Abstract
Perinatal exposure of rats and mice to the typically reported 4mg/g bd wt dose of monosodium glutamate (MSG) results in a complete block in GH secretion as well as obesity, growth retardation and a profound suppression of several cytochrome P450s, including CYP2C11, the predominant male-specific isoform - all irreversible effects. In contrast, we have found that a lower dose of the food additive, 2mg/g bd wt on alternate days for the first 9 days of life results in a transient neonatal depletion of plasma GH, a subsequent permanent overexpression of CYP2C11 as well as subnormal (mini) GH pulse amplitudes in an otherwise normal adult masculine episodic GH profile. The overexpressed CYP2C11 was characterized by a 250% increase in mRNA, but only a 40 to 50% increase in CYP2C11 protein and its catalytic activity. Using freshly isolated hepatocytes as well as primary cultures exposed to the masculine-like episodic GH profile, we observed normal induction, activation, nuclear translocation and binding to the CYP2C11 promoter of the GH-dependent signal transducers required for CYP2C11 transcription. The disproportionately lower expression levels of CYP2C11 protein were associated with dramatically high expression levels of an aberrant, presumably nontranslated CYP2C11 mRNA, a 200% increase in CYP2C11 ubiquitination and a 70–80% decline in miRNAs associated, at normal levels, with a suppression of CYP2C expression. Whereas the GH-responsiveness of CYP2C7 and CYP2C6 as well as albumin was normal in the MSG-derived hepatocytes, the abnormal expression of CYP2C11 was permanent and irreversible.
Keywords: CYP2C11, growth hormone, MSG, sexual dimorphism, SOCS2, STAT5b, GHR
INTRODUCTION
The cytochrome P450 monooxygenases (CYP) are an ancient family of hemoenzymes that catalyze a large variety of essential metabolites, which include the synthesis and the deactivation of adrenal, gonadal and thyroid hormones, eicosanoids, bile acids and fatty acids, as well as the detoxification of innumerable drugs and environmental chemicals - hence their ubiquitous presence throughout all species where hundreds of isoforms have evolved to catalyze a countless number of substrates (Hannemann et al., 2007; Nebert and Dieter, 2000). In the case of vertebrates, the CYPs are most prominent in the liver where they can be expressed as dozens of different isoforms in each species. Those species examined, including trout, chickens, ferrets, dogs, goats, pigs, hamsters, cattle, mice, rats (cf. Shapiro et al., 1995) and not surprisingly humans (Elfarra et al., 1998; O’Malley et al., 1971), all exhibit varying degrees of sex differences in adult drug metabolism. Studies extended to the molecular level have shown that the sexual dimorphisms in adult drug metabolism are due to the existence of multiple forms of sex-dependent hepatic CYPs whose sexually dimorphic expression is regulated by growth hormone (GH) (Dhir et al., 2006; Legraverend et al., 1992; Ryan and Levin, 1990; Shapiro et al., 1995). In fact, the only endogenous factor known to maintain sexually dimorphic expression of hepatic CYPs is GH (Legraverend et al., 1992; Shapiro et al., 1995). More specifically, it is not the amount of circulating GH per se, but rather the sexually dimorphic ultradian rhythms in plasma GH that regulate sex-dependent isoforms of CYP. Although there are some variations between species, in general the adult male GH profile is referred to as “episodic” and is characterized by several daily bursts of hormone separated by lengthy undetectable or barely detectable GH concentrations. In contrast, the adult female GH profile is considered “continuous” as there are far more secretory bursts of hormone, often at lower amplitudes, separated by briefer interpulse periods generally containing measurable levels of GH. The fundamental regulatory difference between male and female GH profiles in all species examined is the much longer GH-devoid interpulse period observed in adult males (Agrawal and Shapiro, 2001; Legraverend et al., 1992; Pampori and Shapiro, 1996; Waxman et al., 1991). In addition to sex differences in CYP expression, this masculine GH secretory signal is responsible, at least in part, for sex differences in growth rates, lean body mass; cardiovascular, bone, adipose, and muscle function; protein, carbohydrate, lipid and electrolyte metabolism (Bengtsson, 1999; Burman et al., 1997; Hubina et al., 2004; Jørgensen and Christiansen, 2005; Kuromaru et al., 1998; Span et al., 2001) and expression levels of hepatic insulin-like growth factor-1 (IGF-1), IGF binding protein and GH binding protein (Hubina et al., 2004; Jansson et al., 1985; Johansson, 1999; Johansson et al., 1999; Jørgensen and Christiansen, 2005; Soares et al., 2004).
In spite of this clear dimorphism in hormonal secretory patterns, GH regulation of sex-dependent CYPs is rather complex. While expression of some of the sexually dimorphic CYPs are dependent upon exposure to the masculine plasma GH profile, others are regulated by the feminine GH profile, while still others respond, albeit at different levels, depending upon their gender, to both profiles and some sex-dependent CYP isoforms are best expressed in the absence of GH. In addition to their inductive effects, the sexually dimorphic circulating GH profiles can be suppressive. That is, some of the isoforms are suppressed by the masculine GH profile, others by the feminine profile, and still others, to different degrees, by both profiles (Legraverend et al., 1992; Pampori and Shapiro, 1999; Waxman, 1992). Another layer of complexity is observed in the fact that each sex-dependent CYP isoform appears to be expressed or suppressed by different signaling elements in the GH profiles. These signals have been identified as the amplitudes, frequencies, and/or durations of the GH pulse and interpulse periods as well as the mean plasma concentration (Agrawal and Shapiro, 2000; Pampori and Shapiro, 1996, 1999). The cellular mechanism by which each CYP “discriminator” recognizes its selective signal in the GH profiles is under nascent investigation.
CYP2C11 is the predominant CYP isoform in male rats, accounting for at least 50% of the total content of hepatic CYP (Morgan et al., 1985). Whereas exposure to the feminine plasma GH profile of continuous GH secretion completely suppresses CYP2C11 expression, elimination of GH from the circulation (i.e. hypophysectomy, HYPOX) permits a modest expression level of 20–25% of normal (Pampori and Shapiro, 1996, 1999). It is, however, exposure to the masculine profile of episodic GH secretion that is solely responsible for the high levels of CYP2C11 mRNA and protein expressed in male rat liver (Agrawal and Shapiro, 2000; Legraverend et al., 1992). More specifically, it is a requisite periodic absence of GH from the circulation observed during the interpulse periods that signal hepatic CYP2C11 transcription (Agrawal and Shapiro, 2001; Waxman et al., 1991). Although essential for male-like expression levels of CYP2C7 and CYP2A1 (Agrawal and Shapiro, 2000), the high amplitude pulses secreted every 3–4h that are so characteristic of the masculine GH secretory profile, are not required for CYP2C11 expression (Agrawal and Shapiro, 2000, 2001). In fact, a reduction in the GH peak heights of 80–90% in an otherwise physiologic masculine profile allows for a modest 20–30% overexpression of CYP2C11 protein and its catalytic activity. Unexpectedly, however, exposure to these mini-GH pulses induces a disproportionate >200% overexpression of the CYP2C11 transcript (Agrawal and Shapiro, 2000, 2001; Pampori and Shapiro, 1994).
Neonatal administration of monosodium glutamate (MSG) at 4mg/g bd wt for 5 injections every other day for the first 9 days of life destroys the arcuate nucleus, permanently preventing GH secretion and CYP2C11 expression (Pampori et al., 1991a; Shapiro et al., 1989). In contrast, smaller neonatal doses (i.e., 4mg/g bd wt on only days 1 and 3 of life or 2mg/g bd wt for the full 5 injections) of the food additive to male pups results in the permanent secretion of mini-GH pulses (10–20% normal amplitudes) in otherwise normal masculine profiles and a concomitant overexpression of CYP2C11; mRNA almost 300% above normal and protein and catalytic activity about 20–40% above normal (Pampori et al., 1991a; Pampori and Shapiro, 1994). Furthermore, an undetermined portion of the transcript is composed of a presumably nontranslated, intron-retained variant containing a premature stop codon which leads to nonsense-mediated RNA decay and/or translation of a truncated protein highy vulnerable to swift degradation (Pampori and Shapiro, 2000).
In the present study, we have investigated the mechanism(s) by which perinatal exposure to the lower dose of MSG (2mg/g bd wt) results in the overexpression of CYP2C11. In particular, we wanted to determine whether the overexpression of CYP2C11 is a result of responsive, but reversible alterations in its dependent signal transduction pathway induced by the GH mini pulse profile or, in addition to the abnormal GH secretory profile, are there other irreversible defects in the mechanism(s) of CYP2C11 expression programmed by perinatal MSG?
MATERIALS AND METHODS
Animals
Rats were housed in the University of Pennsylvania Laboratory Animal Resources facility under the supervision of certified laboratory animal medicine veterinarians and were treated according to a research protocol approved by the Institutional Animal Care and Use Committee of the University. Housing conditions as well as breeding and treatment protocols were reported previously (Pampori et al., 1991a; Shapiro et al., 1989). Basically, newborn male Sprague-Dawley rats [Crl:CD(SD)BR] were treated with MSG, 2mg/g bd wt (Sigma Chemical Co., St. Louis, MO, USA) on alternate days, starting within 24 h of birth for a total of 5 s.c. injections. Controls received an equivalent molar amount of NaCl diluent (12µl/g bd wt) or no treatment at all. The Lee index and hexobarbital sleep times were determined as described previously (Pampori et al., 1991a; Shapiro et al., 1989).
Catheter Implantation, Serial Blood Collection and GH Determination
Repetitive blood samples (40µl) were obtained at 15 min intervals from unrestrained, unstressed and completely conscious neonatally untreated, MSG- and NaCl-treated adult rats outfitted with our mobile catheterization apparatus (Pampori et al., 1991b) for 8 continuous hours. In addition, trunk blood was collected from individual pups, in the morning from 09:00 to 10:00, representing all litters, at 2, 5, 8 and 11 days of age from both MSG- and vehicle-treated male rats. Circulating plasma GH profiles were determined by a sensitive sandwich ELISA method according to Steyn et al. (2011) with modifications by us (Das et al., 2013).
Primary Hepatocyte Culture
Preparation of hepatocytes from control and neonatally MSG-treated adult rats were performed with minor modifications (Thangavel et al., 2006; Thangavel and Shapiro, 2007) by in situ perfusion of collagenase through the portal vein of the anesthetized rats following standard protocol (Strom et al., 1996). The viability of the initial cell suspension of hepatocytes was typically between 80–90% (with trypan blue). Some of the hepatocytes were flash frozen (preplated cells) while the remaining cells were plated at a density of 3 × 106 viable cells per well in six-well plates previously coated with matrigel. Media and culture conditions were reported previously (Thangavel et al., 2006; Thangavel and Shapiro, 2007).
Hormonal Conditions
To replicate the masculine episodic GH profile, hepatocytes were exposed to recombinant rat GH (0.2 ng/ml) (NHPP, Torrance, CA, USA) for 30 min followed by two careful washings with GH-free media that remained in the wells for 11.5 h, at which time the cells were again washed and exposed to the next 30 min pulse of GH (Thangavel et al., 2006; Thangavel and Shapiro, 2007). On the 6th day, cells were harvested 30 min following the addition of the last GH pulse. To replicate the feminine continuous GH profile, hepatocytes were constantly exposed to a 2ng/ml concentration of the rat GH for 12 h, after which time the cells were washed and exposed for another continuous 12 h of the hormone (Thangavel et al., 2004; Thangavel and Shapiro, 2008). Cells were harvested on the 6th day, 30 min after the final GH treatment. Instead of GH, control cells were exposed to GH vehicle, but otherwise identical protocols.
Western Blot and Immunoprecipitation
Whole cell lysates and nuclear fractions (Thangavel and Shapiro, 2007) were extracted from freshly isolated preplated hepatocytes as well as cultured primary hepatocytes 30 min after the last GH pulse and the protein concentrations of the cell lysates were measured by using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Whole cell lysate protein was electrophoresed and electroblotted onto nitrocellulose membranes for immunoblotting. Accordingly, the blots were probed with antibodies against CYP2C11 (Detroit R & D, Inc., Franklin, MI, USA), anti-CYP2C7 (a gift from Dr. An Huang, New York Medical College, Valhalla, New York, USA), anti-rat CYP2C6, anti-rat albumin, anti-rat GH receptor (GHR) and anti-suppressors of cytokine signaling 2 (SOCS2) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Signals were normalized to the expression of β-actin (Sigma Chemical Co., St. Louis, MO, USA). Nuclear fractions were immunoprecipitated with signal transducers and activators of transcription 5b (STAT5b) antibodies (Santa Cruz Biotechnology) as described previously (Thangavel and Shapiro, 2007). Next, the immunoprecipitates were probed with anti-phosphotyrosine (EMD Millipore, Billerica, MA, USA). The protein signals were scanned and the densitometric units were obtained as integrated density values quantitated by using a FluorChem IS-8800 Imager (Alpha Innotech, San Leandro, CA, USA) software supplied with the gel documentation system.
qRT-PCR
CYP2C11, CYP2C7, GHR and albumin gene expressions were determined by qRT-PCR using the TaqMan® assay (Rn10502203_m1, Rn10529602_Mh, Rn00567298_m1 and Rn0592480_m1) and PSMC4 (Rn00821599_g1) as the housekeeping gene on an Applied Biosystem step-one plus q-PCR instrument as per the usual manufacturer’s recommended protocol (Life Technologies, Grand Island, NY, USA). Using custom prepared probes and primers (Integrated DNA Technologies, Coralville, IA, USA) the intron retained-CYP2C11 (Probe: 5’-/56-FAM/TAC TGC CTC/ZEN/AGG TCT CCT CCA CTC C/3IABkFQ/-3’; Primer 1: 5’-CGT GTT AGT GGA TTC TGG GAG-3’; Primer 2: 5’-TTG TTC CCC TCC ATT AAG CC-3’), and CYP2C6 (Probe: 5’-/56-FAM/TGC CAA CCA /ZEN/CAC GAT CAA TCT CTT CC/3IABkFQ/-3’; Primer 1: 5’AAC ACT TCT ACT GTG ACC AAC C-3’; Primer 2: 5’-TGA ATC ATG GCA TCT GTG TAG G-3’) were measured by qRT-PCR with PPIA (Rn00690933_m1) as the housekeeping gene. SOCS2 expression was determined using a SYBR green qRT-PCR assay with cyclophilin A as the housekeeping gene. The PCR primers for SOCS2 (F: 5’-TTA AAA GAG GCG CCA GAA GGA -3’; R: 5’-GCC CGG CTG ATG TCT TAA CA-3’) and cycophilin A (F: 5’-CGA CTG TGG ACA GCT CTA AT -3’; R: 5’-CCT GAG CTA CAG AAG GAA TG -3’) were synthesized by Integrated DNA Technologies on an Applied Biosystem step-one plus q-PCR instrument.
Total RNA from freshly isolated hepatocytes as well as hepatocyte cultures were isolated using Trizol ® reagent (Life Technologies) purified with the Qiagen RNeasy mini kit and treated with DNase I in order to remove any trace of genomic DNA using RNase-Free DNase Set (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. RNA concentrations and purity were determined by UV spectrophotometry (A260/280 > 1.8 and A260/230 > 1.7) and integrity was verified by the intensities of 28S and 18S rRNA bands on a denaturing agarose gel visualized on a FluorChem IS-8800 Imager (Alpha Innotech). cDNA synthesis was completed using the High Capacity RNA-to-cDNA kit (Life Technologies) as per instructions with appropriate no-reverse transcription (−RT) and non-template controls.
Chromatin Immunoprecipitation (ChIP) Assays
A ChIP assay was performed on cultured hepatocytes 30 min after the last episodic hormone pulse on the 6th day in culture as described (Thangavel and Shapiro, 2007) with slight modifications. Briefly, for the cross-linking reaction hepatocytes were treated with 1% formaldehyde for 10 min at room temperature and the reaction was stopped by adding glycine to a final concentration of 0.125M. The cells were then harvested and washed three times with ice-cold Dulbecco’s PBS buffer containing 5 mM EDTA. The nuclei were subsequently isolated and lysed. The lysate was sonicated to generate DNA fragments with an average length of 100–1000bp. After removal of cell debris by centrifugation, the chromatin concentration was measured and about 10% of the chromatin was kept as an input, and the rest of the chromatin was diluted 3-fold. Equal concentrations of chromatin were precleared with protein A agarose beads in the presence of 1 mg/ml BSA and 2µg of sonicated salmon sperm DNA to reduce the nonspecific background. After removal of beads by centrifugation, 2µg of STAT5b-specific antibody (Santa Cruz Biotechnology, Inc.) was added and kept at 4 C overnight on a rotary platform. The immune complexes were collected by centrifugation after adding protein A agarose beads and kept at 4 C for 1 h. The immunoprecipitates were washed sequentially and eluted. To reverse the formaldehyde cross-linking elutes were heated at 65 C for 6 h. DNA fragments were purified by QIAquick Spin kit (Qiagen, Germantown, MD, USA). DNAs were analyzed by semiquantitative PCR as previously reported (Thangavel and Shapiro, 2007) by specific and nonspecific primers of the STAT5b binding region of the CYP2C11 promoter.
Ubiquitination
As previously described (Wang et al., 2011), hepatic whole cell lysate protein (200mg) from neonatally NaCl or MSG treated adult primary hepatocytes exposed to the masculine episodic GH profile or vehicle alone were incubated overnight at 4 C with the highly specific anti-CYP2C11 used in the western blotting in 100µl of modified immunoprecipitation buffer followed by precipitate binding, centrifugation and washings (Banerjee et al., 2013). Non-specific IgG control was also used as a pull-down control to confirm the binding specificity. Proteins were eluted from the gel by boiling for 10 min in 30µl of loading buffer, electrophoresed and immunoblotted with mouse anti-Ub IgG (Santa Cruz Biotechnology).
MicroRNA (miRNA) Isolation and qPCR Array
Total RNA including miRNA was prepared by using Qiazol and miRNeasy mini kit (Qiagen), with DNase treatment. The concentration of total RNA and the RNA quality (260/280 absorbance ratio) of the samples were measured using spectrophotometer and cDNA were synthesized using the miScript II RT kit (Qiagen). The expression analysis of miRNA were determined by the SYBR green-based real-time miRNA qPCR assay method using Rat miFinder RT2 miRNA PCR 96-well plate array (MAR-001A) (SA Biosciences) according to the manufacturer’s guidelines. The array contained a thoroughly researched panel of 84 primers most abundantly expressed and best characterized miRNAs in miRBase, plus a set of six normalization controls as well as controls to asses RNA recovery, reverse transcription performance, and PCR performance. The data were analyzed by using SA Biosciences miRNA PCR Array Data Analysis Software using the ΔΔCT method.
Testosterone Measurement
Plasma testosterone concentrations in adult MSG-treated and control rats were measured by a sensitive competitive ELISA method according to the manufacturer’s recommended protocol (R&D Systems, Minneapolis, MN, USA)
Catalytic Activity
Of the 3 sex-dependent CYPs measured at the mRNA and protein level, CYP2C11, CYP2C7 and CYP2C6, only the former has been shown to exhibit a specific catalytic activity, i.e., CYP2C11-dependent testosterone 2α-hydroxylase (Waxman, 1991) which we measured by our previously reported method (Agrawal et al., 1995a).
Statistics
All the data were subjected to ANOVA. Significant differences were determined with t statistics and the Bonferroni procedure for multiple comparisons.
RESULTS
Control Treatments
The study includes 2 control treatment groups; neonatal rats treated with MSG-vehicle, and neonatal rats that received no treatment. Since there were no significant differences in the measured parameters between the 2 groups, results from the former, i.e. MSG-vehicle treated, were chosen as controls in all figures.
Lee Index
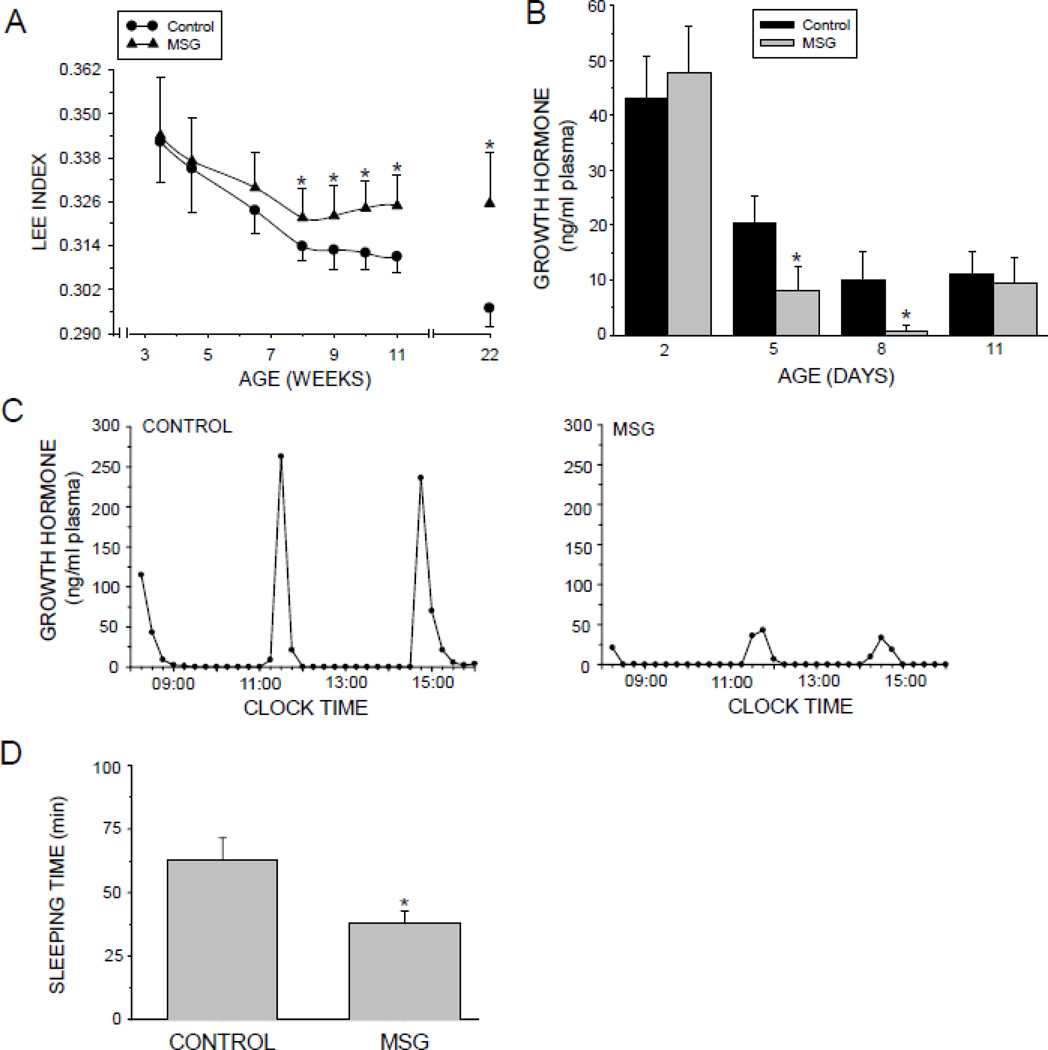
The Lee index is a measure of obesity; the higher the value, the greater the obesity. At 8 weeks of age the Lee index of the neonatally MSG-treated rats became significantly greater than controls with this trend continuing through the conclusion of the study at 22 weeks of age (Fig. 1A). While the cause of obesity can be very complex including genetic, hormonal, metabolic, dietary and environmental factors, GH deficiency can be a contributory factor (Gathercole et al., 2013).
Fig. 1.
Developmental effects of neonatal MSG on obesity, growth hormone secretion and drug action. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7, and 9. A. Post-weaning maturational Lee index profile of male rats treated neonatally with MSG. Results are presented as the mean ±SD of at least 10 rats/data point. *P<0.01 vs controls. B. Plasma growth hormone of male rats pups being treated with either MSG or vehicle. Results are presented as the mean ±SD of at least 7 pups/growp. * P<0.01 vs Control pups. C. Plasma growth hormone profiles in individual, representative adult male rats that were either neonatally administered MSG or vehicle. Plasma levels of circulating growth hormone were obtained from individual undisturbed catheterized rats for 8 continuous hours at 15 min intervals. Similar results were obtained from 4 to 5 additional animals in each treatment group. D. Hexobarbital-induced sleeping times in adult male rats that were either neonatally administered MSG or vehicle. Sleeping times were determined following an ip injection of hexobarbital (150mg/kg bd wt). Results are presented as the means ±SD of at least 10 rats/group. *P<0.01 vs Control rats.
Neonetal GH Levels
Plasma GH concentrations were determined in pups at 2, 5, 8, and 11 days of age while being treated with either MSG or NaCl- vehicle (Fig.1B). In agreement with earlier reports (Kacsoh et al., 1989; Oliver et al., 1982), plasma GH levels in control pups were maximum the first few postpartum days of life, thereafter declining by 50% (5 days of age) to 75% (8 and 11days of age). Neonetal MSG treatment had no observable effect on plasma GH at 2 days of age, but did cause a dramatic decline in circulating GH at 5 and 8 days of life to barely detectable levels, returning to normal concentrations at 11 days of age.
Circulating GH Profiles
Plasma GH profiles are presented as schematic representations of the actual circulating profiles (Fig. 1C). The control rats secreted the typical masculine profile (Agrawal et al., 1995b) characterized by episodic pulses containing amplitudes of ~200–250 ng/ml with durations of ~1 h separated by interpulse periods of 2 to 3 h devoid of any measurable hormone. Neonatally MSG-treated rats exhibited typical masculine profiles of GH release, except that the amplitudes of the ultradian pulses were reduced to about 10 to 20% (i.e., 20–50 ng/ml) of normal male levels. Otherwise, like control males, the peaks occurred around every 3 to 4 h and the intervening 2 to 3 h troughs had undetectable concentrations of hormone.
Serum Testosterone
At around 22 weeks of age, serum concentrations of testosterone were measured in 6 rats from each neonatal treatment group. We observed no statistically significant differences in circulating testosterone levels between NaCl controls (5.02 ± 0.78 ng/ml) and MSG-treated rats (5.97 ± 1.09 ng/ml; x̄ ± sd), indicating that abnormalities in the expression of male-dependent CYP2C11 are not attributable to testosterone.
Sleeping Times
Hexobarbital-induced sleep time is an in vivo measure of drug metabolism competency (MacLeod et al., 1987; Ryan and Levin, 1990). When given the same per kg bd wt dose of hexobarbital, adult rats neonatally treated with MSG slept only 60% as long as control rats suggesting a surfeit in the CYP isoforms contributing to hexobarbital metabolism (Fig. 1D).
CYP2C11
CYP2C11 is the predominant male-dependent form comprising >50% of the total CYPs in male rat liver (Morgan et al., 1985; Waxman, 1991). With the exception of measurements using highly sensitive detection procedures, e.g. qRT-PCR, CYP2C11 is basically undetectable in female rat liver explaining its designation as male-specific (Agrawal and Shapiro, 2000; Pampori and Shapiro, 1996; Ryan and Levin, 1990; Waxman, 1991). Moreover, CYP2C11 is the principal contributor to hexobarbital metabolism in the rat (Ryan and Levin, 1990).
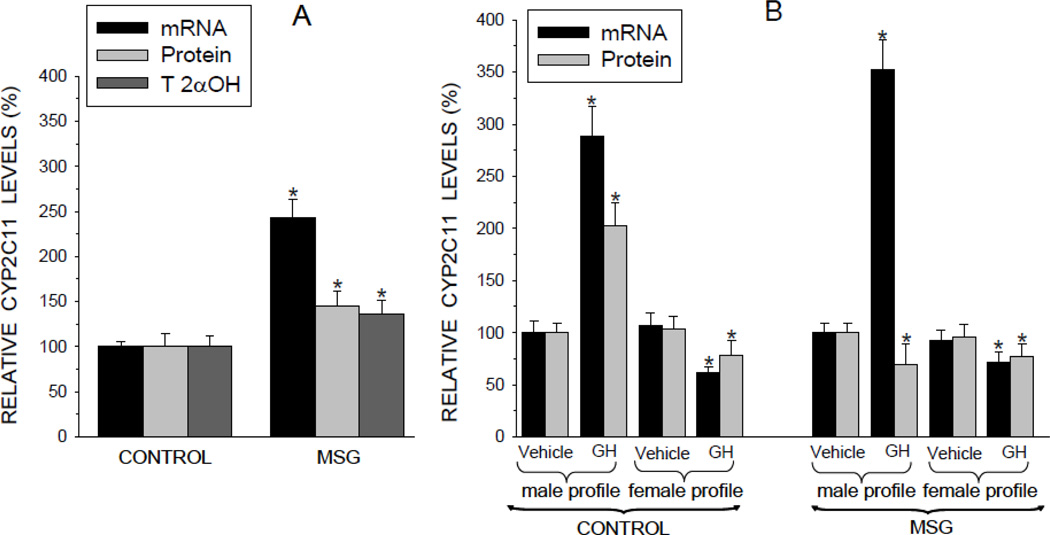
CYP levels in freshly isolated hepatocytes reflected in vivo concentrations (Pampori et al., 1991a; Pampori and Shapiro, 1994). That is, expression levels of CYP2C11 were significantly elevated in the MSG-treated rats explaining, at least in part, the shortened hexobarbital induced sleep times in the affected animals. However, whereas transcript levels of CYP2C11 were increased 2.5 times above controls, protein concentrations and CYP2C11-dependent testosterone 2α-hydroxylase activity levels were elevated only 40 to 50% above normal (Fig. 2A).
Fig. 2.
Relative expression levels [mRNA, protein and testosterone 2α-hydroxylase (T 2αOH) catalytic activity] of the paramount male-specific CYP2C11 isoform in hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7, and 9. A. CYP2C11 levels in freshly isolated preplated hepatocytes. Results are presented as a percentage of mRNA or protein in hepatocytes from control rats arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs control rats. B. Hepatocytes from the same animal were exposed to either the episodic male-like GH profile or the hormone’s vehicle, or the continuous female-like GH profile or vehicle for 6 days. Results are presented as a percentage of mRNA or protein in hepatocytes exposed to vehicle administered in an episodic male-like profile, arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic male-like vehicle profile from rats of the same neonatal treatment, i.e. Control or MSG.
As expected (Thangavel et al., 2006; Thangavel and Shapiro, 2007), after 6 days of exposure to the male-like episodic GH profile, CYP2C11 expression levels (mRNA and protein) in primary hepatocytes derived from control rats more than doubled above baseline concentrations (Fig. 2B). In agreement with its repressive effects (Pampori and Shapiro, 1999), exposure to the female-like continuous GH profile was either ineffective or suppressive. The response of hepatocyte CYP2C11 from the MSG males was similar to that of controls in all measures with the exception of a small suppression in CYP2C11 protein levels following exposure to the episodic GH profile. That is, while the masculine-like in vitro GH profile induced a 3.5 fold increase in CYP2C11 mRNA levels, protein levels of the isoform were reduced ~30%. Similar to controls, exposure to the continuous female-like GH profile suppressed hepatocyte CYP2C11 mRNA and protein concentrations.
Intron-Retained CYP2C11 mRNA
Previously, we had reported (Pampori and Shapiro, 1994) that a submaximal dose of MSG (4 mg MSG/g bd wt injected on days 1 and 3 of life) resulted in an adult secretory profile of GH characterized by mini pulses similar to that reported here, and an accompanying 200% elevation in CYP2C11 mRNA, but only a 30 to 40% increase in protein concentrations. Moreover, we reported that an undetermined amount of the elevated CYP2C11 mRNA was due to the expression of a presumably nontranslated intron-retained transcript associated with a 50% reduction in nuclear splicing capacity (Pampori and Shapiro, 2000).
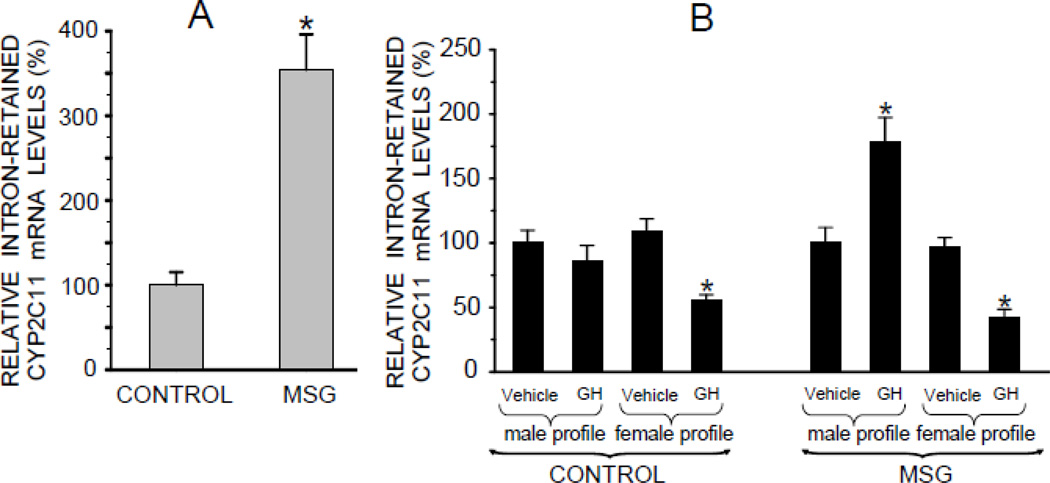
Since the probe used to detect CYP2C11 mRNA (Fig. 2A and B) could not differentiate between the normal and intron-retained transcript, we designed a probe to selectively recognize the last (i.e., retained) intron.
We measured levels of the intron-retained CYP2C11 mRNA in the MSG-derived preplated cells and observed a 3 to 4-fold increase in the concentration of the aberrant transcript as compared to controls (Fig. 3A). Perhaps, then, the dramatic increase in CYP2C11 mRNA in MSG-derived hepatocytes exposed to the episodic GH profile (Fig. 2B) was due to an overexpression of this nontranslatable mRNA variant, explaining the absence of an equally accompanying elevation in CYP2C11 protein levels? In fact, the results do suggest that this is at least a partial explanation. Hepatocytes from the MSG rats exposed to the episodic GH profile exhibited an almost 2-fold increase in levels of the intron-retained CYP2C11 mRNA in contrast to the unresponsiveness of cells from the neonatal controls (Fig. 3B). For the purpose of comparing the effects of the masculine and feminine GH profiles on the levels of the intron-retained CYP2C11 mRNA in the NaCl and MSG-treated rats, transcript concentrations in hepatocytes exposed to the GH diluent were arbitrarily designated 100% in both groups. In fact, however, the aberrant mRNA levels in diluent-treated cells from NaCl and MSG rats reflected the preplated concentrations, i.e., several fold higher in the cultured hepatocytes from the MSG-treated rats (data not presented). This finding suggests that the episodic GH induced several fold increase in CYP2C11 mRNA of the MSG-derived hepatocytes (Fig. 2B) is likely a result of a considerable overexpression of the aberrant transcript.
Fig. 3.
Relative mRNA expression levels of an intron-retained male-specific CYP2C11 isoform in hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7 and 9. A. Intron-retained CYP2C11 levels in freshly isolated preplated hepatocytes. Results are presented as a percentage of mRNA in hepatocytes from control rats arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs control rats. B. Hepatocytes from the same animal were exposed to either the episodic male-like GH profile or the hormone’s vehicle, or the continuous female-like GH profile or vehicle for 6 days. Results are presented as a percentage of mRNA in hepatocytes exposed to vehicle administered in an episodic male-like profile, arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic male-like vehicle profile from rats of the same neonatal treatment, i.e. Control or MSG.
GHR
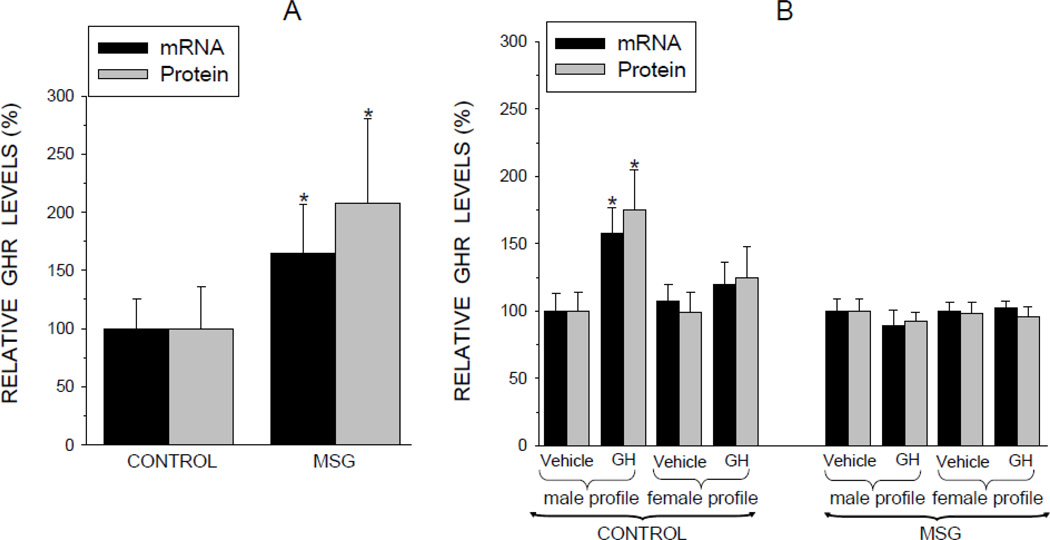
The high expression levels of CYP2C11 found in the male rat are dependent on the episodic GH profile which in turn requires functional GHR. In vivo exposure to the mini GH pulses characteristic of our neonatally MSG-treated adult male rats resulted in a 60 to 100% overexpression of GHR mRNA and protein, respectively, as reflected in the preplated cells (Fig. 4A). The large standard deviations are characteristic of the variable levels of the GHR observed during the different phases (i.e., pulse and interpulse periods) of the circulating GH profile (Bick et al., 1992).
Fig. 4.
Relative expression levels (mRNA and protein) of growth hormone receptor (GHR) in hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7 and 9. A. GHR levels in freshly isolated preplated hepatocytes. Results are presented as a percentage of mRNA or protein in hepatocytes from control rats arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs control rats. B. Hepatocytes from the same animal were exposed to either the episodic male-like profile or the hormone’s vehicle, or the continuous female-like GH profile or vehicle for 6 days. Results are presented as a percentage of mRNA or protein in hepatocytes exposed to vehicle administered in an episodic male-like profile arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic male-like vehicle profile from rats of the same neonatal treatment, i.e. Control or MSG.
In vitro exposure of control cells to the masculine-like GH profile increased GHR expression levels by over 50% whereas the feminine-like GH profile had no inductive effect (Fig. 4B). GHR expression levels in primary hepatocytes from the MSG-treated rats were unresponsive to both sex-dependent GH profiles. It is worth noting that the in vitro protocol was developed to mimic the normal physiologic masculine GH profile in cell culture. In the case of the MSG-treated rats, the “normal” profile is composed of mini pulses, possibly explaining why the GHR in the affected hepatocytes were unresponsive to the in vitro protocol, whereas in vivo levels were above normal.
Although episodic GH was unable to induce above baseline concentrations of GHR in the MSG-treated hepatocytes, it was still possible that the cells expressed sufficient GHR levels to mediate the action of the hormone. In fact, depending on the in vitro treatment, GHR expression levels were up to ~50 to 100% greater in the hepatocytes derived from the MSG-treated rats as compared to controls reflecting preplated concentrations (data not presented). Accordingly, we examined the in vivo and in vitro responsiveness of other GH-dependent CYPs in the MSG-treated rats.
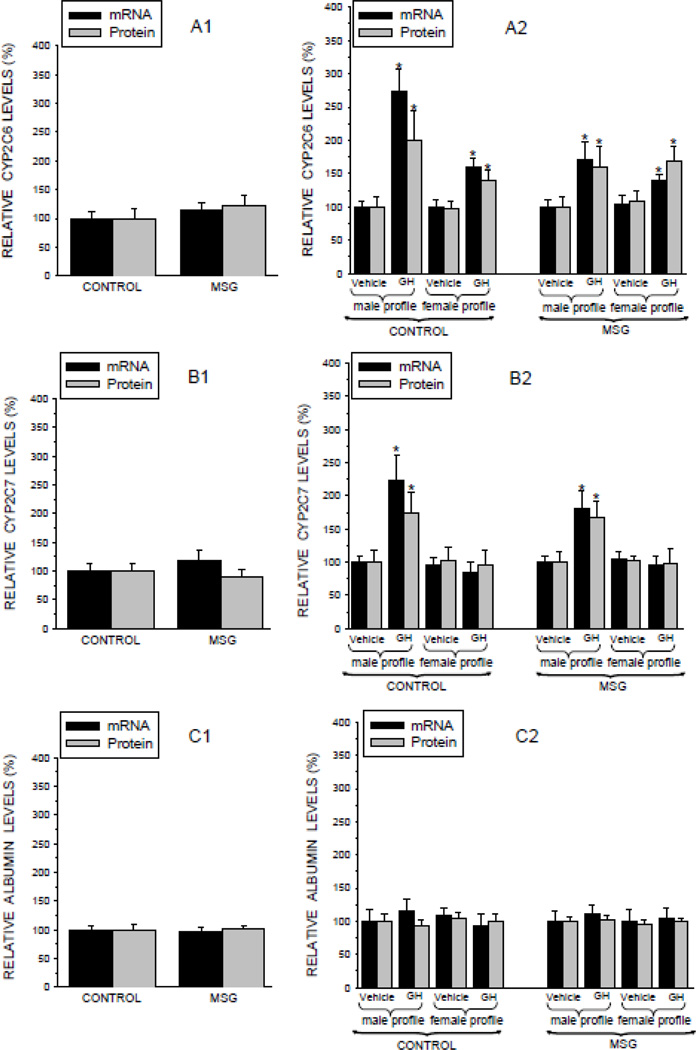
CYP2C6, CYP2C7 and Albumin
CYP2C6 and CYP2C7 are major hepatic isoforms designated female-predominant because they are expressed at greater concentrations in female than male rats. Regardless, both isoforms are GH-dependent in both sexes. It is just that the feminine continuous GH profile is more inductive in females than is the masculine episodic GH profile in males (Pampori and Shapiro, 1999; Thangavel et al., 2004). In vivo-like levels of CYP2C6 (Fig. 5A1) and CYP2C7 (Fig. 5B1) in the preplated hepatocytes were similar in NaCl- and MSG-treated rats. In vitro expression levels (mRNA and protein) of both isoforms were significantly increased in hepatocytes from NaCl (controls) and MSG rats exposed to the male-like GH profile (Figs. 5A2 and 5B2). Exposure to the female-like GH profile induced similar expression levels of CYP2C6 in hepatocytes derived from both NaCl- and MSG-treated rats, though to a lesser degree than that induced by the episodic GH profile (Fig. 5A2). In contrast, CYP2C7 levels were unresponsive to the continuous GH profile in cells obtained from both neonatal treatment groups.2 These results indicate that the long term effects of neonatal exposure to the 2mg/g bd wt dose of MSG on adult drug metabolism appear to be limited to defects in CYP2C11 expression. Moreover, our finding that adult expression levels of hepatic albumin, both sex-and GH-independent (Sharma et al., 2012), were unaffected by neonatal MSG (Figs. 5C1 and 5C2) further supporting the specificity of the MSG-induced defect to mechanisms regulating CYP2C11 expression.
Fig. 5.
Relative expression levels (mRNA and protein) of two CYP isoforms and albumin in hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7 and 9. Female-predominant CYP2C6 (A1), female-predominant CYP2C7 (B1), and sex-independent albumin (C1) levels in freshly isolated preplated hepatocytes. Results are presented as a percentage of mRNA or protein in hepatocytes from control rats arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs control rats. Hepatocytes from the same animal were exposed to either the episodic male-like GH profile or the hormone’s vehicle, or the continuous female like-GH profile or vehicle for 6 days. CYP2C6 (A2), CYP2C7 (B2) and albumin (C2) results are presented as a percentage of mRNA or protein in hepatocytes exposed to vehicle administered in an episodic male-like profile arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic male-like vehicle profile from rats of the same neonatal treatment, i.e. Control or MSG.
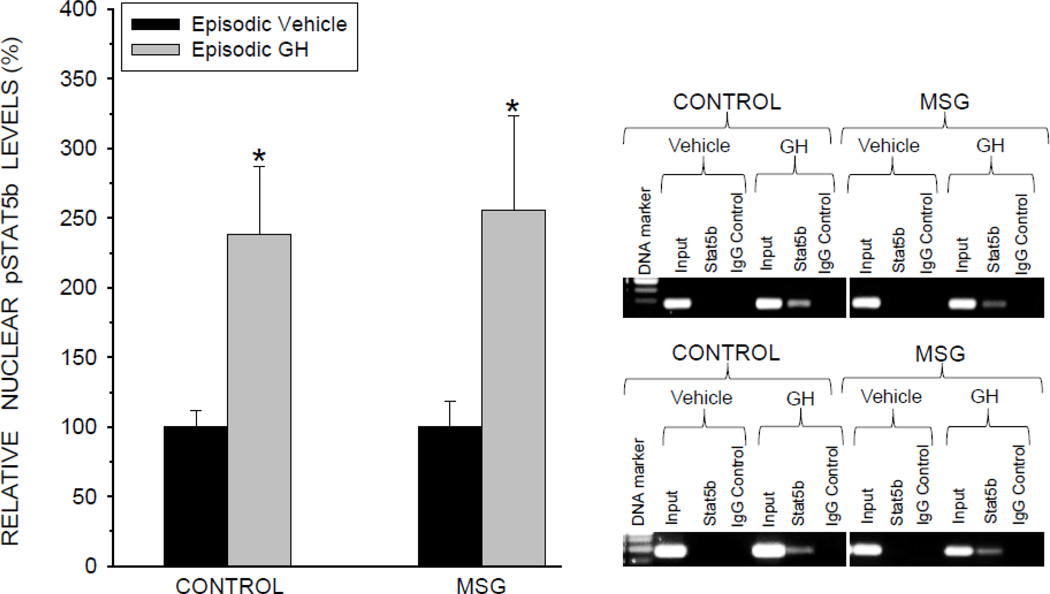
STAT5b
The terminal transducer in the signaling transduction pathway mediating episodic GH induction of CYP2C11 is STAT5b. This transcription factor must be activated (i.e. phosphorylated), homodimerized, translocated to the nucleus where it binds to a promoter site initiating transcription of the CYP2C11 gene (Waxman and Frank, 2000). Primary hepatocytes from both control and MSG-treated male rats exposed to the presence of the masculine episodic GH profile responded with a 2.5-fold increase in activated nuclear pSTAT5b concentrations (Fig. 6). Next, the binding of pSTAT5b to the CYP2C11 promoter was evaluated by ChIP assays. In agreement with the expected sequence of signaling events, binding of pSTAT5b to the promoter occurred in hepatocytes from both control and MSG-treated rats exposed to episodic GH (Fig. 6, right panel). PCR amplification of a negative control using primers flanking the CYP2C11 promoter at a genomic sequence not including the STAT5b binding site was undetectable, demonstrating no measurable non-specific binding (not presented). The binding of pSTAT5b to the CYP2C11 promoter is requisite for CYP2C11 transcription and its detection is in agreement with our finding of GH induction of CYP2C11 mRNA in both the NaCl and MSG-treated rats (Fig. 2B).
Fig. 6.
Nuclear levels of activated signal transducers and activators of transcription (pSTAT5b) in cultured primary hepatocytes derived from male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected either with MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7 and 9. A. Hepatocytes from the same animal were exposed to either the episodic male-like GH profile or the episodic profile containing GH vehicle alone for 6 days. Results are presented as a percentage of nuclear pSTAT5b of hepatocytes from Control treated rats exposed to the episodic vehicle profile arbitrarily designated 100%. Values are means ±SD of at least 5 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic vehicle profile from rats of the same pre-adult treatment, i.e. Control or MSG. Insert: Representative ChIP blots demonstrating episodic GH regulation of pSTAT5b binding to the CYP2C11 promoter in hepatocytes derived from adult male rats neonatally treated with either MSG or vehicle (Control). As described above, the cells were exposed to either the episodic male-like GH profile or the vehicle containing profile. ChIP assays were performed on 5 rats of each treatment group with the same results as that presented in the representative blots.
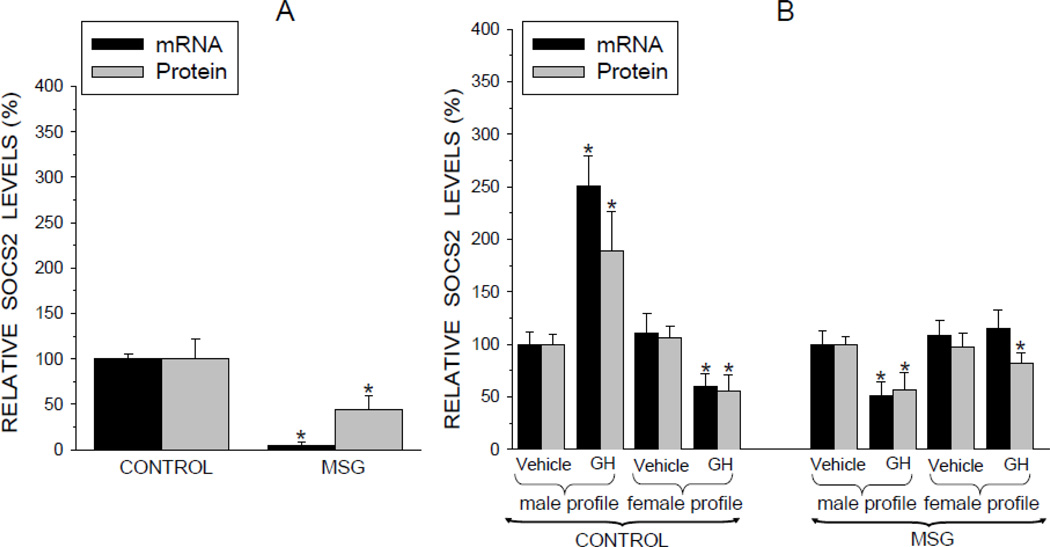
SOCS2
The SOCS family of inhibitory proteins are negative regulators of GH signaling. Their actions are particularly important in regulating the effects of episodic GH on such male-dependent parameters as growth and CYP expression (Flores-Morales et al., 2006; Waxman and Frank, 2000). The in vivo-like preplated hepatocytes from the neonatally MSG-treated rats contained only a fraction of SOCS2 levels observed in controls (Fig. 7A). Whereas protein concentrations of SOCS2 were reduced almost 60%, mRNA levels were barely detectable. When the control cells were treated with the episodic GH profile, SOCS2 concentrations doubled, while the female GH profile suppressed SOCS2 expression by almost half (Fig. 7B). In contrast, exposure of hepatocytes to both sex-dependent GH profiles suppressed SOCS2 expression in the MSG-treated rats - the episodic profile considerably more so than the continuous profile. Moreover, the absolute SOCS2 concentrations in the control and MSG cultured cells reflected the same comparable levels as observed in the freshly isolated preplated cells (data not presented).
Fig. 7.
Relative expression levels (mRNA and protein) of suppressors of cytokine signaling 2 (SOCS2) in hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pup were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7 and 9. A. SOCS2 levels in freshly isolated preplated hepatocytes. Results are presented as a percentage of mRNA or protein in hepatocytes from control rats arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs control rats. B. Hepatocytes from the same animal were exposed to either the episodic male-like GH profile or the hormone’s vehicle, or the continuous female-like GH profile or vehicle for 6 days. Results are presented as a percentage of mRNA or protein in hepatocytes exposed to vehicle administered in an episodic male-like profile arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic male-like vehicle profile from rats of the same neonatal treatment, i.e. Control or MSG.
CYP2C11 Ubiquitination
In an attempt to understand the dissimilar response of hepatic CYP2C11 mRNA and protein to in vitro GH induction of 2 mg MSG treated rats, we examined one likely step in CYP2C11 protein degradation, i.e., ubiquitination. Using 7 rats/treatment group, we found an almost 2-fold increase in ubiquitin-CYP2C11 levels in hepatocytes derived from 6 neonatally MSG-treated male rats exposed in culture to the masculine-like episodic GH profile suggesting a possible contributing factor for the selective reduction in protein levels of the isoform in the face of elevated transcript concentrations. Control cells exposed to the GH vehicle or the male-like GH profile expressed similar relative (%) ubiquitination levels, 100 ± 8 vs 96 ± 13 (x̄ ± sd), respectively. In contrast, ubiquitin-CYP2C11 concentrations of hepatocytes derived from the 2mg MSG rats, exposed to vehicle or GH, were 94 ±5 and 178 ±27 (p<0.01), respectively. A single rat of 7 neonatally MSG-treated males appears to be the exception that proves, or at least supports the rule. Although exhibiting a typical obese-like Lee index, hepatocytes from this one rat responded to GH-induced levels of CYP2C11 and SOCS2 (mRNA and protein) no differently from that of neonatal controls. Moreover, like hepatocytes from control males, hepatic cells from this one rat exhibited no GH increase in ubiquitination levels, possibly explaining the concurring inductive levels of CYP2C11 mRNA and protein in this one rat.
miRNA
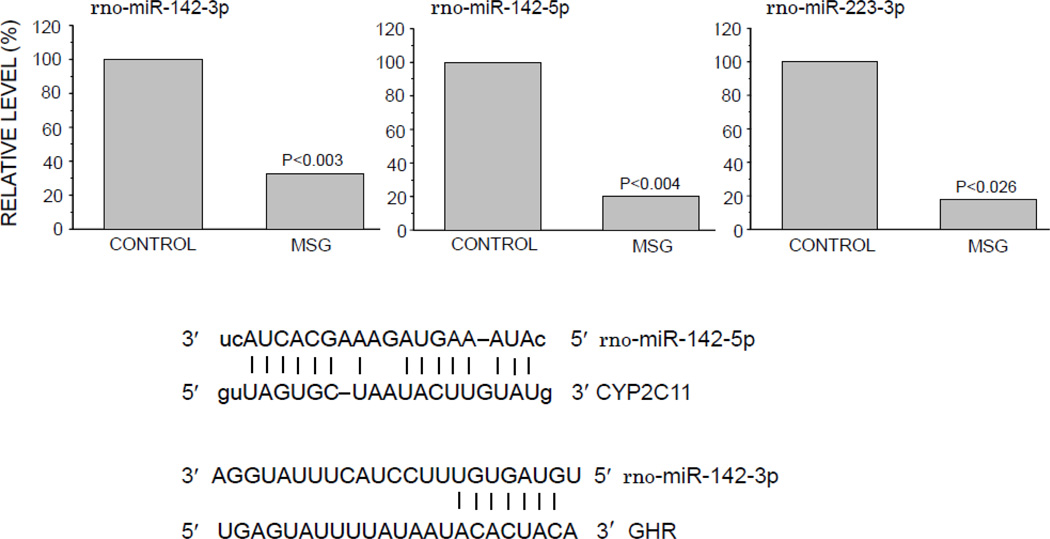
miRNA are naturally occurring, small, single-stranded non-coding RNA molecules involved in post-transcriptional control of gene expression. In animals, miRNA imperfectly hybridize, through partial sequence homology, to the 3’-UTR of target mRNAs causing a block in translation and/or mRNA degradation (Bartel, 2009). Prediction of miRNA target mRNA was carried out by our use of the algorithms miRanda (John et al., 2004) and TargetScan (Lewis et al., 2003). Of the 84 miRNAs measured, only 3 exhibited a statistically significant reduction, i.e., miR-142-3p, miR-142-5p and miR-223-3p, in the freshly isolated hepatocytes of the MSG-treated rats (Fig. 8). Comparing these 3 miRNAs in control cells, we found that miR-142-3p was clearly the most abundant. miR-223-3p concentrations were only 35.3% (p<0.002) of that of miR-142-3p and mi142-5p was only 10.2% (p<0.001) of miR-142-3p levels. Following statistical analyses, miR-142-3p was reported to effectively suppress human CYP2C19 mRNA (Rieger et al., 2013). Although miR-142-3p does not have a high predictability pairing score for target regions on CYP2C11 mRNA, it does exhibit a high potential to bind to the rat GHR mRNA (Fig. 8). While the binding capacity of miR-142-5p has not been investigated with human CYP mRNAs, its potential binding is predicted to be very high for CYP2C11 mRNA (miRanda). Lastly, the predictability pairing score of miR-223-3p to CYP2C11 mRNA is poor and its effects on the expression of other CYP mRNAs has not been reported.
Fig. 8.
Relative expression levels of 3 microRNAs and their complementary sites in the 3’-UTR of CYP2C11 and rat growth hormone receptor in freshly isolated preplated hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (control) on postnatal days 1, 3, 5, 7 and 9. Results are presented as a percentage of microRNA in hepatocytes from control rats arbitrarily designated 100%. Values are means of at least 3 rats/data point.
DISCUSSION
Neonatal administration of submaximal doses of the food additive MSG (2mg/g bd wt × 5d) produced the same long term defects in circulating GH and CYP2C11 expression in freshly isolated hepatocytes as previously observed in vivo (Pampori et al., 1994; 2000). That is, the secretory GH profiles of the affected rats were typical of the masculine episodic profile with the exception of pulse amplitudes, which were permanently reduced to 10–20% of normal. Moreover, CYP2C11 mRNA levels were overexpressed ~2.5 times above controls whereas CYP2C11 protein was elevated only 40 to 50% above normal. While the present study has shown that much of the CYP2C11 transcript is composed of a presumably nontranslated, intron-retained mRNA variant, the smaller increased protein concentrations were functional as judged by a similar increase in the specific CYP2C11-dependent testosterone 2α-hydroxylase activity and CYP2C11-predominant hexobarbital hydroxylase activity. This disproportionate expression of mRNA and protein is not unique and has been reported for CYP2B1 as well as CYP2C11 following treatment with subpharmacologic doses of phenobarbital (Agrawal and Shapiro, 1996; Waxman and Azaroff, 1992). Moreover, another inducing agent, Aroclor 1254, has been shown to stimulate the expression of both wild-type and alternatively spliced forms of CYP2B2 (Lacroix et al., 1990) and CYP1A2 (Affolter et al., 1986), in which a partial intron is incorporated into a mature transcript. Additionally, variant forms of constituent CYP2B (Okino et al., 1987; Miles et al., 1988) and CYP2D6 (Denson et al., 2005) with complete or partial introns retained have been found in normal human liver, leading to the conclusion that endogenous factors can be responsible for aberrant splicing of precursor transcripts. In contrast to CYP2C11, neonatal MSG exposure had no long term effect on the vivo-like masculine expression levels of GH-dependent CYP2C6 and CYP2C7 as well as sex-independent albumin suggesting that the defect may be limited to the GH-CYP2C11 axis.
Previously, our laboratory had investigated the effects of circulating GH pulse amplitudes on expression of CYP isoforms in adult GH-depleted (i.e. HYPOX) male rats (Agrawal and Shapiro, 2000; 2001). In this regard, we reported that renaturalization in HYPOX rats of the circulating mini GH profiles found in the neonatal MSG-treated rats (i.e., mini pulses in an otherwise masculine profile) also replicated the same effects observed on CYP2C11. Perhaps, most significantly, of the 6 sex- and GH-dependent CYPs measured at both the transcript and protein level in the GH-treated HYPOX rats, only CYP2C11 exhibited a dramatic disproportional expression of mRNA and protein concentrations. The question, then, is whether the CYP2C11 defect in the neonatal MSG-treated rats was simply a result of the subnormal GH pulse amplitudes, or was there a permanent developmental defect (i.e., imprinting) in the mechanism of GH regulation of CYP2C11 induced by the amino acid? In this regard, if there was no MSG-induced irreversible imprinting defect(s) in adult CYP2C11 expression, then cultured hepatocytes from the affected rats would respond to GH in the same way as hepatocytes from normal rats. Accordingly, we examined the effects of the masculine-like episodic GH profile on cultured hepatocytes from control and neonatal MSG-treated adult male rats.
Basically, the response of cultured primary hepatocytes to the masculine-like episodic GH profile was in many ways quite different between control and MSG-treated rats. Whereas the episodic GH profile increased CYP2C11 mRNA and protein concentrations several fold at comparable levels in control hepatocytes, only the transcript concentrations were elevated in MSG-derived cells. Moreover, while the control hepatocytes contained very low levels of the intron-retained mRNA which was GH unresponsive, CYP2C11 mRNA levels in the MSG-derived cells appeared to be predominantly composed of the aberrant transcript which was GH inducible. Although the response of CYP2C7 and CYP2C6 in the GH-treated primary hepatocytes was somewhat muted in the MSG-derived cells, the response patterns to the masculine- and feminine-like GH profiles were very similar to controls, further evidence that the defect seemed limited to CYP2C11 expression.
Although GHR expression in the cultured hepatocytes from the MSG-treated rats was unresponsive to episodic GH induction, the cells still contained greater concentrations of the receptor than the GH-responsive control cells. Not only were GHR levels adequate to assure the relatively normal responsiveness of CYP2C7 and CYP2C6 to GH, they were also sufficient for the activation of the episodic GH mediated signal transduction pathway regulating CYP2C11 expression in the MSG-treated cells. In this regard, GH signaling in liver by the episodic profile (in contrast to the continuous profile) is initiated by hormone binding to and the resulting activation of GHR on the surface of the target cells. This allows for the recruitment and/or activation of two molecules of Janus kinase 2 (JAK2), which then cross-phosphorylate each other as well as phosphorylating the receptor on key tyrosine residues. STAT5b, a latent transcription factor, binds to these phosphorylated receptor docking sites, is in turn phosphorylated, homodimerizes, and translocates to the nucleus where it binds to promoter sites initiating transcription of GH-regulated genes. An important negative regulatory mechanism of GH signaling, the SOCS family of inhibitory proteins, inhibit GH signaling by inactivating JAK2, and/or depending upon the protein, by competing with STAT5b for common tyrosine-binding sites on the intracellular tail of the GHR, and/or by a proteasome dependent mechanism that results in the degradation of the (GH-GHR-JAK2)•SOCS complex (Greenhalgh and Alexander, 2004; Landsman and Waxman, 2005; Waxman and Frank, 2000).
This signaling transduction pathway mediating GH induction of CYP2C11 appears to be intact in the hepatocytes of the MSG-treated rats as evidenced by normal STAT5b activation, nuclear translocation and binding to the CYP2C11 promoter, explaining the observed increase in CYP2C11 mRNA. However, in addition to an overexpression of normal transcript levels, there appears to be a permanent defect in transcript processing, resulting in the expression of large amounts of an aberrant, likely nontranslated intron-retained CYP2C11 mRNA. High expression levels of this variant, along with our observation of a 2-fold increase in ubiquitin-CYP2C11 concentrations in the GH exposed MSG-derived hepatocytes explains, at least in part, the disproportionately lower CYP2C11 protein expression in the face of a several fold increase in transcript levels.
The possible involvement of miRNAs in the overexpression of CYP2C11 in the MSG-treated rats was also examined. Although a nascent area of biological investigation, our miRNA findings suggest the possibility that neonatal MSG-induced adult depletion of miR-142-5p may be implicated in the overexpression of CYP2C11. Since miR-142-3p has been reported to be a normally occurring suppressor of CYP2C19 (Rieger et al., 2013), it seems reasonable that a reduction in hepatocyte levels of the miRNA might allow for the overexpression of the isoform. In this regard, miR-142-5p, with a high predictability bonding score for CYP2C11 mRNA, may be a normally occurring repressor of CYP2C11 expression similar to the miR-142-3p repression of CYP2C19. Not only do CYP2C11 and CYP2C19 share a high degree of amino acid homology, they are both differentially regulated by sex-dependent GH profiles and are both expressed at sexually dimorphic levels (Dhir et al., 2006; Shapiro et al., 1995). Although speculative, our observed 80% reduction in miR-142-5p concentrations in MSG-derived hepatocytes may be another factor involved in the permanent overexpression of CYP2C11.3
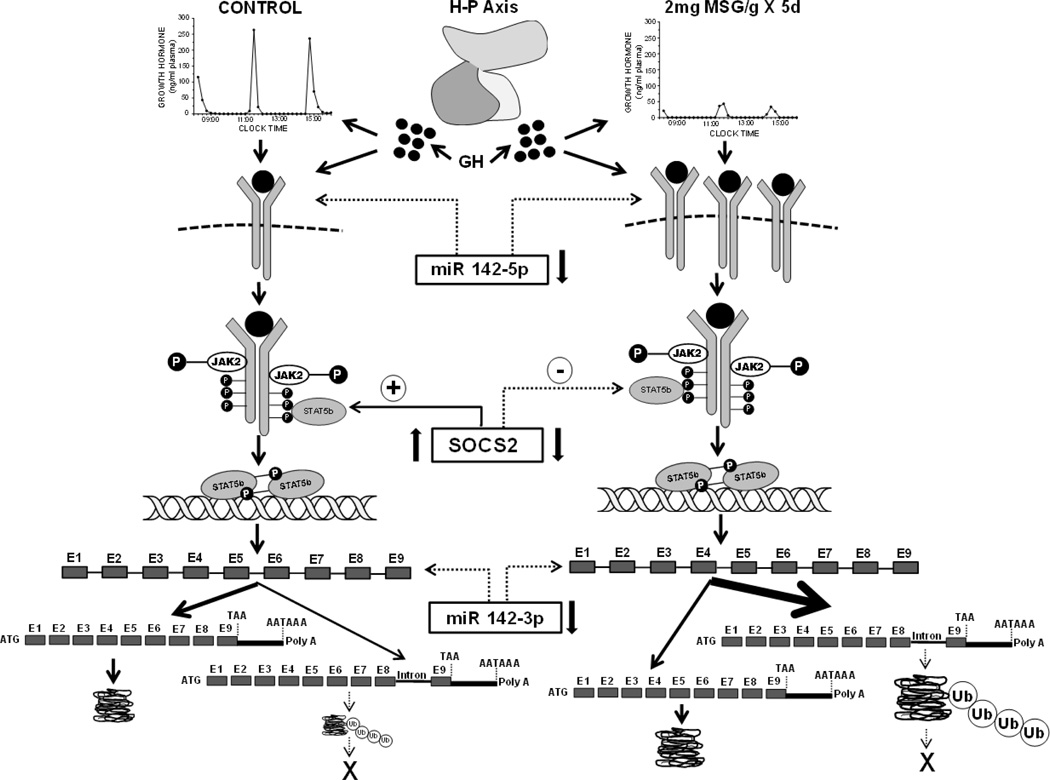
The role, if any, for the dramatic suppression of baseline SOCS2 levels and its unresponsiveness to GH induction on the resulting abnormal CYP2C11 expression of the MSG hepatocytes is unclear. Episodic GH activation of STAT5b is not just requisite for CYP2C11 expression, but for SOCS2 expression as well (Greenhalgh and Alexander, 2004; Waxman and Frank, 2000). Shortly following peak levels of pSTAT5b binding to the CYP2C11 promoter, SOCS2 mRNA concentrations peak, subsequently terminating GH induction of CYP2C11 transcription and allowing for the re-induction by the next episodic GH pulse (Thangavel and Shapiro, 2007; Waxman and Frank, 2000). Although exposure to the episodic GH profile results in STAT5b activation (i.e. phosphorylation), nuclear translocation and binding to the CYP2C11 promoter in the hepatocyte of the MSG-treated rats, unexpectedly, this activated signaling pathway had no inductive effect on SOCS2 expression in vivo (freshly isolated hepatocytes) or in vitro (primary culture). Since it has been proposed (Thangavel and Shapiro, 2007; Waxman and Frank, 2000) that normal masculine levels of CYP2C11 expression are dependent on STAT5b driven SOCS2 expression which intermittently terminates the GH-GHR-JAK2-STAT5b pathway, it is difficult to explain how the MSG-treated rats were capable of overexpressing the isoform. Though speculative, there are several possible explanations. It has been reported that depending upon its concentration, SOCS2 can have dual effects as an enhancer or suppressor of GH signaling (Greenhalgh et al., 2002). In the case of GH-dependent body growth, a SOCS2 deficiency produces excessive growth (LeRoith and Nissley, 2005; Rico-Bautista et al., 2005) and by a possible extension, an overexpression of the CYP2C11 transcript, particularly the aberrant form. In addition, it is possible that other members of the SOCS family can substitute for SOCS2 (Ram and Waxman, 2000; Thangavel and Shapiro, 2007; Tollet-Egnell et al., 1999) and/or there are mechanisms independent of GH-GHR-JAK2-STAT5b-SOCS2 capable of regulating CYP2C11 expression (Verma et al., 2005). The possible mechanism(s) responsible for the overexpression of CYP2C11 in the MSG-treated rats is summarized in schematic Fig. 9.
Fig. 9.
Hypothetical mechanism(s) describing the permanent effect of neonatal “low” dose MSG treatment on adult male CYP211 expression. Neonatal administration of 2mg MSG/g bd wt disrupts the development of the hypothalamic-pituitary (H-P) axis resulting in the adult secretion of mini-GH pulses in an otherwise normal masculine GH profile contributing to the permanent overexpression of CYP2C11. The mini GH secretory profile, alone, and/or in combination with reduced hepatic concentrations of miR142-5p, a putative small, single-stranded non-coding RNA suppressor of GHR transcription, results in the overexpression of the transmembrane hepatic GHR. The overexpressed GHR along with decreased levels of SOCS2, an inhibitor of GH activation of the GHR●JAK2●STAT5b complex, causes an enhanced expression of CYP2C11. This overexpression of CYP2C11 may be supported by the reduced concentrations of hepatic miR142-3p, a putative suppressor of CYP2C11 transcription. In contrast to the normal (CONTROL) condition (left side of schematic) where only a small fraction of the CYP2C11 transcript retains the terminal intron, the bulk of the CYP2C11 transcript (noted by arrow thickness) retains the intron separating exons 8 and 9. Although not studied in this report, the dramatic overexpression of the intron-retained transcript may be due, at least in part, to a reduction in nuclear splicing capacity in the affected rats (Pampori and Shapiro, 2000). The absence of a detectable translation product from the intron-retained CYP2C11 mRNA in both treatment groups (depicted by X) is likely a result of the presence of a premature stop codon leading to the immediate degradation of a likely truncated protein by ubiquitination; the catabolic enzyme complex greatly overexpressed in the MSG-treated male rats. The 40 to 50% overexpression of CYP2C11 protein in the MSG rats is likely due to a similar percent elevation in normal transcript levels.
While it initially appeared that the abnormal overexpression of the principal CYP2C11 in the adult, neonatally MSG-treated male rats was simply a result of the circulatory mini pulses in an otherwise masculine GH profile, the present findings indicate a permanent imprinting defect(s) in both the regulation of CYP2C11 expression and GH responsiveness. Renaturalization of the same mini GH profile in HYPOX rats produced the same degree of overexpression in CYP2C11 mRNA, protein and testosterone 2α-hydroxylase activity as observed in the MSG-treated rats (Pampori and Shapiro, 1994; 2000). However, unlike the MSG-treated rats, renaturalization of the normal masculine episodic GH profile, both in vivo (Agrawal and Shapiro 2000; 2001) and in vitro (Thangavel et al., 2006; Thangavel and Shapiro, 2007) restored the normal expression levels of CYP2C11 in HYPOX rats to that observed in the present control animals indicating no irreversible defect(s) in the pituitary ablated rats. Whereas the presently administered submaximal dose of MSG is not nearly as developmentally detrimental as the invariably reported 4 mg/g bd wt dose of MSG4, it does result in a mild obesity in an otherwise normally appearing animal. Nevertheless, this lower dose which produces a transient depletion in plasma GH during the period of hypothalamic-pituitary-hepatic differention (Shapiro, 2005), and likely even doses several fold lower (Pampori et al., 1991b) can programme subtle developmental defects in drug metabolism that are both permanent and irreversible.
The possible long term pharmacologic/toxicologic consequences of perinatal exposure to MSG, as well as aspartame, the ubiquitous artificial sweetener composed of the other excitatory amino acid, aspartate, and phenylalanine, on human development has been reviewed (cf., Shapiro, 2005) and those aspects relating to human exposure levels are annotated here from references cited in the original review.
Like preservatives, some forms of flavor-enhancing chemicals can be found in almost all commercially prepared foods. Although MSG’s teratogenic potential action resulted in its voluntary removal from baby food in 1969, this was a belated action for millions of adults who had already consumed it in childhood. Americans continue to consume several hundred million pounds of it per year, which makes it highly likely that human perinates are still being exposed to MSG. Food manufacturers are now required by the FDA to list “monosodium glutamate” on food labels, but they do not have to label ingredients that contain free glutamic acid, even though it is the main component of MSG. Consequently, there are dozens of labeled ingredients that contain glutamate, but would be unknown from just their names. Nevertheless, independent analyses have shown that such commercially available foods as soups may contain 1.1 g of MSG per 6 oz serving. Moreover, Chinese and Korean restaurants have been found to add 10g of MSG to a single dish.
Neonatal exposure to aspartate induces similar latently expressed toxicologic defects as MSG. The statistics concerning aspartame consumption reflect those regarding MSG. Studies have reported that children may consume nearly 100mg of aspartame per kilogram of body weight per day, a level approaching the adverse doses found in animal studies. Because aspartame’s use (cereals, soft drinks, candies, desserts, etc.) includes the major components of a child’s diet not currently containing glutamate (frozen dinners, processed lunch meats, canned soups, etc.), there has been a justifiable concern that consumption of both aspartame and glutamate could produce developmental defects in children. These concerns have been refuted by demonstrating that aspartate and/or glutamate consumption at the highest possible human dietary levels does not induce congenital anomalies in primates or produce toxic effects in the large majority of the adult population. On the other hand, studies with rats and mice have shown that either amino acid could produce more subtle developmental defects in GH secretion, obesogenesis, and drug metabolism. Accordingly, it is possible that a subset of the population could be highly sensitive to the effects of the food additives. All smokers do not develop lung cancer, and all children exposed in utero to ethanol do not develop the fetal alcohol syndrome. Thus, early exposure to even low levels of food additives like MSG and aspartame from before birth until 5 years of age, when hepatic and neuroendocrine differentiation are still incomplete, could permanently alter the expression of hepatic drug-metabolizing enzymes and/or their response to inducing agents. Such defects could unknowingly affect the efficacy of drug therapy or the susceptibility to chemically induced cancers in adulthood.
HIGHLIGHTS.
A “low” neonatal dose of MSG causes an immediate but transient growth hormone depletion.
Adult circulating growth hormone contains mini pulses in an otherwise male profile.
CYP2C11 is permanently overexpressed >250%; CYP2C6, 2C7 and albumin remain normal.
The bulk of the overexpressed CYP2C11 mRNA consists of an intron-retained form.
SOCS2 and miRNAs are subnormal, ubiquitination is elevated but JAK2/STAT5b is normal.
ACKNOWLEDGEMENTS
This work was supported by the U.S. National Institutes of Health, Eunice Kennedy Shriver National Institute of Health and Human Development (grant HD-061285).
Abbreviations
- CHIP
chromatin immunoprecipitation
- CYP
cytochrome P450
- GH
growth hormone
- GHR
growth hormone receptor
- HYPOX
hypophysectomized
- JAK
Janus kinase
- miRNA
microRNA
- MSG
monosodium glutamate
- pSTAT5b
phospho signal transducers and activators of transcription 5b
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- SOCS2
suppressors of cytokine signaling 2
- STAT
signal transducers and activators of transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although the feminine continuous GH profile, in vivo and in vitro, is far more inductive of CYP2C6 and CYP2C7 in females than the masculine episodic GH profile (Pampori and Shapiro, 1999; Thangavel et al., 2004), the isoforms in the male hepatocyte obtained from both neonatal treatment groups were more responsive to the male-like profile, supporting our previous report that male hepatocytes are intrinsically insensitive to the female GH profile (Thangavel and Shapiro, 2007).
Similarly, the >80% reduction in miR-142-3p concentrations might be implicated in the overexpression of the GHR in the MSG-derived hepatocytes.
In contrast to the presently administered dose, the 4mg/g bd wt dose of MSG on alternate days for the first 9 days of life produces an adult morbid obesity, extreme lethargy and a complete and permanent depletion of both circulating GH and CYP2C11 expression (Dada et al., 1984; Pampori et al., 1991b).
REFERENCES
- Affolter M, Labbé D, Jean A, Raymond M, Noël D, Labelle Y, Parent-Vaugeois C, Lambert M, Bojanowski R, Anderson A. cDNA clones for liver cytochrome P450s from individual Aroclor-treated rats: constitutive expression of a new P-450 gene related to phenobarbital-inducible forms. DNA. 1986;5:209–218. doi: 10.1089/dna.1986.5.209. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. Intrinsic signals in the sexually dimorphic circulating growth hormone profiles of the rat. Mol Cell Endocrinol. 2001;173:167–181. doi: 10.1016/s0303-7207(00)00401-9. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Pampori NA, Shapiro BH. Thin-layer chromatographic separation of regioselective and stereospecific androgen metabolites. Anal Biochem. 1995a;224:455–457. doi: 10.1006/abio.1995.1071. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Pampori NA, Shapiro BH. Neonatal phenobarbital-induced defects in age-and sex-specific growth hormone profiles regulating monooxygenases. Am J Physiol. 1995b;268:E439–E445. doi: 10.1152/ajpendo.1995.268.3.E439. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. Phenobarbital induction of hepatic CYP2B1 and CYP2B2: Pretranscriptional and post-transcriptional effects of gender, adult age and phenobarbital dose. Mol Pharmacol. 1996;49:523–531. [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. Differential expression of gender-dependent hepatic isoforms of cytochrome P450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. J Pharmacol Exp Ther. 2000;292:228–237. [PubMed] [Google Scholar]
- Banerjee S, Das RK, Shapiro BH. Growth hormone-independent suppression of growth hormone-dependent female isoforms of cytochrome P450 by the somatostatin analog octreotide. Eur J Pharmacol. 2013;715:256–261. doi: 10.1016/j.ejphar.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2007;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson BA. Growth hormone. Norwell, MA: Kluwer Academic Publisher; 1999. p. 359. [Google Scholar]
- Bick T, Hochberg Z, Amit T, Isaksson OGP, Jansson J-O. Roles of pulsatility and continuity of growth hormone (GH) administration in the regulation of hepatic GH-receptors, and circulating GH-binding protein and insulin-like growth factor-I. Endocrinology. 1992;131:423–429. doi: 10.1210/endo.131.1.1612023. [DOI] [PubMed] [Google Scholar]
- Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA. Growth hormone (GH)-deficient men are more responsive to GH replacement therapy than women. J Clin Endocrinol Metab. 1997;82:550–555. doi: 10.1210/jcem.82.2.3776. [DOI] [PubMed] [Google Scholar]
- Dada MO, Campbell GT, Blake CA. Effects of neonatal administration of monosodium glutamate on somatotrophs and growth hormone secretion in prepubertal male and female rats. Endocrinology. 1984;115:996–1003. doi: 10.1210/endo-115-3-996. [DOI] [PubMed] [Google Scholar]
- Das RK, Banerjee S, Shapiro BH. Noncanonical suppression of GH-dependent isoforms of cytochrome P450 by the somatostatin analog octreotide. J. Endocrinol. 2013;216:87–97. doi: 10.1530/JOE-12-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson J, Wu Y, Yang W, Zhang J. Inter-individual variation of several cytochrome P450 2D6 splice variants in human liver. Biochem Biophys Res Comm. 2005;330:498–504. doi: 10.1016/j.bbrc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Dhir RN, Dworakowski W, Thangavel C, Shapiro BH. Sexually dimorphic regulation of hepatic isoforms of human cytochrome P450 by growth hormone. J Pharmacol Exp Ther. 2006;316:87–94. doi: 10.1124/jpet.105.093773. [DOI] [PubMed] [Google Scholar]
- Elfarra AA, Krause RJ, Last AR, Lash LH, Parker JC. Species- and sex-related differences in metabolism of trichloroethylene to yield chloral and trichloroethanol in mouse, rat, and human liver microsomes. Drug Metab Dispos. 1998;26:779–785. [PubMed] [Google Scholar]
- Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol Endocrinol. 2006;20:241–253. doi: 10.1210/me.2005-0170. [DOI] [PubMed] [Google Scholar]
- Gathercole LL, Morgan SA, Tomlinson JW. Hormonal regulation of lipogenesis. Vitam Horm. 2013;91:1–27. doi: 10.1016/B978-0-12-407766-9.00001-8. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Alexander WS. Suppressors of cytokine signalling and regulation of growth hormone action. Growth Horm IGF Res. 2004;14:200–206. doi: 10.1016/j.ghir.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, Fabri LJ, Zhang JG, Martin HM, Willson TA, Billestrup N, Nicola NA, Baca M, Alexander WS, Hilton DJ. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem. 2002;277:40181–40184. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- Hannemann F, Bichet A, Ewen KM, Bernhard R. Cytochrome P450 systems -- biological variations of electron transport chains. Biochim Biophys Acta. 2007;1770:330–344. doi: 10.1016/j.bbagen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Hubina E, Kovacs L, Szabolcs I, Szucs N, Toth M, Racz K, Czirjak S, Gorombey Z, Goth MI. The effect of gender and age on growth hormone replacement in growth hormone-deficient patients. Horm Metab Res. 2004;36:247–253. doi: 10.1055/s-2004-814458. [DOI] [PubMed] [Google Scholar]
- Jansson JO, Eden S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- Johansson AG. Gender difference in growth hormone response in adults. J Endocrinol Invest. 1999;22:58–60. [PubMed] [Google Scholar]
- Johansson AG, Engstrom BE, Ljunghall S, Karlsson FA, Burman P. Gender differences in the effects of long term growth hormone (GH) treatment on bone in adults with GH deficiency. J Clin Endocrinol Metab. 1999;84:2002–2007. doi: 10.1210/jcem.84.6.5743. [DOI] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:1862–1879. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgenson JOL, Christiansen JS. Clinical aspects of growth hormone deficiency in adults. Frontiers Horm Res. 2005;33:1–20. doi: 10.1159/000088338. [DOI] [PubMed] [Google Scholar]
- Kacsoh B, Terry LC, Meyers JS, Crowley WR, Grosvenor CE. Maternal modulation of growth hormone secretion in neonatal rat. I. Involvement of milk factors. Endocrinology. 1989;125:1326–1336. doi: 10.1210/endo-125-3-1326. [DOI] [PubMed] [Google Scholar]
- Kuromaru R, Kohno J, Ueyama N, Hassan HM, Honda S, Hara T. Long-term prospective study of body composition and lipid profiles during and after growth hormone (GH) treatment in children with GH deficiency: gender-specific metabolic effects. J Clin Endocrinol Metab. 1998;83:3890–3896. doi: 10.1210/jcem.83.11.5261. [DOI] [PubMed] [Google Scholar]
- Lacroix D, Desrochers M, Lambert M, Anderson A. Alternative splicing of mRNA encoding rat liver cytochrome P450s (P450IIB2) Gene. 1990;8:201–207. doi: 10.1016/0378-1119(90)90280-5. [DOI] [PubMed] [Google Scholar]
- Landsman T, Waxman DJ. Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J Biol Chem. 2005;280:37471–37480. doi: 10.1074/jbc.M504125200. [DOI] [PubMed] [Google Scholar]
- Legraverend C, Mode A, Wells T, Robinson I, Gustafsson J-Å. Hepatic steroid hydroxylating enzymes are controlled by the sexually dimorphic pattern of growth hormone secretion in normal and dwarf rats. FASEB J. 1992;6:711–718. doi: 10.1096/fasebj.6.2.1537461. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Nissley P. Knock your SOCS off! J Clin Invest. 2005;115:233–236. doi: 10.1172/JCI24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Sorenson MP, Shapiro BH. Strain independent elevation of hepatic mono-oxygenenase enzymes in female mice. Xenobiotica. 1987;127:1095–1102. doi: 10.3109/00498258709044208. [DOI] [PubMed] [Google Scholar]
- Miles JS, Spurr NK, Gough AC, Jowett T, McLaren AW, Brook JD, Wolf CR. A novel human cytochrome P450 gene (P450IIB): chromosomal localization and evidence for alternative splicing. Nucleic Acids Res. 1988;16:5783–5795. doi: 10.1093/nar/16.13.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ET, MacGeoch C, Gustafsson J-Å. Hormonal and developmental regulation of expression of the hepatic microsomal steroid 16α-hydroxylase cytochrome P450 apoprotein in the rat. J Biol Chem. 1985;260:11895–11898. [PubMed] [Google Scholar]
- Nebert DW, Dieter MZ. The evolution of drug metabolism. Pharmacology. 2000;61:121–135. doi: 10.1159/000028393. [DOI] [PubMed] [Google Scholar]
- Oliver C, Giraud P, Lissitzky JC, Coté J, Boudoureque F, Gillioz P, Conte-Devolx b. Influence of endogenous somatostatin on growth hormone and thyrotropin secretion in neonatal rats. Endocrinology. 1982;110:1018–1022. doi: 10.1210/endo-110-3-1018. [DOI] [PubMed] [Google Scholar]
- Okino ST, Quattrochi LC, Pendurthi UR, McBride OW, Tukey RH. Characterization of multiple human cytochrome P450 I cDNAs: the chromosomal localization of the gene and evidence for alternative RNA splicing. J Biol Chem. 1987;262:16072–16079. [PubMed] [Google Scholar]
- O'Malley K, Crooks J, Duke E, Stevenson IH. Effect of age and sex on human drug metabolism. Br Med J. 1971;3:607–609. doi: 10.1136/bmj.3.5775.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampori NA, Agrawal AK, Shapiro BH. Renaturalizing the sexually dimorphic profile of circulating growth hormone in hypophysectomized rats. Acta Endocrinology (Copenh) 1991b;124:283–289. doi: 10.1530/acta.0.1240283. [DOI] [PubMed] [Google Scholar]
- Pampori NA, Agrawal AK, Waxman DJ, Shapiro BH. Differentail effects of neonatally administered glutamate on the ultradian pattern of circulating growth hormone regulating expression of sex-dependent forms of cytochrome P450. Biochem Pharmacol. 1991a;41:1299–1309. doi: 10.1016/0006-2952(91)90101-a. [DOI] [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. Over-expression of CYP2C11, the major male-specific form of hepatic cytochrome P450, in the presence of nominal pulses of circulating growth hormone in adult male rats neonatally exposed to low levels of monosodium glutamate. J Pharmacol Exp Ther. 1994;271:1067–1073. [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. Feminization of hepatic cytochrome P450s by nominal levels of growth hormone in the feminine plasma profile. Mol Pharmacol. 1996;50:1148–1156. [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. Gender differences in the responsiveness of the sexdependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology. 1999;140:1245–1254. doi: 10.1210/endo.140.3.6545. [DOI] [PubMed] [Google Scholar]
- Pampori NA, Shapiro BH. Nominal growth hormone pulses in otherwise normal masculine plasma profile induce intron retention of overexpressed hepatic CYP2C11 with associated nuclear splicing deficiency. Endocrinology. 2000;141:4100–4106. doi: 10.1210/endo.141.11.7751. [DOI] [PubMed] [Google Scholar]
- Ram PA, Waxman DJ. Role of the cytokine-inducible SH2 protein CIS in desensitization of STAT5b signaling by continuous growth hormone. J Biol Chem. 2000;275:39487–39496. doi: 10.1074/jbc.M004755200. [DOI] [PubMed] [Google Scholar]
- Rico-Bautista E, Greenhalgh CJ, Tollet-Egnell P, Hilton DJ, Alexander WS, Norstedt G, Flores-Morales A. Suppressor of cytokine signaling-2 deficiency induces molecular and metabolic changes that partially overlap with growth hormone dependent effects. Mol Endocrinol. 2005;19:781–793. doi: 10.1210/me.2004-0040. [DOI] [PubMed] [Google Scholar]
- Rieger JK, Klein K, Winter S, Zanger UM. Expression variability of absorption, distribution, metabolism, excretion-related microRNAs in human liver: influence of nongenetic factors and association with gene expressions. Drug Metab Dispos. 2013;41:1752–1762. doi: 10.1124/dmd.113.052126. [DOI] [PubMed] [Google Scholar]
- Ryan DE, Levin W. Purification and characterization of hepatic microsomal cytochrome P450. Pharmacol Ther. 1990;45:153–239. doi: 10.1016/0163-7258(90)90029-2. [DOI] [PubMed] [Google Scholar]
- Shapiro BH. Perinatal Origins of Adult Defects in Drug Matabolism. In: Yaffe SJ, Aranda JV, editors. Neonatal and Pediatric Pharmacology. Therapeutic Principles in Practice. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 159–171. [Google Scholar]
- Shapiro BH, Agrawal AK, Pampori NA. Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol. 1995;27:9–20. doi: 10.1016/1357-2725(94)00056-5. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, MacLeod JN, Pampori NA, Morrissey JJ, Lapenson DP, Waxman DJ. Signalling elements in the ultradian rhythm of circulating growth hormone regulating expression of sex-dependent forms of hepatic cytochrome P450. Endocrinology. 1989;125:2935–2944. doi: 10.1210/endo-125-6-2935. [DOI] [PubMed] [Google Scholar]
- Sharma MP, Dworakowski W, Shapiro BH. Intrasplenic transplantation of isolated adult rat hepatocytes: sex reversal and/or suppression of the major constituent isoforms of cytochrome P450. Toxicol Pathol. 2012;40:83–92. doi: 10.1177/0192623311425061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DV, Conceicao FL, Brasil RR, Spina LD, Lobo PM, Silva EM, Buescu A, Vaisman M. Insulin-like growth factor I levels during growth hormone (GH) replacement in GH-deficient adults: a gender difference. Growth Horm IGF Res. 2004;14:436–441. doi: 10.1016/j.ghir.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Span JPT, Pieters GFFM, Sweep FGJ, Hermus ARMM, Smals AGH. Gender differences in rhGH-induced changes in body composition in GH-deficient adults. J Clin Endocrinol Metab. 2001;86:4161–4165. doi: 10.1210/jcem.86.9.7815. [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152:3165–3171. doi: 10.1210/en.2011-0253. [DOI] [PubMed] [Google Scholar]
- Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388–401. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Dworakowski W, Shapiro BH. Inducibility of male-specific isoforms of cytochrome P450 by sex-dependent growth hormone profiles in hepatocyte cultures from male but not female rats. Drug Metab Dispos. 2006;34:140–419. doi: 10.1124/dmd.105.007716. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Garcia MC, Shapiro BH. Intrinsic sex difference determine expression of growth hormone-related female cytochrome P450s. Mol Cell Endocrinol. 2004;220:31–39. doi: 10.1016/j.mce.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Shapiro BH. A molecular basis for the sexually dimorphic response to growth hormone. Endocrinology. 2007;148:2894–2903. doi: 10.1210/en.2006-1333. [DOI] [PubMed] [Google Scholar]
- Thangavel C, Shapiro BH. Inherent sexually dimorphic expression of hepatic CYP2C12 correlated with repressed activation of growth hormone-related signal transduction in male rats. Drug Metab Dispos. 2008;36:1884–1895. doi: 10.1124/dmd.108.021451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollet-Egnell P, Flores-Morales A, Stavréus-Evers A, Sahlin L, Norstedt G. Growth hormone regulation of SOCS-2, SOCS-3, and CIS messenger ribonucleic acid expression in the rat. Endocrinology. 1999;140:3693–3704. doi: 10.1210/endo.140.8.6878. [DOI] [PubMed] [Google Scholar]
- Verma AS, Dhir RN, Shapiro BH. Inadequacy of the Janus kinase 2/signal transducer and activator of transcription signal transduction pathway to mediate episodic growth hormone-dependent regulation of hepatic CYP2C11. Mol Pharmacol. 2005;67:891–901. doi: 10.1124/mol.104.005454. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Guan S, Acharya P, Koop DR, Liu Y, Liao M, Burlingame AL, Correia MA. Ubiquitin-dependent proteasomal degradation of human liver cytochrome P450 2EI. Identification of sites targeted for phosphorylation and ubiquitination. J Biol Chem. 2011;286:9443–9456. doi: 10.1074/jbc.M110.176685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ. Rat hepatic P450IIA and P450IIC subfamily expression using catalytic, immunochemical, and molecular probes. Enzymol. 1991;206:249–267. doi: 10.1016/0076-6879(91)06095-k. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P450 gene expression. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Frank SJ. Growth Hormone Action: Signaling via a JAK-STAT-Coupled Receptor. In: Conn MP, Means AR, editors. Principles of Molecular Regeneration. Tototwa, NJ: Humana Press; 2000. pp. 55–83. [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorophic expression of hepatic cytochrome P450. Proc Natl Acad Sci USA. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]