Abstract
OBJECTIVES
Earlier studies have documented a greater mortality risk associated with conventional compared with atypical antipsychotics. Concern remains that the association is not causal, but due to residual confounding by differences in underlying health. To address this concern, we evaluated whether adjustment for prognostic indices specifically developed fornursing home (NH) populations affected the magnitude of the previously observed associations.
DESIGN
Cohort study
SETTING
A merged dataset of Medicaid, Medicare, the Minimum Data Set (MDS), the Online Survey Certification and Reporting system (OSCAR), and the National Death Index in the US for 2001-2005
PARTICIPANTS
Dual eligible subjects ≥ 65 years who initiated antipsychotic treatment in a NH (n=75,445).
MEASUREMENTS
Three mortality risk scores (MRIS, MMRI-R, and ADEPT) were derived for each patient using baseline MDS data, and their performance was assessed using c-statistics and goodness-of-fit tests. The impact of adjusting for these indices in addition to propensity scores (PS) on the antipsychotic-mortality association was evaluated using Cox models with and without adjustment for risk scores.
RESULTS
Each risk score showed moderate discrimination for 6-month mortality with c-statistics ranging from 0.61 to 0.63. There was no evidence of lack of fit. Imbalances in risk scores between conventional and atypical antipsychotic users in the full cohort, suggesting potential confounding, were greatly reduced within PS deciles. Accounting for each score in the Cox model did not change the relative risk estimates: 2.24 with PS only adjustment vs. 2.20, 2.20, 2.22 after further adjustment for the three risk scores.
CONCLUSION
Although causality cannot be proven based on non-randomized studies, this study adds to the body of evidence rejecting alternative explanations for the increased mortality risk associated with conventional antipsychotics.
Keywords: Antipsychotics, nursing homes, mortality, confounding, pharmacoepidemiology
INTRODUCTION
Antipsychotic medications have been used extensively in the elderly to manage behavioral symptoms associated with dementia, despite the lack of strong evidence on effectivenessand concerns about their safety 1-6. Based on analyses of 17 randomized clinical trials (RCTs), the FDA issued black box warnings of increased mortality associated with the use of atypical antipsychotics in older patients with dementia in 20057.Based on evidence from observational studies8, 9, similar warnings were issued for conventional antipsychotics in 200810.Although these studies implied a greater risk associated with conventional than with atypical antipsychotics, FDA did not consider the evidence sufficient at that stage to conclude that conventional antipsychotics are riskier, due to concerns aboutmethodological limitationsof non-randomized studies. Improper use of antipsychotics is especially of concern for nursing home residents who are at higher risk of adverse events due to underlying comorbidities and intensive medication use 11.Nearly one third of nursing home residents in the United States received antipsychotic drugs in 2007 12 and this proportion has remained high despitethe safety warnings issued by the FDA 13. Following the launch of National Partnership to Improve Dementia Careby the Centers for Medicare and Medicaid Services in 2012, the use of antipsychotics in long-stay nursing home residents has decreased, but the absolute proportion remains high, with 21.7% in the first quarter of 201314. A recent study conducted in multiple European countries reported overall prevalence of antipsychotic medication use at 33%.15 While there has been a shift towards the use of the newer atypical antipsychotics, conventional antipsychotics continue to be used extensively in acute and long-term inpatient settings.16, 17
In the absence of evidence from RCTs, observational studies are an important source of information on the comparative safety of antipsychotic medications in the elderly. Advanced epidemiologic methods have been utilized in the design and analysis of studies of antipsychotic medication safety in order to mitigate potential confounding bias and ensure study validity.The techniques implemented include, amongst others, exclusion of patients that are identified as terminally ill, use of high-dimensional propensity scores to adjust for proxies of unmeasured confounders, instrumental variable analyses, and sensitivity analysis and external adjustment for unmeasured confounding18, 19.Regardless, concern that the increased risk of death associated with conventional antipsychotics may be due to residual confounding by differences in patient characteristics such as frailty and terminal illness, and is therefore “not a cause of death, but caused by impending death” remains20. Several of the previously conducted studies in nursing home populations have adjusted for general comorbidity index scores such as the Charlsonor Elixhauser comorbidity measures, which predict 10-year mortality and in-hospital mortality respectively 21, 22,butno study has accounted for prognostic indices that were specifically developed to predict the risk of 180-day mortality (i.e., the outcome in most studies of antipsychotic safety in the elderly) in a nursing home population.
The objective of our study was to examine whether adjustment for such population specific scores supports the premise that the increased mortality risk observed in this population 8, 9 can be attributed to residual confounding rather than a causal association.
METHODS
Study Design
The data source and study population have previously been described in detail23.Briefly, we used a merged dataset of Medicaid and Medicare claims, the Minimum Data Set (MDS), the Online Survey Certification and Reporting (OSCAR) system, and the National Death Index (NDI) in 45 states in the United States for 2001-2005.The members of the study cohortwere aged ≥ 65 years, were dually eligible for Medicare and Medicaid, started treatment with an antipsychotic drug during a stay in a nursing home, and had six months’ continuous Medicaid coverage before treatment initiation.Subjects who filled a prescription for both conventional and atypical antipsychotics on the index date or who had a pre-existing diagnosis for cancer, schizophrenia, or bipolar disorder were excluded since they were likely to have received antipsychotics for other reasons.
Each subject was assigned to a treatment group based on their first prescription for an antipsychotic in the nursing home.Treatment was considered discontinued if there was a gap of 14 days or more after the days of supply of the previous prescription ran out. Antipsychotic drugs included in the analyses were haloperidol, aripiprazole, olanzapine, quetiapine, ziprasidone, and risperidone. The outcome, defined as non-cancer deathwithin 180 days after treatment initiation, was identified using the National Death Index. Follow up was censored at the time of treatment discontinuation, augmentation, switch to a different drug, and admission to a hospital for 10 days or more since medication use during such stays cannot be observed in the data.
Mortality Risk Scores
Prognostic indices or risk scores are frequently used to predict the probability of death for older adults from measured covariate information24. Three composite risk scores developed for nursing home residents using the MDS were identified in the literature. The Mortality Risk Index Score (MRIS) was developed and revised to predict 1-year mortality in newly admitted residents or long-stay residents 25, 26. The MDS Mortality Risk Index (MMRI) and MMRI-R, a revised version of MMRI, were developed to predict 6-months mortality in nursing home residents27, 28. Lastly, the Advanced Dementia Prognostic Tool (ADEPT) was developed to predict 6-months mortality in advanced dementia patients in nursing homes29, 30.MDS items commonly included in these risk scores are reduced appetite, functional ability level, congestive heart failure, male sex, and shortness of breath. The complete list of MDS items included in each score is presented in the Appendix Table 1, and a full description of and rationale behind the development of the risk scores is provided in the original articles 25-30.
The possible rangesfor each of the scores are 0 to 19.45 for MRIS, 0 to 85 for MMRI-R, and 1 to 32.5 for ADEPT. The scores have previously been validated in small independent datasets ranging in size from 130 to 606 subjects31-33, but they have not yet been validated in a large independent dataset.We estimated the scores for subjects in our nursing home population at the time of treatment initiation with antipsychotics using information from the last available observation for each variable, and assessed the performance of the three risk scores to predict 180-day mortality.
Statistical Analysis
Performance characteristics of the risk scores in the study sample
Logistic regression models with each respective score included as a continuous independent variable and 180-day mortality as the dependent variable were constructed to examinethe discrimination of the risk scores by c-statistics (the area under the receiver operating curve). Nonlinear associations using log-transformation between risk scores and mortality were considered, as well as a combination of the three risk scores. The calibration was evaluated by the Hosmer-Lemeshow goodness-of-fit p-value, which evaluates fit of a prediction modelby comparing the observed number of deaths to the expected number of deaths based on deciles of predicted mortality. The statistical significance was evaluated at α=0.05 level, where significant p-values indicate lack of fit. In addition, we plotted the observed risk of mortality by risk score categories to observe association between risk scores and mortality.
Assessment of potential residual confounding
The original study, which assessed the association between antipsychotics and mortality, used propensity score (PS) stratification to adjust for potential confounding variables23. Propensity scores were derived from predicted probabilities of starting a conventional antipsychoticcompared to an atypical antipsychotic using logistic regression models. The list of variables included in the propensity score model is shown in the Appendix Table 2. To evaluate potential residual confounding within PS strata, we assessed the balance of risk scores across deciles of the propensity score. Any observed imbalance would indicate different baseline risk of death between the two comparison groups, after adjusting for propensity score.
Finally, to evaluate the impact of adjusting for the risk scores on the association between different antipsychotic agents and mortality, we repeated the Cox proportional hazards analyses presented in the original study, while adjusting for both propensity score strataand the respective risk scores (included as a continuous variable). The results from three models (one for each of therisk scores) were compared to those from the models adjusted for propensity score strata only. All analyses were conducted using SAS 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
The study cohortincluded 75,445 nursing home residents who started treatment with antipsychotics between 2001 and 2005;15,390 residents (20.4%) died over a 6-month period.
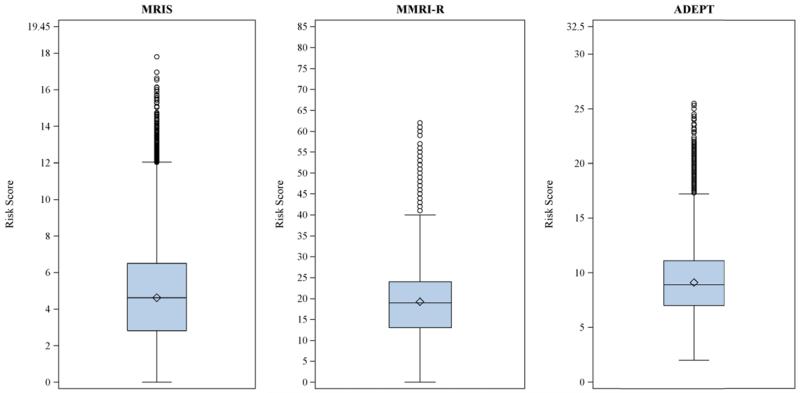
The distribution of the different risk scores as derived from the subjects’ most recent MDS assessment before treatment initiation is shown in Figure 1. The vast majority of the subjects had risk scores in the lower half of the range. The median values were 4.62 for MRIS, 19 for MMRI-R, and 8.9 for ADEPT.The 90th percentile was 8.33 for MRIS, 30 for MMRI-R, and 13.2 for ADEPT.
Figure 1.
Distribution of mortality risk scores in a cohort of 75,445 dually-eligible nursing home residents who initiated treatment with antipsychotic medications after admission. Box range represents interquartile range (IQR; 25th to 75th percentiles); whisker range represents values with distance ≤ 1.5*IQR; dots indicate outliers with distance > 1.5*IQR; horizontal line represents the median; diamonds indicate the means. Y-axis shows the possible range of each score.
MRIS = Mortality Risk Index Scores; MMRI-R = Mortality Risk Index Revised; ADEPT = Advanced Dementia Prognostic Tool
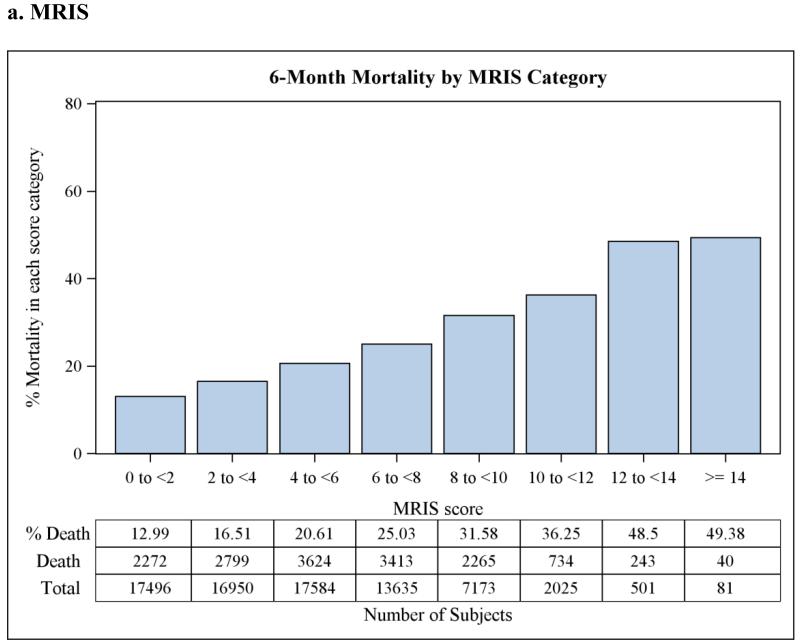
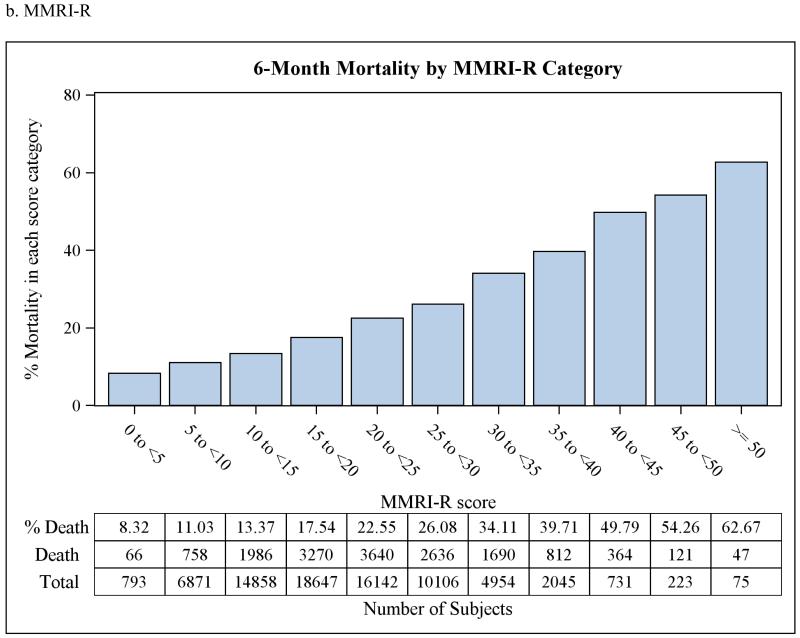
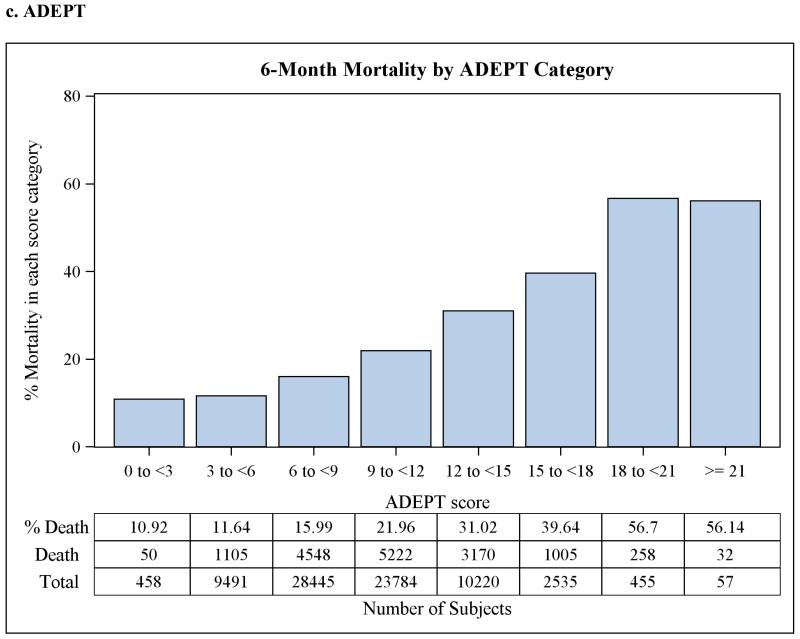
The discrimination statistics, or c-statistics, for each of the three scoreswere 0.61 (95% CI 0.607-0.617) for MRIS, 0.63 (95% CI 0.628-0.638) for MMRI-R, and 0.63(95% CI 0.621-0.630) for ADEPT, indicating moderate discrimination for all three risk scores. C-statistics did not improve when the three risk scores were used together in a single model, or when the average of the three scores was used instead after rescaling them into 0 to 100 scale, or when log transformation of the scores was used. The goodness-of-fit p-valueswere 0.05, 0.18, and 0.50 for MRIS, MMRI-R, and ADEPT, respectively.Although the p-value for MRIS was marginally non-significant, this is likely attributable to the large sample size and not necessarily to a true lack of fit.When categorized, higher risk score category was associated with higher 6-months mortality as shown in Figure 2. This relationship was also present afterstratification by antipsychotic class (data not shown).
Figure 2.
Observed 6-month mortality by risk score category in a cohort of 75,445 dually-eligible nursing home residents who initiated treatment with antipsychotic medications after admission.
MRIS = Mortality Risk Index Scores; MMRI-R = Mortality Risk Index Revised; ADEPT = Advanced Dementia Prognostic Tool
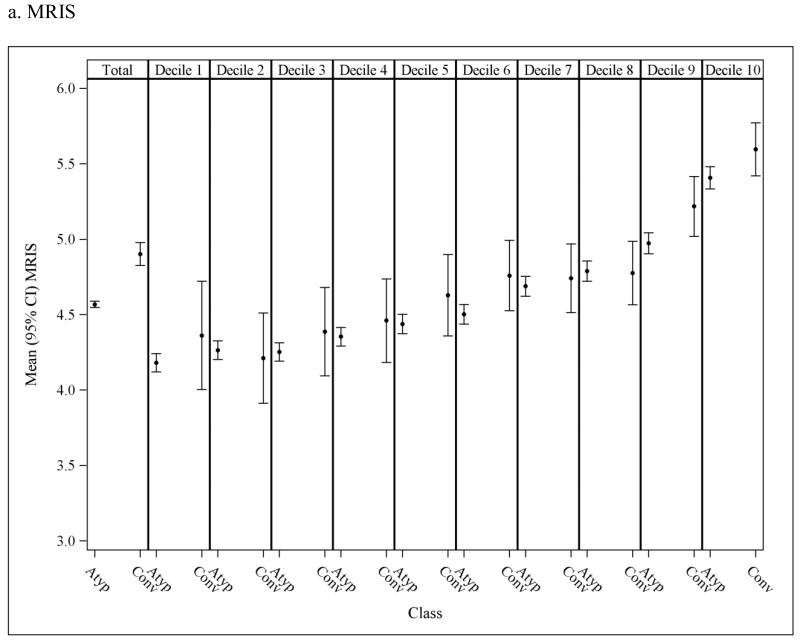
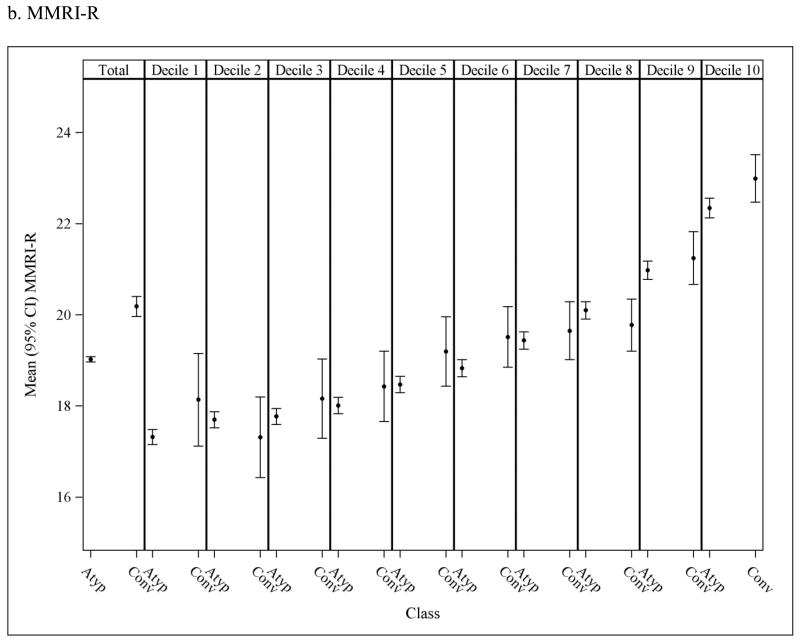
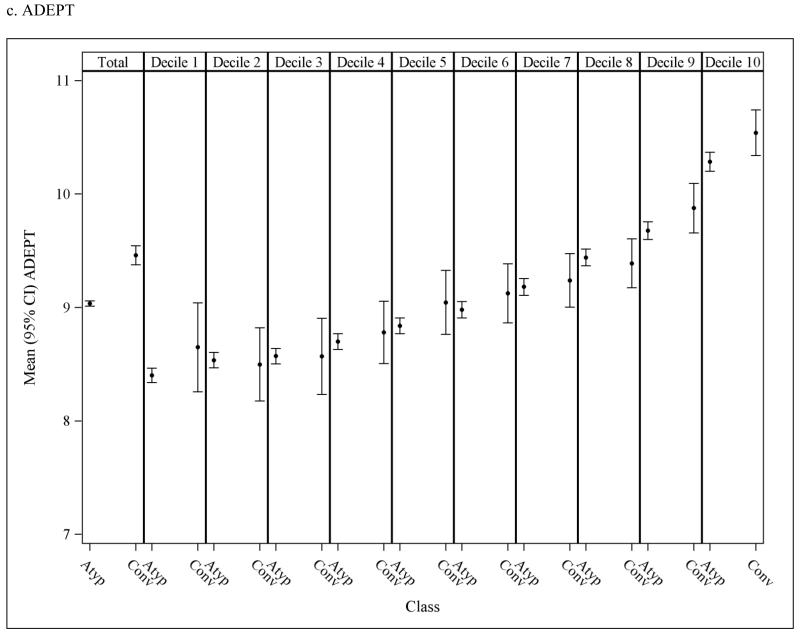
To assess the potential for residual confounding within PS strata, Figure 3 presents the overall andPS stratum-specific risk score distributions. There is some imbalance in risk score in the population overall, suggesting that subjects who initiate conventional antipsychotics are at an increased risk of death at baseline as estimated based on their pre-treatment characteristics. These differences either disappear or are greatly reduced within strata of the PS deciles suggesting that confounding by underlying health differences is accounted for to a large extent through use of the PS, though some residual differencesare observed in the higher deciles.
Figure 3.
Mean (95% CI) risk scores in nursing home residents initiated on atypical and conventional antipsychotic medications; observed means in the overall population (1st column) and in each propensity score (PS) decile (2nd-11th columns). Discrepancy in the mean risk scores between the two medication classes within PS decilesindicates potential residual confounding after adjusting for the PS.The y-axis was truncated to facilitate interpretation; full scale for MRIS = 0 to 19.45; MMRI-R = 0 to 85; ADEPT = 0 to 32.5.
MRIS = Mortality Risk Index Scores; MMRI-R = Mortality Risk Index Revised; ADEPT = Advanced Dementia Prognostic Tool; Atyp = Atypical; Conv = Conventional
The results from PS adjusted proportional hazard models assessing the association between conventional vs. atypical antipsychotics and mortality without and with adjustment for each risk score are shown in Table 1. Accounting for the risk scoresdid not change the relative risk estimates; conventional antipsychotics were associated with more than two fold increase in mortality risk in models adjusted for either PS only or for both PS and risk scores.
Table 1. Hazard Ratios (95% CI) for Non-cancer Death within 180 Days of Start of Treatment in Nursing Home Residents.
| Antipsychotic Class | No. of events |
Unadjusted | Adjusted for PSa | Adjusted for PSa and MRIS26 |
Adjusted for PSa and MMRI-R28 |
Adjusted for PSa and ADEPT30 |
|---|---|---|---|---|---|---|
| Conventionalb | 745 | 2.58 (2.38-2.80) | 2.24 (2.06-2.43) | 2.20 (2.02-2.39) | 2.20 (2.02-2.39) | 2.22 (2.04-2.41) |
PS = Propensity score; MRIS = Mortality Risk Index Score; MMRI-R = MDS Mortality Risk Index Revised; ADEPT = Advanced Dementia Prognostic Tool
PS-adjusted models were stratified across 10th of PS, after truncating 2.5% of patients on either extreme of PS distribution.
Atypical antipsychotics as a group was used as a referent
DISCUSSION
Summary of Main Findings
Using a large independent sample of 75,445 subjects, we validated three mortality risk scores developed specifically for nursing home populations and calculated using MDS data. Discrimination was moderate for all three scores, with c-statisticsranging from 0.61 to 0.63. P values from formal goodness-of-fit tests, as well as graphical exploration of the relation between risk scores and observed mortality indicate adequate calibration of the models using each risk score as a sole independent variable to predict 180-day mortality. Adjusting for potential residual confounding by differences in underlying health using these risk scores, in addition to adjusting for a large number of potential confounders through use of propensity scores, we confirmed previous findingsof an increased risk of 180-day mortality associated with use of conventional antipsychotics compared with atypical antipsychotics (HR=2.24).
In the original development studies, the c-statistics were 0.73 for the MRIS 26, 0.76 for the MMRI-R 28and 0.73 for ADEPT 30. Our c-statistics in this independent sample are lower, but comparable with those from previoussmaller validation studies. Kruse et al. validated MRIS and MMRI-R using a sample from 130 nursing home residents31 and estimated a c-statistic for MRIS of 0.70 (95% CI 0.60-0.81) and for MMRI-R of 0.59 (95% CI 0.46-0.72), but these values are not precisely estimated. In a prospective validation of ADEPT in dementia patients by Mitchell et al., the c-statistic was 0.67 (95% CI 0.62-0.72) 33.Nonlinearity or other modeling issues donot appear to explain the lower c-statistic in our sample. It should be noted that the performance of MRIS or ADEPT scores might have been affected by the differences in patient characteristics or study duration because we did not use the same population (ADEPT) or duration of follow-up (MRIS) used in the original development studies. Nevertheless, when we restricted the analysis to subjects with a recorded diagnosis of dementia, the model performance for the ADEPT score did not change(c-statistic 0.63).
In contrast to the developmental cohorts, most of our subjects had risk scores in the lower half of the score range.We share this characteristic with the validation study by Kruse et al. In their study, 95% of subjects fell in the range of MRIS 3 to 10 and 70% of the subjects had MMRI-R scores less than 26.
Adjusting for the risk scores did not change the effect estimates. This suggests that propensity scores that were developed using the rich information captured in healthcare claims, the MDS and OSCAR already accounted for most of the confounding bias. While the possibility for residual confounding remains in any observational study, it appears unlikely that the findings of increased mortality can be fully explained by residual confounding, independent of all other factors already taken into account.
Utility of Prognostic Scores
There are a small number of studies reporting the use of these risk scores in clinical decision-making processes. In Levy et al., MRIS was used in a program evaluating the end-of-life care outcomes in nursing homes to identify residents at high risk of death34.MRIS and MMRI-I have also been cited in guidelines as clinical indicators for mortality 35. Use ofADEPT has been suggested as an evaluation tool for determining hospice enrollment 36, 37.Though MDS data required to calculate these scores are readily available for most nursing home residents, widespread use of the risk scores in clinical practice has not yet been recommended due to insufficient evidence24.
The present study builds on theclinical utility of theserisk scores and extends their potential use to confounding adjustment in epidemiologic research. Stuart et al recently showed how prognostic-score based balance measures can be used to assess the performance of propensity score methods for reducing bias in non-experimental studies. 38. In their simulations,balance in the prognostic score had the highest correlation in general with bias in the treatment effect, implying that further adjustment with prognostic scores is likely to reduce residual confounding. Another simulation study illustrated the complementary role of propensity and prognostic score methods in reducing bias and improving robustness of models containing both scores 39.
The Role of Delirium
To this date, observational studies have consistently shown increased risk of death associated with conventional antipsychotics compared with atypical antipsychotics or no treatment. One of the alternative explanations other than the hazardous effect of the medication is confounding by indication, in particular delirium20. Delirium isknown to be associated with increased mortality40, 41.Since haloperidol has been considered a drug of choice for patients with delirium42, it has been suggestedthat users of haloperidol have a higher prevalence of delirium and that this cannot be fully controlled for due to unrecognized or undiagnosed delirium in nursing home residents43.
Functional decline in the elderly is also a strong predictor for mortality, and delirium is known to be a predictor for functional decline44, 45.Although therisk scores considered in the current investigation do not include delirium itself, they include functional decline using activities of daily living (ADL) scores. It therefore seems unlikely that our results, which adjust for diagnosed delirium through the propensity score and adjust – at least partially – for undiagnosed delirium through the functional decline measure in the risk scores, can be fully attributed to residual confounding.
Limitations of the Study
Our study has a number of limitations. Two of the three risk scores wereoriginally developed for a slightly different population than was used in our validation study or considered a different duration of follow-up. In particular, ADEPT was developed for advanced dementia patients. Although we did not require a recorded diagnosis of dementia in our main analysis, most subjects are presumably initiated on an antipsychotic medication to address behavioral problems associated with moderate to advanced stages of dementia, since we excluded patients with schizophrenia and bipolar disorder. This is supported by the fact that restricting our population to patients with a recorded dementia diagnosis in sensitivity analysis resulted in very similar findings. Although the exclusion of selected comorbid conditions (i.e., schizophrenia, bipolar disorder, cancer) may reduce the generalizability of our validation results, there is no a priori reason to believe that the validity of the risk scores would be different in this particular subset of the broader target population, nursing home residents. MRIS was developed using data only from newly admitted residents for 1-year mortality. Most of our study population consisted ofnewly admitted residents46, and 6-month mortality is expected to be highly correlated with1-year mortality.
We observed onlya few residents with very high risk scores, which may be explained by the narrower range of patients included in our cohort. Residents with a pre-existing cancer diagnosis were excluded from our study cohort, and a cancer diagnosis is a component in two of the three risk scores. Although this could have affected the discriminative ability of the risk scores, the fact that the discrepancies in risk score distribution are similar to previously reported validation studies in magnitude and direction suggests it is possible that the original prediction models were over-fitted to the development data. While potentially important for clinical decision-making, this should not affect our ability to account for between-group differences in mortality risk in the target population (i.e., patients initiated on antipsychotics without a pre-existing cancer diagnosis).
CONCLUSION
Our study adds to the body of evidencesuggesting that conventional antipsychotics are associated with higher 6-month mortality than atypical antipsychotics,even after further adjustment for baseline mortality risk scores among nursing home residents. To the extent that potential differences in patient characteristics between users of conventional and atypical antipsychotics are properly captured using propensity scores and disease risk scores, it is unlikely that this observed effect of increased mortality can be explained by unmeasured confounding due to underlying differences in health, such as terminal illness, frailtyor delirium.Although causality cannot be proven based on non-randomized studies, this study adds to the growing body of evidence rejecting alternative explanations for the observed association. In light of continued high prevalence of antipsychotics medication use in nursing homes and substantial use of conventional antipsychotics in acute and long-term inpatient settings, comparative safety studies for these medications are clinically relevant. Moreover, the validation results in this large population further support the findings from earlier studies that prognostic risk scores can be used as a guidance tool combined with expert judgment in similar clinical settings.
ACKNOWLEDGMENTS
Funding sources: Y. Park received pharmacoepidemiology training grant support from Harvard School of Public Health. Dr. Schneeweiss is partially supported by grants/contracts from the Patient-Centered Outcomes Research Institute, the FDA, and the National Heart, Lung, and Blood Institute. S. Crystal was supported by CERTS 5 U19 HS02112 Effectiveness and Safety of Antipsychotics – 1 U18 HS017918 T. Gerhard was supported by AHRQ CERTs award U19 HS021112-02 for the Rutgers Center for Education and Research on Mental Health Therapeutics. Dr. Huybrechts was supported by a career development grant K01MH099141 from the National Institute of Mental Health.
J. Franklin received a grant for an unrelated project from Merck. Dr. Schneeweiss is consultant to WHISCON and LLC, and to Aetion, a software manufacturer of which he also owns shares, and is principal investigator of investigator initiated grants to the Brigham and Women’s Hospital from Novartis and Boehringer-Ingelheim unrelated to the topic of this study. T. Gerhard is a board Member of the International Society for Pharmacoepidemiology (ISPE).
Sponsor’s Role: None
Appendix
Appendix Table 1. List of MDS Items Included in the Risk Score Calculation.
| Risk Score | Items from MDS 2.0 |
|---|---|
| MRIS26 | Cancer, Shortness of breath, Congestive heart failure, Bedfast, Male gender, >25% food uneaten, Low functional abilitya, Swallowing problem, Bowel incontinence, BMI < 23 |
| MMRI-R28 | Admission to nursing home (NH) in the past 3 months (Recent NH admission), Weight loss, Renal failure, Congestive heart failure, >25% food uneaten, Male gender, Dehydratedb, Shortness of breath, Cancer and agec, ADL and cognitiond |
| ADEPT30 | Admission to NH in the past 3 months (Recent NH admission), Agee, Male sex, Shortness of breath, At least one pressure ulcer > Stage 2, ADL scoref, Bedfast, Insufficient oral intakeg, Bowel incontinence, BMI <18.5, Weight loss, Congestive heart failure |
Functional ability assessed by ADL score using 7 items from ‘ADL Self-Performance’ measure (bed mobility, transfer, locomotion on unit, dressing, eating, toilet use, and personal hygiene); each variable had 0 to 4 point scale adding up to maximum of 28. If ADL score was greater than the population median, the subject was considered to have ‘low functional ability’.
Dehydration: either ‘Dehydration’ or ‘Insufficient fluid’
Cancer and age: the effect of age stratified across 5-year interval was assessed differently among residents who had cancer and who did not
ADL and cognition: 4 items from ‘ADL Self-Performance’ measure was used to calculate functional ability (locomotion on unit, eating, toilet use, and personal hygiene); each variable had 0 to 4 point scale adding up maximum of 16. This ADL score was modified if the subject also had cognitive decline, assessed by an MDS item ‘Change in cognitive status’.
Age was stratified in 5-year increment.
ADL score was calculated in the same way as it was done in MRIS, and then dichotomized as ADL=28 and ADL<28.
Insufficient oral intake: either ‘>25% food uneaten’ or ‘Insufficient fluid’
Appendix Table 2. List of Variables Included in the Propensity Score.
| Characteristics | Variables in the propensity score |
|---|---|
| Sociodemographic | Age, sex, race, education, and geographical region (state) |
| Clinical | Psychiatric morbidity, cardiovascular morbidity, cerebrovascular disease, other comorbidities (e.g. Parkinson’s disease, epilepsy, diabetes, obesity, functional impairment), the Charlson index, and use of healthcare services potentially predictive of adverse health outcomes in the short term (e.g. number of days in hospital, number of distinct prescriptions for drugs excluding antipsychotics) |
| Nursing home facility | Facility size, occupancy rate, availability of special care units, staffing levels, ownership, resident characteristics (e.g. proportion with dementia, depression) and quality indicators (e.g. proportion bedbound) |
Footnotes
Author Contributions:
Study concept and design: Park, Huybrechts
Acquisition of data: Crystal, Schneeweiss
Analysis and interpretation of data: Park, Franklin, Schneeweiss, Levin, Crystal, Gerhard, Huybrechts
Drafting of manuscript: Park, Huybrechts
Critical revision of the manuscript for important intellectual content: Park, Franklin, Schneeweiss, Levin, Crystal, Gerhard, Huybrechts
REFERENCES
- 1.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 2.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: Meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 3.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 4.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 5.Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: A systematic review and meta-analysis. JAMA. 2011;306:1359–1369. doi: 10.1001/jama.2011.1360. [DOI] [PubMed] [Google Scholar]
- 6.Ballard C, Hanney ML, Theodoulou M, et al. The dementia antipsychotic withdrawal trial (DART-AD): Long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8:151–157. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed November 1, 2013];Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. 2005 FDA (online). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm.
- 8.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176:627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed November 1, 2013];Information for Healthcare Professionals: Conventional Antipsychotics. 2008 FDA (online). Availalbe at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124830.htm.
- 11.Aparasu RR, Chatterjee S, Mehta S, et al. Risk of death in dual-eligible nursing home residents using typical or atypical antipsychotic agents. Med Care. 2012;50:961–969. doi: 10.1097/MLR.0b013e31826ec185. [DOI] [PubMed] [Google Scholar]
- 12.Rochon PA, Stukel TA, Bronskill SE, et al. Variation in nursing home antipsychotic prescribing rates. Arch Intern Med. 2007;167:676–683. doi: 10.1001/archinte.167.7.676. [DOI] [PubMed] [Google Scholar]
- 13.Dorsey ER, Rabbani A, Gallagher SA, et al. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170:96–103. doi: 10.1001/archinternmed.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Accessed July 7, 2014];Press Release: New data show antipsychotic drug use is down in nursing homes nationwide. 2013 CMS (online). Available at: http://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-Releases/2013-Press-Releases-Items/2013-08-27.html.
- 15.Foebel AD, Liperoti R, Onder G, et al. Use of Antipsychotic Drugs Among Residents With Dementia in European Long-Term Care Facilities: Results From the SHELTER Study. J Am Med Dir Assoc. 2014 Sep 24; doi: 10.1016/j.jamda.2014.07.012. pii: S1525-8610(14)00471-X. doi: 10.1016/j.jamda.2014.07.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Zirker W, Dorokhine I, Knapp CM, et al. Haloperidol overdosing in the treatment of agitated hospitalized older people with delirium: A retrospective chart review from a community teaching hospital. Drugs Aging. 2013;30:639–644. doi: 10.1007/s40266-013-0087-7. [DOI] [PubMed] [Google Scholar]
- 17.Swan JT, Fitousis K, Hall JB, et al. Antipsychotic use and diagnosis of delirium in the intensive care unit. Crit Care. 2012;16:R84. doi: 10.1186/cc11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huybrechts KF, Brookhart MA, Rothman KJ, et al. Comparison of different approaches to confounding adjustment in a study on the association of antipsychotic medication with mortality in older nursing home patients. Am J Epidemiol. 2011;174:1089–1099. doi: 10.1093/aje/kwr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 20.Luijendijk H, Bruin Nd, Koolman X. Haloperidol in elderly users: Not a cause of death, but caused by impending death?; Society for Epidemiologic Research 46th Annual Meeting 2013 Abstract 011; Available at: http://www.epiresearch.org/meeting/online/abstract_detail.php?i=84eb13cfed01764d9c401219faa56d53. Accessed November 1, 2013. [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: Population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yourman LC, Lee SJ, Schonberg MA, et al. Prognostic indices for older adults: A systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flacker JM, Kiely DK. A practical approach to identifying mortality-related factors in established long-term care residents. J Am Geriatr Soc. 1998;46:1012–1015. doi: 10.1111/j.1532-5415.1998.tb02759.x. [DOI] [PubMed] [Google Scholar]
- 26.Flacker JM, Kiely DK. Mortality-related factors and 1-year survival in nursing home residents. J Am Geriatr Soc. 2003;51:213–221. doi: 10.1046/j.1532-5415.2003.51060.x. [DOI] [PubMed] [Google Scholar]
- 27.Porock D, Oliver DP, Zweig S, et al. Predicting death in the nursing home: Development and validation of the 6-month Minimum Data Set mortality risk index. J Gerontol A Biol Sci Med Sci. 2005;60:491–498. doi: 10.1093/gerona/60.4.491. [DOI] [PubMed] [Google Scholar]
- 28.Porock D, Parker-Oliver D, Petroski GF, et al. The MDS Mortality Risk Index: The evolution of a method for predicting 6-month mortality in nursing home residents. BMC Res Notes. 2010;3:200. doi: 10.1186/1756-0500-3-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell SL, Kiely DK, Hamel MB, et al. Estimating prognosis for nursing home residents with advanced dementia. JAMA. 2004;291:2734–2740. doi: 10.1001/jama.291.22.2734. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell SL, Miller SC, Teno JM, et al. The advanced dementia prognostic tool: a risk score to estimate survival in nursing home residents with advanced dementia. J Pain Symptom Manage. 2010;40:639–651. doi: 10.1016/j.jpainsymman.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruse RL, Parker Oliver D, Mehr DR, et al. Using mortality risk scores for long-term prognosis of nursing home residents: Caution is recommended. J Gerontol A Biol Sci Med Sci. 2010;65:1235–1241. doi: 10.1093/gerona/glq120. [DOI] [PubMed] [Google Scholar]
- 32.van der Steen JT, Mitchell SL, Frijters DH, et al. Prediction of 6-month mortality in nursing home residents with advanced dementia: Validity of a risk score. J Am Med Dir Assoc. 2007;8:464–468. doi: 10.1016/j.jamda.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell SL, Miller SC, Teno JM, et al. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304:1929–1935. doi: 10.1001/jama.2010.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy C, Morris M, Kramer A. Improving end-of-life outcomes in nursing homes by targeting residents at high-risk of mortality for palliative care: Program description and evaluation. J Palliat Med. 2008;11:217–225. doi: 10.1089/jpm.2007.0147. [DOI] [PubMed] [Google Scholar]
- 35.Davidson KM. Evidence-based practice guideline family preparedness and end-of-life support before the death of a nursing home resident. J Gerontol Nurs. 2011;37:11–16. doi: 10.3928/00989134-20110106-02. [DOI] [PubMed] [Google Scholar]
- 36.Doberman DJ, Yasar S, Durso SC. Would you refer this patient to hospice? An evaluation of tools for determining life expectancy in end-stage dementia. J Palliat Med. 2007;10:1410–1419. doi: 10.1089/jpm.2007.9838. [DOI] [PubMed] [Google Scholar]
- 37.Jayes RL, Arnold RM, Fromme EK. Does this dementia patient meet the prognosis eligibility requirements for hospice enrollment? J Pain Symptom Manage. 2012;44:750–756. doi: 10.1016/j.jpainsymman.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66:S84–S90. e81. doi: 10.1016/j.jclinepi.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leacy FP, Stuart EA. On the joint use of propensity and prognostic scores in estimation of the average treatment effect on the treated: A simulation study. Stat Med. 2014;33:3488–3508. doi: 10.1002/sim.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weddington WW., Jr. The mortality of delirium: An underappreciated problem? Psychosomatics. 1982;23:1232–1235. doi: 10.1016/S0033-3182(82)73269-4. [DOI] [PubMed] [Google Scholar]
- 41.van Hemert AM, van der Mast RC, Hengeveld MW, et al. Excess mortality in general hospital patients with delirium: A 5-year follow-up of 519 patients seen in psychiatric consultation. J Psychosom Res. 1994;38:339–346. doi: 10.1016/0022-3999(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 42.Conn DK, Lieff S. Diagnosing and managing delirium in the elderly. Can Fam Physician. 2001;47:101–108. [PMC free article] [PubMed] [Google Scholar]
- 43.Clegg A, Westby M, Young JB. Under-reporting of delirium in the NHS. Age Ageing. 2011;40:283–286. doi: 10.1093/ageing/afq157. [DOI] [PubMed] [Google Scholar]
- 44.Hjaltadottir I, Hallberg IR, Ekwall AK, et al. Predicting mortality of residents at admission to nursing home: A longitudinal cohort study. BMC Health Serv Res. 2011;11:86. doi: 10.1186/1472-6963-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: A prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 46.Huybrechts KF, Gerhard T, Franklin JM, et al. Instrumental variable applications using nursing home prescribing preferences in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2014;23:830–838. doi: 10.1002/pds.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]