Abstract
Common marmoset (Callithrix jacchus) monkeys when compared to rhesus macaques (Macaca mullatta) present several advantages for disease modeling, especially transgenic initiatives, as they commonly give birth to twins, which increases sample size, have accelerated development and a shorter life span that facilitates the analysis of the onset of age-related diseases. Yet, no tools are currently available to assess marmoset neurodevelopment during the initial first month of life. Here we report the creation of a novel Primate Postnatal Neurobehavioral Assessment Scale for marmoset monkeys (PPNAS-M) that was based on currently available scales for human and rhesus monkeys. Twenty-four healthy marmoset infants (12 females, 12 males) from 12 families were evaluated. The infant assessments involved 10-minute testing administered at 15 and 30 days after birth. The PPNAS-M consists of 41 noninvasive tests grouped into 5 testing categories: visual orienting, auditory and spatial orienting, motor responses, righting and body strength, and temperament tests. Testing at these two ages did not affect the overall health of the infants, suggesting that the PPNAS-M is a non-invasive testing tool. Significant maturation was demonstrated by increased scores in each of the five testing categories from postnatal day 15 to 30, with developmental patterns unique to marmosets. Principal component analysis defined 4 item groups (Orientation, State Control, Motor Maturity and Sensory Sensitivity) with 5 variables each. Orientation and State Control factors were highly similar at both ages and correlated highly with previous item groupings used with rhesus macaques. Our results indicate that the PPNAS-M is a useful assessment tool for detecting neuromotor, attention, and temperament status of infant marmosets and that it is sensitive to developmental effects. Further studies to validate the PPNAS-M for the assessment of normal development versus early effects of developmental perturbations associated to prenatal exposures and transgenesis are warranted.
Keywords: Neurodevelopment, Marmoset, Neurobehavior
INTRODUCTION
Common marmoset (Callithrix jacchus) monkeys are becoming increasingly important as nonhuman primate models for human diseases, particularly neurodegenerative disorders [Tardif et al. 2011; ‘t Hart et al. 2012]. Marmosets frequently give birth to twins, increasing the number of subjects per birth, and have both a shorter life span and accelerated development compared to the traditional nonhuman primate model, rhesus monkeys, thus facilitating studies where age may impact disease onset. The application of transgenic techniques in marmosets [Sasaki et al. 2009] holds the possibility to assess the impact of genetic mutations through the evaluation of the onset of motor, cognitive, and autonomic signs and pathology. The neurodevelopmental assessment methods needed to evaluate future disease models for early detection of abnormal traits requires the ability to assess discrete developmental changes, focusing on transitional periods, and aiming to identify maturity deficits that may not be evident through behavioral observation alone. Thus, there is a true need for tools to detect and quantify normal marmoset neurodevelopment.
Missler and colleagues [1992] and Yamamoto [1993] independently created descriptive scales categorizing marmoset development from birth through adulthood. Later, de Castro Leão et al. [2009] utilized body weight to identify discrete stages in marmoset growth. As multiple births are common in marmosets, and births greater than twins frequently result in at least one infant death, [Heger and Neubert, 1988; Windle et al. 1999] Tardif and colleagues [2002] developed an assessment scale to forecast survival of marmoset neonates. Yet, none of these scales thoroughly evaluate the initial first month of marmoset development or quantify marmoset neurodevelopmental milestones as described for other primate species, revealing a lack of sensitive developmental testing methods and therefore data for marmosets.
The human neonatal period refers to the period immediately succeeding birth and continuing through the first 28 days of extrauterine life [e.g.: Stedman's Medical Dictionary 2000]. This term has been traditionally applied to rhesus, although it is well established that rhesus mature much faster than humans. Evaluation during that first month allows for comparisons between species. Moreover, the first month of postnatal life is a critical period for neurodevelopment in primates [King et al. 1974; Missler et al. 1992; Tardif et al. 2002]. In humans, scales developed for evaluation of newborns such as Brazelton Newborn Behavioral Assessment Scale (NBAS) [Als et al. 1977; Brazelton and Nugent 1995], and for infants and toddlers, Bayley Mental Development (MDI) and Psychomotor Development Indices (PDI) [Bayley, 1993] quantify important early developmental changes. These scales are used to describe normal human neurodevelopment as well as to assess neurodevelopmental outcomes related to premature birth [Greene et al. 2012].
Based on the Brazelton Newborn Behavioral Assessment Scale and Bayley Scales of Infant Development, Schneider and Suomi [1992] created an assessment scale for infant rhesus monkeys. These tests measure dimensions of state modulation or arousal, orientation or attention, and neuromotor maturity, all of which have important ramifications for later cognitive and emotional functioning. After testing, four composite scores are computed for each infant based on factor analytical studies that parallel composite scores in the Brazelton scale for human infants. This neurodevelopmental scale is useful in studies investigating prenatal factors such as maternal stress [Schneider et al. 1999] and maternal alcohol consumption effects on rhesus macaque offspring [Schneider et al. 1997]. Furthermore, Schneider and Coe [1993] adapted this scale to assess neurodevelopment in squirrel monkeys suggesting the scale is not limited for use on Old World species of nonhuman primates.
Here we report the use of a novel Primate Postnatal Neurobehavioral Assessment Scale for marmosets (PPNAS-M) that was based on the Schneider and Suomi [1992] scale. The results are presented as individual developmental change from 15 to 30 days for each separate item that composes the scale, as well as grouped by testing categories and by principal component analysis. The multiple formats were performed to minimize limitations of the specific analysis [Sameroff et al. 1978; Als, 1978], compare marmosets results with previous publications in rhesus monkeys [Coe et al. 2010] and facilitate test application and interpretation by investigators beyond the field of neurodevelopment because, as we mentioned before, marmosets are being proposed as subjects for transgenic models of disease [Okano et al. 2012].
METHODS
Subjects
Twenty-four (n=12 males; n=12 females) healthy newborn common marmoset monkeys (Callithrix jacchus) from 12 different families were used in the present study (Table 1). Animals were housed with their family in cages (0.6 × 0.9 × 1.8m or 0.6 × 1.2 × 1.8m) or in large group enclosures (4.3m2 or 9.5m2). They were maintained on a 12-hour light/dark schedule, with temperature and relative humidity ranges of 24-30 C and 30-70% respectively. Water was continuously provided and feedings (Mazuri Callitrichid High Fiber Diet, Land O Lakes, Mazuri, Brentwood, MO) were twice daily ad lib, supplemented once daily with fruit, vegetables, or mealworms. General monitoring during the study included condition, appearance, weight, food intake and feces output.
Table 1.
Demographics of common marmoset monkey subjects evaluated using the Primate Postnatal Neurodevelopmental Assessment Scale for Marmosets.
| Litter Type | Monkey# Family Letter | Weight (g) | Gender | Overall Score (%) | ||
|---|---|---|---|---|---|---|
| Day 15 | Day 30 | Day 15 | Day 30 | |||
| Single Birth | 1A | 56 | 65 | female | 50.96 | 77.26 |
| Twin (with 1 death) | 2B | 42 | 55 | female | 53.84 | 75.27 |
| 3C | 39 | 56 | male | 47.03 | 84.81 | |
| Twin | 4D | 40 | 45 | male | 58.34 | 79.16 |
| 5D | 42 | 46 | male | 58.00 | 70.83 | |
| 6A | 37 | 58 | female | 59.23 | 68.02 | |
| 7A | 35 | 60 | female | 35.67 | 70.69 | |
| 8E | 44 | 54 | female | 63.48 | 69.06 | |
| 9E | 40 | 52 | female | 55.52 | 80.54 | |
| Triplet (with 1 death) | 10F | 38 | 68 | female | 60.27 | 79.28 |
| 11F | 40 | 68 | male | 56.91 | 82.94 | |
| 12G | 38 | 62 | male | 49.04 | 69.94 | |
| 13G | 38 | 61 | male | 46.24 | 65.08 | |
| 14H | 35 | 55 | female | 32.56 | 65.08 | |
| 15H | 34 | 52 | male | 49.34 | 78.48 | |
| 16I | 46 | 59 | female | 61.27 | 63.08 | |
| 17I | 42 | 54 | male | 60.54 | 72.40 | |
| 18J | 45 | 64 | female | 44.47 | 78.09 | |
| 19J | 44 | 62 | male | 48.25 | 76.65 | |
| 20K | 45 | 65 | male | 50.60 | 73.97 | |
| 21L | 42 | 49 | female | 45.56 | 82.16 | |
| 22L | 42 | 53 | male | 47.38 | 71.21 | |
| Living Triplets | 23H | 34 | 41 | female | 49.71 | 69.97 |
| 24H | 33 | 38 | male | 48.44 | 76.09 | |
The present study was performed in strict accordance with the recommendations in the National Research Council Guide for the Care and Use of Laboratory Animals (2011) and the American Society of Primatologists principles for the ethical treatment of nonhuman primates in an AAALAC accredited facility (Wisconsin National Primate Research Center, Graduate School, University of Wisconsin-Madison). The Institutional Animal Care and Use Committee at the University of Wisconsin-Madison approved the experimental protocol (permit G00679). All efforts were made to minimize the number of animals used and to ameliorate any distress.
Behavioral Evaluations
The infant assessments with the PPNAS-M took place from August, 2012 through May, 2013 at the Wisconsin National Primate Research Center. Each involved an approximate 10-minute testing period administered at days 15±1 and 30±3 after birth. Although testing prior to two weeks might be useful, an attempt was made to minimize disturbance while the infant gained the ability to independently thermoregulate. Marmosets mature quickly, remaining within the infancy stage up until 12 weeks, compared to 12 months in rhesus macaque [Pryce et al. 2011]. Consequently, tests utilized in the rhesus PNNAS [Schneider and Suomi, 1992], which are meant to theoretically assess neonatal stages, are applied to the first month of life in marmosets; however, these tests will be of particular importance when utilizing the PPNAS-M to assess marmoset disease models as they may be especially sensitive to developmental deficits or delays.
The lead author (M.S.) of the Schneider and Suomi assessment scale for rhesus monkeys trained all authors on test administration. After performing pilot evaluations on four infant marmosets (different from the ones reported in this manuscript), the tests were modified and some items deleted, to best address measurement success with marmosets. Once the assessment was agreed upon, the same evaluator (K.B.) performed the testing on all the subjects. Inter-rater reliability was done using two evaluators (K.B. and N.S-D.) simultaneously assessing the same infants. The assessments were performed in three available infants: two infants 15 day old and one 30 day old. For any assessments that required the evaluator to feel the reaction (e.g. grasping, resistance, active power, etc.) each evaluator repeated the assessment with the infant. Inter-rater scores averaged 0.99 for the 15 day old infants’ evaluation and 0.89 for the 30-day old [Kippendorff's alpha interval; Freelon, 2013].
Testing occurred between 0800-1000, after adult morning feeding was completed. To perform the assessment, each baby was wrapped in a soft blanket and taken to a quiet, dim-lit room with parents out of sight and earshot. The testing materials included a soft cloth ball used as a temporary surrogate, a bright green ball with a drawn face for visual orienting, a pen tapping on the testing surface to create a repetitive and consistent form of auditory stimulation, and a cotton tipped applicator for tactile stimulation. As with other human and nonhuman primate newborn evaluations [Als et al. 1977; Schneider and Suomi, 1992; Brazelton and Nugent, 1995], conditions for examination strived to be optimal in order to obtain the best performance of each subject and if necessary, the observation was briefly extended to appropriately score each item.
Primate Postnatal Neurobehavioral Assessment Scale for Marmosets (PPNAS-M)
The PPNAS-M consists of 41 noninvasive tests grouped into 5 testing categories, which were organized based upon the order of test item administration and a priori selection by the authors according to clinical judgment, knowledge of marmoset behavior and studies with rhesus macaques [see Schneider et al. 1991].
The testing categories are: visual orienting, auditory and spatial orienting, motor responses, righting and body strength, and temperament tests (Table 2). Visual orienting tests assess the percentage of time and ability of the infant to maintain focus on a bright object. Likewise, the auditory and spatial orientation tests focus on the infant's ability to orient towards a novel, repetitive auditory stimulus and ability of the marmoset to maintain spatial awareness when physically manipulated. Motor responses tests assess palmar and plantar grasping, rooting and visual-vestibular response, which can be measured through items such as, and labyrinthian head righting. Body righting and strength tests are aimed at evaluation of muscle tone. Temperament ratings include the infant's general state, quality of responses, calming behavior measures and are based upon examiner evaluation of the infant throughout the testing session. Specific neuromotor functions include ratings of muscle tonus, coordination, tremulousness, response speed, and spontaneous motor activity [Schneider and Suomi 1992]. Infant vocalizations are also assessed during a specific one-minute time point before temperament evaluations are recorded. While assessing marmoset vocalizations the number of bouts, in addition to the presence or absence of specific call types, are noted. These call types include: long and short phee calls, which are used to announce and establish contact to other individuals that are not nearby and may not be visible; Er-er calls, which are used as a threatening or aggressive communication to nearby individuals; chirps, which are quiet calls used as intimate contact communication; and infant distress calls, which are a very distinct, high frequency version of the phee call [Norcross and Newman, 1993; Jones, 1994; Miller and Wang, 2006; Barbosa and Mota, 2014] (see Table 4).
Table 2.
Description of the Primate Postnatal Neurodevelopmental Assessment Scale for Marmosets. The scale consists of five testing categories: Visual Orienting, Auditory and Spatial Orienting, Motor Responses, Righting and Body Strength, and Temperament. A description of the evaluation for each item, numerical scores and specific descriptions are listed.
| Visual Orienting Category | ||
|---|---|---|
| Tests | Description | Score |
| Visual Orientation | Eyes orient towards a plastic brightly colored toy placed 45 degrees in each periphery; left, right, up, down. The toy is withdrawn between each direction. | 0= no orient 1= direct brief contact 2= direct prolonged contact |
| Visual Following | Assess visual following of the bright toy in both vertical and horizontal directions. | 0= no follow 1= starts to follow, then stops 2= complete follow |
| Reach and Grasp | Assess the ability of the monkey to reach for the toy during the visual orienting and following tests. | 0= no reach/grasp 1= swat, no finger flexion 2= grasp with finger flexion |
| Duration of Looking | Assess the length of looking during the visual orienting and following tests. | 0= no looking 1= brief looking, <1 seconds 2= prolonged looking, 1-2 seconds |
| Distractibility | Rate distractedness during the visual orienting and following tests. | 0= definitely distracted 1= slightly distracted 2= not distracted |
| Attention | Rate attentiveness during the visual orienting and following tests. | 0= not attentive, <25% time 1= slight attention, 25% time 2= definite attention, 75% time |
| Auditory and Spatial Orienting Category | ||
|---|---|---|
| Tests | Description | Score |
| Startle to Auditory | Tap pen on table surface loudly, assess any response to the sudden sound. | 0= no startle or whole body jerk 1= slight startle, eye jerk 2= moderate startle, head jerk |
| Pen Tapping | Lightly tap pen on table surface repeatedly and assess the monkey's orientation towards the stimulus. | 0= no orienting 1= partial head turning 2= full head turn with visual inspection |
| Inversion | Invert the monkey and assess aversion to the test. | 0= definite aversion 1= slight aversion 2= no aversion |
| Rotation | Spin monkey in a controlled movement left and right. Assess eye/head turn into the direction of the spin. | 0= absent eye/head turn 1= weak eye/head turn 2= strong eye/head turn |
| Motor Responses Category | ||
|---|---|---|
| Tests | Description | Score |
| Tactile Response | Run a cotton tipped applicator along all four extremities; assess the response to the tactile stimulation. Run the applicator against the direction of the hair, distally to proximally. | 0= no response or exaggerated response 1= barely discernable response 2= easily apparent response |
| Galant's Response | Run a cotton tipped applicator laterally along each side of the vertebral column with the direction of the hair. Assess any change in torso shape. | 0= no response or exaggerated response 1= slight curving of the spine 2= definite curving of the spine |
| Palmar Grasp | Induce hand grasp with the end of the cotton tipped applicator, sneak into palm while on the attachment object. | 0= grasp and release 1= grasp, digits stay closed 2= strong digit grasp without voluntary release |
| Plantar Grasp | Induce foot grasp with the end of the cotton tipped applicator, sneak into foot while on the attachment object. | 0= grasp and release 1= grasp, digits stay closed 2= strong digit grasp without voluntary release |
| Rooting Response | Apply light tactile stimulus with the cotton-tipped applicator at the corner of the mouth and assess head turning towards the stimulus. | 0= response is absent 1= weak turn of head towards stimulus 2= full turn of head and lip grasp of stimulus |
| Parachute Response | Assess the monkey's upper extremity limb extension during a headfirst descent towards a surface. | 0= no extension of arms 1= partial extension of arms 2= definite extension of arms/digits |
| Labyrinthian Righting | Tilt the monkey's body 45 degrees sideways and assess realignment of the head. | 0= head stays in the plane of the body 1= head partially rights 2= head aligns with vertical plane |
| Response Speed | Assess the speed of motor responses. | 0= 25% of responses are quick 1= 75% of responses are quick 2= all responses are quick |
| Response Intensity | Assess the response intensity of the tests, focusing on the quality of vocal responses. | 0= extremely loud/shrill vocalizations 1= vocalizations are mild in intensity 2= vocalizations are moderate/average |
| Righting/Body Strength Category | ||
|---|---|---|
| Tests | Description | Score |
| Body Righting | Place the monkey on its back and assess the time needed to turn from supine to prone. | 0= does not turn over 1= turns over, but requires more than 2 seconds to do so 2= turns over instantaneously, <2 seconds |
| Passive Range of Motion (muscle tonus) | Flex and extend each arm to assess the degree of resistance. | 0= barely discernable resistance or exaggerated rigidity 1= mild resistance 2= moderate resistance |
| Active Power | In conjunction with the above test, assess the strength of the muscles when actively contracting. | 0= cannot withstand slight resistance or exaggerated resistance 1= withstands mild resistance 2= withstands moderate resistance |
| Distress to Limitations | Restrict movement for 10 seconds and assess the degree of resistance/vocalizations. | 0= continuous or complete lack of resistance/vocalizations 1= resistance/vocalizations 10% time 2= resistance/vocalizations approximately 25% time |
| Head Posture Prone | Hold monkey horizontally, prone. Assess the ability of the monkey to hold its head up. | 0= flaccid head, hanging down 1= head lifted but not maintained 2= sustained head with semiflexion |
| Head Posture Supine | Hold monkey horizontally, supine. Assess the ability of the monkey to hold its head up. | 0= flaccid head, hanging down 1= head lifted but not maintained 2= sustained head with semiflexion |
| Coordination | Assess the quality of movement. | 0= clumsy movements 1= adequate movements 2= agile movements |
| Spontaneous Crawl/Locomotion | Rate the quality of locomotion. | 0= absent 1= weak attempt 2= coordinated locomotion |
| Tremulousness | Assess the shakiness of the monkey. | 0= 3-4 events 1= 1-2 events 2= no tremulousness |
| Temperament Category | ||
|---|---|---|
| Tests | Description | Score |
| Aversion on Back | Assess any aversion to the Body Righting test. | 0= no vocals/distress 1= slight vocals/distress 2= definite vocals/distress |
| Self-Calming | Assess the ability of the monkey to calm itself down after distress. | 0= easy (upset 10% of time) 1= moderate (upset 25% of time) 2= harder to calm (upset ≥50% of time) |
| Motor Activity | Assess any spontaneous motor activity during testing. | 0= low activity, in motion 25% of time 1= high activity, continuous motion 2= moderate activity, in motion 50% of time |
| Irritability | Assess the amount of distress, including distress calls. | 0= distress apparent >25% of time 1= distress not apparent 2= distress slightly apparent, approximately 25% of time |
| Consolability | Assess the ability of the examiner to console/calm the monkey. | 0= console with ease 1= console using swaddling and stabilizing 2= console with difficulty |
| Fearfulness | Assess the presence of fear with fear grimace to confirm the emotion. | 0= definite fear (grimace present >1 occurrence) 1= slight fear grimace 2= no fear/bold |
| Struggle during Testing | Assess the monkey's struggle/squirming during testing. | 0= squirming <25% of time 1= squirming approximately 50% of time 2= squirming >50% of time |
| Predominant State | Assess the state of the monkey during testing. | 0= highly agitated 1= alert, somewhat agitated 2= alert, awake, aware |
| Bright Eyed | Assess the alertness of the monkey during testing. | 0= dull-eyed 1= intermediate-eyed 2= bright-eyed |
| Aggression | Assess aggression, using biting to confirm the emotion. | 0= continuous aggression/biting 1= 1-2 attempts to bite 2= aggression is absent |
| Drowsiness | Assess the drowsiness of the monkey during testing. | 0= definite sleepiness, >50% testing session 1= slight drowsiness, 25% testing session 2= no drowsiness |
| Cuddliness | Assess how often the monkey molds to the hands of the examiner. | 0= definite molding towards the examiner 1= slight molds 2= no molding, push against the examiner |
| Self-Mouthing | Assess the monkey's placement of hands/feet in their mouth. | 0= definite mouthing 1= 2-3 attempts to self-mouth 2= none/slight mouthing |
Table 4.
Number of monkeys making specific call types at 15 and 30 days postnatal. Long and short phee refers to calls used to announce and establish contact to other individuals that are not nearby and may not be visible; Er-er calls are used as a threatening or aggressive communication to nearby individuals; chirps are quiet calls used as intimate contact communication; and infant distress calls are a very distinct, high frequency version of the submissive nga-nga call [Norcross and Newman, 1993; Jones, 1994; Miller and Wang, 2006; Barbosa and Mota, 2014].
| Type of Call | Day 15 | Day 30 |
|---|---|---|
| Long Phee | 1 | 7 |
| Short Phee | 21 | 18 |
| Er-er | 0 | 8 |
| Chirp | 6 | 3 |
| Distress | 1 | 3 |
Each of the 41 tests is scored on a three-point scale with 0.5 increments (0, 0.5, 1, 1.5, 2). The individual tests, outlined in Table 2, include specific behavioral descriptions associated with each numerical score. To avoid the tendency for the examiner to score tests as low/medium/high responses (potential biased scoring) exact test descriptions are utilized when assigning scores.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 5.0b, GraphPad Software) and R 3.0.2 [R Core Team, 2014]. A P<0.05 was accepted as significant. Comparison over time of the different weight data sets were done using repeated measures ANOVA and corrected with Bonferroni multiple comparison tests.
As each of the five testing categories has a different number of tests, and therefore a different total highest possible score, in order to compare scores between testing categories, a ratio of the total score to the highest possible total score for each category at 15 and 30 days was obtained for each monkey. The normalized dataset was then averaged per time-point. Scores for individual tests within the testing categories were kept at the maximum possible score of 2. Comparisons between the scores obtained at the two time points were performed using matched paired samples Wilcoxon test, which is the nonparametric equivalent of paired samples t-test.
Principal Components Analysis
Principal components analysis was performed to assess how the marmoset factors fit with previous reports in rhesus monkeys [Schneider et al. 1991; Coe et al. 2010]. First, the variables at 15 and 30 days were inspected and those items with zero variance or with greater than 75% of animals scoring at one value (at either time point) were removed. Next, a principal components analysis with varimax rotation was applied separately at each age. Examination of the scree plots showed a maximum of 5 factors. The fifth factor at each age was uninterpretable, therefore a four factor solution was fit at each age. Because of the limited sample size these results should be regarded as somewhat tentative, although the Schneider et al. [1991] principal components analysis had only 23 animals and this study has 24.
RESULTS
The 24 subjects remained healthy throughout testing, and maintained this health status during a 6-month follow up observational period. At 15 days the average weight for all 24 animals was 40.46g (SE=1.02; range: 33-46g) and at 30 days 55.92g (SE=1.65; range: 41-58g) (Table 1). The weights of males and females showed no statistical difference at both timepoints (15 days, P=0.6074; 30 days, P=0.5806). The mean weight gain for both sexes from 15 to 30 days was 15.5g (SE=1.54), a typical healthy gain for captive marmosets based on the database information reported by Smucny and colleagues [Smucny et al. 2004] . In the following pages we describe the results first as they emerged during testing, which will be useful for clinical application. Next, we described groupings based on principal factor analysis to see how well the marmoset factors fit with those of rhesus.
Overall PPNAS-M scores
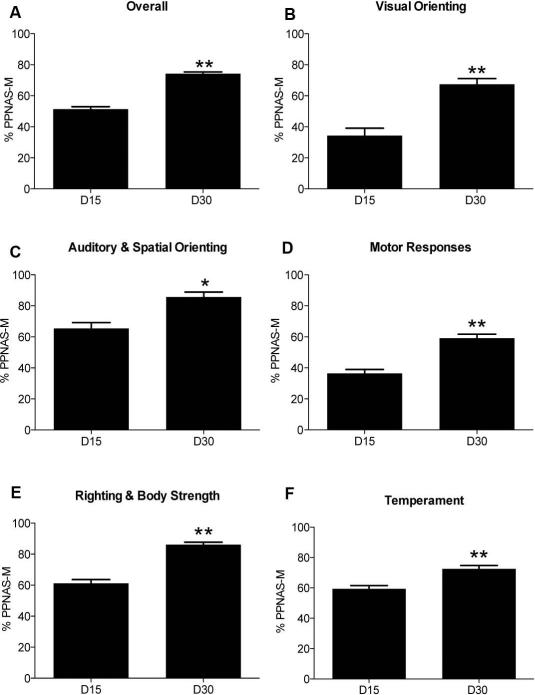
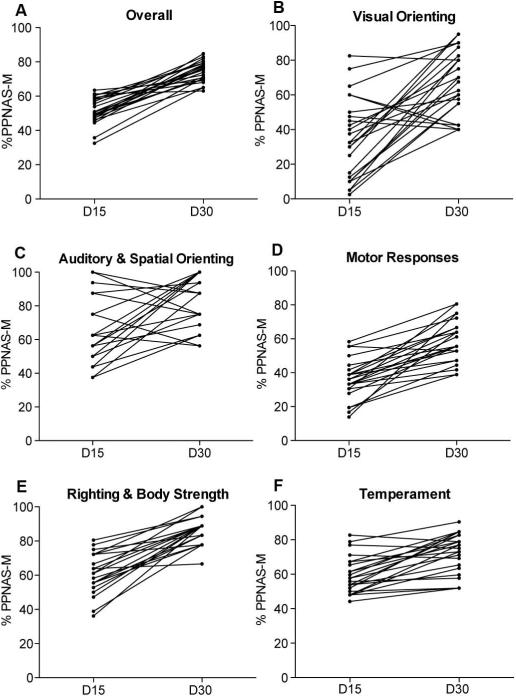
Figure 1 shows percentage of maximal scores achieved overall and for each of the five testing categories. The total PPNAS-M scores demonstrated significant increase overtime, thus maturation, achieving a mean of 51.4% (SE=1.6) of the total possible highest score at day 15 and 74.2% (SE=1.2) at day 30 (Figure 1A). Figure 2 illustrates the change in percentage of total maturity score for each test subject within each category as well as the individual maturation rate between time points. The average difference in individual overall scores between day 15 and day 30 is 22.8% (SE=1.9).
Figure 1.
Graphs illustrating the change in percentage (±SE) of the total maturity score for each testing category. A ratio of the total score to the highest possible total score for each monkey at 15 and 30 days was obtained and this normalized dataset was then averaged per time point. Statistical comparisons between the two time points were performed using a Wilcoxon matched pairs singed rank test. *P≤0.001, **P≤0.0001. (A) Overall PPNAS-M Maturation (B) Visual Orienting category (C) Auditory and Spatial Orienting category (D) Motor Responses category (E) Righting and Body Strength category, (F) and Temperament evaluations.
Figure 2.
Graphs illustrating the change in percentage of the total maturity score for each test subject within each testing category. Slopes of horizontal lines indicate the rate of change of individual scores at postnatal Day 15 and Day 30. Note the lower rate of maturation in individuals with high Day 15 scores, compared to the higher rate of maturation in individuals with low Day 15 scores. (A) Overall PPNAS-M Maturation (B) Visual Orienting category (C) Auditory and Spatial Orienting category (D) Motor Responses category (E) Righting and Body Strength category (F) and Temperament evaluations.
No significant differences were detected between the total composite scores of males (mean±SE; 15 d, 51.8±1.48; 30d, 75.1±1.64; P>0.9999) vs. females (15d, 51.0±2.88; 30d, 73.0±1.87; P=0.3394), confirming that the test is measuring overall developmental changes without the confounding factor of infant sex. Yet significant differences between male and female responses to individual tests were found (see testing category results), suggesting PPNAS-M is sensitive to subtle sex differences. Statistics of individual tests and for each testing category are summarized in Table 3. Below we report the results for each individual testing category, followed by factor analysis results.
Table 3.
Significant values for each of the 41 tests in the PPNAS-M organized by testing categories. A two-tailed Wilcoxon matched pairs signed rank test was used to compare time points. The total testing category percentage is the percentage of the sum of the averaged individual tests divided by the total possible score (TPS) within each category. A maximum score of 2 is possible for each individual test aside from visual orienting with a maximum score of 8 (up, down, right, left) and visual following with a maximum score of 4 (horizontal, vertical).
| Testing Categories | Day 15 Mean±SE | Day 30 Mean±SE | Significance W Value, P Value |
|---|---|---|---|
| Visual Orienting (TPS=20) | |||
| Visual Orienting (× 4) | 3.31±0.53 | 7.06±0.24 | 233.0, <0.0001 |
| Visual Follow (× 2) | 1.29±0.26 | 2.58±0.25 | 198.0, 0.0006 |
| Reach and Grasp | 0.04±0.04 | 0.17±0.12 | 4.0, 0.5 |
| Duration of Looking | 0.83±0.13 | 1.25±0.11 | 96.0, 0.0147 |
| Distractibility | 0.73±0.13 | 1.17±0.13 | 101.0, 0.0259 |
| Attention | 0.67±0.12 | 1.25±0.12 | 171.0, 0.0036 |
| Total Category (%) | 34.37±4.73 | 67.4±3.75 | 262.0, <0.0001 |
| Auditory & Spatial Orienting (TPS=8) | |||
| Startle to Auditory | 0.63±0.19 | 1.42±0.18 | 93.0, 0.0056 |
| Pen Tapping | 1.19±0.16 | 1.71±0.12 | 85.0, 0.0056 |
| Inversion | 1.75±0.10 | 1.77±0.08 | 6.0, 0.75 |
| Rotation | 1.67±0.11 | 1.96±0.04 | 40.0, 0.0195 |
| Total Category (%) | 65.36±3.85 | 85.68±3.22 | 227.0, 0.0006 |
| Motor Responses (TPS=18) | |||
| Tactile Response | 0.79±0.17 | 1.13±0.20 | 32.0, 0.4027 |
| Galant's Response | 0.42±0.16 | 0.79±0.19 | 41.0, 0.1797 |
| Palmar Grasp | 0.90±0.12 | 1.06±0.15 | 31.0, 0.2412 |
| Plantar Grasp | 1.02±0.12 | 0.90±0.13 | −19.0, 0.5737 |
| Rooting Response | 0.83±0.17 | 1.79±0.09 | 167.0, <0.0001 |
| Parachute Response | 0.33±0.13 | 1.94±0.05 | 253.0, <0.0001 |
| Labyrinthian Righting | 0.54±0.10 | 0.52±0.14 | −9.0, 0.8495 |
| Response Speed | 0.42±0.09 | 1.19±0.11 | 194.0, <0.0001 |
| Response Intensity | 1.31±0.09 | 1.33±0.12 | 7.0, 0.7617 |
| Total Category (%) | 36.46±2.46 | 59.14±2.54 | 289.0, <0.0001 |
| Righting & Body Strength (TPS=18) | |||
| Body Righting | 1.25±0.14 | 2.0±0.0 | 136.0, <0.0001 |
| Passive Range of Motion (muscle tonus) | 1.5±0.15 | 1.80±0.10 | 38.0, 0.2217 |
| Active Power | 0.83±0.18 | 1.67±0.13 | 111.0, 0.0014 |
| Distress to Limitations | 0.58±0.19 | 0.67±0.18 | 6.0, 0.7656 |
| Head Posture Prone | 1.96±0.04 | 2.0±0.0 | 1.0, >0.9999 |
| Head Posture Supine | 2.0±0.0 | 2.0±0.0 | X |
| Coordination | 0.92±0.11 | 1.96±0.04 | 231.0, <0.0001 |
| Spontaneous Crawl/Locomotion | 0.0±0.0 | 1.42±0.18 | 171.0, <0.0001 |
| Tremulousness | 1.98±0.02 | 2.0±0.0 | 1.0, >0.9999 |
| Total Category (%) | 61.22 ±2.37 | 86.11±1.57 | 300.0, <0.0001 |
| Temperament (TPS=26) | |||
| Aversion on Back | 0.92±0.10 | 1.08±0.12 | 29.0, 0.2275 |
| Self-Calming | 0.83±0.15 | 1.04±0.12 | 48.0, 0.1869 |
| Motor Activity | 1.17±0.09 | 1.33±0.13 | 41.0, 0.1908 |
| Irritability | 0.52±0.15 | 1.25±0.15 | 135.0, 0.0019 |
| Consolability | 1.33±0.19 | 1.71±0.14 | 38.0, 0.125 |
| Fearfulness | 1.81±0.10 | 2.0±0.0 | 10.0, 0.125 |
| Struggle during Testing | 0.60±0.13 | 1.19±0.15 | 126.0, 0.0014 |
| Predominant State | 1.52±0.13 | 1.38±0.13 | −21.0, 0.5701 |
| Bright Eyed | 1.48±0.15 | 1.71±0.12 | 22.0, 0.2109 |
| Aggression | 0.0±0.0 | 0.42±0.12 | 45.0, 0.0039 |
| Drowsiness | 1.75±0.08 | 1.96±0.04 | 28.0, 0.0156 |
| Cuddliness | 1.5±0.10 | 1.79±0.07 | 85.0, 0.0207 |
| Self-Mouthing | 2.0±0.0 | 2.0±0.0 | X |
| Total Category (%) | 59.34±2.14 | 72.52±2.31 | 300.0, <0.0001 |
Visual Orienting Testing Category
During the visual orienting tests, marmosets demonstrated a significant increase in overall scores over time. This increase was the largest of all categories, achieving at 15 days 34.4% (SE=4.7) and at 30 days 67.4% (SE=3.8) of the total possible score (Figure 1B, 2B). The ability to orient towards and follow the green ball improved significantly, the duration in which the marmoset maintained eye contact with the green ball increased, and overall distractibility significantly decreased, while attentiveness increased. No significant changes were observed in the reach and grasp test. Responses differed between sexes only for the duration of looking test at 15 days, in which males maintained eye contact with the green ball significantly longer than females (P=0.0038).
Auditory and Spatial Orienting Testing Category
Auditory and spatial orienting tests showed a significant increase between the overall score at 15 days, 65.4% (SE=3.9) and 30 days, 85.7% (SE=3.2) (Figure 1C, 2C). All but one test in this category significantly improved: the ability of the marmosets to orient towards a repetitive pen tapping stimulus and the eye movement into the direction of a body rotation increased, and the exaggerated responses to an unexpected auditory stimulus weakened by day 30. Responses to being temporarily inverted were minimal, short-lived and did not change between the two time points. Significant differences between male and female performances were not found in this category.
Motor Responses Testing Category
The motor responses tests showed an overall significant increase in score between 15 and 30 days, from 36.5% (SE=2.5) to 59.1% (SE=2.5) (Figure 1D, 2D). Analysis of the individual tests demonstrated persistence of the rooting response, which was noted as significantly more pronounced at 30 days. The parachute response emerged at 15 days in 2 of the animals; by 30 days it was present in all 24 subjects, which translated into a significant increase scores. The tactile response, Galant's response, palmar and plantar grasping, labyrinthian righting tests and response intensity of the tests completed within the motor responses testing category were present in most of the animals at 15 days and persisted over time. With respect to sex differences, it should be noted that the only two infants that had positive responses to the parachute test at 15 days were both male (P<0.0001). Males also had significantly stronger rooting responses at 30 days (P=0.0006) and labyrinthian righting responses at both 15 and 30 days (P<0.0001).
Righting and Body Strength Testing Category
Significant maturational changes were observed in the righting and strength tests, increasing in mean scores from 61.2% (SE=2.37) at 15 days to 86.11% (SE=1.57) at 30 days (Figure 1E, 2E). The strength with which marmosets actively flexed their arms, without response exaggeration, and their ability to right their bodies when placed in a supine position significantly increased. In addition, significant improvements in overall coordination and ability to spontaneously crawl were seen. Although marmosets achieved an overall significant increase in the righting and strength category, passive range of motion (muscle tonus), distress to limitations, and head posture prone and supine did not significantly improve between time points, as the scores were already high at day 15. This is particularly relevant when testing the posture of the head/neck, as in both the prone and supine positions the responses were optimal in almost every infant tested at each time point. Lastly, slight tremulousness was only observed in one 15-day-old infant. No significant differences between male and female performances were found in this testing category.
Temperament Testing Category
The temperament category showed statistically significant change overtime, at 15 days achieving 59.34% (SE=2.14) and at 30 days 72.52% (SE=2.31) (Figure 1F, 2F). Within individual tests, significant improvements included increases in irritability, struggle during the testing session, aggression, as well as decreases in drowsiness and cuddliness. Significant maturation was not observed in self-calming, consolability, fearfulness, bright-eyed, and predominant state at 30 days. Self-mouthing was not observed in any monkey at any timepoint. Responses differed between sexes only in the irritability test, with females significantly more irritable at 30 days than males (P=0.0006).
Vocalizations
During the PPNAS-M testing sessions we also assessed the number of bouts and presence or absence of vocalizations during a one-minute period (Table 4). At 15 days we recorded 8.6 mean vocalization bouts (SE=1.2) and at 30 days, 9.9 (SE=1.1). At this later timepoint, we noted the appearance of Er-er calls and an increased number of infants producing distress calls and longer phee calls.
Principal Components Analysis
The principal components analysis identified four groupings that were labeled as Orientation, State Control, Motor Maturity and Sensory Sensitivity based on the items grouped and how they matched previous reports in rhesus monkeys [Schneider et al. 1991; Coe et al. 2010]. Table 5 shows the factor loadings at each age for the four-factor solution. The four-factor solution accounted for 52% and 53% of the total variance at 15 and 30 days of age respectively. Below we describe the content of each factor and compare to the rhesus measure.
Table 5.
Principal components analysis loadings for the four factor fit after rotation using 27 individual test items at 15 and 30 days postnatal. Factor loadings greater than or equal to 0.60 are shown in bold with darker shading to highlight the more reliable items with larger loadings. Factor loadings greater than or equal to 0.45 are indicated in italicized font with lighter shading to highlight items considered to be lesser components of that factor. Empty cells contain items that had factor loadings less than 0.30.
| Factor 1: Orientation | Factor 2: State Control | Factor 3: Motor Maturity | Factor 4: Sensory Sensitivity | |||||
|---|---|---|---|---|---|---|---|---|
| Day 15 | Day 30 | Day 15 | Day 30 | Day 15 | Day 30 | Day 15 | Day 30 | |
| Visual Orientation | 0.84 | 0.86 | ||||||
| Visual Following | 0.81 | 0.85 | 0.32 | |||||
| Distractibility | 0.62 | 0.75 | 0.44 | 0.33 | ||||
| Attention | 0.79 | 0.77 | −0.3 | |||||
| Duration during Looking | 0.82 | 0.6 | ||||||
| Response Speed | 0.35 | 0.65 | 0.45 | 0.38 | 0.51 | |||
| Irritability | 0.53 | 0.63 | ||||||
| Consolability | 0.39 | 0.68 | ||||||
| Struggle during Testing | −0.31 | 0.77 | 0.69 | |||||
| Predominant State | 0.33 | −0.79 | −0.6 | 0.53 | ||||
| Cuddliness | 0.79 | 0.79 | ||||||
| Self Calming | −0.59 | 0.5 | 0.58 | −0.65 | ||||
| Pen Tapping | 0.5 | 0.92 | ||||||
| Palmar Grasp | −0.8 | 0.61 | ||||||
| Plantar Grasp | 0.47 | −0.48 | 0.52 | |||||
| Bright Eyed | −0.42 | 0.4 | 0.51 | 0.63 | ||||
| Response Intensity | 0.67 | |||||||
| Aversion to Back | −0.51 | 0.59 | 0.42 | |||||
| Distress to Limitations | −0.36 | 0.4 | ||||||
| Rooting Response | 0.32 | 0.33 | 0.75 | |||||
| Active Power | 0.49 | −0.44 | 0.69 | |||||
| Startle to Auditory | 0.41 | −0.73 | ||||||
| Tactile Response | −0.42 | −0.42 | 0.66 | |||||
| Labyrinthian Righting | −0.41 | 0.62 | ||||||
| Motor Activity | 0.46 | 0.39 | 0.33 | 0.3 | 0.57 | |||
| Passive Range of Motion | 0.43 | 0.32 | −0.52 | |||||
| Galant's Response | 0.43 | −0.41 | 0.56 | |||||
Orientation Factor
The first factor explained 16% of the total variance at day 15 and 19% at day 30, in the 27 PPNAS-M items (see Table 5). It contained high factor loadings at both time points for visual orienting (loading = day 15, 0.84; day 30, 0.86), visual following (loading = day 15, 0.81; day 30, 0.85), distractibility (loading = day 15, 0.62; day 30, 0.75), attention (loading = day 15, 0.79; day 30, 0.77), duration during looking (loading = day 15, 0.82; day 30, 0.62) and a high factor loading at day 30 for response speed (loading = day 30, 0.65). The first factor strongly mirrored the rhesus Orientation factor and was labeled accordingly.
State Control Factor
The second factor explained 15% of the total variance at both 15 and 30 days, in the PPNAS-M items. It contained high factor loadings at both time points for struggle during testing (loading = day 15, 0.77; day 30, 0.69), predominant state (loading = day 15, -0.79; day 30, -0.6), cuddliness (loading = day 15, 0.79; day 30, 0.79) and high factor loadings at day 30 for irritability (loading = day 30, 0.63) and consolability (loading = day 30, 0.68). This factor also contained moderately high factor loadings at both time points for self-calming (loading = day 15, 0.5; day 30, 0.58) and at day 15 for consolability (loading = day 15, 0.53). This second factor mirrored the rhesus State Control factor and was labeled likewise.
Motor Maturity Factor
The third factor explained 12% of the total variance at day 15 and 10% at day 30, in the PPNAS-M items. It contained high factor loadings at both time points for palmar grasping (loading = day 15, −0.8; day 30, 0.61), for pen tapping at day 30 (loading = day 30, 0.92) and for response intensity at day 15 (loading = day 15, 0.67). This factor also contained moderately high factor loadings at both time points for plantar grasping (loading = day 15, −0.48; day 30, 0.52), at day 15 for pen tapping (loading = day 15, 0.5), at day 30 for bright eyed (loading = day 30, 0.51), at day 15 for aversion to back (loading = day 15, 0.59), and at day 15 for active power (loading = day 15, 0.49). The third factor contained some items that overlap with the rhesus motor factors and was designated as Motor Maturity.
Sensory Sensitivity Factor
The fourth factor explained 9% of the total variance at both day 15 and day 30, in the PPNAS-M items. It contained high factor loadings at day 15 for startle to auditory (loading = day 15, −0.73), tactile response (loading = day 15, 0.66), and Labyrinthian righting (loading = day 15, 0.62). It also contained moderately high factor loadings for motor activity at day 15 (loading = day 15, 0.57), passive range of motion at day 15 (loading = day 15, −0.52), and Galant's response at day 30 (loading = day 30, 0.56). This fourth factor was labeled as Sensory Sensitivity.
Comparison of Marmoset Principal Components Analysis Factors to Rhesus
In order to more directly compare the marmoset factors to the rhesus results, we calculated factor scores for the marmoset data a) from the principal components analysis reported here, b) by the Coe et al. [2010] method, and c) by the Schneider et al. [1991] method. In all cases the highest loading items were averaged to create the factor scores. Table 6 shows the correlations of factor scores both within method and between the factor methods. The relationship was strong for the first three factors at day 15 and for the first two factors at day 30. The current Orientation factor highly correlated at both time points to Coe et al. [2010] (day 15 R=0.97, p<0.001; day 30 R=0.95, p<0.001) and Schneider et al. [1991] (day 15 R=0.97, p<0.001; day 30 R=0.95, p<0.001). The current State Control factor correlated similarly highly to Coe et al. [2010] (day 15 R=0.82, p<0.001; day 30 R=0.8, p<0.001) and Schneider et al. [1991] (day 15 R=0.8, p<0.001; day 30 R=0.88, p<0.001). The current Motor Maturity factor significantly correlated at day 15 with Coe et al. [2010] (R=0.65, p<0.001) and with Schneider et al. [1991] (R=0.45, p<0.05). Lastly, the current fourth factor, Sensory Sensitivity, significantly correlated at both day 15 and day 30 with the same Coe et al. [2010] factor (day 15 R=0.44, p<0.05; day 30 R=0.57, p<0.01). The relative independence of the current factors is additionally exemplified as unlike factors within and between the PPNAS-M, Schneider et al. [1991] and Coe et al. [2010] do not correlate well.
Table 6.
Comparison of marmoset factor scores for the PPNAS-M with previously published factors derived from the evaluation of rhesus at (A) 15 days and (B) 30 days post-natal. All the data utilized for the analysis is from the PPNAS-M evaluation in marmoset monkeys. The upper panels for A and B show the correlations of the factor scores for the PPNAS-M. The middle panels show the relationship of the PPNAS-M to the Coe et al. [2011] rhesus factors. The lower panels show the relationship of the PPNAS-M to the Schneider et al. [1991] rhesus factors.
| A | Four Factor Solution | |||
|---|---|---|---|---|
| Day 15 | F1 | F2 | F3 | F4 |
| Braun et al. | ||||
| B1. Orientation | ||||
| B2. State Control | −0.03 | |||
| B3. Motor Maturity | 0.19 | 0.12 | ||
| B4. Sensory Sensitivity | 0.03 | 0.16 | 0.27 | |
| Coe et al. (2010) Factors | ||||
| C1. Orientation | 0.97*** | 0.002 | 0.21 | 0.09 |
| C2. State Control | −0.13 | 0.82*** | 0.33 | 0.1 |
| C3. Motor Activity | 0.51* | 0.22 | 0.65*** | 0.4 |
| C4. Sensory Sensitivity | 0.1 | 0.13 | 0.32 | 0.44* |
| Schneider et al. (1991) Factors | ||||
| S1. Orientation | 0.97*** | 0.05 | 0.14 | 0.02 |
| S2. State Control | 0.18 | 0.81*** | 0.08 | 0.16 |
| S3: Motor Maturity | 0.18 | −0.03 | 0.45* | 0.26 |
| S4. Activity | 0.49* | 0.4* | 0.26 | 0.08 |
| B | Four Factor Solution | |||
|---|---|---|---|---|
| Day 30 | F1 | F2 | F3 | F4 |
| Braun et al. | ||||
| B1. Orientation | ||||
| B2. State Control | −0.06 | |||
| B3. Motor Maturity | −0.17 | 0.31 | ||
| B4. Sensory Sensitivity | 0.18 | −0.02 | −0.14 | |
| Coe et al. (2010) Factors | ||||
| C1. Orientation | 0.95*** | −0.004 | −0.03 | 0.1 |
| C2. State Control | −0.26 | 0.8*** | 0.4 | −0.14 |
| C3. Motor Activity | 0.24 | 0.41* | 0.2 | −0.09 |
| C4. Sensory Sensitivity | −0.22 | −0.41* | −0.26 | 0.57** |
| Schneider et al. (1991) Factors | ||||
| S1. Orientation | 0.95*** | −0.05 | −0.24 | 0.1 |
| S2. State Control | 0.005 | 0.88*** | 0.39 | −0.007 |
| S3: Motor Maturity | 0.48* | −0.05 | 0.12 | 0.58** |
| S4. Activity | −0.002 | 0.58** | 0.07 | −0.14 |
P<0.05
P<0.01
P<0.001.
DISCUSSION
Our results indicate that the PPNAS-M is a useful assessment tool to characterize infant common marmoset monkeys’ neurobehavioral status and early developmental changes.
Testing at these two ages did not affect health and weight of the infants, and overall, marmoset infants did not show much distress during testing, as measured by presence of fear grimace and/or infant distress calls, assuring us that the PPNAS-M is useful as a non-invasive tool. Although other measures of distress can be used (e.g.: increased circulating levels of catecholamines), fear grimace and/or infant distress calls are typical signs of distress in marmosets. This reaction may be unique to marmosets as, in comparison, rhesus monkeys may freeze under conditions aiming to elicit stress by human interaction (Kalin and Shelton, 1998). In addition, changes in weight and overall health are simple and quantifiable signs of impact on development and parent-infant bond, as an infant would not feed and lose weight or become sick if severely distressed.
Tailoring the Testing Paradigms to Marmoset Infants
Based on a pilot study to assess the feasibility of the testing paradigms, we identified nine tests used for evaluation of human and rhesus development that are not useful or measurable for marmosets. These tests include passivity, locomotion, nystagmus response, fine motor manipulations, balance while sitting, maintenance of balance, pull-to-sit, placing response, and the Moro reflex [Schneider and Suomi, 1992].
Passivity, scored as the percentage of time spent inactive, is not useful as a 15 to 30 day old marmoset is constantly active when detached from his/her carrier. Locomotion, scored as quality of locomotion, is not optimal as the test does not distinguish type of motion and was thoroughly addressed in the attempt to crawl and body righting tests. Nystagmus, scored using the duration and excursion length of post-rotary nystagmus following rotation (10/20 s) on rotary board, is not practical because an infant marmoset will not sit still on a rotary board long enough to adequately assess presence or absence of nystagmus. Additionally, the visuo-vestibular system is adequately evaluated using the rotation test.
Fine motor manipulations, scored as the amount of time engaged in fine motor manipulations, are unfeasible because infant marmosets are adapted to grasping (probably because marmosets, unlike rhesus, have claws instead of nails) and are lacking in dexterity compared to the infant rhesus. An indirect measure could be the reach and grasp test. Three other tests, balance while sitting, maintenance of balance, and the pull-to-sit test are not appropriate as marmosets do not sit like a rhesus or human and when attempting these tests marmosets immediately rights themselves to a prone position and retract their arms. The placing response, scored as the infant places the hand/foot on a surface following tactile stimulus of the dorsum, was eliminated from the PPNASM after it failed to produce a response and was difficult to assess with pilot marmoset infants. The Moro reflex, scored via the degree of abduction and extension in upper extremities following flexion of head upon sudden withdrawal of support while supine, was ineffective in pilot infants because infants immediately right themselves to a prone position. Instead, the parachute reflex, a variation of the Moro reflex, was successfully performed with the infant marmosets. The differences in feasible behavioral tests between rhesus and marmosets highlight the disparities in orientation, locomotion, and developmental patterns between these two species.
Of the 24 infants participating in this study only one displayed slight tremulousness at 15 days of age and self-mouthing was not observed in any of the infants at either time point. These two items, although not apparently present in healthy infants, are included in our scale because they represent testable and observable marmoset behaviors and may prove to be important when assessing illness, immaturity, or neurodevelopmental deficits in transgenic marmoset models. Presence or absence of self-mouthing has not been previously evaluated and may not be a behavior that appears at all in marmosets, or alternatively, it may appear prior to 15 days of age and then decrease due to neural maturation and thus is not measured in our study.
Developmental Differences between Infant Marmosets and Rhesus Found During Testing
Compared to rhesus monkeys, reduction in the traditionally considered primitive reflexes was not always observed in marmosets. A waning grasping reflex is observed in macaques, but was not seen in marmosets as the palmar and plantar grasp did not significantly change between both testing days. This may be a result of the necessary maintenance of the palmar and plantar grasping abilities to allow infant marmosets to remain continuously attached to a carrier's back without falling off until the marmoset begins leaving its carriers at 6 weeks of age [Missler et al. 1992].
The primitive rooting response appears to become stronger at one month than at two weeks in marmosets, contrasting with a waning rooting reflex in rhesus [Schneider and Suomi, 1992]. The increased score on the rooting response may be an adaptation to ensure appropriate feeding and survival. Compared to infant rhesus that are solely cared by their mothers and are usually in a frontal position with their faces adjacent to the mother's nipples, infant marmosets must move from the dorsal attachment of the caregiver to the nipples of the mother, usually being transferred from caregiver to the mother. The mother carries the infants much less often than other caregivers and mainly for nursing; therefore we speculate that the reflex is stronger at the infant stage in marmosets compared to rhesus monkeys.
The Labyrinthian righting test proved difficult to assess in marmosets, yet we were able to evaluate it and found no significant improvement within the first month, contrasting to the clear presence and development in rhesus neonates. We interpret this as a possible marmoset delayed response in order to adapt to being intensely jostled in many directions while carried by arboreal family members during the first month of life. Marmoset infants cling onto the caregivers (i.e. mother, father or an older sibling) without any help or support and are expected to maintain position without falling off, as a fall from a high tree branch would mean certain death. Since locomotion in marmosets can be in multiple directions, keeping position with the caregiver without trying to align with an absolute external reference point is key to survival. Therefore, we interpreted that the labyrinthian righting response may interfere with the ability of the infant marmoset to keep the caregiver as the point of reference. Consequently, in marmosets this response may not be fully matured until the infants begin leaving their carriers and become more independent in movement [Stevenson and Poole, 1976; Missler et al. 1992; Yamamoto, 1993; de Castro Leão et al. 2009]. Interestingly, males demonstrated a significantly more pronounced Labyrinthian-righting response than females at both 15 and 30 days.
The parachute response, a variation of the Moro reflex, becomes more visible at one month compared to two weeks in marmosets, and may be a key measurable response exhibitive of normal motor neurodevelopment [Segawa, 2007]. Similar to the labyrinthian righting, responses to the parachute test at 15 days were significantly stronger in males than females.
Results within the righting and body strength category reflect the expected increase in muscle tone and coordination within the first month of development. The ability of the infant to right its body or flip over from a supine position improves, while struggles during the distress to limitations test remain similar in intent, disregarding the increase in strength at 30 days. In addition, coordination improves, which promotes increased spontaneous crawling. Although infant muscle tone increases between 15 and 30 days, beginning before two weeks, the marmoset is already able to completely support its own head and neck in both a prone and supine position, suggestive of significant motor responses before 15 days.
The temperament evaluations, although highly variable between individuals, produced a few universal trends: marmosets become less drowsy at 30 days, and irritability and aggression are heightened. Interestingly, while marmosets remain difficult to console at 30 days, rhesus monkeys become more consolable [Schneider and Suomi, 1992] most likely indicating emerging marmoset independence. Interestingly, female marmosets demonstrate significantly heightened aggression at 30 days when compared to males. As with all the sex-related findings, follow up evaluation of these responses will confirm if the results are truly sex-specific.
As expected, we observed improvements during the first month in the ability of the marmoset to orient towards a visual stimulus. This is indicative of the early development of the marmoset visual system [Hendrickson et al. 2006] including maturation in visual cortex, [Warner et al. 2012; Oga et al. 2013] and retina. This increasing ability to orient towards a visual stimulus also indicates a large improvement in attentiveness and reduction in distractibility, which correlates with the onset of marmoset head cocking and the ability to fixate upon a single object [Menzel, 1980; Kaplan and Rogers, 2006]. The significant improvements in auditory/spatial orienting and in motor responses tests within the first month of life may be related to the marmoset infant being carried by a family member for the majority of the first six weeks of its life and resulting developmental processes as the infant is required to right itself within variable spatial orientations [Ingram, 1977]. Accordingly, the most significant changes between day 15 and day 30 were observed in the visual orienting category, righting and strength category, and the motor responses category with a 33%, 25% and 23% maturation, respectively, emphasizing their importance during the first month of marmoset development.
Compelling maturation was additionally noted in the types of vocalizations between two and four weeks of age, which is unique to marmosets. While no significant change in the number of bouts was observed within the allotted one-minute period, we did observe a shift from a majority of infants using short “phee” calls to more complex combinations of calls; including, long ‘Phee’ calls, an increase in distress calls, and the appearance of ‘Er-er’ calls, which are meant to be aggressive and threatening as would be expected in marmosets as they mature [Abbott and Hearn, 1978, Stevenson and Poole, 1976; Pistorio, et al. 2006].
Applying the PPNAS-M
The marmoset tests highly correlated with rhesus evaluations for the factors of Orientation and State Control indicating the importance and validity of these groupings. The third factor in the PPNAS-M, Motor Maturity, correlates well with Coe's third factor, motor activity, and Schneider's third factor, motor maturity, at post-natal age day 15. However this is not the case at post-natal age day 30, which may emphasize the independence of these two distinct developmental stages in a marmoset. Overall, the third and fourth marmoset factors, Motor Maturity and Sensory Sensitivity, do not correlate as well as the first two factors did with the rhesus factors. These two factors may be marmoset-specific, representative of the accelerated and unique callithrix post-natal maturation.
Like the Brazelton Newborn Behavioral Assessment Scale [1995] and the Schneider and Suomi [1992] assessment for rhesus monkeys, the PPNAS-M measures dimensions of state modulation or arousal, orientation or attention, and neuromotor maturity, all of which are important measures of early neurodevelopment [Als et al. 1977; Schneider and Suomi, 1992; Bayley, 1993]. The PPNAS-M both complements and expands upon the three marmoset developmental scales of Missler et al. [1992], Yamamoto [1993], and de Castro Leao et al. [2009] and illustrates well the numerous and sometimes very striking transitions during the initial one-month infant period.
For physical and neuromotor assessment, the PPNAS-M supports similar findings by Tardif et al. [2002] for necessary motor development required in order to survive early critical days and expands upon this early motor scale by combining motor, sensory, vestibular, and temperament tests to assess development beyond the first few days to one month of age.
It should be noted that as the goal of this project was to create a scale to evaluate normal neurobehavioral development, we strived to minimize the impact on the mother-infant bond by limiting the number of testing sessions, thus we were unable to assess the fleeting onset and offset of reflexes. Studies with repetitive testing on a nearly daily basis and possibly hand rearing [i.e. Schneider et al. 1991] are needed in order to definitively identify milestones in the rapidly developing marmosets.
Specific items showed some variability among the individuals that were tested, suggesting that subtle developmental distinctions were detected. An interesting finding was that individuals with low day 15 scores had a higher maturation rate (i.e. tended to improve more rapidly) to day 30, whereas high scores at day 15 tended to remain more stable until day 30, suggesting a ceiling effect and the need of additional testing tools for older juveniles.
To conclude, the PPNAS-M is the first step in developing a suitable instrument for descriptive studies aiming to detect discreet differences on specific neurobehavioral measures as well as to create a larger assessment utilizing the different groupings. Further studies to validate its application and to assess differences between different experimental groups of animals, including its use for the assessment of normal and transgenic disease models (e.g. Parkinson's Disease, Fragile X) in marmoset monkeys are warranted.
Acknowledgements
This research was supported by NIH grant P51 P51OD011106 (Wisconsin National Primate Research Center, University of Wisconsin-Madison), the Welton Sophomore Scholarship (KB), and Honors Senior Thesis Award (KB). This research was conducted at a facility constructed with support from Research Facilities Improvement Program grants RR15459-01 and RR020141-01. The authors would like to thank Dr. Alejandro Muñoz del Río for expert statistical advice.
Footnotes
The authors of this manuscript declare no conflict of interest.
References
- Abbott DH, Hearn JP. Physical, hormonal and behavioral aspects of sexual development in the marmoset monkey, Callithrix jacchus. The Journal of the Society for Reproduction and Fertility. 1978;53:155–166. doi: 10.1530/jrf.0.0530155. [DOI] [PubMed] [Google Scholar]
- Als H. II. Assessing an assessment: Conceptual considerations, methodological issues, and a perspective on the future of the Neonatal Behavioral Assessment Scale. Monographs of the Society for Research in Child Development. 1978;43:14–28. [PubMed] [Google Scholar]
- Als HT, Tronick E, Brazelton TB. The Brazelton Neonatal Behavioral Assessment Scale (BNBAS). Journal of Abnormal Child Psychology. 1977;5:215–229. doi: 10.1007/BF00913693. [DOI] [PubMed] [Google Scholar]
- Barbosa MN, Mota MTS. Do newborn vocalizations affect the behavioral and hormonal responses of nonreproductive male common marmosets (Callithriz jacchus)? Primates. 2014 doi: 10.1007/s10329-013-0404-0. DOI 10.1007/s10329-013-0404-0. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales Development of Infant. The Psychological Corporation; San Antonio: 1993. p. 374. [Google Scholar]
- Brazelton TB, Nugent JK. Neonatal Behavioral Assessment Scale. MacKeith Press; London: 1995. p. 137. [Google Scholar]
- de Castro Leão A, Neto ADD, de Sousa MBC. New developmental stages for common marmosets (Callithrix jacchus) using mass and age variables obtained by K-means algorithm and self-organizing maps (SOM). Computers in Biology and Medicine. 2009;39:853–859. doi: 10.1016/j.compbiomed.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Crispen HR, Shirtcliff EA, Schneider ML. Challenges to maternal wellbeing during pregnancy impact temperament, attention, and neuromotor responses in the infant rhesus monkey. Developmental Psychobiology. 2010;52:625–37. doi: 10.1002/dev.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freelon D. ReCal OIR: Ordinal, interval, and ratio intercoder reliability as a web service. International Journal of Internet Science. 2013;8:10–16. [Google Scholar]
- Greene MM, Patra K, Nelson MN, Silvestri JM. Evaluating preterm infants with the Bayley-III: Patterns and correlates of development. Research in Developmental Disabilities. 2012;33:1948–1956. doi: 10.1016/j.ridd.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 't Hart BA, Abbot DH, Nakamura K, Fuchs E. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discovery Today. 2012;17:1160–1165. doi: 10.1016/j.drudis.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger W, Neubert D. Maintenance and breeding of Callithrix jacchus in a colony in Berlin. In: Neubert, Merker, Hendrickxx, editors. Non-human Primates – Developmental Biology and Toxicology. Uebrreuter Wisenschaft; Berlin: 1988. pp. 51–64. [Google Scholar]
- Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. The Journal of Comparative Neurology. 2006;497:270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Troilo D, Djajadi H, Possin D, Springer A. Expression of synaptic and phototransduction markers during photoreceptor development in the marmoset monkey. Callithrix jacchus. 2009;512:218–231. doi: 10.1002/cne.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JC. Interactions between parents and infants, and the development of independence in the common marmoset (Callithrix jacchus). Animal Behavior. 1977;25:811–822. [Google Scholar]
- Jones BS. Vocal behavior of the common marmoset: Structure and function of selected calls. Primate Eye. 1994;53:22–23. [Google Scholar]
- Kalin NH, Shelton SE. Ontogeny and stability of separation and threat-induced defensive behaviors in rhesus monkeys during the first year of life. American Journal of Primatology. 1998;44:125–35. doi: 10.1002/(SICI)1098-2345(1998)44:2<125::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Rogers LJ. Head-cocking as a form of exploration in the common marmoset and its development. Developmental Psychobiology. 2006;48:551–560. doi: 10.1002/dev.20155. [DOI] [PubMed] [Google Scholar]
- King JE, Fobes JT, Fobes JL. Development of early behaviors in neonatal squirrel monkeys and cotton-top tamarins. Developmental Psychobiology. 1974;7:97–109. doi: 10.1002/dev.420070202. [DOI] [PubMed] [Google Scholar]
- Menzel MR. Head-cocking and visual perception in primates. Animal Behavior. 1980;28:151–159. doi: 10.1016/s0003-3472(80)80020-0. [DOI] [PubMed] [Google Scholar]
- Miller CT, Wang X. Sensory-motor interactions modulate a primate vocal behavior: antiphonal calling in common marmosets. Journal of Comparative Physiology. 2006;192:27–38. doi: 10.1007/s00359-005-0043-z. [DOI] [PubMed] [Google Scholar]
- Missler M, Wolfe, Rothe H, et al. Developmental biology of the common marmoset: proposal for a “postnatal staging”. Journal of Medical Primatology. 1992;6:285–298. [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Context and gender-specific differences in the acoustic structure of common marmoset. American Journal of Primatology. 1993;30:37–54. doi: 10.1002/ajp.1350300104. [DOI] [PubMed] [Google Scholar]
- Oga T, Aoi H, Sasaki T, Fujita I, Ichinohe N. Postnatal development of layer III pyramidal cells in the primary visual, inferior temporal, and prefrontal cortices of the marmoset. Frontiers in Neural Circuits. 2013;7:31. doi: 10.3389/fncir.2013.00031. doi: 10.3389/fncir.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H, Shikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Seminars in Fetal Neonatal Medicine. 2012;17:336–340. doi: 10.1016/j.siny.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Pistorio AL, Vintch B, Wang X. Acoustic analysis of vocal development in a New World primate: the common marmoset (Callithrix jacchus). Journal of Acoustical Society of America. 2006;120:1655–1670. doi: 10.1121/1.2225899. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postanatal social stress on physiology, behavior and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology. 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. http://www.R-project.org. [Google Scholar]
- Sameroff AJ, Krafchuk EE, Bakow HA. IV. Issues in grouping items from the Neonatal Behavioral Assessment Scale. Monographs of the Society for Research in Child Development. 1978;43:46–59. [PubMed] [Google Scholar]
- Saucedo A, Morales PR. Basics of macaque pediatrics. The Veterinary Clinics of North America: Exotic Animal Practice. 2012;15:289–98. doi: 10.1016/j.cvex.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Suemizo H, Shimada A, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–528. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behavior and Development. 1992;15:155–177. [Google Scholar]
- Schneider ML, Coe CL. Repeated social stress during pregnancy impairs neuromotor development of the primate infant. Journal of Developmental and Behavioral Pediatrics. 1993;14:81–87. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Suomi SJ, Champoux MB. Laboratory assessment of temperament and enviornmental enrichment in rhesus monkey infants (Macaca mulatta). American Journal of Primatology. 1991;25:137–155. doi: 10.1002/ajp.1350250302. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induce attention and neuromotor impairments in primate Infants. Child Development. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Development. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Segawa M. Pathophysiology of Down Syndrome: Consideration from Clincial Neurology. Neuropathology. 2007;13:301–303. [Google Scholar]
- Springer AD, Troilo D, Possin D, Hendrickson AE. Foveal cone density shows a rapid postnatal maturation in the marmoset monkey. Visual Neuroscience. 2011;28:473–484. doi: 10.1017/S0952523811000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman's Medical Dictionary 27th Edition. Lippincott Williams and Wilkins; Baltimore, MD, USA.: 2000. [Google Scholar]
- Stevenson MF, Poole TB. An ethogram of the common marmoset (Calithrix jacchus jacchus): General behavioural repertoire. Animal Behaviour. 1976;24:428–451. doi: 10.1016/s0003-3472(76)80053-x. [DOI] [PubMed] [Google Scholar]
- Smucny DA, Abbott DH, Mansfield KG, Schultz-Darken NJ, Yamamoto ME, Alencar AI, Tardif SD. Reproductive output, maternal age, and survivorship in captive common marmoset females (Callithrix jacchus). American Journal of Primatology. 2004;64:107–121. doi: 10.1002/ajp.20065. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Layne DG, Cancino L, Smucny DA. Neonatal behavioral scoring of common marmosets (Callithrix jacchus): Relation to physical condition and survival. Journal of Medical Primatology. 2002;31:147–151. doi: 10.1034/j.1600-0684.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken N, Yamamoto ME. Reproduction in captive common marmosets (Callithrix jacchus). Comparative Medicine. 2003;53:364–368. [PubMed] [Google Scholar]
- Tardif SD, Abee CR, Mansfield KG. Workshop summary: Neotropical primates in biomedical research. ILAR Journal. 2011;52:386–392. doi: 10.1093/ilar.52.3.386. [DOI] [PubMed] [Google Scholar]
- Warner CE, Kwan WC, Bourne JA. The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. The Journal of Neuroscience. 2012;32:17073–17085. doi: 10.1523/JNEUROSCI.3269-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle CP, Baker HF, Ridley RM, Oerke A-K, Martin RD. Unrearable litters and prenatal reduction of litter size in the common marmoset (Callithrix jacchus). Journal of Medical Primatology. 1999;28:73–83. doi: 10.1111/j.1600-0684.1999.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. From dependence to sexual maturity: the behavioral ontogeny of Callithrichidae. In: Rylands A, editor. Marmosets and Tamarins: Systematics, Behavior and Ecology. Oxford University Press; London: 1993. pp. 235–254. [Google Scholar]