Abstract
Despite modern combination antiretroviral therapy (CART), distal neuropathic pain (DNP) continues to affect many individuals with HIV infection. We evaluated risk factors for new onset DNP in the CNS Antiretroviral Therapy Effects Research (CHARTER) study, an observational cohort. Standardized, semi-annual clinical evaluations were administered at six U.S. sites. DNP was defined by using a clinician-administered instrument standardized across sites. All participants analyzed were free of DNP at study entry. New onset DNP was recorded at the first follow-up visit at which it was reported. Mixed effects logistic regression was used to evaluate potential predictors including HIV disease and treatment factors, demographics, medical comorbidities and neuropsychiatric factors. Among 493 participants, 131 (27%) reported new DNP over 2,306 visits during a median follow-up of 24 months [interquartile range (IQR) 12-42]. In multivariable regression, after adjusting for other covariates, significant entry predictors of new DNP were older age, female sex, current and past antiretroviral treatment, lack of virologic suppression, and lifetime history of opioid use disorder. During follow-up, more severe depression symptoms conferred a significantly elevated risk. The associations with opioid use disorders and depression reinforce the view that the clinical expression of neuropathic pain with peripheral nerve disease is strongly influenced by neuropsychiatric factors. Delineating such risk factors might help target emerging preventive strategies, for example, to individuals with a prior history of opioid use disorder, or might lead to new treatment approaches such as the use of tools to ameliorate depressed mood.
Introduction
Sensory neuropathy (SN) is a common cause of chronic neuropathic pain that contributes to disability, unemployment, depression, medication overuse and frequent medical provider visits. HIV frequently leads to SN and attendant distal neuropathic pain (DNP) [17; 22]. DNP typically appears first in the toes and feet and is often described with words such as “stabbing”, “burning” and “aching” [9; 36]. DNP is the most frequent source of disability in HIV-SN and often requires daily analgesics or other pain-modifying therapies. HIV-SN persists and can occur de novo even during successful (i.e., virologically suppressive) treatment with modern treatment with combination antiretroviral therapies (CART) [22]. In a previously reported, prospective, cross-sectional analysis [17] of data collected at six US academic medical centers between 2002-2007, we found prevalent HIV-associated sensory neuropathy (HIV-SN) in 881 of 1539 participants (57%). Of these, 38% reported DNP. DNP was significantly associated with disability in daily activities, unemployment, and reduced quality of life, even when rated mild in severity. Risk factors for neuropathic pain were use of specific, neurotoxic, dideoxynucleoside analogue antiretrovirals (“D-drugs”) and higher CD4 nadir. Additionally, in a previous analysis of a subset of these study participants published in Pain [37], we demonstrated that the presence of DNP and paresthesias had 95% sensitivity for detecting objective evidence of neuropathy when clinical examination was supplemented by quantitative sensory testing (QST) and nerve conduction studies (NCS). However, many individuals with HIV-SN, defined as one or more clinical signs of diminished vibration or sharp sensation in the legs and feet, or reduced ankle reflexes in a distal, symmetrical pattern, experienced no neuropathic pain. This begs the question of what differentiates individuals who do and do not experience DNP, given that they have the same evidence of injury to distal peripheral nerves.
Factors that predict new onset DNP in HIV are unknown. Neuropsychiatric conditions such as substance use and mood disorders have been shown to influence the prevalence and incidence of neuropathic pain in other diseases [27; 31]. Thus we wished to evaluate their impact on new onset DNP in HIV, particularly with modern treatments. Additionally, based on previous findings and known risk factors for concurrent DNP, we expected that other significant predictors of new onset DNP would include HIV disease and treatment factors (nadir CD4 [39], viral load, exposure to neurotoxic antiretrovirals [12; 33]), comorbidities (diabetes mellitus [21], hepatitis C virus infection [20], hypertriglyceridemia [7] and demographic factors (age, sex, stature) [11].
Methods
Study Design
CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is a prospective, cross-sectional and longitudinal, observational cohort study designed to examine the effects of HIV and antiretroviral (ARV) therapy on the nervous system, conducted at six US academic sites. Institutional Review Boards at each site approved this research and each participant provided written informed consent. Data were collected according to a protocol of comprehensive neuromedical, neurobehavioral, psychiatric, and laboratory assessments that were standardized across sites. Participants completed evaluations at study entry and follow-up visits every six months.
Study Participants
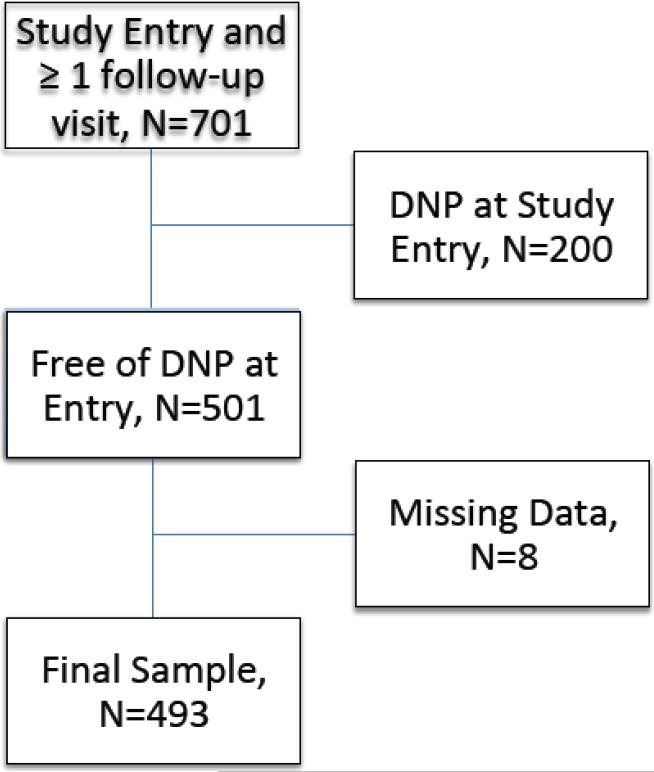
All participants were ambulatory and underwent evaluation in outpatient research centers. Eligibility criteria included the ability to undergo a structured clinical interview to provide details of CART use and a standardized examination for symptoms and signs of HIV-SN. The CHARTER Study inclusion criteria were broad; individuals were excluded only for active opportunistic infections, uncontrolled major psychiatric disorders such as schizophrenia or inability to cooperate with the clinical evaluation. Comorbidities such as HCV infection and substance use disorders were permitted. Figure 1 presents a flow chart explaining how participants were selected for this analysis. Of the 1,583 participants enrolled, 701 had at least one follow-up visit. Of these, 501 were free of DNP at study entry. After excluding five additional participants with missing data on one or more variables, 493 participants enrolled between September 4, 2003, and January 14, 2010 remained in the analysis.
Figure 1.
Flow diagram showing selection of cases for this analysis
Clinical Evaluations for HIV Sensory Neuropathy
Clinicians including physicians and nurses were trained to perform and document a standardized, targeted interview and examination to capture HIV-SN signs and symptoms. Distal neuropathic pain (DNP), the primary outcome in this analysis, was defined by a standardized clinician-administered assessment [17; 28; 37] and recorded on a case report form. For analysis, DNP severity grades were collapsed into a single, dichotomous outcome, present or absent, based on our previous work in which we showed that even DNP rated by clinicians as “mild” was associated with a reduced quality of life, disability in daily activities, and unemployment [17]. DNP was considered present based on self-report whether or not the clinical examination found signs of SN. Because neuropathic pain in HIV is known to wax and wane in severity [38], and may even remit and then recur, we also asked participants to estimate the duration of their DNP in spans of time: 4 days to 4 weeks, 1 month to 1 year, 1 to 10 years, greater than 10 years. SN signs were bilateral diminished vibratory sensation at the great toes, bilateral reduced sharp-dull discrimination in the feet and toes and reduced or absent ankle reflexes. Symmetric bilateral presence of at least one sign was required as evidence of SN.
HIV Disease and Treatment Characteristics
The following clinical correlates and risk factors for DNP were evaluated by using interviews and laboratory assessments where appropriate. Self-reported details of past and current antiretroviral (ARV) use were captured on a structured case report form. CART and use of specific, neurotoxic, dideoxynucleoside analogue antiretrovirals (“D-drugs”) were classified as current, past, or never used. Nonfasting blood samples were collected by venipuncture. Blood was used to quantify plasma HIV viral load (VL) by reverse transcription polymerase chain reaction ultrasensitive assay (nominal lower quantitation limit, 50 copies/mL [Amplicor; Roche Diagnostic Systems, Indianapolis, Indiana]). In this cohort, all participants with undetectable plasma VL took CART and nearly all participants off CART had detectable plasma VLs. Current CD4 levels were measured by flow cytometry within 1 week of the clinical assessment. Nadir CD4 levels were collected by self-report, as previously validated [15; 25]. After clotting, serum was separated and sent to a CLIA (Clinical Laboratory Improvement Amendments of 1988; http://wwwn.cdc.gov/clia) -certified laboratory for measurement of triglycerides and glucose. Type 2 diabetes mellitus was recorded if diagnosed by primary care physicians or by self-report of use of anti-diabetic medications.
Psychiatric Measures
The Beck Depression Inventory-II [BDI-II; [8]] is a standardized questionnaire that rates severity of depressive symptoms during the previous 14 days. The scores were categorized into four groups, as follows: 0-13 (no depression); 14-19 (mild depression); 20-28 (intermediate depression); 29-63 (severe depression). In addition, past and current DSM-IV diagnoses of major depressive disorder (MDD) and substance use disorders (abuse and dependence) were captured using the Composite International Diagnostic Interview (CIDI), a fully structured, lay-administered instrument [45]. Additional details on these measures are described in [25].
Statistical analyses
Mixed effects logistic regression models, a subclass of the generalized linear mixed models (GLMM)[14], were applied to evaluate predictors of new onset DNP. Because neuropathic pain may remit and recur, GLMM was deemed more appropriate than survival analysis. GLMM is an extension of linear regression that allows for non-normally distributed responses and both fixed and random effects in the linear predictors. In our case we assumed a binomial distribution for the binary outcome, and a logit link function, conditional on the subject-specific random effects. Predictor variables were modeled as fixed effects. The approach incorporated a random intercept that modeled risk differences among different individuals, plus a shared slope, representing the change in risk over time in the entire sample. Since all participants were pain-free at study entry, the pain outcomes were considered from the first visit (month 6) on.
We evaluated two classes of DNP predictors: those measured at study entry, as well as time-dependent predictors assessed during follow-up. Predictors measured at study entry were hypothesized to reflect cumulative past markers of nerve injury and psychiatric history up to the time of study entry, while time-dependent variables reflected the success of ongoing antiretroviral treatment and concurrent mood state. Pre-specified study entry predictors were the presence or absence of neuropathy signs, CART use, including separate variables for study entry and past exposure to D-drugs; HIV disease markers (plasma VL, self-reported nadir CD4 and measured study entry CD4); hepatitis C virus (HCV) serology; and study entry and past MDD. These same variables, with the exception of past CART use and past MDD, were reassessed at follow-up visits and coded as time-dependent covariates. VLs were log10 transformed for analysis and were analyzed both continuously and categorically (undetectable, ≤ 50 c/ml vs. detectable, >50 c/ml).
Univariable Analysis
For each predictor two random-intercept mixed effects logistic regression models were fit, one with additive time and predictor effects on DNP, and the other also including an interaction between the predictor and time. The likelihood ratio test was used to determine which of the two was the better model for each predictor. Furthermore, the likelihood ratio test was used to conclude whether or not the covariate was a significant predictor of pain in the additive model. CD4 variables were dichotomized at a clinically relevant cut point (e.g., CD4 < 200 versus >=200). Each odds ratio and its confidence interval indicate the extent to which the relevant predictor increases or decreases the likelihood of DNP, and its significance. Predictors with likelihood ratio p-values less than 0.05 were classified as significant.
Multivariable Analysis
All covariates that had a p-value of 0.10 or lower in univariable analyses were entered into a multivariable model, which constituted the initial model. Backwards model selection was then performed and one covariate was removed at a time, using the Akaike information criterion. Interaction terms were kept in the model only if significant at the 0.05 level. Adjusted odds ratios and 95% confidence intervals using the Wald test were computed.
Sensitivity Analyses
To test of the robustness of our findings to model assumptions, we performed 3 sensitivity analyses under conditions that varied the criteria for being at risk or reaching the endpoint of new onset DNP. First, to evaluate the possibility that mild pain was responsible for the significance of the regression predictors, we performed a secondary analysis in which the endpoint was moderate or severe DNP (versus any DNP). Second, to determine if individuals without clear evidence of neuropathy were responsible for the significance of the regression predictors, we limited the analysis to the subset of individuals who also had clinical exam findings consistent with neuropathy (bilaterally reduced distal vibration, reflexes, pin sensation) at the time they developed DNP. A third and final check on robustness limited the at risk pool to individuals with signs of neuropathy and also required that they have pain of at least moderate severity.
Results
Study entry characteristics of the 493 initially DNP-free study participants are provided in Table 1. Participants were mostly male (81%) and mostly either African-American (44%) or Caucasian (42%). The average age was 42 years (range, 18 to 66). The median nadir CD4 was 192 (interquartile range [IQR] 52 to 342). Most participants (68%) were prescribed CART. Of those on CART, 201 (60%) had an undetectable plasma VL, and the median current CD4 was 442 (IQR 290-639) cells/mm3. Among the 156 not taking CART, 106 were CART naïve and the remainder had some prior exposure to ART but had discontinued it. As expected, CART naïve participants had higher nadir CD4 counts than those taking CART and those who had discontinued CART (median [IQR] 400 [300, 559] for CART naive vs. 117 [25, 219] for currently using CART and 289 [162, 410] for discontinued CART). One third (167; 34%) had a history of prior exposure to potentially neurotoxic drugs (stavudine, didanosine); 71 (14%) took these medications at entry. Comorbid diabetes mellitus was diagnosed in 39 participants (8%) and hepatitis C virus seropositivity in 125 (25%). A lifetime history of substance use disorder (abuse or dependence) by DSM-IV criteria was present for 356 (73%) participants. Among those meeting criteria for lifetime disorders, most were remote (333; 94%) rather than current (in the last 30 days; N=23; 6%). Alcohol use disorders were the most common (270, 55%), followed by cocaine (40%), methamphetamine (18%) and opioids (17%). Among the 131 participants with a history of opioid use disorders, 13 (9.9%) took opioid medications at study entry. The median study entry BDI-II score was 9 (IQR 4 to 18), indicating minimal current depressive symptomatology on average.
Table 1.
Study entry demographic and clinical characteristics of study participants according to whether or not they subsequently reported DNP. P-values listed compare participants with and without new onset DNP.
| All Participants | No New Pain | With New DNP | p-value | |
|---|---|---|---|---|
| N | 493 | 362 | 131 | new vs. not |
| Age, mean (SD) | 42.4 (8.6) | 41.8 (8.8) | 44.0 (7.7) | 0.012 |
| Male, No. (%) | 400 (81%) | 301 (83%) | 99 (76%) | 0.068 |
| Ethnicity, No. (%) | 0.640 | |||
| Black | 217 (43.8%) | 161 (44%) | 56 (43%) | |
| White | 209 (42.3%) | 148 (41%) | 61 (47%) | |
| Hispanic | 52 (11%) | 41 (11%) | 11 (8%) | |
| Other | 15 (3.0%) | 12 (3%) | 3 (2%) | |
| Height (inches), mean (SD) | 68.8 (3.8) | 68.9 (3.6) | 68.5 (4.2) | 0.300 |
| CART use, No. (%) | 0.002 | |||
| Current | 337 (68%) | 244 (67%) | 93 (71%) | |
| Naïve | 106 (22%) | 89 (25%) | 17 (13%) | |
| Past | 50 (10%) | 29 (8%) | 21 (16%) | |
| D-drug use, No. (%) | 0.0 94 | |||
| Current | 71 (14%) | 52 (14%) | 19 (15%) | |
| Naïve | 255 (52%) | 197 (54%) | 58 (44%) | |
| Past | 167 (34%) | 113 (31%) | 54 (41%) | |
| CD4 nadir, median (IQR), cells/uL | 192 (52-342) | 199 (54-34) | 158 (39-297) | 0.100 |
| CD4 current, median (IQR), cells/uL | 442 (290-641) | 441 (295-643) | 458 (267-634) | 0.300 |
| Plasma VL, median (IQR), log10 copies/mL | 2.28 (1.70-3.99) | 2.30 (1.70-4.02) | 2.14 (1.70-3.87) | 0.620 |
| HCV seropositive, No. (%) | 125 (25%) | 85 (23%) | 40 (31%) | 0.130 |
| Diabetes, No. (%) | 39 (8.0%) | 30 (8%) | 9 (7%) | 0.710 |
| Triglycerides, median (IQR) | 129 (92-187) | 132 (92-187) | 128 (94-178) | 0.850 |
| BDI-II, median (IQR) | 9 (4-18) | 8 (3-16) | 12 (6-21) | 0.002 |
| Lifetime Substance Use Disorder-Any, No. (%) | 356 (73%) | 252 (70%) | 104 (79%) | 0.040 |
| Alcohol | 270 (55%) | 191 (53%) | 79 (60%) | 0.180 |
| Methamphetamine | 89 (18%) | 66 (18%) | 23 (18%) | 0.900 |
| Cocaine | 197 (40%) | 138 (38%) | 59 (45%) | 0.210 |
| Opioids | 85 (17%) | 53 (15%) | 32 (24%) | 0.015 |
Among the 493 participants initially free of pain, 131 (27%) participants reported new DNP over a median follow-up of 24 months [IQR 12-42] (1,961 visits). Among these 131, only 1 (0.76%) took opioid analgesics at the new onset visit. The rate of new DNP in the entire cohort was 16.4 per 100 person-years of follow-up (PYFU); among those on CART with undetectable VL at study entry, the rate was 14.8 per 100 PYFU. The duration of DNP reported at the new onset visit was more than one year in 59 participants (45%), one month to one year in 59 (45%) and less than one month for 13 (10%). Of the 131 participants with new DNP, 85 (65%) had one or more signs of neuropathy (symmetric distal reduction in reflexes or in pin or vibration sensation) at the new DNP visit.
Table 2 summarizes the results of univariable analyses for selected predictor variables according to those for which time interactions were not significant (Table 2a) and those for which time interactions were significant (Table 2b). Significant variables in Table 2 were then carried forward (as described above in Methods) into the multivariable analysis presented in Table 3.
Table 2a.
Univariable mixed effects logistic regression analysis for variables predicting new onset DNP without time interaction. All variables are measured at study entry unless otherwise specified. N=493 subjects, 2306 visits.
| Covariate | Level | OR | 95% CI | LRT P-value |
|---|---|---|---|---|
| Age | per yr | 1.051 | (1.013, 1.091) | 0.0078 |
| Gender | Women | Ref | 0.048 | |
| Men | 0.47 | (0.22, 0.989) | ||
| Ethnicity | White | Ref | 0.54 | |
| Black | 0.71 | (0.37, 1.37) | ||
| Hispanic | 0.48 | (0.16, 1.48) | ||
| Other | 0.76 | (0.12, 4.98) | ||
| CART Use at Study Entry | Never | Ref | 0.00 043 | |
| Current | 3.34 | (1.41, 7.89) | ||
| Past | 9.02 | (2.90, 28.04) | ||
| D-drug Use | Never | Ref | 0.13 | |
| Current | 1.064 | (0.36, 3.11) | ||
| Past | 1.84 | (0.981, 3.44) | ||
| CD4 < 200 at Study Entry | >= 200 | Ref | 0.47 | |
| < 200 | 1.36 | (0.59, 3.13) | ||
| Nadir CD4 < 200 | >= 200 | Ref | 0.32 | |
| < 200 | 1.33 | (0.75, 2.37) | ||
| Plasma HIV VL at Study Entry | Undetectable | Ref | 0.84 | |
| Detectable | 1.066 | (0.57, 2.01) | ||
| Time-updated Detectable Plasma HIV VL | Undetectable | Ref | 0.053 | |
| Detectable | 1.49 | (0.9928, 2.25) | ||
| Hepatitis C Serostatus | Negative | Ref | 0.19 | |
| Positive | 1.55 | (0.81, 2.95) | ||
| Diabetes Mellitus | No DM2 | Ref | 0.95 | |
| DM2 | 0.961 | (0.31, 2.98) | ||
| Triglycerides | per unit | 0.99903 | (0.9954, 1.0027) | 0.6 |
| Time-updated Depressive Symptoms (BDI-II) | 0-13 | Ref | 0.0091 | |
| 14-19 | 1.62 | (0.919, 2.85) | ||
| 20-28 | 1.89 | (1.047, 3.41) | ||
| 29-63 | 3.3 | (1.58, 6.88) | ||
| Lifetime History of Any Substance | No | Ref | 0.036 | |
| Abuse/Dependence | ||||
| Yes | 2.13 | (1.033, 4.41) | ||
| Alcohol: History of Ab/Dep | No | Ref | 0.085 | |
| Yes | 1.73 | (0.923, 3.23) | ||
| Methamphetamine: History of Ab/Dep | No | Ref | 0.66 | |
| Yes | 0.83 | (0.37, 1.89) | ||
| Cocaine: History of Ab/Dep | No | Ref | 0.12 | |
| Yes | 1.64 | (0.88, 3.07) | ||
| Opioids: History of Ab/Dep | No | Ref | 0.008 | |
| Yes | 2.87 | (1.31, 6.28) |
Table 2b.
Univariable mixed effects logistic regression analysis with significant time interaction
| Covariate | Level | Effect at 1 year: OR (95% CI) | Yearly Change: OR (95% CI) | Interaction p-value |
|---|---|---|---|---|
| Current CART Use | Never | Ref | 1.80 (1.095, 2.95) | 0.038 |
| Current | 3.58 (1.21, 10.63) | 1.0043 (0.90, 1.13) | ||
| Past | 4.73 (1.42, 15.76) | 0.82 (0.58, 1.18) | ||
| Current CD4 < 200 | >= 200 | Ref | 1.053 (0.939, 1.18) | 0.043 |
| < 200 | 1.98 (0.989, 3.95) | 0.77 (0.57, 1.030) | ||
| Height | 66in (25th %-ile) | 1.39 (1.087, 1.77) | 0.919 (0.81, 1.045) | 0.0024 |
| 69in (50th %-ile) | Ref | 1.048 (0.938, 1.17) | ||
| 71in (75th %-ile) | 0.80 (0.68, 0.946) | 1.14 (1.0021, 1.30) | ||
| HIV Duration | 48mo (25th %-ile) | 0.74 (0.55, 1.015) | 1.26 (1.013, 1.56) | 0.0079 |
| 120mo (50th %-ile) | Ref | 1.082 (0.944, 1.24) | ||
| 180mo (75th %-ile) | 1.28 (0.988, 1.65) | 0.953 (0.85, 1.075) | ||
| Depressive Symptoms at Study Entry | BDI-II 0-13 | Ref | 1.16 (1.0076, 1.34) | 0.022 |
| BDI-II 14-19 | 3.36 (1.30, 8.70) | 0.76 (0.56, 1.016) | ||
| BDI-II 20-28 | 3.33 (1.36, 8.11) | 1.0072 (0.79, 1.28) | ||
| BDI-II 29-63 | 3.07 (0.975, 9.70) | 0.81 (0.59, 1.103) |
BDI-II, Beck Depression Inventory, Second Edition.
* Covariates with LRT p-value < 0.10. ~ Covariates not significant. Ref: reference level (OR=1)
Table 3.
Multivariable predictors of new onset DNP in the mixed effects logistic regression. Covariate effects are adjusted for other variables in the model. No covariate-time interactions were significant. N=493 subjects, 2306 visits.
| Covariate Effect | ||||
|---|---|---|---|---|
| Covariates1 | Covariate Levels | OR | 95 % CI | p |
| Time* | NA | 0.992 | (0.88, 1.12) | 1.000 |
| Age | Per year | 1.037 | (1.00, 1.08) | 0.064 |
| Gender | Male vs Female | 0.47 | (0.22, 0.980) | 0.150 |
| Plasma VL at entry | Undetectable | Ref 2 | -- | 0.038 |
| Detectable | 1.54 | (1.007, 2.35) | -- | |
| CART Use at study entry | Never Used | Ref 2 | -- | 0.0072 |
| Current | 2.44 | (1.026, 5.80) | -- | |
| Past Use | 4.59 | (1.51, 13.97) | -- | |
| Lifetime history of Opioid Abuse/ Dependence | No | Ref 2 | -- | -- |
| Yes | 2.31 | (1.078, 4.95) | 0.014 | |
| Time-updated Severity of depressive symptomatology | BDI-II 0-13 | Ref 2 | -- | 0.0017 |
| BDI-II 14-19 | 1.66 | (0.922, 3.00) | -- | |
| BDI-II 20-28 | 1.89 | (1.026, 3.49) | -- | |
| BDI-II 29-63 | 2.99 | (1.41, 6.31) | -- | |
All covariates entered into this model were significant (α = 0.15) in the univariable analysis
Odds ratios calculated by comparison to a reference group comprising individuals on CART with undetectable plasma VL at study entry and all follow-up visits (i.e., optimally treated), without depressed mood at study entry and having no history of substance abuse or dependence.
ref: reference level (OR=1)
BDI-II, Beck Depression Inventory, Second Edition.
Multivariable Analysis
Table 3 shows the final selected multivariable model. No variables interacted with time. Significant predictors of new onset DNP after adjusting for other variables in the model were current (OR 2.44 versus naive) and past (OR 4.59 versus naive) CART use at study entry, detectable plasma viral load during follow-up (OR 1.54 versus undetectable), lifetime history of opioid use disorders (OR 2.31 versus no opioid use disorder) and more severe depression symptoms at follow-up (OR 1.66, 1.89, and 2.99 for mild, intermediate, and severe depression versus no depression). Participant age and sex were retained in the model based on the AIC criterion; older age had higher risk than younger age (OR 1.20 for 50 years versus 45 years, p-value = 0.064); men (81% of the cohort) had lower risk than women, at a p-value (0.15). Variables not included in the multivariable model were race/ethnicity, history of d-drug use, current and nadir CD4, estimated duration of HIV infection at study entry, hepatitis C serostatus, diabetes mellitus, serum triglycerides [7; 43], and lifetime history of alcohol or other non-opioid substance use disorders.
Sensitivity analyses
To test of the robustness of our findings, as described in Methods we performed 3 sensitivity analyses under conditions that varied the criteria for being at risk or reaching the endpoint of new onset DNP. In both the primary and the 3 sensitivity analyses, a past history of opioid use disorder remained significant as a predictor of new onset DNP. Additionally, age remained significant in all models. Time-updated depression severity as measured by the BDI was significant in all sensitivity analyses except the third, and most stringent, where power was reduced due to the smaller number of endpoints reached.
Discussion
We observed new onset DNP -- defined as bilateral distal leg and foot pain with neuropathic qualities in participants who did not report this at study entry -- in one quarter of HIV-infected individuals over an average of 2 years. Independent of other risk factors, older age was associated with higher rates of new DNP. Since DNP is the principal reason for seeking medical attention among patients with HIV-SN, and since pain contributes significantly to reduced quality of life [17], and to medical costs [26], our findings have substantial implications for an aging population of HIV-infected men and women on long-term ART.
Neuropsychiatric factors, specifically opioid use disorders and worsening depressed mood, were associated with increased rates of new DNP. Sensitivity analyses demonstrated that these risk factors were robust to model assumptions, since they remained significant when limited only to individuals with clear clinical evidence of sensory neuropathy and when the endpoint was limited to those moderate or severe DNP. DNP was associated with past opioid abuse, rather than recent use of opioids. This implies that new onset DNP reflects a shared vulnerability, perhaps mediated by brain reward circuits. Indeed, reciprocal projections between the nucleus accumbens, a key brain reward hub, and prefrontal cortex subserve important aspects of both pain and addiction [2; 4; 6; 18]. For example, opioid addiction is associated with increased dopaminergic tone between the ventral tegmental area and nucleus accumbens, leading to long-term downregulation of dopamine receptors in the accumbens and enhanced glutamatergic transmission in prefrontal-accumbens pathways [5]. These same circuits process emotional and cognitive aspects of sensory information [1; 35]. Thus in individuals with SN and a past history of opioid abuse, aberrant sensory input from damaged peripheral nerves might be processed differently by brain reward circuits in vulnerable individuals, predisposing to the clinical phenotype of DNP.
We found also that during follow-up worse depressive symptoms predicted new DNP. This was not simply due to depressed individuals having poorer antiretroviral medication adherence [16; 32], since multivariable regression showed that depression remained significant after adjusting for adherence. Previous studies have demonstrated that, regardless of the cause of the underlying pain state, pain and depression are reciprocally reinforcing [3; 13; 26]. In depression, altered brain activity in the insular cortex may impair the ability to modulate pain experience [41; 42] leading to allodynia, the experience of pain in response to stimuli that are not ordinarily painful.
HIV disease and treatment status significantly affected new DNP rates in this study. Thus the highest rate occurred in a relatively small group of individuals (N=50) who had prior CART exposure, but had discontinued antiretroviral therapy. These individuals had a history of more advanced immunosuppression, as indexed by lower nadir CD4 counts, than ART-naïve participants. Also they had lower current CD4s than those with virologic suppression on CART. The lowest rate was seen in ARV naïve individuals, likely reflecting their less advanced HIV disease. Among those taking CART, rates of new DNP were intermediate between the two previously mentioned groups. Additionally, individuals with detectable plasma HIV viral loads at entry had a higher risk of new DNP. Since 30 to 40% of HIV-infected persons on CART in the U.S. do not maintain durable virologic suppression due to transfer of care, poor antiretroviral adherence, and other factors [24], our findings suggest that DNP will be a persistent or increasing clinical problem.
Overall these findings indicate that the determinants of new onset DNP in the current treatment era are different from those for DNP as described in previous studies. For example, whereas in previous studies CD4 nadir was a robust correlate of DNP [17; 40], we did not find it to correlate with new onset DNP in the present study. Also, previous studies have demonstrated that exposure to neurotoxic antiretroviral agents such as stavudine increased the risk of DNP [12; 33], but we did not find such exposure to predict new DNP in the current era. In previous studies taller stature [11] and comorbid diabetes mellitus [30] correlated with DNP. Although height was a significant predictor in our univariable analysis, it was no longer significant when the multivariable analysis adjusted for other predictors. Although we did not find diabetes mellitus to be a significant predictor, the number of diabetics in this cohort was relatively small (39; 7.8%). Alcohol is a peripheral neurotoxin that can cause DNP; nutritional deficiencies are known to amplify alcohol's neurotoxicity [10; 29]. Although we did not observe an association between alcohol use disorders and DNP, the magnitude of alcohol-related effects may have been attenuated in these community dwelling U.S. participants in care who were unlikely to suffer from substantial nutritional deficiencies.
This study has several limitations. Clinicians were trained to assess whether subjects' pain was likely to be neuropathic by virtue of the use of descriptors such as “stabbing”, “aching” and “burning”. These particular descriptors were chosen from a much longer list of adjectives often used by individuals with pain due to distal neuropathy. Although these terms were presented as guidelines, rather than formal definitions, the limitation to this particular short list of descriptors was otherwise expedient and it is possible that under-ascertainment of neuropathic occurred, since some patients use alternative descriptors. All prospective cohort studies are subject to bias related to factors not observed or measured. A strength of the present analysis was its extensive characterization of disease and treatment indicators and comorbid conditions. Nevertheless it remains possible that important factors were not assessed. In our study, some of those with incident DNP did not have objective findings of distal sensory loss or reduced tendon reflexes. However, the clinical neurological examination is incompletely sensitive to the presence of neuropathy, and in clinical practice neuropathy is frequently diagnosed by using more sensitive measures of nerve function such as quantitative sensory testing (QST) and nerve conduction studies (NCS). One reason for the lesser sensitivity of the clinical examination is that it does not adequately assess the function of small fibers, including C and delta fibers, which subserve pain perception [19]. Ancillary testing improves sensitivity to small fiber injury. While ancillary testing is prohibitively expensive to carry out in a large cohort such as this one, we did perform QST and NCS on a subset of these same study participants. In a previous analysis published in Pain [37], we demonstrated that the presence of DNP and paresthesias had 95% sensitivity for detecting objective evidence of neuropathy with such tests. Indeed, early reviews that included autopsy studies found evidence of peripheral nerve damage in more than half of those dying with AIDS — even though only 40% had symptoms [23; 44]. Notwithstanding these considerations, we performed a sensitivity analysis limiting the sample at risk to those who did show signs of neuropathy on examination. This sensitivity analysis demonstrated that the predictors were robust to model assumptions.
It might be argued that DNP in some of our subjects was relatively mild therefore of questionable clinical significance. However, we have previously shown in this same cohort that DPN intensity is not as strongly related to quality of life as is commonly assumed [28]. Thus even mild pain was associated with substantial distress and reductions in life quality. To further address the concern that milder degrees of DNP were “driving” the observed relationships, we re-analyzed our data after excluding cases with clinician-rated “mild” DNP. In the remaining subjects with moderate or severe DNP, we found the same significant predictors.
Neuropathic pain can wax and wane in severity [38]. Also, DNP can remit, either spontaneously or as a result of treatment, and might even recur at a later time. The present study was not designed to distinguish between recurrent and incident DNP. However, 90% of participants reported that the duration of their neuropathic pain was at least one month, and almost half indicated that it had been present for longer than a year. Future work is needed to develop instruments to carefully discriminate between recurrent and incident neuropathic pain.
Characterizing predictors of new DNP is important since some of these individuals will ultimately transition to chronic pain, a condition which affects tens of millions of people in the United States, is often refractory to medical therapy, and is associated with disability, reduced quality-of-life, high health care costs and premature death. Determining the rate at which new DNP still occurs in individuals who are growing older on antiretroviral therapy is important to estimate the likely burden of chronic pain for the US healthcare system. Future studies might address prevention strategies to reduce new DNP among individuals at high-risk, such as those with opioid use disorders or depression. A recent opinion piece highlighted the tension between the challenges of chronic pain and addiction[34]. This paper and ours together point to the dilemma faced by clinicians managing DNP of whether to use opiates for pain in patients with a prior history of opioid abuse. Imaging of brain structure and function as well as characterization of peripheral nerve function in participants before and after new HIV DNP might help to clarify brain influences on DNP and identify potential new therapeutic targets.
Supplementary Material
Summary.
Key predictors of new onset distal neuropathic pain in HIV+ individuals during follow-up were past opioid use disorders and worsening depression symptoms.
Acknowledgements
The authors wish to acknowledge the contributions of Nicole Duarte, Ph.D., who administered the standardized psychiatric evaluations for mood disorders and substance abuse.
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER; https://www.charterresource.ucsd.edu) is supported by awards N01 MH22005, HHSN271201000036C and HHSN271201000030C from the National Institutes of Health.
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D.; Laboratory and Virology Component: Scott Letendre, M.D. (Co-P.I.), Davey M. Smith, M.D. (Co-P.I.).; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Matthew Dawson; Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J Taylor, Ph.D., Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D.; Johns Hopkins University Site: Justin McArthur (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Kaori Phillips, B.S.N.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
Dr. Vaida receives ongoing research support from NIH P30 MH62512, NIH P50 DA26306, NIH R01 MH083552, NIH R01 AI47033, NIH U01 AI74521, NIH R01 MH085608, HHSN271201000030C, HHSN271201000036C, Precision Photonics Corporation AI068543. Dr. Vaida has also served on a data safety and management board for Ardea Biosciences, Inc.
Dr. Atkinson is a consultant for Eli Lilly Pharmaceuticals, and is funded by the Department of Veterans Affairs, and NIH grants 2 P30 MH62512-06; 5P01DA012065-09; R01 MN73419; R01 MH61146;
Dr. Simpson receives research support from the NIH (NINDS and NIMH). He provided consultancy to GlaxoSmithKline and Gilead.
Dr. Marra receives research support from the NIH (NINDS and NIMH). She receives royalties from Lippincott Williams and Wilkins and from UptoDate.
Dr. Clifford is supported by NIH grants NS077384; AI69495; DA022137; HHSN271201000036C; NR012907; Alzheimer Association; He has also received research support from Lilly, Roche, Pfizer, Bavarian Nordic, and Biogen. In addition, Dr. Clifford has provided scientific advisory or consulting to Amgen, Biogen Idec, Drinker, Biddle and Reath (PML Consortium Scientific Advisory Board), Quintiles, Roche, Genentech, Novartis, GlaxoSmithKline, Millennium, Bristol Meyers Squibb, Genzyme, and Pfizer.
Dr. Gelman receives support for NIH Grants U24MH100930-01, R01NS079166, R01NS072005, 1R01MH101017, and HHSN271201000036C.
Dr. Grant receives ongoing research support from NIH P30 MH62512, NIH P50 DA26306, NIH P01 DA12065 NIH U01 MH83506, NIH R01 MH78748, NIH R01 MH83552, NIH/University of Nebraska P01 DA026146, HHSN271201000030C and, HHSN271201000036C. He has also received honoraria from Abbott Pharmaceuticals as part of their Educational Speaker Program.
Dr. Ellis received consultant fees from NeurogesX and is funded by NIH grants R01MH058076, U01MH83506, P30MH62512, R01MH83552, P50DA26306, R01MH095621, 2U01NS32228, and HHSN271201000036C.
Footnotes
Conflicts of Interest
Ms. Malvar has no conflicts.
Ms. FitzSimons-Sanders has no conflicts.
Dr. Duarte has no conflicts.
Mr Bohannon has no conflicts.
Dr. Keltner has no conflicts.
Dr. Robinson-Papp has no conflicts.
References
- 1.Ahn S, Phillips AG. Dopaminergic correlates of sensory-specific satiety in the medial prefrontal cortex and nucleus accumbens of the rat. J Neurosci. 1999;19(19):RC29. doi: 10.1523/JNEUROSCI.19-19-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S. Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav. 2013;112:34–41. doi: 10.1016/j.pbb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890–897. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci. 2013;33(41):16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee S, McCutchan JA, Ances BM, Deutsch R, Riggs PK, Way L, Ellis RJ. Hypertriglyceridemia in combination antiretroviral-treated HIV-positive individuals: potential impact on HIV sensory polyneuropathy. AIDS. 2011;25(2):F1–6. doi: 10.1097/QAD.0b013e328341dd68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 9.Belgrade MJ. Following the clues to neuropathic pain. Distribution and other leads reveal the cause and the treatment approach. Postgrad Med. 1999;106(6):127–132, 135-140. doi: 10.3810/pgm.1999.11.770. [DOI] [PubMed] [Google Scholar]
- 10.Bosch EP, Pelham RW, Rasool CG, Chatterjee A, Lash RW, Brown L, Munsat TL, Bradley WG. Animal models of alcoholic neuropathy: morphologic, electrophysiologic, and biochemical findings. Muscle Nerve. 1979;2(2):133–144. doi: 10.1002/mus.880020208. [DOI] [PubMed] [Google Scholar]
- 11.Cherry CL, Affandi JS, Imran D, Yunihastuti E, Smyth K, Vanar S, Kamarulzaman A, Price P. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology. 2009;73(4):315–320. doi: 10.1212/WNL.0b013e3181af7a22. [DOI] [PubMed] [Google Scholar]
- 12.Cherry CL, McArthur JC, Hoy JF, Wesselingh SL. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol. 2003;26(2):195–207. doi: 10.1016/s1386-6532(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 13.Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med. 2005;35(9):1275–1282. doi: 10.1017/S0033291705004952. [DOI] [PubMed] [Google Scholar]
- 14.Diggle P. Analysis of longitudinal data. Oxford University Press; Oxford ; New York: 2002. [Google Scholar]
- 15.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 17.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67(5):552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27. doi: 10.1016/j.pneurobio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N, Lewis RA, Low PA, Fisher MA, Herrmann D, Howard JF, Lauria G, Miller RG, Polydefkis M, Sumner AJ. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1(1):14–22. doi: 10.1016/j.pmrj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Estanislao LB, Morgello S, Simpson DM. Peripheral neuropathies associated with HIV and hepatitis C co-infection: a review. AIDS. 2005;19(Suppl 3):S135–139. doi: 10.1097/01.aids.0000192082.41561.49. [DOI] [PubMed] [Google Scholar]
- 21.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB. Peripheral Neuropathy in HIV: prevalence and risk factors. AIDS. 2011 doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25(7):919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin JW, Crawford TO, Tyor WR, Glass JD, Price D, Cornblath DR, McArthur JC, Griffin JW, et al. Predominantly sensory neuropathy in AIDS: distal axonal degeneration and unmyelinated fiber loss. Neurology. 1991;41(3(suppl 1)):374. [Google Scholar]
- 24.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, Gray KM, Cohen SM, Mermin J, Skarbinski J. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 25.Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IOM . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 27.Jain R, Jain S, Raison CL, Maletic V. Painful diabetic neuropathy is more than pain alone: examining the role of anxiety and depression as mediators and complicators. Curr Diab Rep. 2011;11(4):275–284. doi: 10.1007/s11892-011-0202-2. [DOI] [PubMed] [Google Scholar]
- 28.Keltner JR, Vaida F, Ellis RJ, Moeller-Bertram T, Fitzsimmons C, Duarte NA, Robinson-Papp J, Dworkin RH, Clifford DB, McArthur JC, Simpson DM, Collier AC, Marra CM, Atkinson JH, Grant I. Health-Related Quality of Life ‘Well-Being’ in HIV Distal Neuropathic Pain is More Strongly Associated with Depression Severity than with Pain Intensity. Psychosomatics. 2012;53(4):380–386. doi: 10.1016/j.psym.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koike H, Sobue G. Alcoholic neuropathy. Curr Opin Neurol. 2006;19(5):481–486. doi: 10.1097/01.wco.0000245371.89941.eb. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40(1):148–157. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- 31.Lucey BP, Clifford DB, Creighton J, Edwards RR, McArthur JC, Haythornthwaite J. Relationship of depression and catastrophizing to pain, disability, and medication adherence in patients with HIV-associated sensory neuropathy. AIDS Care. 2011;23(8):921–928. doi: 10.1080/09540121.2010.543883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, Grant I, The Hnrc G, The Tmarc G. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 2012 doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RD, Wong WM, Keruly JC, McArthur JC. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS. 2000;14(3):273–278. doi: 10.1097/00002030-200002180-00009. [DOI] [PubMed] [Google Scholar]
- 34.Olsen Y, Sharfstein JM. Chronic Pain, Addiction, and Zohydro. N Engl J Med. 2014 doi: 10.1056/NEJMp1404181. [DOI] [PubMed] [Google Scholar]
- 35.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 36.Paice JA, Ferrans CE, Lashley FR, Shott S, Vizgirda V, Pitrak D. Topical capsaicin in the management of HIV-associated peripheral neuropathy. J Pain Symptom Manage. 2000;19(1):45–52. doi: 10.1016/s0885-3924(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 37.Robinson-Papp J, Morgello S, Vaida F, Fitzsimons C, Simpson DM, Elliott KJ, Al-Lozi M, Gelman BB, Clifford D, Marra CM, McCutchan JA, Atkinson JH, Dworkin RH, Grant I, Ellis R. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain. 2010;151(3):732–736. doi: 10.1016/j.pain.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, Hillman S, Brizz B, Cohn DL. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA. 1998;280(18):1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- 39.Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66(11):1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 40.Smith CJ, Sabin CA, Lampe FC, Shah S, Tyrer M, Youle MS, Cropley I, Johnson MA, Phillips AN. The relationship between CD4 cell count nadirs and the toxicity profiles of antiretroviral regimens . Antiviral Therapy. 2005;10:459–467. [PubMed] [Google Scholar]
- 41.Strigo IA, Matthews SC, Simmons AN. Decreased frontal regulation during pain anticipation in unmedicated subjects with major depressive disorder. Translational psychiatry. 2013;3:e239. doi: 10.1038/tp.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Archives of general psychiatry. 2008;65(11):1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 44.Wiley CA. Neuromuscular diseases of AIDS. FASEB J. 1989;3(13):2503–2511. doi: 10.1096/fasebj.3.13.2553521. [DOI] [PubMed] [Google Scholar]
- 45.Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. Br J Psychiatry. 1991;159:645–653, 658. doi: 10.1192/bjp.159.5.645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.