the vehicle control group (Fig. 4B, n=5-9, p≤0.05).
Figure 4. Effect of DEHP on the antral follicle mRNA expression of downstream apoptosis factors.
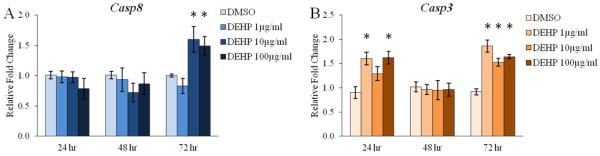
Antral follicles were isolated from adult CD-1 mice and were cultured with vehicle (DMSO) or DEHP (1-100μg/ml) for 24-72 hr. Following each 24 hr time-point, antral follicles were pooled per treatment group and were subjected to qPCR for the measurements of Casp8 (panel A) and Casp3 (panel B). All values were normalized to Actb. Graph represents means ± SEM from 3-9 separate experiments, with 10-16 follicles/treatment group in each experiment. Asterisks (*) represent significant difference from vehicle control (p ≤ 0.05).
Effect of DEHP on antral follicle-produced sex steroid hormone levels
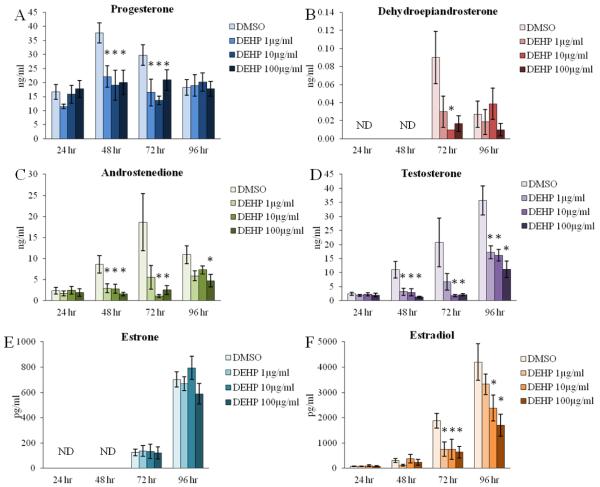
Previous studies have determined that DEHP exposure decreases estradiol levels both in vivo [47-50] and in vitro [52, 53]. Similar to previous studies, we have reported that DEHP exposure for 96 hr decreases estradiol levels in an in vitro antral follicle culture system [54]. However, the direct effects of DEHP at earlier time-points of exposure and on the estradiol precursor sex steroid hormones are relatively unknown. Thus, we conducted a time-course experiment to investigate the effects of DEHP upstream of estradiol production and to observe the initial effects of DEHP on antral follicle steroidogenesis. DEHP exposure significantly decreased the levels of progesterone following 48 and 72 hr at all selected doses of DEHP when compared to the vehicle control group (Fig. 5A, n=5-9, p≤0.05). DEHP exposure also significantly decreased the levels of DHEA following 72 hr at the 10μg/ml dose when compared to the vehicle control group (Fig. 5B, n=5-9, p≤0.05). Similarly, androstenedione levels were significantly decreased following 48 hr at all selected doses of DEHP, following 72 hr at the 10 and 100μg/ml doses, and following 96 hr at the 100μg/ml dose when compared to the vehicle control group (Fig. 5C, n=5-9, p≤0.05). Likewise, DEHP exposure significantly decreased the levels of testosterone following 48 hr at all selected doses of DEHP, following 72 hr at the 10 and 100μg/ml doses, and following 96 hr at all selected doses of DEHP when compared to the vehicle control group (Fig. 5D, n=5-9, p≤0.05). Similar to our previously published study [54], DEHP exposure significantly decreased estradiol levels following 96 hr at the 10 and 100μg/ml doses when compared to the vehicle control group; but interestingly and not previously reported, DEHP exposure for 72 hr also decreased estradiol levels at all selected doses of DEHP when compared to the vehicle control group (Fig. 5F, n=5-9, p≤0.05). In contrast, DEHP exposure had no effect on the levels of estrone at each time-point tested when compared to the vehicle control group (Fig. 5E, n=3-9).
Figure 5. Effect of DEHP on antral follicle-produced sex steroid hormone levels.
Antral follicles were isolated from adult CD-1 mice and were cultured with vehicle (DMSO) or DEHP (1-100μg/ml) for 24-96 hr. Following each 24 hr time-point, media were pooled per treatment group and were subjected to ELISAs for the measurements of progesterone (panel A), dehydroepiandrosterone (panel B), androstenedione (panel C), testosterone (panel D), estrone (panel E), and estradiol (panel F). Graph represents means ± SEM from 3-9 separate experiments, with medium from 10-16 wells/treatment group in each experiment. Hormones with ND (not detectable) at certain time-points had levels of that hormone below threshold detection. Asterisks (*) represent significant difference from vehicle control (p ≤ 0.05).
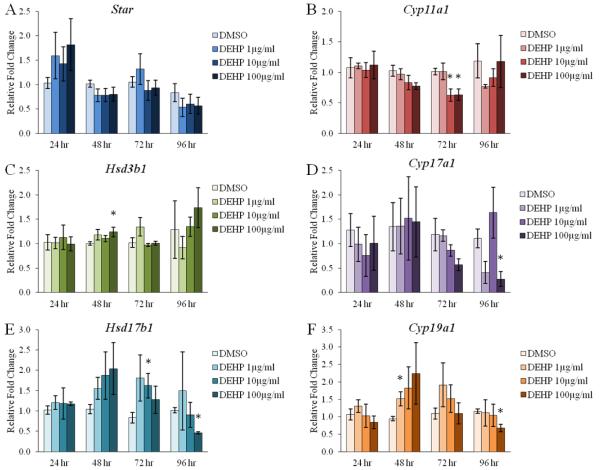
Effect of DEHP on the antral follicle mRNA expression of key steroidogenic enzymes
Previous studies have determined that DEHP exposure decreases the ovarian mRNA levels of Cyp19a1, also known as aromatase, in vivo [47, 76] and in an in vitro antral follicle culture system following 96 hr [54]. Similar to the effects on the levels of steroid hormones, not much is known about the direct effects of DEHP on the steroidogenic enzymes upstream of Cyp19a1. Because we observed decreases in the levels of estradiol and its precursor sex steroid hormones across multiple time-points, we investigated the initial effects and potential mechanisms by which DEHP inhibits steroidogenesis. Specifically, antral follicles were cultured with vehicle control (DMSO) or DEHP (1-100μg/ml) for 24-96 hr. Following each 24 hr time-point, antral follicles were subjected to qPCR to investigate the direct effects of DEHP on the estradiol biosynthesis enzymes (Star, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b1, and Cyp19a1), which convert cholesterol to estradiol in a step-wise manner. DEHP exposure significantly decreased the mRNA levels of Cyp11a1 following 72 hr at the 10 and 100μg/ml doses when compared to the vehicle control group (Fig. 6B, n=5-9, p≤0.05). Beginning at 48 hr, DEHP exposure significantly increased the mRNA levels of Hsd3b1 at the 100μg/ml dose when compared to the vehicle control group (Fig. 6C, n=5-8, p≤0.05). Further, DEHP exposure significantly decreased the mRNA levels of Cyp17a1 following 96 hr at the 100μg/ml dose when compared to the vehicle control group (Fig. 6D, n=5-9, p≤0.05). Meanwhile, there was a trend for a significant decrease in the mRNA levels of Cyp17a1 following 72 hr at the 100μg/ml dose and following 96 hr at the 1μg/ml dose when compared to the vehicle control group (Fig. 6D, n=5-9). Beginning at 48 hr, there was a trend for a significant increase in the mRNA levels of Hsd17b1 at all selected doses tested, and this trend persists through 72 hr with statistical significance at the 10μg/ml dose (Fig. 6E, n=5-9, p≤0.05). However, by 96 hr, the mRNA levels of Hsd17b1 were significantly decreased at the 100μg/ml dose when compared to the vehicle control group (Fig. 6E, n=5-9, p≤0.05). Similarly, there was a trend for a significant increase in the mRNA levels of Cyp19a1 at all selected doses tested following 48 hr, with a statistical significance at the 1μg/ml dose when compared to the vehicle control group (Fig. 6F, n=5-8, p≤0.05). By 96 hr, there was a significant decrease in the mRNA levels of Cyp19a1 at the 100μg/ml dose (Fig. 6F, n=5-9, p≤0.05). In contrast, DEHP exposure had no effect on the mRNA levels of Star at each time-point tested when compared to the vehicle control group (Fig. 6A, n=3-9).
Figure 6. Effect of DEHP on the antral follicle mRNA expression of key steroidogenic enzymes.
Antral follicles were isolated from adult CD-1 mice and were cultured with vehicle (DMSO) or DEHP (1-100μg/ml) for 24-96 hr. Following each 24 hr time-point, antral follicles were pooled per treatment group and were subjected to qPCR for the measurements of Star (panel A), Cyp11a1 (panel B), Hsd3b1 (panel C), Cyp17a1 (panel D), Hsd17b1 (panel E), and Cyp19a1 (panel F). All values were normalized to Actb. Graph represents means ± SEM from 3-9 separate experiments, with 10-16 follicles/treatment group in each experiment. Asterisks (*) represent significant difference from vehicle control (p ≤ 0.05).
DISCUSSION
We utilized an in vitro antral follicle culture system to investigate the effects of DEHP, a ubiquitous endocrine disrupting chemical, on antral follicle growth, atresia, and steroidogenesis. Our main findings suggest that DEHP exposure alters the follicular mRNA levels of cyclins, a cyclin dependent kinase, and a cyclin dependent kinase inhibitor to potentially impact the previously observed effect of DEHP-induced inhibition of antral follicle growth [54, 55]. Further, our work demonstrates that DEHP exposure directly induces antral follicle atresia as evident via histological evaluation and increased mRNA levels of caspases, and that alterations in the mRNA levels of BCL2 family members may be responsible for the DEHP-induced onset of atresia. Additionally, we have shown that DEHP directly impacts the production of sex steroid hormones upstream of estradiol and alters the mRNA levels of the estradiol biosynthesis enzymes upstream of Cyp19a1. Importantly, we have established a time-course of the DEHP-induced defects on antral follicles, and we have determined the initial effects of DEHP on antral follicle growth, atresia, and steroidogenesis.
The use of this antral follicle culture system is vital in understanding the direct effects of DEHP on the most mature, and only, follicle type that is capable of ovulation and synthesis of steroid hormones. Previous work has investigated the effects of DEHP exposure on antral follicle growth and steroidogenesis in vivo showing that DEHP decreased antral follicle diameter, and decreased the levels of serum estradiol produced by the antral follicles [47-50]. The limitation of these previous studies is that they did not determine whether the effects on antral follicle size and estradiol production were direct effects on the follicle or were indirect effects caused by toxicity in other organ systems, namely the hypothalamus-pituitary axis. To combat this limitation, previous studies have shown that DEHP and MEHP decreased estradiol production and Cyp19a1 levels in murine and human granulosa cell cultures in vitro [53, 77-80]. However, the limitation of these studies is that they only utilized one somatic follicular cell type. Antral follicle growth, survival from atresia, and steroidogenesis rely on the multi-directional communication between the oocyte, granulosa cells, and theca cells [18, 22, 72, 73]. Thus, although previous studies have been instrumental in understanding the defects caused by DEHP, the in vitro whole antral follicle culture system has proved to be a novel approach in understanding the direct effects of DEHP on antral follicle functionality [57, 81].
Our results indicate that DEHP directly inhibits antral follicle growth and dysregulates the expression of cell cycle regulators. We have previously reported that DEHP exposure decreased the antral follicle mRNA levels of Ccnd2 and Cdk4 following 96 hr [54]. Expanding upon these findings, we have established the time-course effects of DEHP on the antral follicle levels of several cyclins, Cdk4, and Cdkn1a. Contrary to the findings at 96 hr, DEHP exposure prior to 96 hr increased the mRNA levels of Ccnd2, Cdk4, Ccne1, Ccna2, and Ccnb1. Further, DEHP exposure decreased the mRNA levels of the inhibitor Cdkn1a. The earliest effect of DEHP on the regulators of the cell cycle was observed following 24 hr of exposure, where DEHP exposure increased the mRNA levels of Ccnd2 and Ccna2, but only at the lowest dose. The most striking finding is that DEHP exposure increased Ccnb1 following 48 and 72 hr at all selected doses. That, as well as the increases in the other cyclins and Cdk4, and decreases in Cdkn1a suggests that early exposure to DEHP may result in an initial push for cell cycle progression and proliferation to combat the toxicity of DEHP. This potentially is why we do not observe growth inhibition prior to 72 hr of DEHP exposure. This compensatory response, however, is not enough to prevent the DEHP-induced inhibition of growth following 72 hr, and this compensation is completely ablated by 96 hr of exposure because follicle growth is further inhibited and the mRNA levels of Ccnd2 and Cdk4 are decreased in response to DEHP treatment [54]. This compensation, nevertheless, may explain why we do not observe complete antral follicle growth inhibition, but rather the growth of the DEHP treated follicles is inhibited by roughly 15% of control treated follicles [55]. Interestingly, the 1μg/ml dose of DEHP is the only dose that does not inhibit antral follicle growth following 72 hr. This is likely because the 1μg/ml dose is the only dose to have compensatory increases in cyclins at the earliest time-point tested, while both the 10 and 100μg/ml doses show no signs of compensation at 24 hr of exposure.
For an antral follicle to function properly, it must remain viable by escaping atresia [18]. Atresia is a natural process by which follicles undergo demise via apoptosis [22]. Following 96 hr of treatment, DEHP exposure at the 10 and 100μg/ml doses directly increased the number of apoptotic bodies found in the antral follicles leading to an increase in the atresia rating. This increase in atresia dramatically affects the health of the antral follicle and possibly can have lasting impacts on reproductive and non-reproductive health. This expands upon previous studies, where exposure to DEHP and its metabolite MEHP induced apoptosis in ovarian cells in vivo [82, 83] and in vitro [84-86].
Follicular atresia is primarily regulated by the balance of pro-apoptotic factors (such as Bad, Bax, and Bok) and anti-apoptotic factors (such as Bcl2 and Bcl2l10) [23-26, 69]. Our findings that DEHP exposure may affect the expression of pro-apoptotic and anti-apoptotic factors expand upon previous studies that suggest DEHP causes oxidative stress in antral follicles following 24 hr of exposure [55]. Further, oxidative stress has been shown to be a regulator of atresia [87-92]. Therefore, it appears that DEHP exposure initially induces oxidative stress and increases pro-apoptotic factors or congruently induces oxidative stress and increases pro- and anti-apoptotic factors following 24 hr of exposure. Following 48 hr of exposure, the two anti-apoptotic factors tested are elevated to combat the initial increase in pro-apoptotic factors, and this occurs in the general absence of an increase in pro-apoptotic factors. However, pro-apoptotic factors again became elevated following 72 hr of exposure. It appears that the compensatory increases in Bcl2 and Bcl2l10 occur after the onset of oxidative stress and increases in pro-apoptotic factors, and this compensation is not enough to alleviate the atresia observed at the 10 and 100μg/ml doses following 96 hr of exposure. However, increases in BCL2 can potentially have pro-apoptotic implications. When BCL2 is cleaved by caspases, studies have reported that BCL2 may acquire pro-apoptotic properties [93]. In support of this hypothesis, both Casp8 and Casp3 are increased following DEHP treatment in the present study. Thus, even though DEHP increases anti-apoptotic factors, it is possible that these factors have pro-apoptotic activities, and DEHP increased downstream apoptotic caspases further suggesting an increase in apoptosis. Interestingly, atresia is not observed at the 1μg/ml dose, and this is likely attributed to the decrease in the Bax/Bcl2 ratio following 48 hr of exposure and the absence of DEHP-induced oxidative stress in the previously mentioned study at the 1μg/ml dose [55].
Proper regulation of antral follicle steroidogenesis is essential for reproductive and non-reproductive health because defects in hormone production, even upstream of estradiol, can lead to anovulation, infertility, premature ovarian failure, cardiovascular disease, osteoporosis, mood disorders, and even premature death [29-46]. We have previously reported that DEHP exposure directly decreased estradiol levels in the antral follicle culture system following 96 hr at the 10 and 100μg/ml doses, but few studies have investigated the effects of DEHP upstream of estradiol. [54]. Expanding upon previous findings and to understand the mechanism by which DEHP disrupts steroidogenesis, we established the time-course effects of DEHP on the levels of antral follicle-produced sex steroid hormones. Prior to the observed decrease in estradiol levels, DEHP exposure decreased the levels of several precursor steroid hormones. The earliest time-point of the inhibition of steroidogenesis occurs following 48 hr of exposure, where DEHP decreased the levels of progesterone, androstenedione, and testosterone at all selected doses tested. This decrease persists following 72 hr, where DEHP exposure decreased the levels of progesterone, DHEA, androstenedione, testosterone, and even estradiol. Similarly, DEHP exposure following 96 hr decreased the levels androstenedione, testosterone, and estradiol. Estradiol production requires the presence of these upstream steroid hormones to be converted to downstream hormones and ultimately estradiol [18, 72, 73]. Thus, it is likely the lack of precursor hormones following 48 hr of exposure that leads to the decrease in estradiol levels following 72 hr and ultimately 96 hr of exposure.
Estradiol biosynthesis involves the strict coordination of the steroidogenic enzymes present in the theca and granulosa cells that convert the precursor sex steroid hormones to estradiol [72, 73]. We have previously reported that DEHP exposure directly decreased the antral follicle mRNA levels of Cyp19a1, which is the downstream enzyme that converts androgens to estrogens [54]. However, few studies have investigated the effects of DEHP upstream of Cyp19a1. Thus, we established the time-course effects of DEHP on the mRNA levels of each steroidogenic enzyme responsible for generating estradiol. The initial effects of DEHP exposure on the mRNA levels of the steroidogenesis occurred following 48 hr of exposure. DEHP exposure increased the mRNA levels of Hsd3b1 (which converts pregnenolone to progesterone and DHEA to androstenedione) at the 100μg/ml dose and Cyp19a1 at the 1μg/ml dose. Interestingly, DEHP increased the mRNA levels of Hsd17b1 (which converts androstenedione to testosterone and estrone to estradiol) following 72 hr of exposure at the 10μg/ml dose. Also following 72 hr of exposure, DEHP decreased the mRNA levels of Cyp11a1 (which converts cholesterol to pregnenolone). Following 96 hr of exposure, DEHP decreased the mRNA levels of Cyp17a1 (which converts pregnenolone to DHEA and progesterone to androstenedione), Hsd17b1, and Cyp19a1 at the 100μg/ml dose.
Initially, it appears that selected steroidogenic enzymes increase transcription to compensate for the decrease in steroid hormones following 48 hr of exposure. This compensation, however, is not adequate to restore the levels of androstenedione, testosterone, and estradiol to control levels. Although there is not a difference in the levels of progesterone following 96 hr, it is not because the toxicity of DEHP on progesterone production is alleviated. In fact, progesterone levels remain constant throughout culture, and it is the vehicle control group that decreases production of progesterone from 72 to 96 hr of exposure. A similar phenomenon occurs with DHEA and androstenedione, where the levels of the hormones in the vehicle control group decrease from 72 to 96 hr of exposure. At this time-point in culture, it is likely that the antral follicles are shifting production of these precursor hormones to favor the production of the downstream hormones, testosterone, estrone, and estradiol. This effect was also seen in previous studies that investigated the time-course effects of bisphenol A on mouse antral follicles in vitro [94, 95]. Following 96 hr of exposure, the compensatory increase in the steroidogenic enzymes is lost at the 100μg/ml dose because of a decrease in the rate limiting enzymes, Cyp17a1, Hsd17b1, and Cyp19a1. However, this does not explain the decrease in estradiol levels at the 10μg/ml dose. It is most likely that the absence of precursor hormones leads to the decrease in estradiol following 96 hr of exposure at the 10μg/ml dose, whereas it is both the absence of precursor hormones and decrease in steroidogenic enzyme levels that lead to the decrease in estradiol levels following 96 hr of exposure at the 100μg/ml dose. Further, in the general absence of decreased steroidogenic enzyme expression, it is possible that ongoing apoptosis in the granulosa cells contributes to a loss of steroidogenically active cells, potentially leading to a decrease in precursor hormone levels. Conversely, even though the levels of precursor hormones are decreased at the 1μg/ml dose, the moderate increases in the mRNA levels of Hsd17b1 and Cyp19a1 following 48 and 72 hr of exposure at the 1μg/ml dose appear to be adequate in restoring estradiol levels to control levels following 96 hr of exposure.
In conclusion, we have utilized the novel antral follicle culture system to establish the direct and temporal effects of DEHP on antral follicle growth, atresia, and steroidogenesis. Collectively, our results indicate that DEHP inhibits follicle growth potentially via dysregulation of the cell cycle, induces atresia potentially via dysregulation of apoptosis, and inhibits steroidogenesis potentially via the lack of upstream sex steroid hormones and disruption of the steroidogenic enzymes. These effects of DEHP are of concern because they can negatively impact ovulation, initiation and maintenance of pregnancy, estrous cyclicity, maintenance of the reproductive tract, and non-reproductive health [29-46]. Although the processes of growth, atresia and steroidogenesis are linked in certain aspects, the mechanism of the temporal relationship of the three aspects of follicular function has yet to be elucidated [18, 73]. Further, studies should be done to explore the reasons for the non-monotonic dose responses observed for some endpoints in response to DEHP.
Supplementary Material
HIGHLIGHTS.
DEHP inhibits antral follicle growth by dysregulating cell cycle regulators
DEHP induces antral follicle atresia by dysregulating apoptosis regulators
DEHP inhibits the production of antral follicle produced sex steroid hormones
ACKNOWLEDGMENTS
The authors thank Dr. Ayelet Ziv-Gal, Dr. Liying Gao, and Shreya Patel for assistance with follicle isolations, and the authors thank all members of Dr. Flaws’ laboratory for technical assistance. This work was supported by the National Institute of Environmental Health Sciences grant R01ES019178 (JAF) and an Interdisciplinary Environmental Toxicology Program Fellowship (PRH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 2.2002 AfTSaDRA . Toxicological profile for Di(2-ethylhexyl)phthalate (DEHP) U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2002. [Google Scholar]
- 3.Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Regul Toxicol Pharmacol. 1999;29:327–357. doi: 10.1006/rtph.1999.1296. [DOI] [PubMed] [Google Scholar]
- 4.Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2002;16:529–653. doi: 10.1016/s0890-6238(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Silva MJ, Reidy JA, Hurtz D, 3rd, Malek NA, Needham LL, Nakazawa H, Barr DB, Calafat AM. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112:327–330. doi: 10.1289/ehp.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Hakansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, Rosskamp E, Schluter C, Seifert B, Ullrich D. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- 9.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environ Int. 2009;35:14–20. doi: 10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Silva MJ, Reidy JA, Herbert AR, Preau JL, Jr., Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol. 2004;72:1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- 12.Wittassek M, Angerer J, Kolossa-Gehring M, Schafer SD, Klockenbusch W, Dobler L, Gunsel AK, Muller A, Wiesmuller GA. Fetal exposure to phthalates--a pilot study. Int J Hyg Environ Health. 2009;212:492–498. doi: 10.1016/j.ijheh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate. 2003;83:22–24. doi: 10.1159/000067012. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Zheng LX, Gu YP, Wang JY, Zhang YH, Song WM. [Levels of environmental endocrine disruptors in umbilical cord blood and maternal blood of low-birth-weight infants] Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:177–180. [PubMed] [Google Scholar]
- 15.Krotz SP, Carson SA, Tomey C, Buster JE. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet. 2012;29:773–777. doi: 10.1007/s10815-012-9775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaul AF, Souney PF, Osathanondh R. A review of possible toxicity of di-2-ethylhexylphthalate (DEHP) in plastic intravenous containers: effects on reproduction. Drug Intell Clin Pharm. 1982;16:689–692. doi: 10.1177/106002808201600908. [DOI] [PubMed] [Google Scholar]
- 18.Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- 19.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 20.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–940. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- 21.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 22.Hirshfield AN. Size-frequency analysis of atresia in cycling rats. Biol Reprod. 1988;38:1181–1188. doi: 10.1095/biolreprod38.5.1181. [DOI] [PubMed] [Google Scholar]
- 23.Ratts VS, Flaws JA, Kolp R, Sorenson CM, Tilly JL. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology. 1995;136:3665–3668. doi: 10.1210/endo.136.8.7628407. [DOI] [PubMed] [Google Scholar]
- 24.Flaws JA, Hirshfield AN, Hewitt JA, Babus JK, Furth PA. Effect of bcl-2 on the primordial follicle endowment in the mouse ovary. Biol Reprod. 2001;64:1153–1159. doi: 10.1095/biolreprod64.4.1153. [DOI] [PubMed] [Google Scholar]
- 25.Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 26.Greenfeld CR, Pepling ME, Babus JK, Furth PA, Flaws JA. BAX regulates follicular endowment in mice. Reproduction. 2007;133:865–876. doi: 10.1530/REP-06-0270. [DOI] [PubMed] [Google Scholar]
- 27.Hsu SY, Hsueh AJ. Tissue-specific Bcl-2 protein partners in apoptosis: An ovarian paradigm. Physiol Rev. 2000;80:593–614. doi: 10.1152/physrev.2000.80.2.593. [DOI] [PubMed] [Google Scholar]
- 28.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 29.Bagur AC, Mautalen CA. Risk for developing osteoporosis in untreated premature menopause. Calcif Tissue Int. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- 30.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 31.Everson SA, Matthews KA, Guzick DS, Wing RR, Kuller LH. Effects of surgical menopause on psychological characteristics and lipid levels: the Healthy Women Study. Health Psychol. 1995;14:435–443. doi: 10.1037//0278-6133.14.5.435. [DOI] [PubMed] [Google Scholar]
- 32.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 33.Armamento-Villareal R, Villareal DT, Avioli LV, Civitelli R. Estrogen status and heredity are major determinants of premenopausal bone mass. J Clin Invest. 1992;90:2464–2471. doi: 10.1172/JCI116138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christiansen C. Prevention and treatment of osteoporosis with hormone replacement therapy. Int J Fertil Menopausal Stud. 1993;38(Suppl 1):45–54. [PubMed] [Google Scholar]
- 35.Mosca L. Estrogen and atherosclerosis. J Investig Med. 1998;46:381–386. [PubMed] [Google Scholar]
- 36.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 37.Dennerstein L, Lehert P, Burger H, Dudley E. Mood and the menopausal transition. J Nerv Ment Dis. 1999;187:685–691. doi: 10.1097/00005053-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Woods NF, Mariella A, Mitchell ES. Patterns of depressed mood across the menopausal transition: approaches to studying patterns in longitudinal data. Acta Obstet Gynecol Scand. 2002;81:623–632. doi: 10.1034/j.1600-0412.2002.810708.x. [DOI] [PubMed] [Google Scholar]
- 39.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couse JF, Korach KS. Exploring the role of sex steroids through studies of receptor deficient mice. J Mol Med (Berl) 1998;76:497–511. doi: 10.1007/s001090050244. [DOI] [PubMed] [Google Scholar]
- 41.Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–3262. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- 42.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 43.Britt KL, Findlay JK. Estrogen actions in the ovary revisited. J Endocrinol. 2002;175:269–276. doi: 10.1677/joe.0.1750269. [DOI] [PubMed] [Google Scholar]
- 44.Findlay JK, Britt K, Kerr JB, O'Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation: the role of oestrogens. Reprod Fertil Dev. 2001;13:543–547. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- 45.Davis VL, Couse JF, Goulding EH, Power SG, Eddy EM, Korach KS. Aberrant reproductive phenotypes evident in transgenic mice expressing the wild-type mouse estrogen receptor. Endocrinology. 1994;135:379–386. doi: 10.1210/endo.135.1.8013372. [DOI] [PubMed] [Google Scholar]
- 46.Fauser BC, Laven JS, Tarlatzis BC, Moley KH, Critchley HO, Taylor RN, Berga SL, Mermelstein PG, Devroey P, Gianaroli L, D'Hooghe T, Vercellini P, et al. Sex steroid hormones and reproductive disorders: impact on women's health. Reprod Sci. 2011;18:702–712. doi: 10.1177/1933719111405068. [DOI] [PubMed] [Google Scholar]
- 47.Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128:216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- 48.Hirosawa N, Yano K, Suzuki Y, Sakamoto Y. Endocrine disrupting effect of di-(2-ethylhexyl)phthalate on female rats and proteome analyses of their pituitaries. Proteomics. 2006;6:958–971. doi: 10.1002/pmic.200401344. [DOI] [PubMed] [Google Scholar]
- 49.Ma M, Zhang Y, Pei X, Duan Z. [Effects of di-(2-ethylhexyl) phthalate exposure on reproductive development and PPARs in prepubertal female rats] Wei Sheng Yan Jiu. 2011;40:688–692. 697. [PubMed] [Google Scholar]
- 50.Liu T, Li N, Zhu J, Yu G, Guo K, Zhou L, Zheng D, Qu X, Huang J, Chen X, Wang S, Ye L. Effects of di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-ovarian axis in adult female rats. Reprod Toxicol. 2014;46:141–147. doi: 10.1016/j.reprotox.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod. 2014;90:136. doi: 10.1095/biolreprod.114.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laskey JW, Berman E. Steroidogenic assessment using ovary culture in cycling rats: effects of bis(2-diethylhexyl)phthalate on ovarian steroid production. Reprod Toxicol. 1993;7:25–33. doi: 10.1016/0890-6238(93)90006-s. [DOI] [PubMed] [Google Scholar]
- 53.Svechnikova I, Svechnikov K, Soder O. The influence of di-(2-ethylhexyl) phthalate on steroidogenesis by the ovarian granulosa cells of immature female rats. J Endocrinol. 2007;194:603–609. doi: 10.1677/JOE-07-0238. [DOI] [PubMed] [Google Scholar]
- 54.Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol. 2010;242:224–230. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol. 2012;258:288–295. doi: 10.1016/j.taap.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gillum N, Karabekian Z, Swift LM, Brown RP, Kay MW, Sarvazyan N. Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicol Appl Pharmacol. 2009;236:25–38. doi: 10.1016/j.taap.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lenie S, Smitz J. Steroidogenesis-disrupting compounds can be effectively studied for major fertility-related endpoints using in vitro cultured mouse follicles. Toxicol Lett. 2009;185:143–152. doi: 10.1016/j.toxlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Mlynarcikova A, Nagyova E, Fickova M, Scsukova S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol In Vitro. 2009;23:371–377. doi: 10.1016/j.tiv.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 59.Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, Mazzeo P, Petraglia F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod. 2003;18:1512–1515. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- 60.Pak VM, Nailon RE, McCauley LA. Controversy: neonatal exposure to plasticizers in the NICU. MCN Am J Matern Child Nurs. 2007;32:244–249. doi: 10.1097/01.NMC.0000281965.45905.c0. [DOI] [PubMed] [Google Scholar]
- 61.Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 2009;12:157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- 62.Sjoberg PO, Bondesson UG, Sedin EG, Gustafsson JP. Exposure of newborn infants to plasticizers. Plasma levels of di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate during exchange transfusion. Transfusion. 1985;25:424–428. doi: 10.1046/j.1537-2995.1985.25586020115.x. [DOI] [PubMed] [Google Scholar]
- 63.Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 64.Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- 65.Craig ZR, Hannon PR, Wang W, Ziv-Gal A, Flaws JA. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod. 2013;88:23. doi: 10.1095/biolreprod.112.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- 67.Cannon JD, Cherian-Shaw M, Lovekamp-Swan T, Chaffin CL. Granulosa cell expression of G1/S phase cyclins and cyclin-dependent kinases in PMSG-induced follicle growth. Mol Cell Endocrinol. 2007;264:6–15. doi: 10.1016/j.mce.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu T, Hirai Y, Miyamoto A. Expression of cyclins and cyclin-dependent kinase inhibitors in granulosa cells from bovine ovary. Reprod Domest Anim. 2013;48:e65–69. doi: 10.1111/rda.12177. [DOI] [PubMed] [Google Scholar]
- 69.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 70.Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol. 1992;43:779–804. doi: 10.1016/0960-0760(92)90307-5. [DOI] [PubMed] [Google Scholar]
- 71.Penning Molecular endocrinology of hydroxysteroid dehydrogenases. Endocrine Reviews. 1997:281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 72.Falck B. Site of production of oestrogen in the ovary of the rat. Nature. 1959;184(Suppl 14):1082. doi: 10.1038/1841082a0. [DOI] [PubMed] [Google Scholar]
- 73.Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60:51–89. doi: 10.1152/physrev.1980.60.1.51. [DOI] [PubMed] [Google Scholar]
- 74.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 75.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- 76.Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt JS, Schaedlich K, Borromeo V. Exposure to di(2-ethyl-hexyl) phthalate (DEHP) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology. 2012;153:937–948. doi: 10.1210/en.2011-1450. [DOI] [PubMed] [Google Scholar]
- 77.Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994;128:224–228. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- 78.Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172:217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- 79.Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201:133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 80.Reinsberg J, Wegener-Toper P, van der Ven K, van der Ven H, Klingmueller D. Effect of mono-(2-ethylhexyl) phthalate on steroid production of human granulosa cells. Toxicol Appl Pharmacol. 2009;239:116–123. doi: 10.1016/j.taap.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 81.Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- 82.Li N, Liu T, Zhou L, He J, Ye L. Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol. 2012;34:869–875. doi: 10.1016/j.etap.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 83.Xu C, Chen JA, Qiu Z, Zhao Q, Luo J, Yang L, Zeng H, Huang Y, Zhang L, Cao J, Shu W. Ovotoxicity and PPAR-mediated aromatase downregulation in female Sprague-Dawley rats following combined oral exposure to benzo[a]pyrene and di-(2-ethylhexyl) phthalate. Toxicol Lett. 2010;199:323–332. doi: 10.1016/j.toxlet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Ambruosi B, Uranio MF, Sardanelli AM, Pocar P, Martino NA, Paternoster MS, Amati F, Dell'Aquila ME. In vitro acute exposure to DEHP affects oocyte meiotic maturation, energy and oxidative stress parameters in a large animal model. PLoS One. 2011;6:e27452. doi: 10.1371/journal.pone.0027452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inada H, Chihara K, Yamashita A, Miyawaki I, Fukuda C, Tateishi Y, Kunimatsu T, Kimura J, Funabashi H, Miyano T. Evaluation of ovarian toxicity of mono-(2-ethylhexyl) phthalate (MEHP) using cultured rat ovarian follicles. J Toxicol Sci. 2012;37:483–490. doi: 10.2131/jts.37.483. [DOI] [PubMed] [Google Scholar]
- 86.Craig ZR, Singh J, Gupta RK, Flaws JA. Co-treatment of mouse antral follicles with 17beta-estradiol interferes with mono-2-ethylhexyl phthalate (MEHP)-induced atresia and altered apoptosis gene expression. Reprod Toxicol. 2014;45:45–51. doi: 10.1016/j.reprotox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 89.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21:219–222. doi: 10.1097/gco.0b013e32832924ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- 92.Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 93.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 94.Peretz J, Flaws JA. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2013;271:249–256. doi: 10.1016/j.taap.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peretz J, Neese SL, Flaws JA. Mouse strain does not influence the overall effects of bisphenol a-induced toxicity in adult antral follicles. Biol Reprod. 2013;89:108. doi: 10.1095/biolreprod.113.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.