Abstract
The severity of tubulointerstitial fibrosis is regarded as an important determinant of renal prognosis. Therapeutic strategies targeting tubulointerstitial fibrosis have been considered to have potential in the treatment of chronic kidney disease. This study aims to evaluate the protective effects of (-)-epigallocatechin-3-gallate (EGCG), a green tea polyphenol, against renal interstitial fibrosis in mice. EGCG was administrated intraperitoneally for 14 days in a mouse model of unilateral ureteral obstruction (UUO). The results of our histological examination showed that EGCG alleviated glomerular and tubular injury and attenuated renal interstitial fibrosis in UUO mice. Furthermore, the inflammatory responses induced by UUO were inhibited, as represented by decreased macrophage infiltration and inflammatory cytokine production. Additionally, the expression of type I and III collagen in the kidney were reduced by EGCG, which indicated an inhibition of extracellular matrix accumulation. EGCG also caused an up-regulation in α-smooth muscle actin expression and a down-regulation in E-cadherin expression, indicating the inhibition of epithelial-to-mesenchymal transition. These changes were found to be in parallel with the decreased level of TGF-β1 and phosphorylated Smad. In conclusion, the present study demonstrates that EGCG could attenuate renal interstitial fibrosis in UUO mice, and this renoprotective effect might be associated with its effects of inflammatory responses alleviation and TGF-β/Smad signaling pathway inhibition.
Keywords: EGCG, UUO mice, renal fibrosis, ECM, EMT, TGF-β/Smad
Introduction
All progressive chronic kidney disease (CKD) will lead to destructive fibrosis (Eddy 2000). Tubulointerstitial fibrosis is considered to be the hallmark of renal disease. The extent of tubulointerstitial fibrosis better correlates with a deterioration of renal function than with glomerular changes, and its severity is regarded as an important determinant of renal prognosis in both humans and animals (Bohle et al. 1994; Eddy 2000). Thus, therapeutic strategies targeting tubulointerstitial fibrosis are considered to hold potential for the treatment of CKD.
Numerous growth factors have been implicated in the pathophysiology of renal fibrosis, with transforming growth factor-β (TGF-β) considered as one of the central mediators of the disease. In addition to understanding the molecular biology of fibrogenesis, increasing attention has been paid to the role of TGF-β signaling in mediating apoptosis and in epithelial-to-mesenchymal transition (EMT) in chronic progressive renal disease. EMT, a process whereby epithelial cells lose their epithelial phenotype and gain attributes of mesenchymal cells, has been implicated in the generation of myofibroblasts and fibroblasts in kidney disease (Liu 2010). TGF-β is considered to be the most potent inducer of EMT (Zavadil and Bottinger 2005) in various types of epithelial cells via both Smad-dependent and -independent mechanisms (Fan et al. 2007; Masszi et al. 2003; Sato et al. 2003). Therefore, targeting TGF-β-mediated EMT may ameliorate the progressive loss of renal function in the kidney.
(-)-epigallocatechin-3-gallate (EGCG) is the most abundant polyphenol able to be extracted from green tea. A number of studies have reported that EGCG harbors anti-oxidative, anti-inflammatory and anti-carcinogenic effects (de Mejia et al. 2009; Katiyar and Elmets 2001; Katiyar 2003; Melgarejo et al. 2010; Yang 1997; Yang et al. 2006). Specifically, EGCG is protective against renal injury induced by numerous factors. In the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway, EGCG inhibits the development of obstructive nephropathy (Zhou et al. 2013). In addition, by targeting redox and inflammatory pathways, EGCG reverses the progression of immune-mediated glomerulonephritis in mice (Peng et al. 2011). Others have shown that EGCG protects the kidneys against ischemia/reperfusion (I/R) injury in rats by increasing the heme oxygenase-1 (HO-1) gene (Kakuta et al. 2011). EGCG can also ameliorate glucose toxicity and renal injury in streptozotocin-induced diabetic rats (Yamabe et al. 2006). EGCG also mitigates experimental fibrosis in various organs, including the liver (Safer et al. 2012; Xiao et al. 2014), heart (Cai et al. 2013; Shen et al. 2012) and lungs (Sriram et al. 2008; You et al. 2014), and some studies have indicated that EGCG regulates TGF-β signaling (Park et al. 2011; Xiao et al. 2014). However, the protective effect of EGCG against renal interstitial fibrosis has not been studied to date.
Based on the beneficial effects of EGCG on other renal injury and nonrenal models of fibrosis, we hypothesized that EGCG would have a beneficial effect on renal fibrosis. Thus, the aim of this study was to evaluate the renal protective effect of EGCG in mice harboring unilateral ureteral obstruction (UUO)-induced progressive tubulointerstitial fibrosis. In addition, we also investigated the effects of EGCG on TGF-β signaling and EMT.
Materials & Methods
Animals
A total of 24 male C57BL/6 mice, aged 6 to 8 weeks and weighing 18 to 22 g, were purchased from the Laboratory Animal Center of China Medical University (Shenyang, China). Animals were acclimated with free access to food and water in a facility with controlled temperature (22C) on a 12/12 hr light/dark cycle for 1 week before experimentation. All experimental protocols were approved by the ethics committee of the China Medical University.
Grouping
The mice were randomly divided into four groups (n=6 in each group): sham-operated group (Sham), EGCG-treated, sham-operated group (Sham + EGCG), UUO control group (UUO), and EGCG-treated with UUO group (UUO + EGCG). The UUO operation was conducted as per the established procedure (Satoh et al. 2001). Briefly, after intraperitoneal anesthesia by 10% chloral hydrate (3 ml/kg), the left ureter was separated and ligated at two locations using 4-0 silk and cut between the ligatures. Mice subjected to sham treatment underwent the same procedure, with their ureters manipulated but not ligated. Mice in the two EGCG groups received an intraperitoneal dose of 5 mg/kg EGCG (Biopurify Phytochemicals Ltd; Chengdu, China) once a day for 14 days whereas mice in the two control groups received the same volume of saline solution. The dose of EGCG used in the study was based on a previous study of the renoprotective effects of EGCG (Zhou et al. 2013) as well as our preliminary study. At day 14 after treatment, the body weight of each mouse was measured, and all mice were euthanized. Renal tissue was harvested and the obstructed kidneys of the mice were weighed.
Histopathological Examination
Kidneys from experimental mice were fixed with 4% paraformaldehyde in 0.01 M PBS buffer overnight. Fixed kidneys were then dehydrated, embedded in paraffin, and cut into 5-μm sections. Paraffin-embedded sections of renal tissues were stained with both Periodic acid-Schiff (PAS) and Masson’s trichrome staining to determine the general histology of the tissue and the amount of interstitial collagen deposited.
Immunohistochemical Analysis
The expressions of fibrosis regulatory proteins in the kidney were identified by immunohistochemistry (IHC) using standard techniques. Sections were incubated overnight at 4C with 1:50 monoclonal anti-F4/80 (Santa Cruz Biotechnology, Dallas, TX), collagen (Col)-I or Col-III antibody (1:100; Wanleibio, Shenyang, China) and then incubated in biotinylated goat anti-rabbit serum (1:200; Beyotime Institute of Biotechnology, Haimen, China) at 37°C for 30 min. Sections were then washed with 1×PBS and incubated with horseradish peroxidase (HRP)-labeled streptavidin (Beyotime Institute of Biotechnology) for 30 min at 37°C. The staining was visualized by diaminobenzidine (Beyotime Institute of Biotechnology) and sections were counterstained with hematoxylin and observed under light microscopy (DP73; Olympus, Tokyo, Japan).
ELISA Assay
Total protein from rat nephridial tissue was extracted using lysis buffer containing Nonidet P-40 and 1% phenylmethanesulfonyl fluoride (Beyotime Institute of Biotechnology). Protein content was then measured using a Bicinchoninic Acid (BCA) protein assay kit (Beyotime Institute of Biotechnology). MCP-1, MCP-3, IL-1β and TNF-α levels were quantified according to the manufacturer’s instructions using commercial ELISA kits (USCN Life Science; Wuhan, China).
Western Blot Analysis
Total protein was extracted as described for the ELISA assay. Protein expression levels were assessed using standard western blot analysis methods. Briefly, proteins were separated by electrophoresis, and then electrophoretically transferred onto PVDF membranes (Millipore, Billerica, MA). Membranes were then blocked using 5% skim milk and incubated overnight at 4C with the corresponding primary antibodies: anti-TGF-β, anti-α-smooth muscle actin (SMA), anti-Col-I, anti-Col-III, anti-E-Cadherin and anti-Snail (1:1000; Wanleibio), anti-Smad 2/3, and anti-p-Smad2/3 (1:500; Biosynthesis Biotechnology, Beijing, China). Membranes were then incubated in HRP-conjugated anti-rabbit IgG antibody (1:5000; Beyotime) for 1 hr at room temperature, followed by enhanced chemiluminescence reagent (7 Sea Pharmtech, Shanghai, China) before exposure onto X-ray film. All blots were quantified using Gel-Pro-Analyzer software (Media Cybernetics; Bethesda, MD) and the grey levels are expressed as a percentage of β-actin levels (loading control, 1:1000; Wanleibio).
Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted using RNA Simple Total RNA Kit (Tiangen; Beijing, China) according to the manufacturer’s protocol. cDNA was synthesized using Super M-MLV Reverse Transcriptase (BioTeke; Beijing, China). Real-time PCR was performed with an Exicycler 96 Real Time PCR System (Bioneer; Daejeon, Korea) using the SYBR Green PCR reagent kit (Solarbio Science & Technology; Beijing, China). The expression of each gene was normalized to that of β-actin. Primers are as follows: E-cadherin, F: 5’- TCAAAGTGGCGACAGACGG-3’,R:5’-GTTGG ATTCAGAGGCAGGGT-3’;α-SMA,F:5’-CTCAT CCACGAAACCACCTAT-3’, R: 5’- CGCCGATCCA GACAG AATA-3’; Snail, F: 5’- TCTGC ACGACCTGCG GAAAG-3’,R: 5’-TTGTGGAGCAAGGACATTCG G-3’; β-actin, F: 5’-CTGTGCCCATCTACGAGGGCTAT-3’, R: 5’- TTTGATGTCA CGCACGATTTCC-3’(Sangon Biotech; Shanghai, China).
Statistical Analysis
Data are presented as the mean ± standard deviation (SD). The results were assessed using a two-way analysis of variance (ANOVA) followed by an LSD test for comparisons among groups. Statistical analyses were conducted using SPSS 11.0 statistical package (Chicago, IL). P values <0.05 were considered to be statistically significant.
Results
Effects of EGCG on Body and Kidney Weight Changes
Table 1 shows the effects of EGCG and UUO on body weight and obstructed kidney weight. UUO had no effect on body weight but significantly lowered obstructed kidney weight of treated mice (p<0.01 versus sham-operated mice). EGCG treatment for 14 days caused a slight but significant increase in kidney weight (p<0.01 versus UUO mice).
Table 1.
Physiological Indices of Mice.
| Groups | Sham | Sham + EGCG | UUO | UUO + EGCG |
|---|---|---|---|---|
| BW (g) | 22.9 ± 1.2 | 23.1 ± 1.2 | 21.3 ± 1.7 | 22.2 ± 1.4 |
| LKW/BW (g/100g) | 1.42 ± 0.21 | 1.39 ± 0.09 | 0.91 ± 0.09# | 1.16 ± 0.09§,* |
p<0.01 versus Sham group; §§p<0.01 versus Sham + EGCG group; **p<0.01 versus UUO group. BW, body weight; LKW, left kidney weight; EGCG, (-)-epigallocatechin-3-gallate. Data are the mean ± SD; n=6.
EGCG Improves Renal Glomerular and Tubular Injury and Interstitial Fibrosis
Renal histological findings in PAS-stained sections are shown in Figure 1. Sham-operated mice showed normal glomeruli and tubule interstitium. However, mice with UUO showed a thickened glomerular basement membrane, atrophic tubules, inflammatory cell infiltration, increased extracellular matrix (ECM) deposition, and sclerosis. The administration of EGCG for 14 days significantly alleviated glomerular and tubular injury in UUO mice. No apparent damage was found in EGCG-treated, sham-operated mice.
Figure 1.
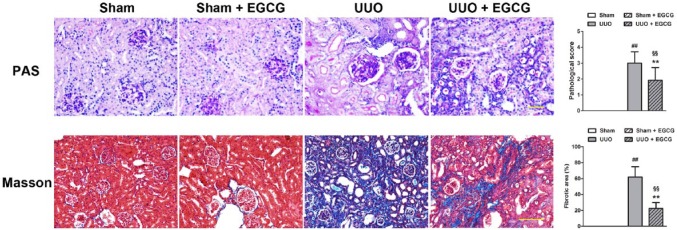
Representative images of histological staining of renal sections. Renal sections were stained with PAS and Masson’s trichrome (Masson) staining. All values are the mean ± SD; n=3. ##p<0.01 versus Sham; §§p<0.01 versus Sham + EGCG group; **p<0.01 versus UUO group. Scale, 100 μm.
Renal fibrosis in mice was also evaluated by Masson’s trichrome staining. No interstitial collagen deposition was found in the Sham group or in the EGCG-treated, sham-operated group. However, compared with mice in the Sham group, UUO mice showed increased collagen deposition and significantly increased interstitial fibrosis. The administration of EGCG markedly decreased the area of interstitial fibrosis in mice in the UUO group.
EGCG Ameliorates Inflammatory Responses in UUO Mice
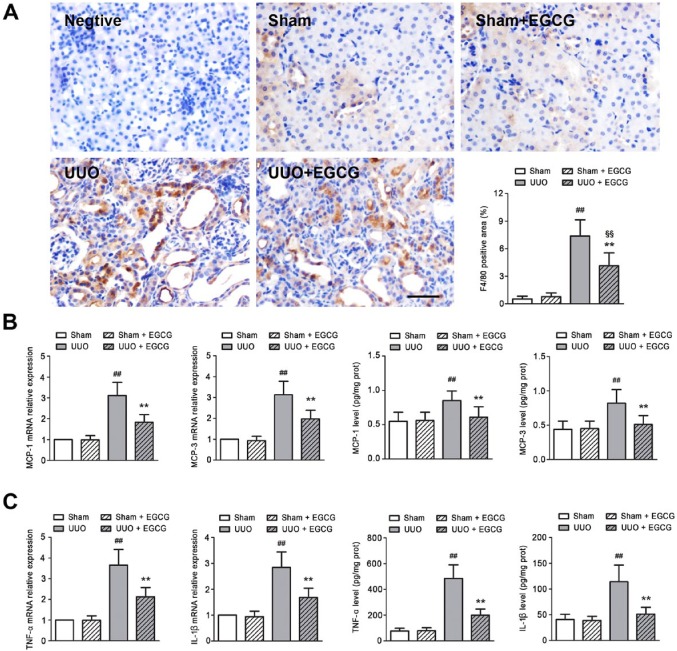
Effects of EGCG on macrophage infiltration and cytokine production were evaluated in UUO mice. A large number of F4/80-positive cells were seen in the UUO kidneys (Fig. 2A), which is in accordance with previous studies (Lai et al. 2013; Kushiyama et al. 2011). EGCG administration dramatically decreased the number of F4/80-positive cells. The enhanced mRNA expression of inflammatory cytokines, including monocyte chemotactic protein (MCP)-1, MCP-3, tumor necrosis factor (TNF)-α and interleukin (IL)-1β, was found in the UUO kidneys, as well as in protein secretions (Fig. 2B, 2C). However, EGCG significantly reduced the production of cytokines. These results indicate that EGCG ameliorates UUO-induced inflammatory responses in the kidney.
Figure 2.
EGCG ameliorates inflammatory responses in the obstructive kidney of UUO mice. (A) F4/80 immunostaining and quantification. F4/80-positive staining, as a macrophage marker, was increased in UUO mice, and this increase could be reversed by EGCG treatment. (B) Chemokine gene expression and protein secretion. (C) Inflammatory cytokine gene expression and protein secretion. UUO induced an increase in the expression and secretion of monocyte chemotactic protein (MCP)-1, MCP-3, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β; EGCG treatment prevented the increases in these genes. All values are the mean ± SD; n=6. ##p<0.01 versus sham group; §§p<0.01 versus sham + EGCG group; **p<0.01 versus UUO group. Scale, 40 μm.
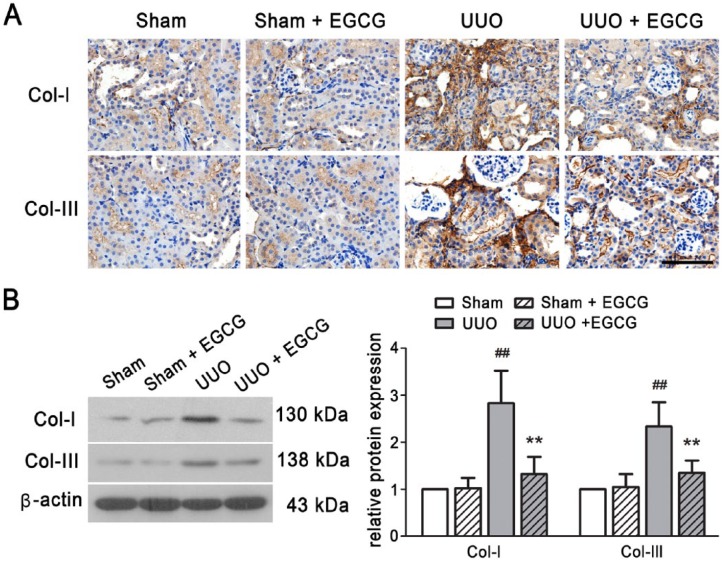
EGCG Inhibits ECM Accumulation and EMT in UUO Kidneys
ECM proteins and markers of EMT were detected in the kidneys of UUO mice. As compared with the sham-operated mice, Col-I and Col-III were significantly increased in the UUO mice, as determined by IHC staining (Fig. 3A) and western blotting (Fig. 3B). In addition, increased α-SMA and decreased E-cadherin expression were found in the UUO kidneys (Fig. 4). Snail mRNA and protein levels were also up-regulated in the UUO mice (Fig. 5). These results suggest that UUO induces the excessive deposition of ECM components and causes EMT in the kidneys of mice. EGCG treatment for 14 days not only inhibited the up-regulated expression of Col-I and Col-III, but also prevented UUO-induced EMT, as indicated by the increase in E-cadherin expression and the decrease in α-SMA expression. Additionally, EGCG significantly reduced Snail gene and protein expression. These results indicate that EGCG attenuates UUO-induced renal fibrosis by inhibiting ECM accumulation and EMT.
Figure 3.
EGCG inhibits ECM accumulation in the kidney of UUO mice. Collagen (Col)-I and Col-III expressions were detected by immunohistochemistry (A) and western blotting (B). All values are the mean ± SD; n=3. ##p<0.01 versus sham-operated group; **p<0.01 versus UUO group. Scale, 100 μm.
Figure 4.
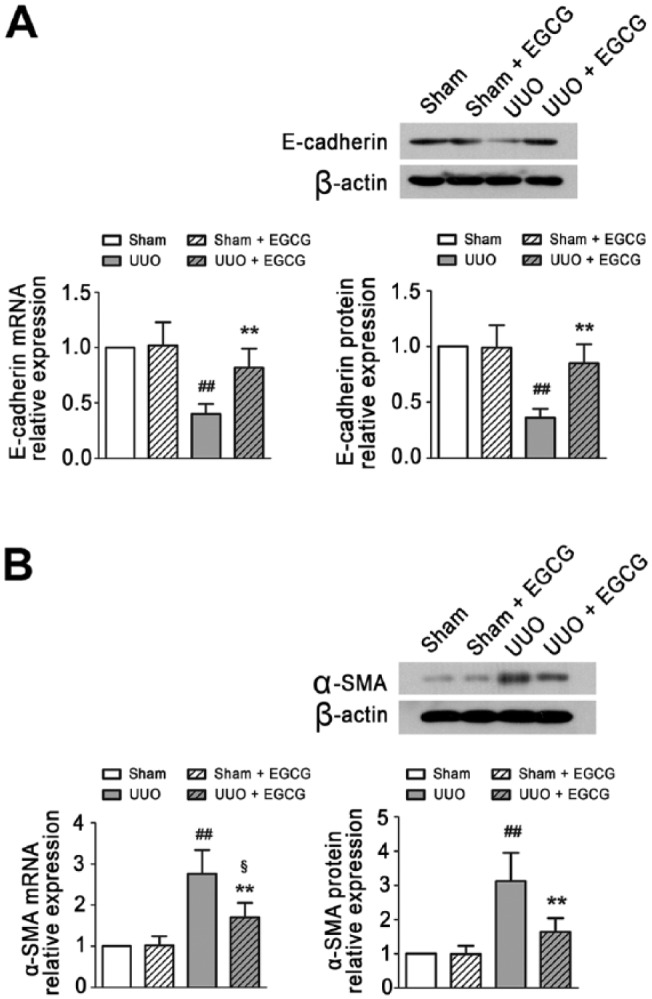
EGCG inhibits the UUO induced EMT in the kidney of UUO mice. Real-time PCR and Western blot analysis demonstrated EGCG inhibits E-cadherin expression (A) and enhances α-SMA expression (B) in the kidney. All values are the mean ± SD; n=3. ##p<0.01 versus sham-operated group; §p<0.05 versus Sham + EGCG group; **p<0.01 versus UUO group. Scale, 40 μm.
Figure 5.
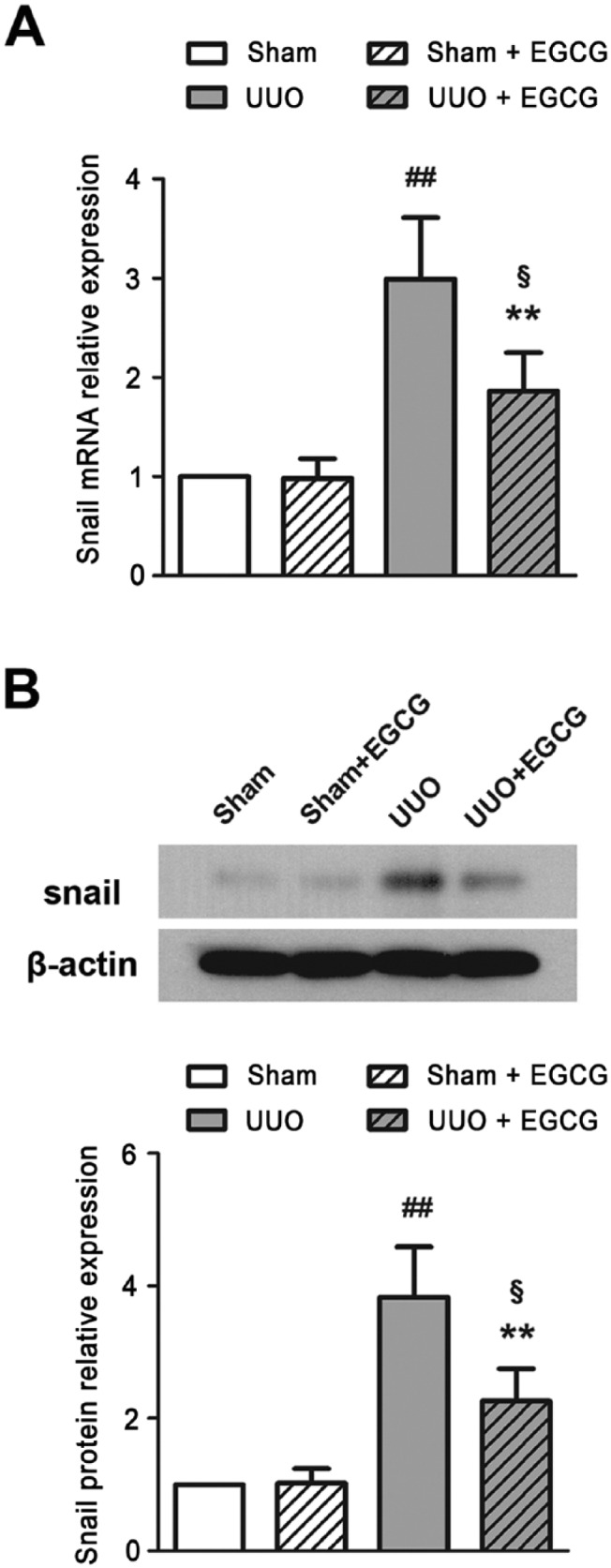
EGCG down-regulates Snail mRNA (A) and protein (B) expression in the kidney of UUO mice. All values are the mean ± SD; n=3. ##p<0.01 versus sham group; §p<0.05 versus sham + EGCG group; **p<0.01 versus UUO group.
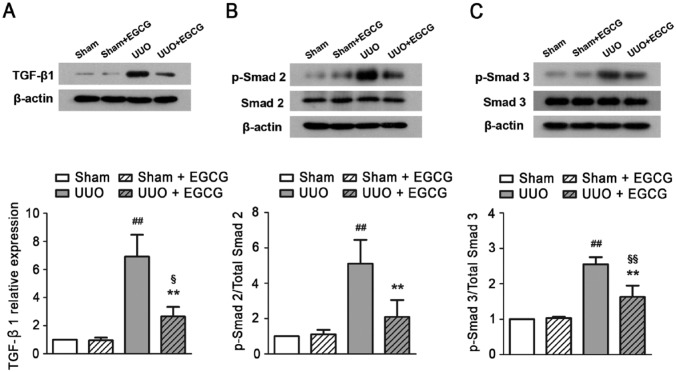
EGCG Regulates TGF-β1/Smad Signaling in the UUO Kidney
Finally, to investigate the possible mechanisms underlying the effects of EGCG, we examined the TGF-β1/Smad signaling pathway in the obstructed kidney. In sham-operated mice, TGF-β1 expression was low (Fig. 6A), and no changes were observed after EGCG treatment. In the UUO mice, TGF-β1 protein expression increased approximately 7-fold as compared with that in the sham-operated mice. Accordingly, Smad 2 and -3, downstream proteins of TGF-β1, were activated following stimulation with the ligand, as shown by the large increases in the proportion of phosphorylated Smad 2 and -3 (Fig. 6B, 6C). In contrast, EGCG treatment markedly reduced TGF-β1 protein levels and therefore decreased the phosphorylations of Smad 2 and -3. The results indicate that EGCG might attenuate UUO-induced renal fibrosis by regulating the TGF-β/Smad pathway.
Figure 6.
EGCG regulates TGF-β signaling pathway in UUO mice. (A) Transforming growth factor (TGF)-β, (B) p-Smad 2, (C) p-Smad 3 were upregulated in the kidney of UUO mice after 14 days of EGCG treatment. All values are the mean ± SD; n=3. ##p<0.01 versus Sham group, §p<0.05 versus Sham + EGCG group; §§p<0.01 versus Sham + EGCG group, **p<0.01 versus UUO group.
Discussion
EGCG, a natural extract from green tea, possesses anti-oxidant (Lambert and Elias 2010), anti-inflammatory, anti-cancer (de Mejia et al. 2009) and anti-infection (Steinmann et al. 2013) properties and these changes have been noted in a broad range of organs, including the heart, liver, lungs, brain, and kidneys. In the present study, we evaluated the anti-fibrotic effect of EGCG in renal tissues in mice with obstructive nephropathy.
Macrophage infiltration is considered to be correlated with the degree of renal fibrosis, and macrophage depletion has been shown to reduce fibrosis (Ricardo et al. 2008). In the present study, macrophage infiltration and increased cytokine production were found in the obstructive kidney of UUO mice and EGCG treatment was able to significantly attenuate these inflammatory responses. Although it is unclear whether inflammation directly contributes to the renal fibrosis observed in the obstructive kidneys in the UUO mice, the amelioration of the observed inflammatory responses is likely to be beneficial to renal repair.
The severity of renal interstitial fibrosis, which is directly correlated with the glomerular filtration rate, is regarded as a single histological marker of the renal dysfunction (Farris et al. 2011; Harris and Neilson 2006). In the normal kidney, the production and assembly of ECM—a major constituent of renal interstitium—is finely controlled, and thus maintains connective tissue homeostasis. Col-I and Col-III are major components of this ECM. In this study, we found an increase in Col-I and Col-III expression in UUO kidneys. Accordingly, our histological examination showed large accumulation of ECM components as well as tubulointerstitial fibrosis, and glomerular and tubular injury. Collectively, these results indicate a disorder in ECM balance in vivo, which might thereby disrupt the normal structure of the kidney and lead to renal failure. The effect of EGCG on the ECM has been reported in a variety of fibrotic models in vivo and in vitro, such as I/R-induced renal injury (Kakuta et al. 2011), carbon tetrachloride (CCl4)-induced chronic liver fibrosis (Tipoe et al. 2010), ovariectomy-induced bladder dysfunction (Juan et al. 2012), and TGF-β-induced dermal fibroblasts (Dooley et al. 2010). In this study, consistent with previous studies, we found that ECM accumulation and tubulointerstitial fibrosis could be alleviated by EGCG, and these effects were shown as a decrease in the expression of Col-I and Col-III. Thus, we provide morphological and molecular evidence that EGCG exerts anti-fibrotic effects in renal tissue by suppressing the excessive production and deposition of ECM.
Excess ECM deposition is a key component of interstitial fibrosis. Under pathological conditions in the kidney, α-SMA-positive myofibroblasts are responsible for the excess accumulation of ECM. Accordingly, renal interstitial fibrosis is characterized by the de novo activation of myofibroblasts (Badid et al. 2002). The origin and activation of myofibroblasts include interstitial fibroblasts, circulating precursor cells, perivascular smooth muscle cells, as well as the process of EMT (Liu 2004). Among these sources, EMT is considered to be a crucial component of renal fibrogenesis and has thus been given the most attention by researchers in the field. It is unlikely that EMT-independent activation of myofibroblasts can be sustained (Yang et al. 2002). Indeed, in the absence of EMT, myofibroblast accumulation is not only diminished but also reversed (Yang et al. 2002). The presence of EMT in renal fibrosis was first demonstrated by Strutz et al. in 1995. During EMT, tubular epithelial cells lose their expression of epithelial markers, such as E-cadherin, gain the expression of mesenchymal markers, such as α-SMA, and produce interstitial matrix proteins, such as Col-I and Col-III (Iwano et al. 2002; Yang and Liu 2001). Consistent with previous studies (Vidyasagar et al. 2008; Yang and Liu 2001), here we found an up-regulation in the expression of α-SMA and a down-regulation in the expression of E-cadherin in the UUO mice. Moreover, we observed an increase in the expression of Snail—a zinc-finger transcription factor that mediates the EMT process (Nieto 2002)—in the kidneys of UUO mice. All these changes could be prevented by EGCG treatment, as indicated by the decrease in α-SMA and Snail and the increase in E-cadherin; these findings are in agreement with those found by Xiao and colleagues in samples of non-alcoholic fatty liver disease (Xiao et al. 2014) and by De Amicis and colleagues using human thyroid carcinoma cell lines (De Amicis et al. 2013). Thus, our observations indicate that the renoprotective effect of EGCG may, in part, stem from suppressing the EMT process.
Activation of TGF-β signaling occurs in almost all kidney disease. TGF-β binding to its cognate receptors results in the phosphorylation and activation of Smad 2 and -3 (ten Dijke et al. 2000). Phosphorylated Smad 2/3, in turn, partner with Smad 4 and then translocate to the nucleus to control the transcription of target genes (Bottinger and Bitzer 2002). Besides the Smad pathway, TGF-β is also capable of inducing EMT through other pathways, such as the mitogen-activated protein kinase (MAPK), Akt, Wnt, and Notch pathways, among others (Zavadil and Bottinger 2005). Smad signaling appears to have a pivotal role in TGF-β-induced EMT; therefore, in this study, we mainly focused our investigation on the intracellular signal transduction on TGF-β/Smad pathway.
The overexpression of Smad 2 and Smad 3 promotes a TGF-β-stimulated accumulation of EMT and ECM proteins in human lens epithelial cells (Li et al. 2014). Silencing Smad 3 gene prevents TGF-β-induced E-cadherin downregulation, whereas silencing Smad 3 and Smad 2 suppresses the TGF-β-induced increase in α-SMA expression in porcine bladder urothelial cells (Li et al. 2014). In primary tubular epithelial cells derived from kidneys of Smad3-knockout mice, TGF-β failed to induce EMT and was unable to induce the expression of key transcriptional regulators of EMT (Zavadil et al. 2004). Furthermore, in experimental animal models, Smad3 gene knockout ameliorated epithelial degeneration and fibrosis in mice with UUO-induced tubulointerstitial fibrosis (Sato et al. 2003). Certain drugs that inhibit TGF-β/Smad signaling can also attenuate EMT and renal fibrosis in experimental animals (Pan et al. 2013). In this study, we found an up-regulation of TGF-β1 protein and phosphorylated Smad 2 and -3. The results suggest that UUO-induced renal fibrosis might be mediated by the activation of the TGF-β1/Smad signaling pathway. However, EGCG could prevent TGF-β1 up-regulation and Smad phosphorylation. These findings indicate that EGCG may attenuate UUO-induced renal fibrosis via the TGF-β1/Smad signaling pathway.
In summary, EGCG administration for 14 days attenuated renal fibrosis by preventing ECM accumulation and EMT, which might be associated with its anti-inflammatory properties and TGF-β1/Smad signaling regulation. Other pathways are likely to also contribute to the renoprotective effects of EGCG and these pathways will also need to be studied in the future.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the Special Foundation for Science and Technology Innovation of Shenyang City (No.: F13-318-1-23), the Social Development Project of Liaoning Province (No.: 2013225049), and the General Scientific Research Foundation of Department of Education, Liaoning Province (No.: L2014313).
References
- Badid C, Desmouliere A, Babici D, Hadj-Aissa A, McGregor B, Lefrancois N, Touraine JL, Laville M. (2002). Interstitial expression of alpha-SMA: an early marker of chronic renal allograft dysfunction. Nephrol Dial Transplant 17:1993-1998. [DOI] [PubMed] [Google Scholar]
- Bohle A, Strutz F, Muller GA. (1994). On the pathogenesis of chronic renal failure in primary glomerulopathies: a view from the interstitium. Exp Nephrol 2:205-210. [PubMed] [Google Scholar]
- Bottinger EP, Bitzer M. (2002). TGF-beta signaling in renal disease. J Am Soc Nephrol 13:2600-2610. [DOI] [PubMed] [Google Scholar]
- Cai Y, Yu SS, Chen TT, Gao S, Geng B, Yu Y, Ye JT, Liu PQ. (2013). EGCG inhibits CTGF expression via blocking NF-kappaB activation in cardiac fibroblast. Phytomedicine 20:106-113. [DOI] [PubMed] [Google Scholar]
- De Amicis F, Perri A, Vizza D, Russo A, Panno ML, Bonofiglio D, Giordano C, Mauro L, Aquila S, Tramontano D, Andò S. (2013). Epigallocatechin gallate inhibits growth and epithelial-to-mesenchymal transition in human thyroid carcinoma cell lines. J Cell Physiol 228:2054-2062. [DOI] [PubMed] [Google Scholar]
- de Mejia EG, Ramirez-Mares MV, Puangpraphant S. (2009). Bioactive components of tea: cancer, inflammation and behavior. Brain Behav Immun 2009;23:721-731. [DOI] [PubMed] [Google Scholar]
- Dooley A, Shi-Wen X, Aden N, Tranah T, Desai N, Denton CP, Abraham DJ, Bruckdorfer R. (2010). Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatology 49:2024-2036. [DOI] [PubMed] [Google Scholar]
- Eddy AA. (2000). Molecular basis of renal fibrosis. Pediatr Nephrol 15:290-301. [DOI] [PubMed] [Google Scholar]
- Fan L, Sebe A, Péterfi Z, Masszi A, Thirone AC, Rotstein OD, Nakano H, McCulloch CA, Szászi K, Mucsi I, Kapus A. (2007). Cell contact-dependent regulation of epithelial-myofibroblast transition via the rho-rho kinase-phospho-myosin pathway. Mol Biol Cell 18:1083-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. (2011). Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 22:176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RC, Neilson EG. (2006). Toward a unified theory of renal progression. Ann Rev Med 57:365-380. [DOI] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. (2002). Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Inv 110:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan YS, Chuang SM, Lee YL, Long CY, Wu TH, Chang WC, Levin RM, Liu KM, Huang CH. (2012). Green tea catechins decrease oxidative stress in surgical menopause-induced overactive bladder in a rat model. BJU Int 110:E236-E244. [DOI] [PubMed] [Google Scholar]
- Kakuta Y, Okumi M, Isaka Y, Tsutahara K, Abe T, Yazawa K, Ichimaru N, Matsumura K, Hyon SH, Takahara S, Nonomura N. (2011). Epigallocatechin-3-gallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Transpl Int 24:514-522. [DOI] [PubMed] [Google Scholar]
- Katiyar SK. (2003). Skin photoprotection by green tea: antioxidant and immunomodulatory effects. Curr Drug Targets Immune Endocr Metabol Disord 3:234-242. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Elmets CA. (2001). Green tea polyphenolic antioxidants and skin photoprotection. Int J Oncol 18:1307-1313. [DOI] [PubMed] [Google Scholar]
- Kushiyama T, Oda T, Yamada M, Higashi K, Yamamoto K, Sakurai Y, Miura S, Kumagai H. (2011). Alteration in the phenotype of macrophages in the repair of renal interstitial fibrosis in mice. Nephrology (Carlton) 16:522-535. [DOI] [PubMed] [Google Scholar]
- Lai CF, Chen YM, Chiang WC, Lin SL, Kuo ML, Tsai TJ. (2013) Cysteine-rich protein 61 plays a proinflammatory role in obstructive kidney fibrosis. PloS One 8:e56481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. (2010). The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yuan X, Li J, Tang X. (2014). Implication of Smad2 and Smad3 in Transforming Growth Factor-beta-induced Posterior Capsular Opacification of Human Lens Epithelial Cells. Curr Eye Res 9:1-12. [DOI] [PubMed] [Google Scholar]
- Liu Y. (2004). Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15:1-12. [DOI] [PubMed] [Google Scholar]
- Liu Y. (2010) New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21:212-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A, Di Ciano C, Sirokmány G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. (2003). Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol 284:F911-F924. [DOI] [PubMed] [Google Scholar]
- Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. (2010). Targeting of histamine producing cells by EGCG: a green dart against inflammation? J Physiol Biochem 66:265-270. [DOI] [PubMed] [Google Scholar]
- Nieto MA. (2002). The snail superfamily of zinc-finger transcription factors. Nature Rev Mol Cell Biol 3:155-66. [DOI] [PubMed] [Google Scholar]
- Pan MM, Zhang MH, Ni HF, Chen JF, Xu M, Phillips AO, Liu BC. (2013). Inhibition of TGF-beta1/Smad signal pathway is involved in the effect of Cordyceps sinensis against renal fibrosis in 5/6 nephrectomy rats. Food Chem Toxicol 58:487-494. [DOI] [PubMed] [Google Scholar]
- Park SJ, Jeong JM, Jeong HS, Park JS, Kim NH. (2011). Effects of Epigallocatechin-3-Gallate on the Expression of TGF-beta1, PKC alpha/betaII, and NF-kappaB in High-Glucose-Stimulated Glomerular Epithelial Cells. Chonnam Med J 47:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A, Ye T, Rakheja D, Tu Y, Wang T, Du Y, Zhou JK, Vaziri ND, Hu Z, Mohan C, Zhou XJ. (2011). The green tea polyphenol (-)-epigallocatechin-3-gallate ameliorates experimental immune-mediated glomerulonephritis. Kidney Int 80:601-611. [DOI] [PubMed] [Google Scholar]
- Ricardo SD, van Goor H, Eddy AA. (2008). Macrophage diversity in renal injury and repair. J Clin Inv 118:3522-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer AM, Afzal M, Nomani A, Sosamma O, Mousa SA. (2012). Curative propensity of green tea extract towards hepatic fibrosis induced by CCl(4): A histopathological study. Exp Ther Med 3:781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Kashihara N, Yamasaki Y, Maruyama K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K, Makino H. (2001). Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 12:317-325. [DOI] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. (2003). Targeted disruption of TGF-beta1/Smad3 signaling protects against tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Inv 112:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CL, Samathanam C, Graham S, Dagda RY, Chyu MC, Dunn DM. (2012). Green tea polyphenols and 1-alpha-OH-vitamin D(3) attenuate chronic inflammation-induced myocardial fibrosis in female rats. J Med Food 15:269-277. [DOI] [PubMed] [Google Scholar]
- Sriram N, Kalayarasan S, Sudhandiran G. (2008). Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biol Pharma Bull 31:1306-1311. [DOI] [PubMed] [Google Scholar]
- Steinmann J, Buer J, Pietschmann T, Steinmann E. (2013). Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol 168:1059-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. (1995). Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 130:393-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin CH. (2000). Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci 25:64-70. [DOI] [PubMed] [Google Scholar]
- Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA. (2010). Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 273:45-52. [DOI] [PubMed] [Google Scholar]
- Vidyasagar A, Reese S, Acun Z, Hullett D, Djamali A. (2008). HSP27 is involved in the pathogenesis of tubulointerstitial fibrosis. Am J Renal Physiol 295:F707-F716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML, Tipoe GL. (2014). Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr 53:187-199. [DOI] [PubMed] [Google Scholar]
- Yamabe N, Yokozawa T, Oya T, Kim M. (2006). Therapeutic potential of (-)-epigallocatechin 3-O-gallate on renal damage in diabetic nephropathy model rats. J Pharmacol Exp Ther 319:228-236. [DOI] [PubMed] [Google Scholar]
- Yang CS. (1997). Inhibition of carcinogenesis by tea. Nature ;389:134-135. [DOI] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. (2006). Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinog 45:431-435. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. (2001). Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159:1465-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Shultz RW, Mars WM, Wegner RE, Li Y, Dai C, Nejak K, Liu Y. (2002). Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J Clin Inv 110:1525-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Wei L, Sun WL, Wang L, Yang ZL, Liu Y, Zheng K, Wang Y, Zhang WJ. (2014). The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int J Mol Med 34:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. (2005). TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 24:5764-5774. [DOI] [PubMed] [Google Scholar]
- Zhou P, Yu JF, Zhao CG, Sui FX, Teng X, Wu YB. (2013). Therapeutic potential of EGCG on acute renal damage in a rat model of obstructive nephropathy. Mol Med Rep 7:1096-1102. [DOI] [PubMed] [Google Scholar]