Abstract
Repetitions that are distributed (spaced) across time prompt enhancement of a memory‐related event‐related potential, compared to when repetitions are massed (contiguous). Here, we used fMRI to investigate neural enhancement and suppression effects during free viewing of natural scenes that were either novel or repeated four times with massed or distributed repetitions. Distributed repetition was uniquely associated with a repetition enhancement effect in a bilateral posterior parietal cluster that included the precuneus and posterior cingulate and which has previously been implicated in episodic memory retrieval. Unique to massed repetition, conversely, was enhancement in a right dorsolateral prefrontal cluster that has been implicated in short‐term maintenance. Repetition suppression effects for both types of spacing were widespread in regions activated during novel picture processing. Taken together, the data are consistent with a hypothesis that distributed repetition prompts spontaneous retrieval of prior occurrences, whereas massed repetition prompts short‐term maintenance of the episodic representation, due to contiguous presentation. These processing differences may mediate the classic spacing effect in learning and memory. Hum Brain Mapp 36:1381–1392, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: repetition, retrieval, posterior parietal
INTRODUCTION
Repetition can prompt either suppression or enhancement effects in neural measures of stimulus processing, including electrophysiological [Grill‐Spector et al., 2006], magnoencephalographic [Huberle and Lutzenberger, 2013], and neuroimaging [Segaert et al., 2013]. Moreover, distributed repetitions, which are spaced across a learning episode, uniquely prompt an enhanced late centro‐parietal positive potential at both encoding and retrieval [Ferrari et al., 2014] that is similar in timing and topography to a classic old–new ERP difference found during a recognition test [Rugg and Curran, 2007], suggesting that distributed, but not massed (contiguous), repetition may prompt spontaneous retrieval of prior occurrences. In this study, we assessed this hypothesis using fMRI to identify distinct neural regions activated during encoding of massed or distributed repetitions.
A number of different mechanisms have been investigated that might mediate the classic finding that repetitions that are distributed in time facilitate both later learning and memory, compared to contiguous massed repetitions [i.e., “the spacing effect”; Cepeda et al., 2006; Glenberg, 1979; Godbole et al., 2014]. Among these are a hypothesis that repetitions that are distributed across time uniquely prompt what was originally called “study‐phase retrieval” [Greene, 1989] or spontaneous retrieval, an involuntary “reminding” of prior occurrences which facilitates later performance [Hintzman, 2004, 2010]. If distributed repetitions prompt spontaneous retrieval, we expected to find functional activity in one or more regions previously implicated in episodic memory uniquely for distributed repetitions.
Neuroimaging studies of episodic memory have reported an extensive network of regions involved in explicit recognition, including regions in frontal and prefrontal cortex, parietal and cingulate cortex, as well as in medial temporal lobe (MTL), including perirhinal and entorhinal cortex [for overview, see Rugg and Vilberg, 2013]. Moreover, enhanced activity in a region of the posterior parietal cortex that includes the precuneus and posterior cingulate (e.g., BA 7/29) has been a reliable index of old–new differences beginning with early neuroimaging (PET and fMRI) reviews of functional activation during episodic recognition [e.g., Rugg and Henson, 2002]. More recent reviews report that enhanced functional activity in posterior parietal cortex, particularly a medial region involving the precuneus, is reliably obtained when recognizing repeated (“old”), compared to new, items during episodic recognition across a wide variety of stimulus materials, modalities, and tasks [see Wagner et al., 2005]. To the extent that activation in these regions signal episodic memory processing, we expected that distributed, but not massed, repetition would elicit functional activation in one or more of these regions.
Whereas enhanced activation is reliably found for old, compared to new, items in a number of cortical regions [for an overview see; Guerin and Miller, 2009; Rugg and Vilberg, 2013; Wagner et al., 2005], in many neural regions the signature of prior experience is not an enhanced BOLD signal for repeated items, but rather suppression effects, in which neural activity is significantly attenuated for repeated, compared to new, items [Kirchhoff et al., 2000; Turk‐Browne et al., 2006; Ward et al., 2013]. Repetition suppression [see Grill‐Spector et al., 2006], is, in fact, a ubiquitous finding in studies of repetition with or without subsequent tests of memory and has been variously interpreted as reflecting perceptual sharpening, neural fatigue, information accumulation, or predictive coding [see Grill‐Spector et al., 2006; Segaert et al., 2013]. In a study that investigated neural suppression during encoding of massed, compared to distributed, presentations of faces, Xue et al. [2011] report more suppression in bilateral fusiform cortex for massed repetitions, prompting their interpretation that encoding may be deficient during massed repetition.
In this study, we assessed repetition suppression and enhancement effects using fMRI in a free viewing context in which natural scenes were presented that were novel, or repeated using massed (3×) or distributed (3×) repetition. The analytic strategy was as follows: (1) To assess whether repetition prompts BOLD activity in regions that are not found when encoding novel pictures, we first determined the set of regions that show a reliable increase in BOLD activity when viewing novel pictures, (2) we next assessed effects of repetition in these “novel picture processing regions,” with the goal of determining whether and how BOLD changes vary depending upon whether repetitions were massed or distributed, and (3) most importantly, we then assessed effects of repetition in regions that were not implicated in novel picture processing, with the specific question of whether distributed repetition uniquely prompts activity in one or more regions previously identified as important in episodic memory processing.
Mixed evidence exists regarding whether the amount of repetition suppression may also vary with the emotional salience of events. In at least one instance, repetition suppression was larger when viewing fearful compared to neutral faces [Ishai et al., 2004], whereas other studies of face perception [e.g., Rotshtein et al., 2001] have not found differential suppression as a function of emotion. Because pictures of facial expressions are generally less psychophysiologically evocative than emotional scenes [Wangelin et al., 2012], and engage different neural circuits [Sabatinelli et al., 2011], effects of emotion on repetition effects might be better elucidated using more evocative scenes. To reassess the relationship between repetition and emotion, we presented pictures of both emotionally arousing (erotica, violence) and neutral scenes, and assessed whether the functional activation patterns found for massed and distributed repetition vary with emotionality.
METHOD
Participants
Twenty‐four students enrolled in general psychology courses at the University of Florida participated for course credit or $20. All participants had normal visual acuity and reported no previous experience of claustrophobia during a phone interview. The study was approved by the local institutional review board and informed consent was obtained prior to the experiment.
MATERIALS AND DESIGN
The stimuli consisted of 24 pictures selected from the International Affective Picture System [IAPS; Lang et al., 2008], consisting of 12 emotionally evocative pictures (six erotica; six violent), and 12 neutral pictures. Of the 24 pictures, 12 (half emotional) were presented four times using massed repetitions and 12 (half neutral) were presented four times using distributed repetitions; the first presentation of each of the 24 pictures served as the 24 novel trials. Thus, for massed repetitions, each of the 12 pictures was repeated three times in a row following the initial novel presentation [i.e., 12 pictures × 3 massed repetitions = 36 trials, half (18) emotional]; for distributed repetition, each of the three repetitions were intermixed with other pictures [lag 24–30 trials between repetitions; 12 pictures × 3 repetitions = 36 trials overall; half (18) emotional].
An additional 12 pictures were presented that followed the last massed presentation of each picture, based on a previous study in which we found that these pictures elicit enhanced attention [Ferrari et al., 2010]. These 12 trials were not included here. Overall, the entire design involved the 196 critical trials (24 pictures × 4 presentations) and 12 filler trials for a total of 208 trials.
Each picture was presented for 3 s followed by a 12 s inter‐trial interval consisting of a black screen with a white fixation‐cross in the center of the screen. All stimuli were backward projected onto a LCD monitor (640 × 480 pixel resolution) situated behind the participant's head, and viewed using a head‐coil mounted mirror (IFIS‐SA, Invivo, Orlando, FL). Stimulus presentation was controlled using a PC‐compatible computer running E‐Prime (Psychology Software Tools, Sharpsburg, PA). Two orders were constructed such that if a picture was presented with massed repetition in one order, it was presented with distributed repetition in the other. In addition, picture presentation order was counterbalanced so that pictures presented in the first half of the experiment in one order were presented in the second half of the experiment in the second order (and vice versa).
PROCEDURE
After entering the scanner, participants were instructed to view each picture while it was on the screen and to maintain their gaze on a centrally presented fixation cross.
Data Acquisition and Reduction
A T1‐weighted anatomical volume was first acquired using a Siemens 3T Allegra MR scanner. Functional volumes were 50 coronal slices (2.5 mm thick, 0.5 mm gap) acquired using a T2* weighted echo planar imaging sequence with a 3 s TR, 35 ms TE, 64 × 64 acquisition matrix, and 160 mV FOV (2.5 × 2.5 inplane voxel resolution) resulting in a total of 565 functional volumes. Data were processed offline using the Analysis of Functional Neuroimages software [AFNI; Cox, 1996] in which structural images were aligned to functional images, and the functional data were then slice‐time adjusted, motion corrected, spatially smoothed (5 mm FWHM Gaussian kernel), and expressed as percent blood oxygen level‐dependent (BOLD) signal relative to the mean BOLD activity across the entire timeseries for each voxel. These data were deconvolved using a general linear regression model that estimated the amplitude of the hemodynamic response to each stimulus category using a top hat function convolved with a standard gamma variate response as a basis function. The model included each of the six conditions (novel, massed, distributed × emotional, neutral) and regressors of noninterest that modeled motion residuals and baseline drift. The resulting beta values for each of the six conditions for each participant were spatially normalized and resampled to a 2.5 mm isotropic voxel size.
Data Analysis
The analytic strategy involved first identifying regions that were active during novel picture processing, regardless of hedonic content. To do so, functional data during novel picture viewing was assessed in an ANOVA which provided separate t‐test statistics testing whether the mean beta value across participants was significantly greater than 0 when viewing emotional and neutral pictures. Each of these two statistical volumes was thresholded at t(23) = 3.8 (uncorrected P < 0.001; false discovery q = 0.006) and corrected for multiple comparisons with a cluster size criterion of 86 voxels (FWE P < 0.01), using the AFNI program 3dClustSim which estimates the probability of a false positive cluster when the noise is thresholded at different levels. A subsequent conjunction analysis retained only voxels that were significant both when viewing novel emotional pictures and when viewing novel neutral pictures. These voxels were neuroanatomically labeled using macrolabels derived from the MNI template [Eickhoff et al., 2005] that had been transformed into Talairach space [Brett et al., 2001]. The extent of activation in each identified region was expressed as the proportion of voxels retained in the conjunction analysis relative to the total number of voxels comprising each region. (To provide a reference for the selected threshold, the extent of activation in Fig. 1 also includes the proportion of voxels activated at a lower statistical threshold, t(23) = 2.84 (uncorrected P < 0.01) and corrected for multiple comparisons with a similar cluster size criterion of 86 voxels.) To assess effects of repetition and emotion in the novel picture processing regions, the mean beta value was averaged over significant voxels in each region for each participant and condition, and submitted to an ANOVA that included repeated measures variables of repetition (novel, massed, distributed) and content (emotional, neutral).
Figure 1.
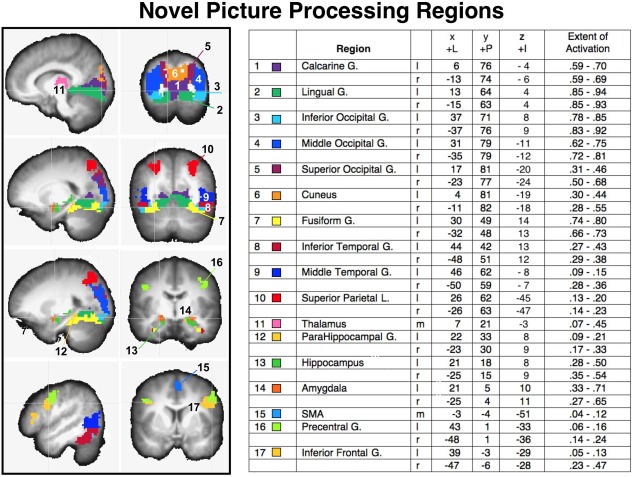
Activation of regions resulting from a conjunction analysis of significant functional activity when viewing novel pictures that were emotional or neutral, overlaid on a structural image representing the average anatomical image across all participants. Left panels: saggital and coronal views depict the voxels in each anatomical region that showed significant BOLD increases when viewing novel pictures. Region number (1–17) and color code is listed next to the region label in the accompanying chart, together with the Talairach coordinates (LPI) for the center of functional activity in each region, and the extent of functional activity, expressed as the proportion of voxels reaching significance as a function of the total number of voxels in each anatomical region, averaged over participants, using a strict threshold for significant BOLD change (P < 0.001 uncorrected, cluster size 86) and a more lenient criterion (P < 0. 01, uncorrected, cluster size = 86). l=left; r=right; m=medial.
Repetition effects in regions other than those involved in picture processing were determined by first masking out the anatomical regions involved in novel picture processing. Then, the main effect of repetition in voxels remaining in the volume was assessed in a repeated measures (novel, distributed, massed) ANOVA that was thresholded at F(1,23) = 4.56 (uncorrected P < 0.01), and corrected for false positives (using the AFNI program 3dClustSim) with a cluster size of 56 voxels (FWE P < 0.01). For the resulting significant functional clusters, the mean beta value was averaged over significant voxels for each participant as a function of repetition condition (novel, massed, distributed) and content (emotional, neutral) and submitted to a two‐way repeated measures ANOVA.
Descriptive statistics used to illustrate the BOLD waveforms during picture viewing as a function of repetition and emotion were computed by deviating the percent BOLD change (following preprocessing) on each TR from the value in a baseline volume that immediately preceded picture onset; these change scores were then averaged over participant, trial, and condition to illustrate mean BOLD activity in specific functional clusters.
RESULTS
Novel Picture Processing Regions
Figure 1 illustrates regions involved in novel picture processing, based on a conjunction analysis in which voxels were retained that showed a significant increase in BOLD activity when viewing emotional picture as well as when viewing neutral pictures. The coordinates for the center of activity in each region, the extent of activation, and the mean BOLD change (beta) in each region when viewing novel pictures are also included.
Not surprisingly, novel picture processing was associated with significant BOLD activity in multiple regions in bilateral occipital, temporal, parietal, and frontal cortex, as well as in thalamic and subcortical regions.1 As expected, activity in regions of the posterior cortex, including those implicated in the ventral visual processing stream was predominant, including bilateral occipital (inferior, middle), temporal (inferior, middle) gyrus and fusiform cortex, as well as calcarine and lingual gyri. Regions implicated in the dorsal visual processing stream were also activated, including bilateral activity in superior occipital cortex and superior parietal cortex, which extended slightly into inferior parietal cortex. As Figure 1 indicates, the center and amplitude of activity in each region in posterior cortex was strikingly similar in the left and right hemispheres.
Beyond the visual cortex, significant activity was found during novel picture processing in the thalamus as well as in subcortical regions that included the bilateral parahippocampus, hippocampus, and amygdala. Of note is that these regions, in surviving the conjunction analysis, show a significant increase in BOLD activity even when processing novel pictures that are not highly emotional, consistent with studies demonstrating both hippocampal [Menon et al., 2000] and amygdala sensitivity to novelty [e.g., Balderston et al., 2011]. The most anterior regions of the cortex consistently activated during novel picture processing included bilateral activity beginning around the precentral gyrus and extending into the inferior frontal gyrus (orbicularis, triangularis) and a small, but reliable, cluster in supplementary motor area (SMA) in the region of the supplementary eye fields.
Novel Picture Processing Regions: Repetition Effects
Table 1 lists the effects of repetition in regions activated during novel picture processing, and, in every instance, repetition prompted significant suppression in which repeated pictures, whether massed or distributed, showed smaller positive BOLD changes than were found during novel picture processing. Suppression effects were found throughout the occipito‐temporal cortex, as well as in the amygdala, hippocampal/parahippocampal regions, thalamus, and all anterior regions. In some of these regions, repetition suppression did not differ for massed and distributed repetition, including the amygdala (see Fig. 2, top right), thalamus, and all anterior regions, whereas repetition suppression was significantly larger for massed, compared to distributed, repetition in fusiform gyrus (see Fig. 2, bottom right), calcarine, inferior and middle occipital gyrus, and inferior temporal gyrus, as well as in the hippocampus, and parahippocampus (see Fig. 2, left). Only two regions implicated in novel picture processing did not show significant repetition effects, and these included SMA and superior parietal cortex.
Table 1.
Mean BOLD change (beta) in each of the regions activated during novel picture processing for novel pictures, and for pictures that were repeated with massed or distributed repetition, the F‐statistic testing the main effect of repetition (novel, massed, distributed), and significant differences in follow up comparisons
| Region | Mean BOLD change | Repetition F (2,22)= | Significant difference | ||
|---|---|---|---|---|---|
| Novel | Dist | Mass | |||
| Calcarine G. | 0.81 | 0.68 | 0.62 | 12.8*** | abc |
| Lingual G. | 0.73 | 0.60 | 0.55 | 13.1*** | ab |
| Inferior occipital G. | 0.82 | 0.71 | 0.65 | 8.7** | abc |
| Middle occipital G. | 0.64 | 0.54 | 0.47 | 13.3*** | abc |
| Superior occipital G. | 0.46 | 0.39 | 0.35 | 7.0** | ab |
| Cuneus | 0.56 | 0.47 | 0.42 | 8.6*** | ab |
| Fusiform G. | 0.74 | 0.58 | 0.52 | 20.7**** | abc |
| Inferior temporal G. | 0.59 | 0.45 | 0.39 | 17.9**** | abc |
| Middle temporal G. | 0.47 | 0.37 | 0.33 | 16.5**** | ab |
| Superior parietal L. | 0.50 | 0.42 | 0.38 | — | — |
| Thalamus | 0.24 | 0.16 | 0.14 | 9.2** | ab |
| ParaHippocampal G. | 0.31 | 0.21 | 0.14 | 18.6**** | abc |
| Hippocampus | 0.26 | 0.16 | 0.12 | 20.0**** | abc |
| Amygdala | 0.32 | 0.19 | 0.14 | 12.0*** | ab |
| SMA | 0.32 | 0.28 | 0.31 | — | — |
| Precentral G. | 0.40 | 0.30 | 0.29 | 8.7** | ab |
| Inferior frontal G. | 0.41 | 0.28 | 0.26 | 23.8**** | ab |
All of the effects of repetition in regions activated during novel picture processing are repetition suppression effects.
****P < 0.0001 ***P < 0.001 **P < 0.01 *P < 0.05.
a, Novel > distributed repetition.
b, Novel > massed repetition.
c, Distributed repetition > massed repetition.
Figure 2.
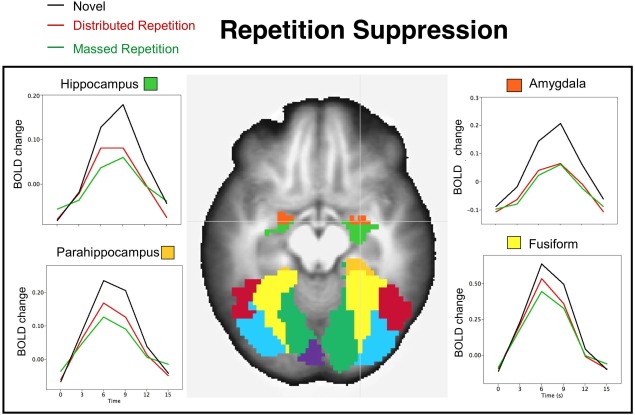
Repetition suppression effects in regions activated during novel picture processing. The BOLD waveforms illustrate the change in BOLD activity (deviated from the baseline volume preceding picture onset) averaged over all participants when viewing novel pictures and those presented with massed or distributed repetition in hippocampus (top left), parahippocampus (bottom left), amygdala (top right), and fusiform (bottom right). An axial slice illustrating each region (z = +8) is overlaid on the anatomical image averaged across all participants. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Repetition Effects: Outside Novel Picture Processing Regions
Figure 3 illustrates the single functional cluster not activated during novel picture processing which showed a positive increase in BOLD activity uniquely for distributed repetition—a bilateral medial cluster in posterior parietal cortex, located along the occipitoparietal junction that included regions of the posterior precuneus (BA 7) and the anterior cuneus (BA 31),2 extending inferiorly to the posterior cingulate (BA 29). BOLD changes in this posterior parietal cluster were significantly enhanced when viewing pictures presented with distributed repetition, compared to either massed repetition, F(1,23) = 10.8, P = 0.003, or to novel presentations, F(1,23) = 16.4, P = 0.0005. As illustrated in Figure 3, enhanced activity in this functional cluster was similar at each of the three distributed repetitions of each picture.
Figure 3.
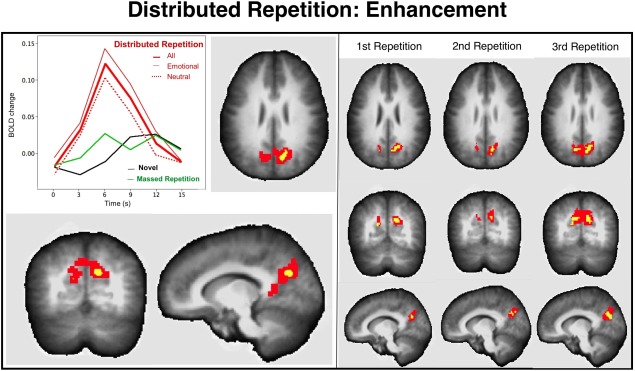
Repetition enhancement effect for distributed repetition in posterior parietal cortex (x 14, y 63, z −26) includes regions of the posterior precuneus, anterior cuneus, and posterior cingulate and is overlaid on the averaged anatomical image. The BOLD waveforms (deviated from the baseline volume preceding picture onset) illustrate that functional activity is enhanced when viewing distributed repetitions, whether emotional or neutral, compared to massed repetitions, or to novel picture viewing. The right panel illustrates that repetition enhancement effects are found in a similar posterior parietal cluster on the first, second, and third distributed repetitions of each scene. The voxel colors on the overlays for the anatomical images indicate significant enhancement effects at P < 0.001 (yellow) and P < 0.05 (red).
For massed repetition, conversely, repetition enhancement was found uniquely in a cluster situated on the lateral aspect of the right superior and middle frontal gyrus (e.g., dorsolateral prefrontal cortex; BA 9/10; see Fig. 4, top panel) which showed significant enhancement during massed repetitions, compared to when viewing novel pictures, F(1,23) = 28, P < 0.0001, or pictures presented with distributed repetition, F(1,23) = 19.3, P = 0.0002.
Figure 4.
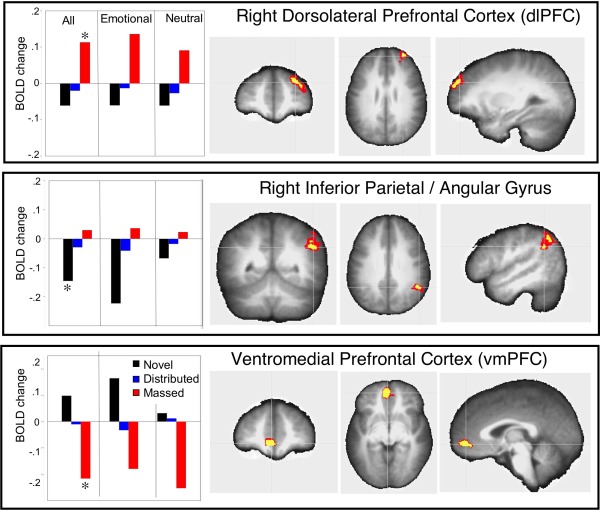
Repetition effects in regions not involved in novel picture processing are overlaid on the averaged anatomical image. Left panels: bar graphs illustrate the mean beta averaged over content for novel and repeated (massed or distributed) pictures, and separately for emotional and neutral scenes. An asterisk (*) in the contrasts involving all pictures indicates that the condition significantly differed from the others. Top panel: enhanced activity in a functional cluster in right dorsolateral prefrontal cortex (x −32 y −50 z −27) was found for massed repetition, regardless of hedonic content. Middle panel: a significant decrease in BOLD activity was found for novel pictures in a cluster in right lateral inferior parietal cortex (x −50 y 51 z −32) which was larger for emotional, compared to neutral pictures. Bottom panel: a significant decrease in BOLD activity was found for massed repetition in a cluster in ventromedial prefrontal cortex (x 5 y −47 −1) regardless of hedonic content. The voxel colors on the overlays for the anatomical images indicate significance of the effects at P < 0.001 (yellow) and P < 0.05 (red). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Two other repetition effects outside the picture processing regions involved decreases in BOLD activity. A cluster located in right inferior parietal cortex (beginning in the angular gyrus and extending to supramarginal gyrus; BA 40/39; see Fig. 4, middle) resulted in a significant decrease in BOLD activity when viewing novel, compared to repeated, pictures (distributed: F(1,23) = 12.6, P = 0.002; massed: F(1,23) = 20.9, P = 0.0001). And, in ventromedial prefrontal gyrus (BA 10), a significant decrease in BOLD activity was found during massed repetition compared to either novel picture viewing F (1,23) = 30.3, P < 0.0001, or to distributed repetition, F(1,23)=15.9, P = 0.0006 (see Fig. 4, bottom).
Effects of Emotion
Differences in functional activity when viewing novel emotional, compared to neutral, scenes replicated previous studies, with enhanced BOLD activity found in the amygdala, as well as throughout lateral occipital cortex (inferior, middle, superior), fusiform gyrus, and inferior frontal regions (see Table 2). Emotional pictures were also associated with enhanced BOLD activity in the hippocampus, precentral gyrus, and middle temporal gyrus. Repetition suppression effects in the novel picture processing regions were generally quite similar in magnitude for emotional and neutral scenes (see Table 3) except that suppression in the superior parietal lobe, which was not found in the overall analysis, was significant for neutral, but not emotional, pictures, during both massed and distributed repetition.
Table 2.
Mean BOLD signal change (beta) in novel picture processing regions when viewing novel emotional and neutral pictures, and the F test and P‐value of the statistical difference (emotional, neutral)
| Region | Mean BOLD change | F(1,23) | P‐value | |
|---|---|---|---|---|
| Emotional | Neutral | |||
| Calcarine G. | 0.84 | 0.79 | 1.6 | 0.22 |
| Lingual G. | 0.76 | 0.71 | 2.2 | 0.15 |
| Inferior occipital G.* | 0.92 | 0.72 | 26.4 | <0.0001 |
| Middle occipital G.* | 0.70 | 0.58 | 15.6 | 0.0006 |
| Superior occipital G.* | 0.50 | 0.43 | 8.4 | 0.008 |
| Cuneus | 0.57 | 0.54 | 1.3 | 0.26 |
| Fusiform G. | 0.78 | 0.70 | 6.8 | 0.02 |
| Inferior temporal G.* | 0.69 | 0.48 | 45.7 | <0.0001 |
| Middle temporal G. | 0.54 | 0.41 | 10.1 | 0.004 |
| Superior parietal L. | 0.55 | 0.45 | 4.9 | 0.03 |
| Thalamus | 0.27 | 0.21 | 2.4 | 0.14 |
| ParaHippocampal G. | 0.31 | 0.31 | <1 | 0.95 |
| Hippocampus | 0.30 | 0.23 | 6.8 | 0.02 |
| Amygdala* | 0.41 | 0.23 | 16.8 | 0.0004 |
| SMA | 0.37 | 0.27 | 4.2 | 0.05 |
| Precentral G. | 0.46 | 0.33 | 10.7 | 0.003 |
| Inferior frontal G. | 0.47 | 0.34 | 8.3 | 0.009 |
Asterisks (*) identify regions in which the emotional‐neutral difference was significant with a Bonferroni correction (P = 0.002).
G = gyrus; l = lobe; SMA = supplementary motor area.
Table 3.
Repetition suppression effects in regions active during novel picture processing separately for emotional and neutral pictures that were presented with massed or distributed repetition, and significance of the t‐test comparing suppression for emotional and neutral scenes
| Region | Amount of suppressiona | |||||
|---|---|---|---|---|---|---|
| Massed | Distributed | |||||
| Emot | Neu | t‐test | Emot | Neu | t‐test | |
| Calcarine G. | 0.15 | 0.24 | * | 0.13 | 0.13 | |
| Lingual G. | 0.14 | 0.23 | * | 0.12 | 0.15 | |
| Inferior occipital G. | 0.15 | 0.19 | 0.08 | 0.14 | ||
| Middle occipital G. | 0.15 | 0.20 | 0.07 | 0.14 | ||
| Superior occipital G. | 0.09 | 0.15 | 0.05 | 0.11 | ||
| Cuneus | 0.11 | 0.17 | 0.09 | 0.10 | ||
| Fusiform G. | 0.18 | 0.26 | 0.13 | 0.19 | ||
| Inferior temporal G. | 0.20 | 0.20 | 0.12 | 0.15 | ||
| Middle temporal G. | 0.13 | 0.16 | 0.09 | 0.12 | ||
| Superior parietal L. | 0.05 | 0.17 | * | 0.01 | 0.15 | * |
| Thalamus | 0.08 | 0.13 | 0.08 | 0.09 | ||
| ParaHippocampal G. | 0.12 | 0.21 | * | 0.11 | 0.09 | |
| Hippocampus | 0.11 | 0.18 | 0.12 | 0.08 | ||
| Amygdala | 0.15 | 0.20 | 0.17 | 0.10 | ||
| SMA | −0.01 | 0.03 | 0.03 | 0.05 | ||
| Precentral G. | 0.10 | 0.12 | 0.09 | 0.10 | ||
| Inferior frontal G. | 0.14 | 0.16 | 0.13 | 0.12 | ||
*P < 0.05
Suppression is calculated as the reduction in mean BOLD signal change (beta value) when functional activity measured during repetition is subtracted from the BOLD signal change measured during novel picture processing.
Outside the novel picture processing regions, enhanced activity in the posterior parietal cortex that occurred uniquely for distributed repetitions was found for both emotional and neutral pictures (see timecourse data in Fig. 3). In addition, the significant enhancement found for massed repetitions in right lateral prefrontal cortex was found for both emotional and neutral pictures (see Fig. 4, top), and the decrease in BOLD activity for massed repetitions in ventromedial prefrontal cortex (vmPFC; see Fig. 4, bottom) was also found for both emotional and neutral pictures. The decrease in BOLD activity in the (right) angular gyrus when viewing novel pictures was significantly larger for emotional, compared to neutral, pictures, F(1,23) = 10.5, P = 0.004 (Fig. 4, middle).
DISCUSSION
Distributed repetition of natural scenes was uniquely associated with increased BOLD activity in a bilateral medial cluster in posterior parietal cortex that included regions of the posterior precuneus and anterior cuneus that extended inferiorly to the posterior cingulate (BA 7/31/29). Enhanced BOLD activity in this medial posterior parietal cluster was not found during massed repetition, or when encoding novel pictures, whereas functional brain activity in these medial regions of the posterior parietal cortex have been previously implicated in memory‐related processes such as episodic retrieval in a number of studies of episodic memory [Donaldson et al., 2001; Guerin and Miller, 2009; Kompus et al., 2011; Wagner et al., 2005; Yassa and Stark, 2008]. For massed repetition, conversely, repetition enhancement was found uniquely in a cluster in right dlPFC, spanning superior and medial frontal gyrus (BA 9/10) that is often found in studies of working memory [e.g., Cohen et al., 1997], suggesting that massed repetition prompts continued maintenance in short‐term memory presumably due to contiguous presentation.
Processing novel pictures, whether emotional or neutral, involved widespread activation in occipital, temporal, and parietal cortex. Activity in the ventral visual stream was relatively spatially contiguous from anterior fusiform cortex to parahippocampal/hippocampal regions to the amygdala, regardless of picture content (Fig. 2). Heightened activation in amygdala and hippocampus during processing of novel pictures is consistent with previous data indicating a general sensitivity to novelty in both regions. And, replicating previous data, emotional pictures were associated with enhanced BOLD activity in many regions involved in novel picture processing, including lateral occipital cortex (inferior, middle), fusiform cortex, inferior frontal gyrus, the hippocampus, and the amygdala [e.g., Bradley et al., 2003; Lang et al., 1998; Phan et al., 2004; Sabatinelli et al., 2011]. We have interpreted enhanced functional activity for emotionally evocative cues as reflecting “natural selective attention” that is engaged by cues that activate fundamental motivational systems of appetite and defense [Bradley, 2009; Lang et al., 1997]. Nonetheless, repetition suppression and enhancement effects were generally similar for emotional and neutral scenes, and most importantly, enhanced posterior parietal activity prompted by distributed repetitions, as well as activation of the dlPFC by massed repetitions, were found regardless of hedonic content, suggesting these effects reflect the operation of a general cognitive mechanism.
Repetition Enhancement
Although suppression is a classic finding in neural studies of repetition, Segaert et al. [2013] review instances in which repetition prompts BOLD enhancement, sometimes interpreted as reflecting the operation of an additional cognitive process [e.g., Henson et al., 2000]. This hypothesis is consistent with our interpretation that enhanced posterior parietal activity for distributed repetition signals the engagement of an additional episodic retrieval process. Enhanced activity in posterior parietal cortex, including the precuneus and posterior cingulate (e.g., BA 7/29) has been noted during explicit recognition tasks [e.g., Rugg and Henson, 2002; Wagner et al., 2005], including when pictures of natural scenes are explicitly recognized [Weymar et al., 2012]. In addition, when functional activity is directly compared during explicit and implicit memory tasks, bilateral activation of the precuneus is among the most prominent regions reliably engaged in both tasks [Donaldson et al., 2001]. Moreover, Nelson et al. [2013] found that enhanced posterior parietal activity was associated with “test‐potentiated learning,” in which items tested (retrieved) during study show better learning than those not tested. Reviewing the many functions of the precuneus, Cavanna and Trimble [2006] conclude that enhanced activity in the posterior precuneus, in particular, is reliably related to episodic memory processes in both explicit and implicit contexts.
Our finding of enhanced posterior parietal activity uniquely for distributed repetitions during free viewing further supports a hypothesis that episodic retrieval in this context is spontaneous or involuntary. This involuntary “reminding” [e.g., Tullis et al., 2014] is sometimes considered the most natural mode of memory retrieval in daily life: cues automatically retrieve related events, enabling judgments of frequency, recency, order, and spacing [Hintzman, 2004, 2010] as well as mediating prospective memory and future actions [Einstein et al., 2005]. Additional support for this interpretation is garnered from Lee et al. [2013] who found that the posterior precuneus was the only region that reliably differentiated between repeated and novel faces in a lie detection task regardless of whether participants were instructed to respond truthfully or to lie about prior occurrence.
To the extent that medial parietal cortex activity indexes spontaneous reminding, a possible link can be made between the current data and the reliable inclusion of the precuneus as a component of the default mode network [Buckner, 2012]. Current conceptualizations of default mode processing hypothesize that it reflects internal‐oriented processing that includes spontaneous episodic retrieval, mental imagery, and future planning, which fits well with the hypothesized role of reminding in relating the present to both the past and the future [e.g., Buckner, 2012; Schacter et al., 2012; Spreng and Grady, 2010]. Spontaneous retrieval could facilitate later memory performance in a number of ways, not least of which is that the act of retrieval itself facilitates learning and memory [e.g., Carrier and Pashler, 1992; Karpicke and Blunt, 2011; Karpicke and Roediger, 2008; Nelson et al., 2013].
Working Memory
Massed repetition was uniquely associated with enhanced activity in right superior/medial frontal gyrus (dlPFC; BA 9/46), a region long implicated in maintenance of information in working memory [Cohen et al., 1997; Pessoa et al., 2002]. An early hypothesis that verbal and nonverbal material may differentially engage left and right prefrontal regions, respectively, was not always supported, as bilateral dlPFC activation was found in a number of studies during active maintenance, regardless of the nature of the materials [Nystrom et al., 2000; Postle et al., 2000]. One possiblity is that the effortful maintenance induced in prototypical working memory tasks, such as the N‐back, recruits both hemispheres because participants attempt to use idiosyncratic verbal codes to assist in task performance, even when targets are nominally nonverbal. In this study, conversely, maintenance in working memory is achieved simply by contiguous repetition of the same stimulus, which is only apparent in the right hemisphere.
Repetition Suppression
The repetition suppression effects found in almost all of the novel picture processing regions when viewing natural scenes are consistent with Kirchhoff et al. [2000] who reported repetition suppression effects in inferior prefrontal, fusiform, and medial temporal lobe, as well as Menon et al. [2000] who found “novelty” effects (e.g., enhanced activation for novel, compared to repeated) in lingual gyrus, parahippocampus, hippocampus, and inferior frontal gyrus (in analyses restricted to these regions) for natural scenes [see also Buckner et al., 1999; Stern et al., 1996; Tulving et al., 1994; Wagner et al., 1998]. The ubiquity of repetition suppression in regions implicated in novel picture processing suggests that functional activity in these regions index processes involved in initial resolution of picture content that may reflect perceptual or semantic priming [Wiggs and Martin, 1998].
Repetition suppression effects were slightly larger for massed, compared to distributed, repetition in some regions (e.g., hippocampal, parahippocampal, fusiform, etc.), raising the question of whether enhanced suppression contributes to the poorer memory for massed repetitions. Ward et al. [2013] found no evidence that amount of suppression was related to subsequent memory, however, and Summerfield et al. [2008] attributed differences in suppression to ease of prediction during encoding. Larsson and Smith [2012] explicitly tested between stimulus expectation and neuronal fatigue accounts of repetition suppression by presenting frequent or infrequent repetitions of faces alone, or in a dual‐task context. When faces were presented alone, repetition suppression was greater for frequent, compared to infrequent, repetitions. When attention was diverted, however, repetition suppression was equivalent for both frequent and infrequent repetitions, presumably because the difficult distracting task eliminated the contribution of active expectation. In the current study, stimulus expectation could play a similar role in enhancing suppression for massed, compared to distributed, repetitions, but would not be strongly implicated in mediating later memory performance.
Reduced BOLD Activity
A significant repetition effect found in right lateral inferior parietal cortex (including angular gyrus; BA 40/39) was primarily due to a decrease in BOLD activity when viewing novel, compared to repeated, scenes and, in general, effects linked with decreased BOLD activity are typically more difficult to interpret [but see Bressler et al., 2007]. Of note is that a similar region in the left lateral parietal cortex, in the region of the angular and supramarginal gyrus, is commonly reported in studies of episodic memory and, in at least some cases, the statistical old–new difference is due to a decrease in BOLD activity for novel stimuli, rather than enhancement for old items [e.g., McDermott et al., 2000; Wheeler and Buckner, 2004]. Because the direction of old–new differences can not be ascertained from statistical maps, it is important to determine whether these effects are indeed due to enhanced BOLD activity for “old” items.
Summary
In a free‐viewing context, distributed repetition of natural scenes uniquely prompts enhanced functional activity in a region in the posterior parietal cortex that includes the posterior precuneus, anterior cuneus, and posterior cingulate cortex, regions previously implicated in episodic memory retrieval. Massed repetition, conversely, was associated with unique enhancement in right dorsolaterial prefrontal cortex that includes superior and medial frontal gryus, regions previously implicated in working memory. These data are consistent with an interpretation that distributed repetition may prompt episodic memory‐related processes such as stimulus‐based spontaneous retrieval of a prior occurrence, which could mediate later facilitated learning and memory. Retrieval‐related processes are not engaged during massed repetition because the episodic representations are continuously maintained in short‐term memory, simply by virtue of contiguous presentation. Taken together, fMRI has helped to elucidate the cognitive processing that occurs when simply encountering novel and repeated scenes in a free viewing (i.e., incidental memory) context, and suggests that differences in spontaneous retrieval may mediate the spacing effect, in which distributed, compared to massed, repetitions benefit both learning and memory.
Footnotes
Significant BOLD activity was also found bilaterally in the cerebellum (mean beta 0.18, left; 0.22, right) and cerebellar vermis (mean beta 0.22) during novel picture processing, with only a small proportion (<5%) showing significant repetition suppression which all bordered regions showing significant suppression effects near the inferior temporal and fusiform cortex. There were no repetition enhancement effects in these regions.
Functional activity found in the posterior cuneus during novel picture processing did not spatially overlap with the anterior cluster that showed enhanced activation for distributed repetitions.
REFERENCES
- Balderston NL, Schultz DH, Helmstetter FJ (2011): The human amygdala plays a stimulus specific role in the detection of novelty. NeuroImage 55:1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM (2009): Natural selective attention: Orienting and emotion. Psychophysiology 46:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King WM, Desai P (2003): Activation of the visual cortex in motivated attention. Behav Neurosci 117:369–380. [DOI] [PubMed] [Google Scholar]
- Bressler D, Spotswood N, Whitney D (2007): Negative BOLD fMRI response in the visual cortex carries precise stimulus‐specific information. PLoS ONE 2:e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J (2001): Using the talairach atlas with the MNI template. NeuroImage 13:S85. [Google Scholar]
- Buckner RL (2012): The serendipitous discovery of the brain's default network. NeuroImage 62:1137–1145. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE (1999): Frontal cortex contributes to human memory formation. Nat Neurosci 2:311–314. [DOI] [PubMed] [Google Scholar]
- Carrier M, Pashler H (1992): The influence of retrieval on retention. Mem Cogn 20:633–642. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D (2006): Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull 132:354–380. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE (1997): Temporal dynamics of brain activation during a working memory task. Nature 386:604–608. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL (2001): Dissociating memory retrieval processes using fMRI: Evidence that priming does not support recognition memory. Neuron 31:1047–1059. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J (2005): Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. J Exp Psychol Gen 134:327–342. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ (2010): Detecting novelty and significance. J Cogn Neurosci 22:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ (2014): Massed and distributed repetition of natural scenes: Brain potentials and oscillatory activity. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Glenberg AM (1979): Component‐levels theory of the effects of spacing of repetitions on recall and recognition. Mem Cogn 7:95–112. [DOI] [PubMed] [Google Scholar]
- Godbole NR, Delaney PF, Verkoeijen PPJL (2014): The spacing effect in immediate and delayed free recall. Memory 22:462–469. [DOI] [PubMed] [Google Scholar]
- Greene RL (1989): Spacing effects in memory: Evidence for a two‐process account. J Exp Psychol Learn Mem Cogn 15:371–377. [Google Scholar]
- Grill‐Spector K, Henson R, Martin A (2006): Repetition and the brain: Neural models of stimulus‐specific effects. Trends Cogn Sci 10:14–23. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB (2009): Lateralization of the parietal old/new effect: An event‐ related fMRI study comparing recognition memory for words and faces. NeuroImage 44:232–242. [DOI] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R (2000): Neuroimaging evidence for dissociable forms of repetition priming. Science 287:1269–1272. [DOI] [PubMed] [Google Scholar]
- Hintzman DL (2004): Judgment of frequency vs. recognition confidence: Repetition and recursive reminding. Mem Cogn 32:336–350. [DOI] [PubMed] [Google Scholar]
- Hintzman DL (2010): How does repetition affect memory? Evidence from judgments of recency. Mem Cogn 38:102–115. [DOI] [PubMed] [Google Scholar]
- Huberle E, Lutzenberger W (2013): Temporal properties of shape processing by event‐related MEG adaptation. NeuroImage 67:119–126. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG (2004): Repetition suppression of faces is modulated by emotion. PNAS 101:9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpicke JD, Blunt JR (2011): Retrieval practice produces more learning than elaborative studying with concept mapping. Science 311:772–775. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Roediger HL (2008): The critical importance of retrieval for learning. Science 319:966–968. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE (2000): Prefrontal‐temporal circuitry for episodic encoding and subsequent memory. J Neurosci 20:6173–6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Eichele T, Hugdahl K, Nyberg L (2011): Multimodal imaging of incidental retrieval: The low route to memory. J Cogn Neurosci 23:974–960. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (1997): Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban MT editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Lawrence Erlbaum Associates; pp 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V (1998): Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 35:199–210. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (2008): International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A‐8. Gainesville, FL: University of Florida. [Google Scholar]
- Larsson J, Smith AT (2012): fMRI repetition suppression: Neuronal adaptation or stimulus expectation? Cereb Cortex 22:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TMC, Leung M‐k, Lee TMY, Raine A, Chan CCH (2013): I want to lie about not knowing you, but my precuneus refuses to cooperate. Sci Rep 3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL (2000): Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: An event‐related fMRI study. J Cogn Neurosci 12:965–976. [DOI] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL (2000): Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp 11:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Arnold KM, Gilmore AW, McDermott KB (2013): Neural signatures of test‐potentiated learning in parietal cortex. J Neurosci 33:11754–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD (2000): Working memory for letters, shapes, and locations: fMRI evidence against stimulus‐based regional organization in human prefrontal cortex. NeuroImage 11:424–446. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini PA, Ungerleider LG (2002): Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron 35:975–987. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I (2004): Functional neuroimaging studies of human emotions. CNS Spectr 9:258–266. [DOI] [PubMed] [Google Scholar]
- Postle BR, Stern CE, Rosen BR, Corkin S (2000): An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. NeuroImage 11:409–423. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Malach R, Hadar U, Graif M, Hendler T (2001): Feeling or features: Different sensitivity to emotion in high‐order visual cortex and amygdala. Neuron 32:747–757. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T (2007): Event‐related potentials and recognition memory. Trends Cogn Sci 11:251–257. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA (2002): Episodic memory retrieval: An (event‐related) functional neuroimaging perspective In: Parker A, Wilding E, Bussey T, editors. The Cognitive Neuroscience of Memory: Encoding and Retrieval. Hove: Psychology Press; pp 3–37. [Google Scholar]
- Rugg MD, Vilberg KL (2013): Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol 23:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J (2011): Emotional perception: Meta‐analyses of face and natural scene processing. NeuroImage 54:2524–2533. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: Remembering, imagining and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert K, Weber K, de Lange FP, Petersson KM, Hagoort P (2013): The suppression of repetition enhancement: A review of fMRI studies. Neuropsychologia 51:59–66. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL (2010): Patterns of brain activity supporting autobiographical memory, prospection, theory‐of‐mind, and their relationship to the default mode network. J Cogn Neurosci 22:1112–1123. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, González RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR (1996): The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93:8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam M‐M, Egner T (2008): Neural repetition suppression reflects fulfilled perceptual expectations. Nat Neurosci 11:1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis JG, Benjamin AS, Ross BH (2014): The reminding effect: Presentation of associates enhances memory for related words in a list. J Exp Psychol Gen 143:1526–1540. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S (1994): Novelty encoding networks in the human brain: Positron emission tomography data. Neuroreport 5:2525–2528. [DOI] [PubMed] [Google Scholar]
- Turk‐Browne NB, Yi D‐J, Chun MM (2006): Linking implicit and explicit memory: Common encoding factors and shared representations. Neuron 49:917–927. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998): Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science 281:1188–1191. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453. [DOI] [PubMed] [Google Scholar]
- Wangelin BC, Bradley MM, Kastner A, Lang PJ (2012): Affective engagement for facial expressions and emotional scenes: The influence of social anxiety. Biol Psychol 91:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EJ, Chun MM, Kuhl BA (2013): Repetition suppression and multi‐voxel pattern similarity differentially track implicit and explicit visual memory. J Neurosci 33:14749–14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymar M, Bradley MM, Sege CT, Lang PJ (2012): Neural activation and memory for natural scenes. Psychophysiology 49:S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL (2004): Functional‐anatomic correlates of remembering and knowing. NeuroImage 21:1337–1349. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A (1998): Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8:227–233. [DOI] [PubMed] [Google Scholar]
- Xue G, Mei L, Chen C, Lu Z‐L, Poldrack R, Dong Q (2011): Spaced learning enhances subsequent recognition memory by reducing neural repetition suppression. J Cogn Neurosci 23:1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL (2008): Multiple signals of recognition memory in the medial temporal lobe. Hippocampus 18:945–954. [DOI] [PubMed] [Google Scholar]


