Abstract
Objective
An estimated 35–50% of lung and head/neck cancer patients are smoking at diagnosis; most try to quit, however a substantial proportion resumes smoking. As cancer treatments improve, attention to the effects of continued smoking on quality of life in the survivorship period is increasing. The current study examines if smoking abstinence following surgical treatment is associated with better quality of life.
Methods
Participants were 134 patients with head/neck or lung cancer who received surgical treatment. Smoking status and indices of quality of life (depressive symptoms, fatigue, and pain) were assessed at the time of surgery (baseline) and at 2, 4, 6, and 12 months post-surgery. Analyses were performed using a Generalized Estimating Equations approach. A series of models examined the correlation between smoking status and post-surgery quality of life while adjusting for demographics, clinical variables, and baseline smoking status and quality of life.
Results
Continuous post-surgery abstinence was associated with lower levels of depressive symptoms and fatigue; however, the relationship with fatigue became non-significant after adjusting for baseline fatigue and income. There was no significant relationship observed between smoking status and pain.
Conclusions
Findings add to a growing literature showing that smoking cessation is not associated with detrimental effects on quality of life and may have beneficial effects, particularly with regard to depressive symptoms. Such information can be used to motivate smoking cessation and continued abstinence among cancer patients and increase provider comfort in recommending cessation.
Keywords: tobacco use, quality of life, head and neck cancer, lung cancer
Background
Cigarette smoking is a primary risk factor for cancer, with the strongest associations for lung and head/neck cancers [1]. An estimated 35–50% of lung and head/neck cancer patients are current smokers at the time of diagnosis [2, 3]. Most will spontaneously quit after diagnosis [4, 5], and smoking cessation interventions for these patients have produced high short-term cessation rates [2]. Less is known about relapse rates in this population, but estimates have ranged from 13% to 60% [5–11]. Relapse prevention among these patients is a priority, as continued smoking has a negative impact on morbidity and mortality [12, 13], including risk for other smoking-related illnesses (e.g. coronary heart disease) and second primary tumors [14], and recurrence [13, 14]. Smoking also has more immediate adverse effects on cancer treatment outcomes, including reduced treatment efficacy [e.g., 15] and higher rates of complications and side effects [16–19], as well as quality of life indices including physical and emotional functioning [e.g., 20, 21, 22].
As cancer treatments improve, attention to potential negative effects of continued smoking on quality of life in the survivorship period is increasing. In particular, quality of life indices that would likely be affected by both 1) cancer diagnosis and treatment and 2) continued smoking, such as pain and fatigue, have received the most attention. Current smokers have generally reported poorer quality of life than never smokers, with former smokers reporting an intermediate level [e.g., 23]. Regarding emotional functioning, continued smoking has been associated with greater depressive symptomatology [e.g., 24]. Limitations of these studies include small sample sizes and assessments that occurred at a single point in time among patients at varying stages of treatment.
We recently conducted a prospective study of relapse trajectories and predictors among lung and head/neck cancer patients who underwent surgical treatment and either 1) had quit smoking after their diagnosis and within six months prior to surgery or 2) were smoking prior to surgery but made a quit attempt immediately following surgery, beginning during their hospital stay when smoking was not permitted [9]. Predictors of relapse in patients who had quit pre-surgery included higher perceived difficulty quitting and lower perceived risks of resuming smoking. For patients who were smoking pre-surgery, lower quitting self-efficacy, higher depression proneness, and greater fears about cancer recurrence were predictive of relapse.
The current analyses extend these findings from our prospective study and examine whether continuous smoking abstinence post-surgery is associated with differences in post-surgery quality of life with respect to both emotional (depressive symptoms) and physical (pain, fatigue) functioning indices. We address limitations of previous research on the relationship between smoking status and quality of life among cancer patients by including a larger sample of patients with two cancer types and using a prospective design with assessments at multiple time points post-surgery (2, 4, 6, and 12 months). We hypothesized that in the year following surgery, patients who were continuously abstinent would report lower levels of depressive symptoms, fatigue, and pain relative to patients who resumed smoking.
Methods
Participants
Please refer to previously published reports for details regarding recruitment, participants, and study procedures [9, 25]. In brief, patients (N =134) were recruited in-person from the thoracic (n = 65) and head and neck (n = 69) clinics at Moffitt Cancer Center (MCC) following a medical chart review for tobacco use history among all patients scheduled for surgery. We screened 353 patients in person, of whom 220 were eligible and 154 enrolled and completed the baseline assessment. Patients (n = 134) included in the current analyses were those who completed at least one of the four follow-up surveys (103 completed all four, 15 completed three, 11 completed two, and 5 completed one) and survived the 1-year follow-up period. Eligible patients had smoked at least 10 cigarettes per day for at least one year prior to diagnosis, received surgical cancer treatment and were abstinent for at least 24 hours; 64.2% smoked during the week prior to surgery. All MCC patients have access to a certified tobacco cessation specialist who is available to provide brief intervention based on the 5A’s model (ask if patient smokes, advise smokers to quit, assess willingness to quit, assist in referring to treatment, arrange follow-up) [see 25]. However, no intervention was provided as part of the study. This study was approved by the Institutional Review Board of the University of South Florida.
Procedure
After eligibility was confirmed, interested patients provided informed consent and then completed a baseline assessment (see measures below). Head/Neck (HN) patients completed the baseline at a pre-operative appointment that usually occurred within one week prior to surgery. Thoracic (TH) patients completed the baseline in their hospital room, generally within two to three days after surgery and when they had recovered sufficiently to consent and participate readily. Telephone follow-up assessments occurred at 2, 4, 6, and 12 months post-surgery. Patients were compensated $25 for each of the 5 assessments.
Measures
Demographics, clinical variables, and smoking history
At baseline, participants reported demographics (gender, age, race, ethnicity, marital status, education, income), alcohol use history (frequency, history of treatment), and smoking history including years smoked, average and lifetime maximum number of cigarettes smoked per day (CPD), and nicotine dependence (Fagerström Test for Nicotine Dependence, FTND) [26], with items worded to reflect pre-quit CPD and dependence for those who had already quit (“when you were a regular smoker”). Cancer stage and treatments received (chemotherapy and/or radiation) were abstracted from patients’ medical records.
Smoking Status
At baseline and all follow-up assessments, patients reported number of cigarettes smoked during the previous 7 days. Self-reports were confirmed via exhaled carbon monoxide (CO) from a subsample who reported abstinence and were seen at a hospital visit [9]. For the current analyses, participants who reported 7-day abstinence at all four follow-ups were classified as abstinent; those who reported smoking at one or more follow-ups were classified as smoking.
Quality of life
Depressive symptomatology during the past week was assessed at baseline with the 20-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) [27] and with a 10-item short version [28] at follow-ups to minimize participant burden. At both baseline and follow-up visits, fatigue was assessed with the 9-item Brief Fatigue Inventory (BFI) [29] and pain, including severity and interference with functioning, was assessed with the 15-item Brief Pain Inventory Short Form (BPI-SF) [30]. Preliminary analyses found that pain severity and interference were highly correlated across all follow-up visits (r’s > .81). Therefore, we performed primary analyses on pain severity only.
Data analysis
Variable-level missing data, less than 9% of all data, were imputed using multiple imputation (MI). Twenty data sets were created using PROC MI in SAS/STAT software version 9.3 (SAS Institute; Cary, NC). A Markov Chain Monte Carlo method [e.g., 31] with adaptive rounding [32] for binary variables (e.g., smoking status) was employed. Fifty-eight variables across the 5 time points were entered in the multiple imputation: 1) smoking status at all time points, 2) quality of life variables at all time points, 3) cancer type, smoking history, and demographic variables that were used as control variables in the models evaluated (see below). For sixteen variables in the third cluster, the interaction with cancer type was also included in the multiple imputation.
Imputed datasets were analyzed using a Generalized Estimating Equations (GEE) framework to accommodate the repeated measurements at unequal time intervals (2, 4, 6, & 12 months). The identity link function was used for all quality of life variables. A first-order autoregressive structure was specified for the working correlation matrix. Robust estimation of standard errors was used. Alpha was set at .05 for all analyses. Preliminary analyses examined the bivariate relationships between baseline measures and quality of life during follow-up. Significant baseline measures as well as significant interactions with either cancer type or month were incorporated in primary analyses.
The goal of the primary analyses was to compare post-surgery quality of life of patients who were abstinent at all follow-ups vs. patients who had smoked during at least one follow-up period. Each quality of life outcome variable (depressive symptoms, fatigue, pain severity) was assessed using a three-step modeling procedure (A, B, and C), increasing the number of variables at each step in order to provide a complete context for the effect of smoking status on quality of life. In the first step, the basic model (Model A) assessed smoking status as a predictor of quality of life with cancer type (HN vs. TH) and follow-up month (2, 4, 6 & 12) in the model. The second step (Model B) added the traditional control variables of gender, age, and cancer stage along with any significant 2-way interactions with Model A variables observed in preliminary analyses. The third step (Model C) added significant predictors observed in preliminary analyses. All possible two-way interactions with cancer type and month were also assessed at each step. Our presentation of multiple models—from basic to traditional to complex with inclusion of statistically-driven predictors—allows for a thoughtful examination of under what circumstances a relationship is evident between smoking status and quality of life as well as the strength of this relationship in the context of other influences on quality of life. Moreover, this analytic strategy allows for examination of significant relationships between disease-related and socio-demographic variables on quality of life.
Results
Participant Characteristics
Table 1 presents descriptive statistics by cancer type. Compared to TH patients, HN patients were less likely to be female and were significantly younger (ps <.01), had smoked for fewer years and were more likely to have smoked during the week prior to surgery (ps < .001), and were more likely to have Stage 1 or 2 cancer and to have received radiation treatment (ps < .001).
Table 1.
Demographic, Smoking, and Clinical Characteristics
| Demographic Variables | All (N=134) | Head/Neck (N=69) | Thoracic (N=65) |
|---|---|---|---|
| Sex: Female ** | 43.3% | 30.5% | 56.9% |
| Age – M (SD) *** | 58.6 (11.2) | 55.3 (9.7) | 62.0 (11.8) |
| Race: | |||
| White/Caucasian | 96.2% | 97.1% | 95.3% |
| Black/African American | 2.3% | 2.9% | 1.6% |
| Other | 1.5% | 0.0% | 3.1% |
| Hispanic | 1.5% | 2.9% | 0.0% |
| Marital Status: | |||
| Married | 53.7% | 50.7% | 56.9% |
| Single | 14.9% | 15.9% | 13.9% |
| Divorced | 21.6% | 24.6% | 18.5% |
| Widowed | 9.7% | 8.7% | 10.8% |
| Education: Less than 12th grade | 20.2% | 23.2% | 16.9% |
| Household Income: Median category | $30K–$40K | $30K–$40K | $30K–$40K |
| Smoking & Alcohol Variables | All | Head/Neck | Thoracic |
|---|---|---|---|
| Years smoking – M (SD) *** | 39.3 (12.8) | 35.1 (11.7) | 43.7 (12.6) |
| CPD average – M (SD) | 24.2 (11.7) | 23.6 (12.7) | 24.9 (10.5) |
| CPD maximum – M (SD) | 34.9 (15.2) | 36.0 (15.6) | 33.7 (14.9) |
| Fagerström Dependence – M (SD) | 5.8 (2.2) | 5.7 (2.3) | 5.9 (2.1) |
| Alcohol consumption: 2+ drinks/week | 43.6% | 44.1% | 43.1% |
| Alcohol abuse or treatment | 24.6% | 26.1% | 23.1% |
| Clinical Variables | All | Head/Neck | Thoracic |
|---|---|---|---|
| Stage 1 or 2 *** | 55.2% | 44.9% | 66.2% |
| Received chemotherapy treatment | 40.3% | 40.6% | 40.0% |
| Received radiation treatment*** | 41.8% | 63.8% | 18.5% |
Note: For comparisons of Thoracic versus Head/Neck patients,
denotes P ≤ .001.
denotes P ≤ .01.
denotes P ≤ .05.
Post-surgical smoking status
The number of abstinent participants varied slightly across the 20 imputed datasets with either 67 or 68 patients (50–51%) designated as abstinent. Smoking abstinence rates were significantly different by cancer type (p < .001). Among TH patients, 43 of 65 (66%) were abstinent. Whereas, among HN patients, 24 or 25 (35–36%) were abstinent. As noted above, cancer type was included in all primary analyses.
Quality of Life Measures
Overall means and standard errors (averaged across the 20 imputed datasets) for depressive symptoms, fatigue, and pain-severity at 2, 4, 6, and 12 months post-surgery are presented in Figure 1. Preliminary analyses found the baseline measure was a significant predictor for each quality of life measure (ps < .001). In addition, income and its interaction with cancer type and month were also significant predictors for each quality of life measure. Therefore, these two variables and the two interaction terms were included in Model C for the primary analyses. No other measure presented in Table 1 was a significant predictor of post-surgery quality of life.
Figure 1.
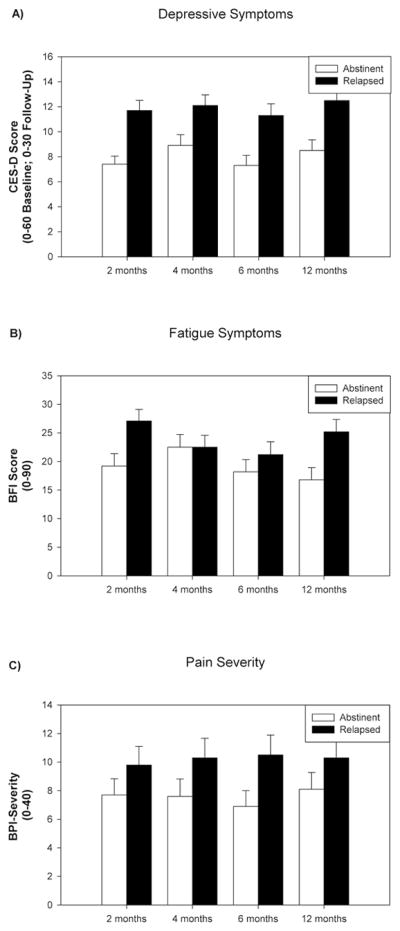
Means (Standard Errors) of Quality of Life Outcomes as a Function of Smoking Status. Means were averaged across the 20 imputed datasets.
The following 3 sections present significant results for Model A (smoking status, month, cancer type, and interactions), then Model B (Model A plus gender, age, cancer stage, and any significant 2-way interactions with the Model A variables), and then Model C (Model B plus baseline measure, income, and the interaction of income with month and cancer type). Detailed results for the models assessing each quality of life measure are shown in Table 2.
Table 2.
Post-Surgical Quality of life for Abstinent versus Smoking Patients
| CES-Depression | Model A | Model B | Model C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p |
| Smoking Status | −3.64 | −5.52, −1.75 | .000 | −2.81 | −4.72, −0.90 | .007 | −1.93 | −3.58,−0.28 | .028 |
| Smoking Status*Month | 0.09 | −0.17, 0.36 | .484 | 0.09 | −0.17, 0.36 | .393 | 0.18 | −0.08, 0.44 | .180 |
| Smoking Status*Cancer Type | −0.00 | −3.81, 3.81 | .867 | 1.71 | −2.20, 5.62 | .486 | −0.80 | −4.19, 2.60 | .653 |
| Month | 0.12 | 0.00, 0.24 | .050 | 0.12 | 0.00, 0.24 | .054 | 0.11 | 0.00, 0.22 | .061 |
| Cancer Type | −1.06 | −2.95, 0.83 | .274 | −1.33 | −3.28, 0.62 | .184 | −1.75 | −3.50,−0.01 | .050 |
| Month*Cancer Type | −0.26 | −0.52, 0.00 | .059 | −0.26 | −0.51, 0.00 | .058 | −0.27 | −0.53,−0.02 | .039 |
|
| |||||||||
| Gender | 2.61 | 0.74, 4.48 | .009 | 1.74 | 0.14, 3.34 | .038 | |||
| Age | −0.48 | −0.14, 0.04 | .340 | 0.00 | −0.07, 0.07 | .762 | |||
| Stage | 0.65 | −0.18, 1.47 | .132 | 0.39 | −0.31, 1.08 | .285 | |||
| Gender*Cancer Type | 4.29 | 0.54, 8.03 | .028 | 2.42 | −0.95, 5.79 | .160 | |||
|
| |||||||||
| Baseline | 0.23 | 0.16, 0.30 | .000 | ||||||
| Income | −0.57 | −0.98,−0.17 | .010 | ||||||
| Income*Month | −0.11 | −0.17,−0.14 | .003 | ||||||
| Income*Cancer Type | 1.05 | 0.20, 1.91 | .019 | ||||||
| BFI-Fatigue | Model A | Model B | Model C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p |
| Smoking Status | −5.72 | −10.3,−1.13 | .018 | −5.73 | −10.4,−1.07 | .021 | −3.05 | −7.32, 1.22 | .172 |
| Smoking Status*Month | −0.31 | −1.00, 0.39 | .389 | −0.30 | −0.99, 0.39 | .396 | −0.17 | −0.90, 0.55 | .651 |
| Smoking Status*Cancer Type | 0.96 | −8.19, 10.1 | .795 | 1.52 | −7.62, 10.7 | .734 | −3.01 | −11.6, 5.56 | .498 |
| Month | −0.09 | −0.40, 0.22 | .572 | −0.09 | −0.41, 0.22 | .554 | −0.11 | −0.42, 0.20 | .482 |
| Cancer Type | −0.69 | −5.25, 3.88 | .793 | −0.30 | −5.29, 4.69 | .840 | −1.39 | −6.09, 3.30 | .566 |
| Month*Cancer Type | −0.23 | −0.92, 0.46 | .521 | −0.23 | −0.92, 0.46 | .512 | −0.27 | −0.97, 0.42 | .444 |
|
| |||||||||
| Gender | 2.35 | −2.27, 6.98 | .321 | 0.77 | −3.47, 5.01 | .726 | |||
| Age | 0.05 | −0.16, 0.27 | .622 | 0.13 | −0.05, 0.30 | .152 | |||
| Stage | 2.23 | 0.26, 4.20 | .030 | 1.58 | −0.24, 3.39 | .089 | |||
| Stage*Cancer Type | −2.25 | −6.22, 1.73 | .279 | −1.46 | −5.11, 2.19 | .442 | |||
|
| |||||||||
| Baseline | 0.37 | 0.22, 0.52 | .000 | ||||||
| Income | −1.22 | −2.41,−0.03 | .050 | ||||||
| Income*Month | −0.15 | −0.34, 0.05 | .173 | ||||||
| Income*Cancer Type | 1.65 | −0.65, 3.95 | .139 | ||||||
| BPI-Severity | Model A | Model B | Model C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p |
| Smoking Status | −1.39 | −4.21, 1.43 | .338 | −0.71 | −3.43, 2.02 | .617 | 1.40 | −1.02, 3.82 | .260 |
| Smoking Status*Month | 0.08 | −0.26, 0.42 | .643 | 0.08 | −0.26, 0.41 | .648 | 0.20 | −0.13, 0.54 | .242 |
| Smoking Status*Cancer Type | 1.41 | −4.21, 7.04 | .627 | 2.72 | −2.46, 7.91 | .311 | 0.48 | −3.67, 4.62 | .799 |
| Month | 0.05 | −0.11, 0.22 | .510 | 0.05 | −0.11, 0.22 | .514 | 0.05 | −0.11, 0.21 | .537 |
| Cancer Type | −3.57 | −6.37, −0.76 | .016 | −2.84 | −5.75, 0.08 | .064 | −4.30 | −6.83, −1.77 | .002 |
| Month*Cancer Type | −0.03 | −0.36, 0.31 | .836 | −0.03 | −0.37, 0.30 | .826 | −0.08 | −0.40, 0.24 | .651 |
|
| |||||||||
| Gender | 2.44 | −0.34, 5.21 | .090 | 1.75 | −0.66, 4.16 | .162 | |||
| Age | −0.11 | −0.25, 0.02 | .118 | −0.04 | −0.13, 0.06 | .482 | |||
| Stage | 1.34 | 0.21, 2.47 | .025 | 0.48 | −0.46, 1.41 | .320 | |||
|
| |||||||||
| Baseline | 0.37 | 0.25, 0.48 | .000 | ||||||
| Income | −0.81 | −1.43, −0.20 | .018 | ||||||
| Income*Month | −0.16 | −0.24, −0.07 | .001 | ||||||
| Income*Cancer Type | 2.28 | 1.15, 3.41 | .000 | ||||||
Notes. Smoking status is coded abstinent at all follow-ups [1] and not abstinent at all follow-ups [0].
CES = Center for Epidemiological Studies, BFI = Brief Fatigue Inventory scale, BPI = Brief Pain Inventory
Model A: The base model assesses abstinent versus not abstinent in the context of Cancer Type (H/N vs. Thoracic) and Month (2, 4, 6, 12).
Model B: This model assesses abstinent versus not abstinent after adding 3 traditional control variables (Age, Gender, & Stage) to the base model (A).
Model C: This model assesses abstinent versus not abstinent after adding 2 variables found to be a predictor of the outcome measure in preliminary analyses – baseline QofL measure and income – to Model B.
Depressive symptoms
Model A showed that abstinent participants exhibited lower CES-D scores (p < .001) as well as an increase in scores over time (p =.05). Model B showed that abstinent participants exhibited lower CES-D scores (p = .007). Model B also showed that women exhibited higher CES-D scores (p = .009) and there was an interaction of gender with cancer type (p = .028) with the gender difference greater for TH patients. Model C found that abstinent participants exhibited lower CES-D scores (p = .028). As observed in the preliminary analyses, baseline CES-D, income, and the interactions of income with month and cancer type were significant predictors (ps < .02). Gender, cancer type, and the interaction of month and cancer type also predicted CES-D scores in this model (ps < .05).
Fatigue
Model A showed that those abstinent exhibited lower BFI scores (p = .018). Model B also showed that abstinent participants exhibited lower BFI scores (p = .021). In addition, Model B showed that later stage patients exhibited higher BFI scores (p = .030). In contrast, Model C did not show a significant effect for smoking status on BFI scores (p = .172) when baseline BFI (p < .001) and income (p = .050) were included. There were no other significant predictors in Model C.
Pain severity
Model A showed that HN patients exhibited greater BPI-S scores (p = .016). Model B showed that those with later stage cancer exhibited greater BPI-S scores (p = .025). Model C showed that cancer type, baseline BPI-S, income, and the interaction of income with month and cancer type were significant predictors (ps < .02). Smoking status was not a significant predictor in any of the 3 models (ps > .25).
Conclusions
In the current study, we examined relationships among smoking status and quality of life indicators in lung and head/neck cancer patients who underwent surgical treatment and had quit smoking within six months prior to surgery or made a quit attempt immediately after surgery. Results revealed that during the year after surgery, patients who maintained abstinence from smoking reported lower levels of depressive symptoms than patients who resumed smoking, even after adjusting for follow-up month, cancer type (lung vs. head/neck), gender, cancer stage, income, and baseline (i.e., at the time of surgery) depressive symptoms.
Most smokers are motivated to quit smoking to improve their long-term physical health, regardless of whether they have been diagnosed with cancer [33, 34]. At the same time, concerns about more immediate negative effects of smoking cessation on quality of life may represent a significant barrier to maintaining abstinence, for both the general population of smokers [35] as well as cancer patients [34]. The current study adds to a growing literature suggesting that smoking abstinence is associated with beneficial, rather than detrimental, effects on depressive symptomatology [36] and extends this important finding to cancer patients. This information may be used to alleviate concerns of both patients and oncology providers that smoking cessation would have a negative effect on mood [34], and to motivate quit attempts and sustained abstinence in these patients, for whom smoking cessation is especially urgent and medically warranted.
Our analyses also revealed that post-surgery smoking abstinence was associated with reduced fatigue after adjusting for follow-up month, cancer type, gender, and cancer stage; however, this relationship was no longer significant after additional adjustment for income and baseline fatigue. Additionally, post-surgery smoking abstinence was not significantly associated with pain severity. Although some previous studies have found that smoking among head/neck and lung cancer patients was associated with increased pain and fatigue [e.g., 20, 21, 23], these studies compared current smokers with former (distant and recent grouped together) and never-smokers, rather than with only recent former smokers who had quit since diagnosis as in the current study.
Models in the current analyses also revealed that higher income was associated with higher quality of life (lower depressive symptoms, fatigue, and pain) at follow-up. Furthermore, there were significant interactions between income and cancer type and between income and follow-up month for depressive symptoms and pain, such that the protective effect of higher income on these variables was more pronounced for HN patients relative to TH patients. The relationship between these variables and income also grew stronger over time, perhaps because quality of life at earlier time points was driven more by factors directly related to surgery (i.e. medical complications) and the protective effects of socioeconomic status did not truly emerge until later. Furthermore, higher income may provide more resources for connecting patients with support services including both social support as well as instrumental supports (e.g., help with daily living tasks) during the survivorship period. These outcomes are consistent with previous literature demonstrating a significant relationship between socioeconomic status and treatment outcomes in head/neck and lung cancer patients, including morbidity, mortality, and quality of life [37, 38].
There are several limitations of the current study that must be acknowledged. First, the sample was predominantly Caucasian and limited to patients diagnosed with head/neck or lung cancer who evidenced baseline differences by cancer type. However, it is important to note that our analytic approach included cancer type as well as any significant interactions between cancer type and baseline variables to account for such differences. Future studies should extend this work to more diverse patients with other types of cancer and appropriately control for differences between cancer types, as was done in the current study. Second, a more comprehensive battery that includes other quality of life indicators such as social, relationship, educational/work, and leisure functioning would also be beneficial. Third, because participants were not randomized to abstain or smoke, the temporal order of the relationship between abstinence and quality of life cannot be determined and causal inferences cannot be made.
Findings from the current study add to a growing body of research indicating that smoking cessation is associated with long-term benefits for quality of life; specifically, the current study found that patients who abstained from smoking for one year after surgical cancer treatment reported reduced depressive symptoms relative to patients who resumed smoking. Prior research reflects a discomfort among oncology providers in discussing smoking cessation that may be in part be due to a reluctance to take away something pleasurable from patients at a time of heightened distress [34]. Thus, our findings may be incorporated into interventions for this population to motivate sustained abstinence among patients as well as changing attitudes and behaviors of providers.
Acknowledgments
Funding Source: This work has been supported by the National Cancer Institute R03 CA 126409 and in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA76292)
Footnotes
Financial Disclosures: There are no financial disclosures for any of the authors.
References
- 1.USDHHS. The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. Contract. [Google Scholar]
- 2.Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98:632–44. doi: 10.1002/cncr.11538. [DOI] [PubMed] [Google Scholar]
- 3.Park ER, Japuntich SJ, Rigotti NA, Traeger L, He Y, Wallace RB, Malin JL, Zallen JP, Keating NL. A snapshot of smokers after lung and colorectal cancer diagnosis. Cancer. 2012 doi: 10.1002/cncr.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gritz ER, Schacherer C, Koehly L, Nielsen IR, Abemayor E. Smoking withdrawal and relapse in head and neck cancer patients. Head Neck. 1999;21:420–7. doi: 10.1002/(sici)1097-0347(199908)21:5<420::aid-hed7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Walker MS, Larsen RJ, Zona DM, Govindan R, Fisher EB. Smoking urges and relapse among lung cancer patients: findings from a preliminary retrospective study. Prev Med. 2004;39:449–57. doi: 10.1016/j.ypmed.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110:1199–202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 7.Cooley ME, Wang Q, Johnson BE, Catalano P, Haddad RI, Bueno R, Emmons KM. Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung Cancer. 2012;76:144–9. doi: 10.1016/j.lungcan.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooley ME, Sarna L, Kotlerman J, Lukanich JM, Jaklitsch M, Green SB, Bueno R. Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent. Lung Cancer. 2009;66:218–25. doi: 10.1016/j.lungcan.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons VN, Litvin EB, Jacobsen PB, Patel RD, McCaffrey JC, Oliver JA, Sutton SK, Brandon TH. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer. 2013;119:1420–7. doi: 10.1002/cncr.27880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davison AG, Duffy M. Smoking habits of long-term survivors of surgery for lung cancer. Thorax. 1982;37:331–3. doi: 10.1136/thx.37.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gritz ER, Nisenbaum R, Elashoff RE, Holmes EC. Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer. Cancer Causes Control. 1991;2:105–12. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 12.Jerjes W, Upile T, Radhi H, Petrie A, Abiola J, Adams A, Kafas P, Callear J, Carbiner R, Rajaram K, Hopper C. The effect of tobacco and alcohol and their reduction/cessation on mortality in oral cancer patients: Short communication. Head Neck Oncol. 2012;4:6. doi: 10.1186/1758-3284-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawahara M, Ushijima S, Kamimori T, Kodama N, Ogawara M, Matsui K, Masuda N, Takada M, Sobue T, Furuse K. Second primary tumours in more than 2-year disease-free survivors of small-cell lung cancer in Japan: the role of smoking cessation. Br J Cancer. 1998;78:409–12. doi: 10.1038/bjc.1998.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Kamdar O, Le W, Rosen GD, Upadhyay D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–46. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zevallos JP, Mallen MJ, Lam CY, Karam-Hage M, Blalock J, Wetter DW, Garden AS, Sturgis EM, Cinciripini PM. Complications of radiotherapy in laryngopharyngeal cancer: effects of a prospective smoking cessation program. Cancer. 2009;115:4636–44. doi: 10.1002/cncr.24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–16. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 18.Moller AM, Pedersen T, Villebro N, Schnaberich A, Haas M, Tonnesen R. A study of the impact of long-term tobacco smoking on postoperative intensive care admission. Anaesthesia. 2003;58:55–9. doi: 10.1046/j.1365-2044.2003.02788_2.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith JB, Fenske NA. Cutaneous manifestations and consequences of smoking. J Am Acad Dermatol. 1996;34:717–32. doi: 10.1016/s0190-9622(96)90002-x. quiz 33–4. [DOI] [PubMed] [Google Scholar]
- 20.Logan HL, Fillingim RB, Bartoshuk LM, Sandow P, Tomar SL, Werning JW, Mendenhall WM. Smoking status and pain level among head and neck cancer patients. J Pain. 2010;11:528–34. doi: 10.1016/j.jpain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, Jacobsen PB. Associations between pain and current smoking status among cancer patients. Pain. 2011;152:60–5. doi: 10.1016/j.pain.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy SA, Ronis DL, Valenstein M, Fowler KE, Lambert MT, Bishop C, Terrell JE. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics. 2007;48:142–8. doi: 10.1176/appi.psy.48.2.142. [DOI] [PubMed] [Google Scholar]
- 23.Garces YI, Yang P, Parkinson J, Zhao X, Wampfler JA, Ebbert JO, Sloan JA. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–41. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 24.Berg CJ, Thomas AN, Mertens AC, Schauer GL, Pinsker EA, Ahluwalia JS, Khuri FR. Correlates of continued smoking versus cessation among survivors of smoking-related cancers. Psychooncology. 2013;22:799–806. doi: 10.1002/pon.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons VN, Litvin EB, Unrod M, Brandon TH. Oncology healthcare providers’ implementation of the 5A’s model of brief intervention for smoking cessation: Patients’ perceptions. Patient Educ Couns. 2012;86:414–9. doi: 10.1016/j.pec.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 29.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–96. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in pain research and therapy, volume 12: issues in pain measurement. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 31.Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- 32.Bernaards CA, Belin TR, Schafer JL. Robustness of a multivariate normal approximation for imputation of incomplete binary data. Stat Med. 2007;26:1368–82. doi: 10.1002/sim.2619. [DOI] [PubMed] [Google Scholar]
- 33.Baha M, Le Faou AL. Smokers’ reasons for quitting in an anti-smoking social context. Public Health. 2010;124:225–31. doi: 10.1016/j.puhe.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Simmons VN, Litvin EB, Patel RD, Jacobsen PB, McCaffrey JC, Bepler G, Quinn GP, Brandon TH. Patient-provider communication and perspectives on smoking cessation and relapse in the oncology setting. Patient Educ Couns. 2009;77:398–403. doi: 10.1016/j.pec.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisinger C, Aadahl M, Toft U, Jorgensen T. Motives to quit smoking and reasons to relapse differ by socioeconomic status. Prev Med. 2011;52:48–52. doi: 10.1016/j.ypmed.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Shahab L, Andrew S, West R. Changes in prevalence of depression and anxiety following smoking cessation: results from an international cohort study (ATTEMPT) Psychol Med. 2013:1–15. doi: 10.1017/S0033291713000391. [DOI] [PubMed] [Google Scholar]
- 37.Demiral AN, Sen M, Demiral Y, Kinay M. The effect of socioeconomic factors on quality of life after treatment in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:23–7. doi: 10.1016/j.ijrobp.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 38.Yang R, Cheung MC, Byrne MM, Huang Y, Nguyen D, Lally BE, Koniaris LG. Do racial or socioeconomic disparities exist in lung cancer treatment. Cancer. 2010;116:2437–47. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]


