Abstract
Syndecans are important cell surface proteoglycans with many functions; yet, they have not been studied to a very large extent in primary human endothelial cells. The purpose of this study was to investigate syndecan-4 expression in cultured human umbilical vein endothelial cells (HUVECs) and assess its role in inflammatory reactions and experimental wound healing. qRT-PCR analysis revealed that syndecan-3 and syndecan-4 were highly expressed in HUVECs, whereas the expression of syndecan-1 and -2 was low. HUVECs were cultured with the inflammatory mediators lipopolysaccharide (LPS) and interleukin 1β (IL-1β). As a result, syndecan-4 expression showed a rapid and strong increase. Syndecan-1 and -2 expressions decreased, whereas syndecan-3 was unaffected. Knockdown of syndecan-4 using siRNA resulted in changes in cellular morphology and focal adhesion sites, delayed wound healing and tube formation, and increased secretion of the pro-inflammatory and angiogenic chemokine, CXCL8. These data suggest functions for syndecan-4 in inflammatory reactions, wound healing and angiogenesis in primary human endothelial cells.
Keywords: angiogenesis, inflammation, primary human endothelial cells, shedding, syndecan-4, wound healing
Introduction
The integrity of the circulatory system is of fundamental importance for all body functions. The endothelial cells are engaged in a variety of processes ranging from regulation of blood pressure and coagulation to extravasation of immune cells during infection and filtration of urine in the kidneys (Sumpio et al. 2002). These cells are continuously exposed to shear stress and have a protective glycocalyx, which has important functions in endothelial cells in vivo (Salmon and Satchell 2012). The glycocalyx is rich in proteoglycans (PGs), as is the underlying basement membrane of endothelial cells. Several types of PGs are expressed by endothelial cells, including the cell-surface glypicans and syndecans, and the extracellular matrix PGs, such as perlecan, biglycan (Couchman and Pataki 2012; Iozzo 2005) and serglycin (Meen et al. 2011). Cell-surface syndecans belong to a family of transmembrane PGs comprising four members. They can be subdivided into two groups: the first comprises syndecans-1 and -3; the second, syndecans-2 and -4 (Bernfield et al. 1992). The syndecans have distinct tissue distributions. Syndecan-1 is expressed on epithelial cells and myeloma cells, whereas syndecan-2 has been reported to be expressed by mesenchymal cells and endothelial cells. Syndecan-3 expression is predominantly in neural crest cells and syndecan-4 is the only family member with ubiquitous distribution (Teng et al. 2012). All syndecans contain an ectodomain to which glycosaminoglycan (GAG) chains are covalently attached. Most of the GAG chains are of the heparan sulfate (HS) type, but chondroitin sulfate or dermatan sulfate can also be attached, depending on cell type and stimuli (Okina et al. 2009). The transmembrane part is conserved, as are the two regions of the cytoplasmic tails. A variable region, located between the two conserved cytoplasmic regions, is unique for each syndecan. The syndecan cytoplasmic domains have been documented to participate in signal transduction and in interactions with the cytoskeleton (Multhaupt et al. 2009). Furthermore, syndecan-4 has been shown to be involved in the formation of focal adhesion sites (Couchman 2010).
Syndecans are multifunctional molecules with the ability to interact through their ectodomains—mostly through their GAG chains—with extracellular matrix and signaling molecules. The interactions between growth factors, such as fibroblast growth factor 2 (FGF-2) (Matsuo and Kimura-Yoshida 2013) and vascular endothelial growth factor (VEGF) has been well documented (Jakobsson et al. 2006). HS chains in the ectodomain are instrumental for such interactions and also in cell adhesion processes (Gopal et al. 2010). The cytoplasmic domain of syndecan-4 has been shown to interact with phosphatidylinositol 4,5-biphosphate leading to binding and activation of protein kinase Cα. Further downstream targets from this activation involve G proteins of the Rho family (Morgan et al. 2007; Dovas et al. 2006). Endocytosis and intracellular trafficking of syndecans can also have effects on cellular signaling and syndecans have been shown to be localized in perinuclear vesicles (Lambaerts et al. 2009) and in the nucleus (Lim and Couchman 2014; Stewart and Sanderson 2014), suggesting multiple intracellular functions for syndecans.
Syndecans residing on cell surfaces can be subjected to regulation at several levels, such as endocytosis (Lambaerts et al. 2009), shedding (Manon-Jensen et al. 2010) and posttranslational modifications of HS chains by enzymes including heparanases (Fux et al. 2009) and sulfatases (Uchimura et al. 2006). This family of cell-surface PGs has multiple functions with relevance to several types of human diseases (Teng et al. 2012). However, deletion of any of the four members using knock-out technology has revealed only mild phenotypes (Alexopoulou et al. 2007). Some of the phenotypes reported involve angiogenesis and wound healing (Echtermeyer et al. 2001) where endothelial cells are important. Several studies on syndecans have focused on epithelial cells (Bernfield et al. 1999) and fibroblasts (Okina et al. 2012), but some also focus on endothelial cells (Fuster and Wang 2010; Ramnath et al. 2014). In such studies, the role of syndecans in angiogenesis, wound healing and inflammation has been addressed. Few studies, however, have used primary human endothelial cells. The purpose of this study was to investigate the effects of inflammatory conditions and wound healing on syndecan-4 expression in primary human umbilical vein endothelial cells (HUVECs) in vitro. The results presented show that syndecan-4 is a major PG in HUVECs and that exposure to lipopolysaccharide (LPS) or interleukin-1β (IL-1β) increase its expression and secretion. Further, syndecan-4 silencing resulted in changes in cellular morphology and delayed capacity of HUVEC wound closure and tube formation.
Materials & Methods
Cell Culture and Treatment
HUVECs were isolated from infant umbilical cords of normal pregnancies, as described (Jaffe 1973). The cells were cultured at 37°C and 5% CO2 in MCDB 131 medium (Sigma-Aldrich; St Louis, MO) with 5 mM glucose and supplemented with 7% fetal calf serum (FCS; Sigma-Aldrich), 10 ng/ml epidermal growth factor (EGF; R&D Systems, Minneapolis, MN), 1 ng/ml basic fibroblast growth factor (FGF-2, R&D Systems), 1 µg/ml hydrocortisone (Sigma-Aldrich), 250 ng/ml fungizone and 50 µg/ml gentamycine (GIBCO, Life Technologies; Carlsbad, CA). The purity of the endothelial cell cultures was verified by microscopic observations of the morphology of each culture as well as regular staining for the endothelial cell marker, von Willebrand factor (vWF). The cells were routinely grown to 80%–90% confluence and cells from passages 1–3 were used in experiments.
For experiments, cells at a density of 75×103 cells/cm2 were plated on 1% gelatin (w/v)-coated 24-well plates in culture medium and grown to confluence. During experiments, cells were incubated with LPS (1 µg/ml; Sigma-Aldrich) in combination with Lipopolysaccharide binding protein (25 ng/ml, R&D Systems), IL-1β (0.5 ng/ml; R&D Systems), or with GM6001 (5 and 25 µM; Calbiochem, San Diego, CA) in culture medium with 2% FCS for the indicated time points.
siRNA Transfection
Knockdown of syndecan-4 gene expression was performed using LipofectamineTM 2000 (Invitrogen; Carlsbad, CA), according to the protocol provided by the manufacturer. Briefly, cells were reversely transfected with syndecan-4 siRNA (sc-36588; Santa Cruz Biotechnology, Dallas, TX) for 5 h followed by a 5 h forward transfection with a 16 h interval between the two transfections. Another siRNA duplex, synthesized and annealed by Ambion (Austin, TX) with the target sequences 5’-AGCCAAUACUUUUCCGGAGTT-3’ and 5’-CUCCGGAAAAGUAUUGGCUTT-3’, was also used to confirm the effect of syndecan-4 knockdown in HUVECs. The syndecan-4 siRNA from Santa Cruz Biotechnology was used in all syndecan-4 knockdown experiments in this study. The control siRNA used was Silencer Negative Control #1 siRNA (AM4635; Ambion). SiRNA-transfected cells were used for experiments approximately 96 h after the introduction of siRNA. The syndecan-4 silencing efficiency at the mRNA and protein levels was determined by qRT-PCR and ELISA, respectively. The morphology of HUVECs in the control (siControl)- and syndecan-4 siRNA (siSDC4)-treated cells was investigated at confluence by light microscopy with a 10× objective.
In vitro Scratch Wound Healing Assay
Wound healing rates were assessed using a scratch assay. HUVECs seeded in 24-well or 48-well plates were cultured until confluence. For experiments with LPS and IL-1β, the cells were exposed to these agents for 24 h in medium containing 2% FCS prior to wounding. Two scratches were then perpendicularly introduced to the HUVEC monolayer with a 200 µl sterile pipette tip. Cells were then washed to remove debris and incubated in fresh medium containing LPS or IL-1β. Images from two different scratch areas in each culture well were systematically obtained using Olympus CKX41 microscope (Olympus; Tokyo, Japan) with a CachN 10× objective. The distance between wound edges was manually measured at three to six points on each image, and the values were used to calculate the percent gap closure at 8 h relative to that at 0 h.
Quantitative Real-time PCR, qRT-PCR
HUVECs were incubated with LPS and IL-1β or transfected with siRNA targeting syndecan-4. After the various treatments, total RNA was isolated and purified by using E.Z.N.A. Total RNA kit 1 (R6834-02; Omega Bio-Tek, Norcross, GA) according to the manufacturer’s instructions. RNA concentrations were measured using Nano-drop ND-1000 Spectrophotometer (Thermo Scientific; Wilmington, DE). Equal amounts of RNA from each sample were then reverse-transcribed in a total volume of 20 µl using High Capacity RNA-to-cDNA Kit (Applied Biosystems; Foster City, CA). For quantitative PCR, gene amplification was performed on an ABI Prism 7900HT Real-Time PCR System (Applied Biosystems) using TaqMan Gene Expression Master Mix, and predesigned TaqMan Gene Expression Assays, as listed in Table 1. In all siRNA-treated samples, the housekeeping gene β-actin was used as internal control, whereas RPL30 was used as housekeeping gene for LPS- and IL-1β-treated samples. The cycling conditions for amplification reactions were as follows: 50°C for 2 min before the 1st cycle, initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The threshold cycles (Ct) for each sample, run in duplicates, were determined using the dedicated ABI Prism 7900HT SDS software. Gene expression levels were normalized to that of internal control genes, and the relative expression of target genes in treated samples compared to control samples were calculated using the ΔΔCt method, as previously described (Livak and Schmittgen 2001).
Table 1.
TaqMan Gene Expression Assays used in qRT-PCR Analyses.
| Gene | Gene Name | Assay ID |
|---|---|---|
| ACTB | β-actin | Hs00265497_m1 |
| RPL30 | 60S ribosomal protein L30 | Hs99999903_m1 |
| SDC1 | Syndecan-1 | Hs00896423_m1 |
| SDC2 | Syndecan-2 | Hs00299807_m1 |
| SDC3 | Syndecan-3 | Hs00206320_m1 |
| SDC4 | Syndecan-4 | Hs00161617_m1 |
| VEGFA | Vascular endothelial growth factor A | Hs00900055_m1 |
| VEGFB | Vascular endothelial growth factor B | Hs00173634_m1 |
| FGF2 | Fibroblast growth factor 2 (basic) | Hs00266645_m1 |
| PDGF-A | Platelet-derived growth factor alpha polypeptide | Hs00964426_m1 |
| TGFB2 | Transforming growth factor, beta 2 | Hs00234244_m1 |
Flow Cytometry
Flow cytometry was performed on HUVECs treated with LPS, IL-1β or SDC4 siRNA, as described. The Fc receptors were blocked using Human TruStain FcX (BioLegend; San Diego, CA). Syndecan-4 was stained using clone 5G9 (Santa Cruz Biotechnology) and goat anti-mouse IgG (H+L) Alexa Fluor 488 (Invitrogen). Purified mouse IgG2a was used as an isotype control (Biolegend). Fixability Viability Dye (eBioscience; San Diego, CA) was used to discern between live and dead cells. Samples were analyzed on the BD FACSCanto (BD Biosciences; Franklin Lakes, NJ).
Western Blot Analysis
Levels of cellular β-actin and the loading control, tubulin, after syndecan-4 knock-down were determined by western blotting. Control siRNA- or syndecan-4 siRNA-treated cells were grown in 24-well plates for 24 h. The medium was then removed and the cells were washed with PBS and lysed in RIPA buffer. Protein concentrations were measured by the BCA kit from Bio-Rad (Hercules, CA). Equal amounts of protein from cell lysates were then subjected to SDS-PAGE on 4%–20% gradient gels and electroblotted onto PVDF membranes (EMD Millipore, Billerica, MA) using the Criterion™gel system (Bio-Rad). The membranes were blocked in TBST with 5% dry milk, washed, incubated with mouse anti-β-actin antibody (Sigma-Aldrich; 1:1000) diluted in TBST with 1% dry milk for 2 h, and then incubated with secondary antibodies conjugated to HRP (GE Healthcare; Buckinghamshire, UK). For loading control, the membranes were stripped in Restore Plus Western Blot stripping buffer (Thermo Scientific) and then re-blotted with rabbit polyclonal anti-tubulin antibody (Abcam; 1:20,000). Chemiluminescence detection kit ECL Western Blotting Detection Reagents (GE-Healthcare) was used for protein visualization and the signal was detected by exposing to films (Amersham Hyperfilm™ ECL).
ELISA
After transfection with syndecan-4 siRNA, or after incubation with LPS or IL-1β for the indicated time periods, the conditioned medium was harvested and cells were washed with PBS and lysed in RIPA buffer. The concentrations of syndecan-4 were measured by Syndecan-4 ELISA kit (IBL International GmbH, Hamburg, Germany), according to instructions of the manufacturer. Secreted levels of CXCL1 and CXCL8 were determined using human chemokine (C-X-C motif) ligand 1 (CXCL1) DuoSet DY275 and human CXCL8 DuoSet DY208 (R&D Systems), respectively. The measurements were performed either in single wells or in duplicate wells, and the results are presented as the mean concentration ± SEM from 4–7 different donors of primary HUVECs.
Immunocytochemistry
For the immunofluorescence experiments, HUVECs were grown on gelatin-coated Lab-Tek chamber slides (Nalge Nunc, Rochester, NY) to sub-confluence. After incubation with the indicated reagents for 24 h, cells were washed with PBS and fixed in 4% paraformaldehyde (PFA) for 10 min. Cells were then washed three times in PBS before quenching of background fluorescence in 50 mM NH4Cl for 10 min at room temperature. Fixed cells were permeabilized using 0.1% Triton X-100 in PBS for 10 min. Nonspecific binding of antibodies was blocked by pre-incubation with 1% BSA in PBS for 30 min. Cells were then incubated with mouse anti-vinculin (clone hVIN-1, Sigma-Aldrich; 1:200) or mouse anti-von Willebrand factor (Dako, Carpinteria, CA; 1:600) antibodies (diluted in 1% BSA in PBS) for 1 h at room temperature. Cells were further incubated for 30 min at room temperature with Alexa Fluor 488- or Alexa Fluor 546-conjugated secondary antibodies (Invitrogen, 1:600). For staining of F-actin filaments, cells were incubated with Rhodamine Phalloidin or Alexa Fluor 488 Phalloidin (Invitrogen; 1:200) together with the secondary antibody. The cells were mounted using SlowFade Gold antifade reagent with DAPI (Invitrogen) and examined with a confocal microscope Olympus FluoView FV1000 (Olympus). The images were processed using Adobe Photoshop CS4 (Adobe Systems Inc., San Jose, CA).
Cell Proliferation Assay
After treatment with siRNA, cells were plated at a density of 10,000 cells/well on a microtiter plate and cultured for 24 h. The cell proliferation assay was performed using CyQuant Direct cell proliferation assay kit following instructions from the manufacturer (Life Technologies). The fluorescence intensity values were used to calculate the percent proliferation of siSDC4 cells relative to siControl cells. The assay was performed on HUVECs from five different donors.
In Vitro Tube Formation Assay
To assess the effects of syndecan-4 knockdown on endo-thelial tube formation, we used the In Vitro Angiogenesis assay kit (Trevigen, Inc., Gaithersburg, MD), according to the manufacturer’s instructions. Control and syndecan-4 siRNA-transfected cells were trypsinized, resuspended in MCDB 131 medium with or without (negative control) growth supplements and plated (10,000 cells/well) on top of growth factor-reduced basement membrane extract (BME) gel. The cells were incubated for 4 h in a CO2 incubator at 37°C before staining with calcein for 30 min. Images were recorded with a Leica DM IL inverted contrast micro-scope with Leica DFC420 digital color camera (Leica Microsystems; Wetzlar, Germany). For quantitative evaluation, representative pictures were analyzed with WimTube (Wimasis; Munich, Germany). The total tube lengths and loop numbers were determined.
Morphometry
Cell shape can be quantified as the degree of roundness. We expressed this parameter as the relationship between the short and the long diameter of the cells. A perfect circle will give the number 1, or 100%, whereas a lower number will indicate a more elongated cell shape. These parameters were determined by visual inspection of pictures acquired from siControl cells and siSDC4 cells. Images were obtained with confocal microscope Olympus FluoView FV1000 from HUVECs prepared from four different donors. We assessed five cells from each of 1–3 different pictures.
Statistical Analysis
Cells from at least three donors were included in all experiments performed. A comparative analysis of the data was carried out using the Student’s paired t-test. The significance levels were *p≤0.05 (5%); **p≤0.01 (1%); ***p≤0.001 (0.1%). Data are presented as the mean ± SEM of the number (n) of indicated experiments.
Results
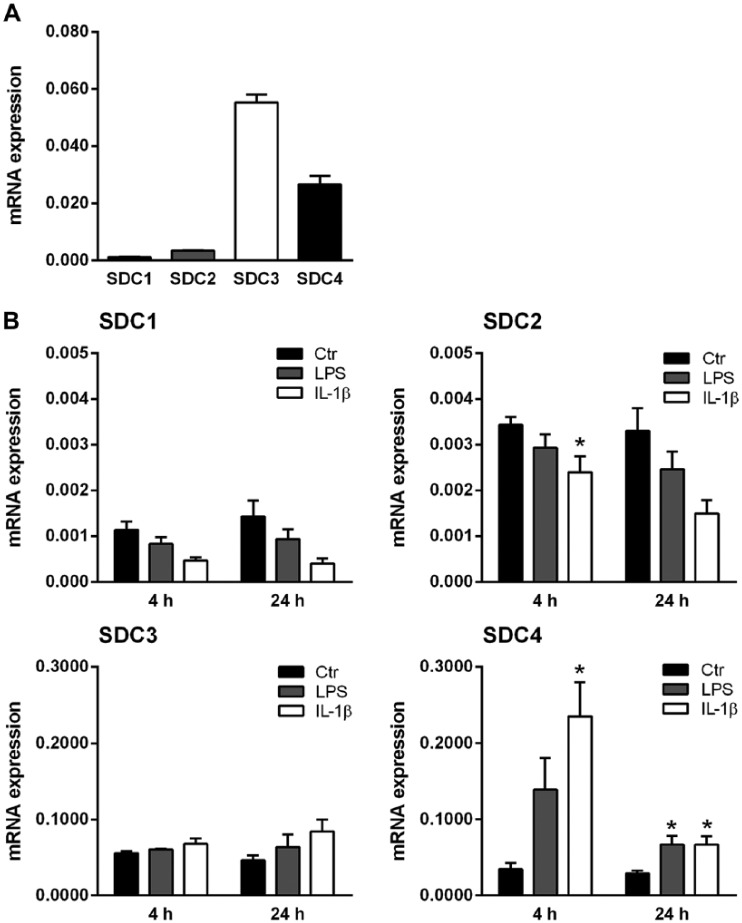
Primary HUVECs were cultured in vitro and the expression levels of the different syndecans were determined using qRT-PCR. Figure 1A shows that syndecan-3 was expressed at the highest levels of all of the four syndecans investigated, followed by syndecan-4. The expression levels of both syndecan-1 and syndecan-2 were low. To address the possible importance of syndecans in the inflammatory response, HUVECs were cultured in the presence of two major inflammatory mediators, LPS and IL-1β. Syndecan mRNA expression levels were determined after 4 and 24 h. As can be seen from Figure 1B, there were obvious differences in the expression levels of the four syndecans after such treatments. Syndecan-1 and syndecan-2 expression levels were down-regulated in a time-dependent manner by LPS, and more so by IL-1β. Syndecan-3 expression, on the other hand, was increased, although not significantly. In contrast, the expression of syndecan-4 showed an early and strong response, with a 4-fold increase with LPS and an 8-fold increase with IL-1β as compared with the control at 4 h. Again, the effect was more pronounced after exposure to IL-1β than to LPS. Furthermore, the stimulation persisted after 24 h, with a significant 2-fold increase in expression relative to control, albeit, at a lower level than that after 4 h; this indicated a transient increase in syndecan-4 expression after stimulation. With the concentrations used, IL-1β treatment of HUVECs was more effective than LPS in regulating syndecan expression. These results show a striking effect of stimulation on syndecan-4 expression. In further studies, we therefore focused on syndecan-4.
Figure 1.
Syndecan mRNA expressions in HUVECs and their response to inflammatory stimuli. (A) Gene expression levels of syndecans (SDC)-1, -2, -3 and -4 were analyzed by qRT-PCR and normalized to the internal control gene RPL30 (n=3). (B) Gene expression of SDC-1, -2, -3 and -4 in untreated cells (Ctr) or after incubation with LPS or IL-1β for 4 and 24 h. Data are presented as the mean ± SEM (n=3–4). A value of p<0.05 is considered statistically significant between treatment and control groups. *p ≤0.05.
Syndecan-4 cell-surface expression in HUVECs was analyzed by flow cytometry in control and LPS/IL-1β-stimulated cells. As can be seen in Figure 2, syndecan-4 expression was increased with both stimulators, and again to a larger extent with IL-1β than with LPS. Furthermore, syndecan-4 levels in conditioned medium were slightly but significantly increased after 4 h of LPS treatment (Fig. 3A). After 24 h, both LPS and IL-1β stimulation resulted in a significant increase in secretion (2.9 and 3.4 times higher, respectively).
Figure 2.
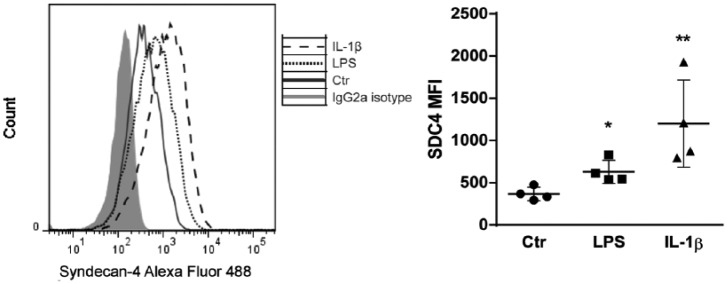
Effect of LPS and IL-1β on syndecan-4 cell-surface expression. HUVECs were cultured with LPS, IL-1β, or left untreated (Ctr) for 24 h. Syndecan-4 cell-surface expression was measured using flow cytometry. Results are presented as mean median fluorescence intensity (MFI) ± SEM. *p<0.05, **p<0.01 indicate statistically significant differences.
Figure 3.
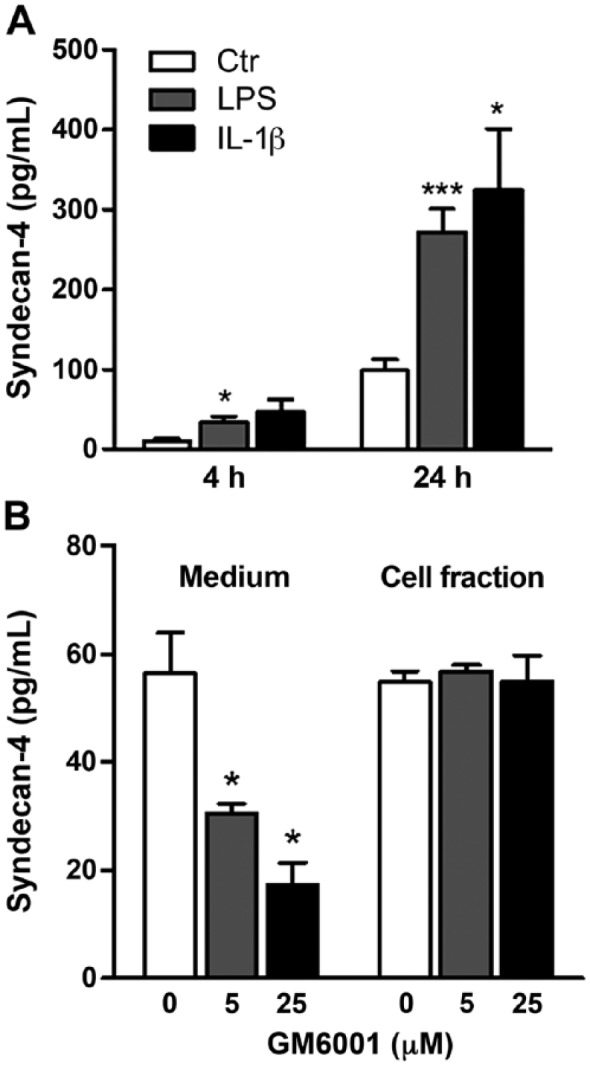
Effect of LPS and IL-1β and matrix metalloproteinase inhibitor, GM6001, on syndecan-4 shedding. (A) HUVECs were exposed to LPS or IL-1β for 4 and 24 h. Supernatants were harvested and the amount of syndecan-4 in the conditioned media was quantified by ELISA (n=6). (B) Syndecan-4 levels in the conditioned media and in cell lysates were determined by ELISA after HUVECs received a 24-h incubation with 5 or 25 µM GM6001 (n=4). A value of p<0.05 is considered a statistically significant difference between the treatment and control. *p<0.05, ***p<0.001.
Syndecan secretion is the result of shedding, involving different types of proteases (Manon-Jensen et al. 2010). To investigate this further, we made use of a wide-range inhibitor of matrix metalloproteinases (MMPs), the compound GM6001. Treating HUVECs with GM6001 resulted in a dose-dependent and significant decrease in syndecan-4 release, as can be seen in Figure 3B. This suggests that a major part of syndecan-4 release from HUVECs in vitro is due to shedding by MMPs. MMP-9, but not MMP-2, gene expression and secretion was increased in stimulated cells, supporting a role for MMP-9 in syndecan-4 shedding in HUVECs (results not shown).
As syndecan-4 is expressed at a high level and stimulated by both LPS and IL-1β, we further investigated if exposures to these two agents would interfere with wound healing using scratch wound assays in cultured HUVECs. However, we could not observe any significant stimulating or inhibitory effects of LPS or IL-1β treatments on the time of wound closure in cultured HUVECs, as shown in Figure 4A and 4B.
Figure 4.
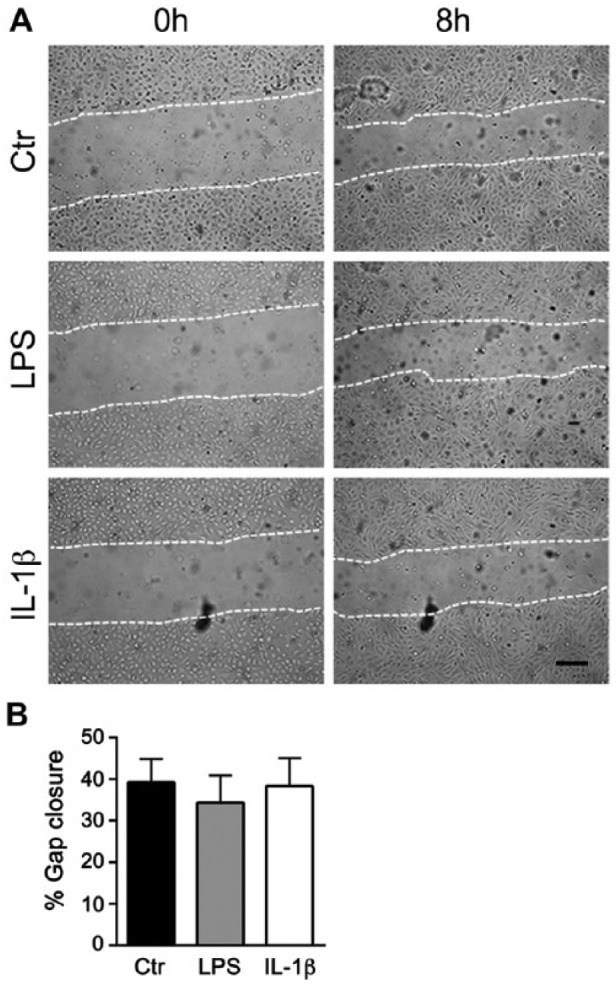
Effect of LPS and IL-1β on wound healing in HUVECs. (A) Representative phase contrast images of scratch wounds at 0 and 8 h after wounding in cells pretreated with LPS or IL-1β for 24 h. (B) Percent gap closure of HUVECs upon treatment with LPS and IL-1β after 8 h (n=3). Scale, 200 µm.
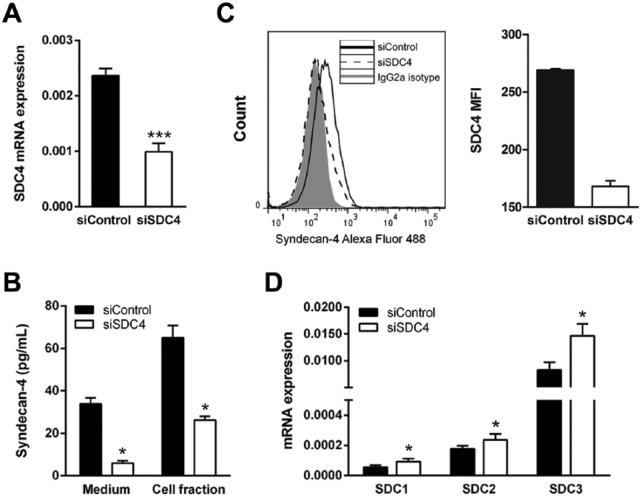
Syndecan-4 has been shown to be present in focal adhesion sites (Couchman 2010). To study the possible roles of syndecan-4 in HUVECs in more detail, we first investigated if LPS and IL-1β treatment could have any effect on focal adhesions. HUVECs were cultured with and without the two stimulating agents, stained for vinculin and actin with an anti-vinculin antibody and phalloidin, respectively. The vinculin staining of focal adhesion sites was generally quite similar in control and stimulated cells, with a trend toward somewhat weaker staining in the stimulated cells. Furthermore, only minor differences were observed after actin staining (data not shown). To investigate further the possible functions of syndecan-4 in HUVECs, we used siRNA to knock-down its expression. We achieved a significant decrease in expression of approximately 40% of that of control siRNA-transfected cells (Fig. 5A). Cell-associated, cell-surface expression and shed syndecan-4 were also significantly decreased (Fig. 5B and 5C). The decrease in syndecan-4 expression resulted in a significant increase in the expression of each of the other three syndecans, as can be seen in Figure 5D. The viability of HUVECs after siRNA treatment was not affected, as determined by trypan blue exclusion (not shown).
Figure 5.
Syndecan-1, -2 and -3 gene expressions in syndecan-4 knockdown HUVECs. Cells were transfected with syndecan-4 siRNA or control siRNA, as described in the Materials & Methods. (A) qRT-PCR analysis of syndecan-4 transcripts in siSDC4 cells (n=9). (B) Syndecan-4 in conditioned media and cell fractions was determined by ELISA (n=3). (C). Cell-surface expression was determined by flow cytometry (n=2). (D) qRT-PCR analysis of the expression of the other syndecan family members after knock down of syndecan-4 (n=10). Results are presented as the mean ± SEM. A value of p<0.05 is considered a statistically significant difference between the treatment and control. *p<0.05 and ***p<0.001.
HUVECs treated with siRNA for syndecan-4 were then further analyzed by light microscopy. The results showed that the morphology of the siSDC4-treated cells differed from that of the control siRNA-treated cells. The decreased expression of syndecan-4 resulted in a more elongated and spread-out morphology, as demonstrated in Figure 6A. Treating HUVECs with a second siRNA sequence resulted in a similar change (Fig. 6B), confirming the effect of syndecan-4 knockdown on HUVEC morphology. Quanti-fication of the morphological changes to cells from the four donors is presented in Figure 6C, with a decrease in average roundness observed after syndecan-4 knockdown. How-ever, treating HUVECs with syndecan-4 siRNA did not affect the expression of the well-established endothelial marker, von Willebrand factor (Fig. 6D), suggesting a rearrangement of the HUVEC cytoskeleton as opposed to changes in differentiation or phenotype.
Figure 6.
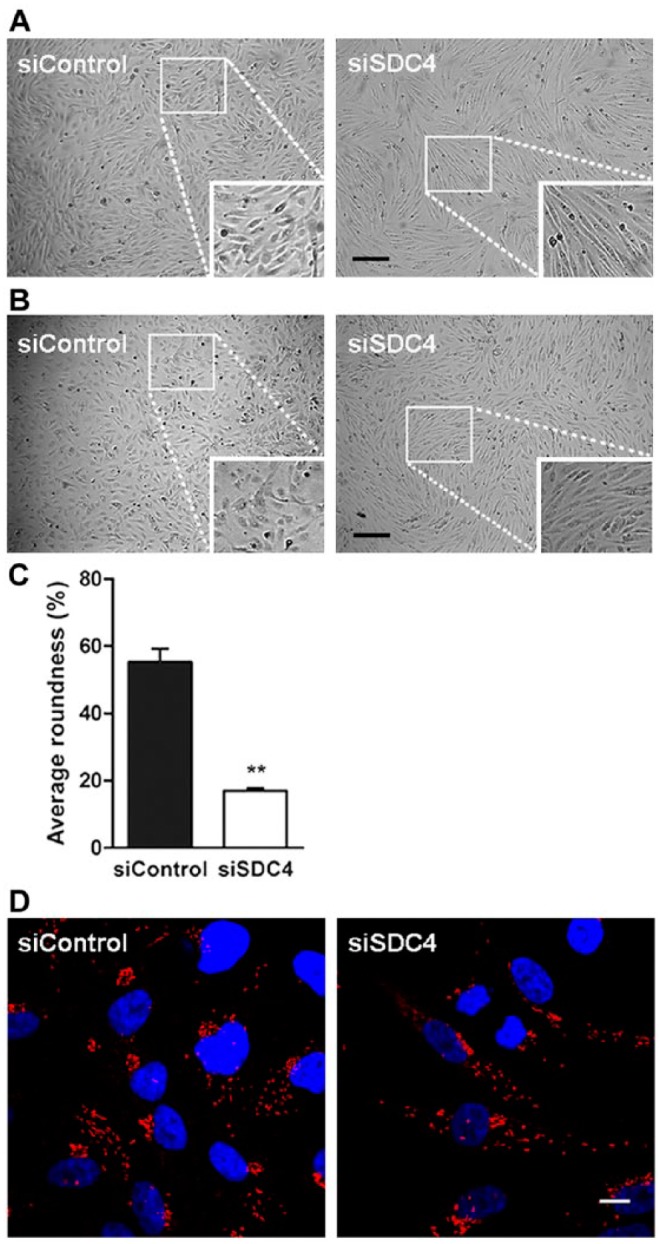
Morphology of HUVECs after syndecan-4 knock down. (A) and (B) show light microscopy images of siRNA-transfected cells from two individual cultures. The cells were transfected with control siRNA and either syndecan-4 siRNA from Santa Cruz (A) or syndecan-4 siRNA from Ambion (B), Scale, 200 µm. (C) Quantification of morphological changes, presented as the % of roundness (n=4). (D) Immunostaining of siRNA-transfected cells for the endothelial marker, von Willebrand factor (vWF), showing the same phenotype in both siControl and siSDC4 cells. Scale, 10 µm. The results show one representative out of three donors. A value of p<0.05 is considered a statistically significant difference. **p<0.01.
To investigate further the effects of decreased syndecan-4 expression on focal adhesions in HUVECs, the cells were stained for vinculin and actin. We observed a change in the distribution of focal adhesions (Fig. 7A). Whereas vinculin in the control cells was present in distinct and clearly visible focal adhesion sites at the cell cortex, the syndecan-4 siRNA-treated cells contained less pronounced focal adhesions that were more evenly distributed throughout the cells. Also, the actin filaments associating with vinculin at focal adhesion sites were affected by syndecan-4 siRNA treatment. From thick bundles with a somewhat heterogeneous pattern in the control cells, the actin filaments in the syndecan-4 knockdown cells became thinner in appearance and aligned in parallel with the cell axis (Fig. 7B). Western blot analysis showed that knockdown of syndecan-4 did not affect the cellular protein levels of actin (Fig. 7C), indicating that the reduction of actin bundles might be due to a disassembly/rearrangement of F-actin as a consequence of decreased syndecan-4 expression.
Figure 7.
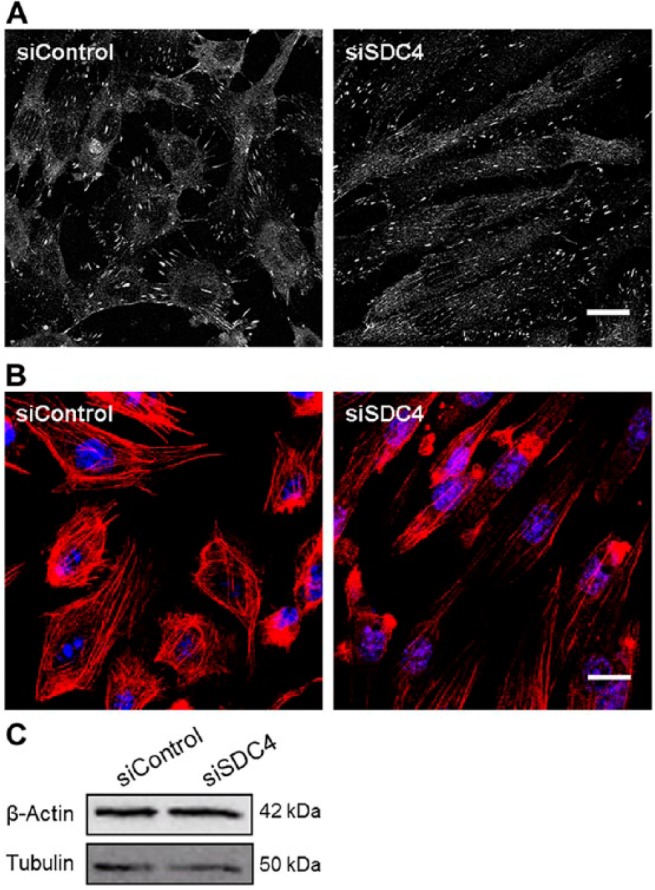
Effect of syndecan-4 knockdown on focal adhesions and actin cytoskeleton in HUVECs. HUVECs treated with siRNA against syndecan-4 (siSDC4) or control (siControl) were plated on gelatin-coated chamber slides, fixed, permeabilized and immunostained for vinculin (A) or stained with rhodamine-phalloidin for F-actin (B). Images are representative for stainings of HUVECs from three different donors. (C) Western blot analysis of cell lysates from siSDC4 and siControl for the expression of β-actin. Tubulin was used as a loading control. Scale, 10 µm.
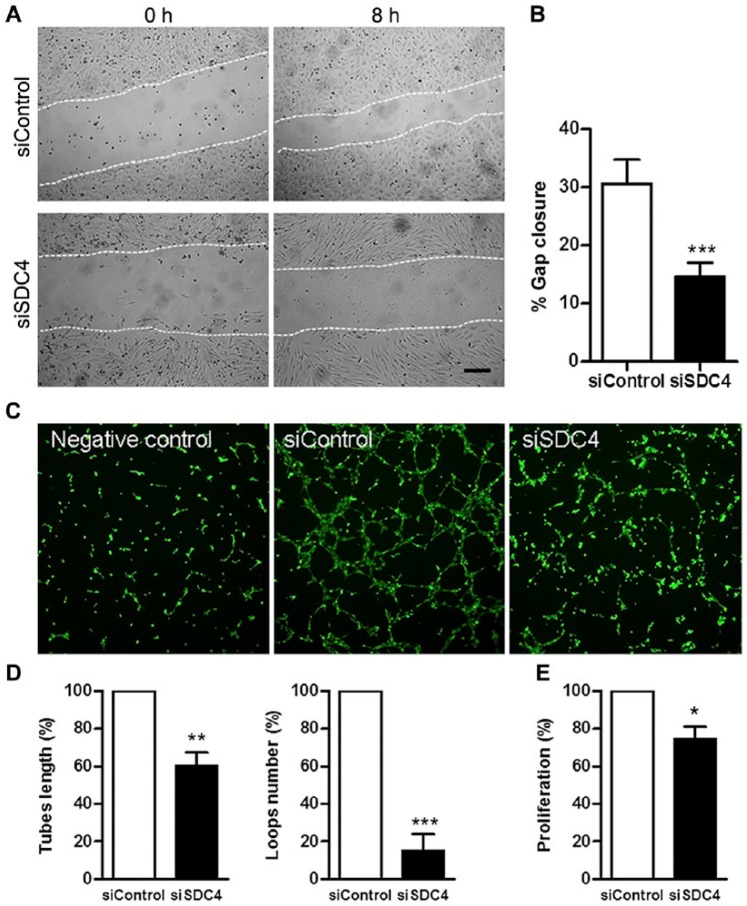
The changes observed in cellular morphology and adhesion in HUVECs with decreased syndecan-4 levels could possibly have effects on other types of cellular behavior. To test this, HUVECs were analyzed by light microscopy at 8 and 24 h after scratch wounds were created in confluent cells. The closure of wounds was delayed in HUVECs with lowered syndecan-4 expression, as demonstrated in Figure 8A and 8B. After 8 h, there was a reproducibly larger area not covered by endothelial cells after syndecan-4 siRNA treatment, suggesting a role for syndecan-4 in wound healing in HUVECs. Furthermore, in vitro tube formation assays showed an inhibition of endothelial angiogenesis in HUVECs with decreased syndecan-4 expression, as demonstrated by a significant reduction in both tube lengths and loop numbers (Fig. 8C and 8D). Also, HUVEC proliferation was reduced in syndecan-4 siRNA-treated cells (Fig. 8E)
Figure 8.
Migration, tube formation, and proliferation of HUVECs in syndecan-4 knock down cells. (A) Phase contrast images of scratch wound assays on confluent monolayers of cells treated with control siRNA (siControl) or siRNA against syndecan-4 (siSDC4) at 0 h and after 8 h. (B) Bar diagram showing the percent gap closure (n=10) of siControl and siSDC4 cells 8 h after wounding. (C) In vitro tube formation assay with negative control (without growth factors; left panel), siControl and siSDC4 cells. (D) Quantification of tube length (left) and loop numbers (right) of siControl and siSDC4 cells formed in basement membrane extract (BME) gels by Wimasis Image Analysis. Data are expressed as the percent of the control (n=5). (E) Cell proliferation assay. HUVECs were treated with siSDC4 or siControl and cultured in microtiter plates for 24 h. Data are expressed as a percent of the control (n=4). All values are the mean ± SEM. *p<0.05, **p<0.01 and ***p<0.001 indicate a significant difference from the control. Scale, 200 µm.
These effects observed in HUVECs with reduced syndecan-4 expression suggest that decreased cell-surface expression can affect both cell growth and migration. It is also possible that lowered shedding of syndecan-4 can also affect the secretion of PG-binding chemokines and growth factors. CXCL1 and CXCL8 are chemokines shown to be involved in the regulation of angiogenesis (Martin et al. 2009; Heidemann et al. 2003; Miyake et al. 2013). Surprisingly, the secretion of the chemokine CXCL8 was significantly increased in the syndecan-4 knockdown cells, as shown in Figure 9A. There was also a trend for an increase in CXCL1. Also, the transcript expressions of growth factors involved in endothelial angiogenesis were shown to be significantly up-regulated after syndecan-4 knock down (Fig. 9B).
Figure 9.
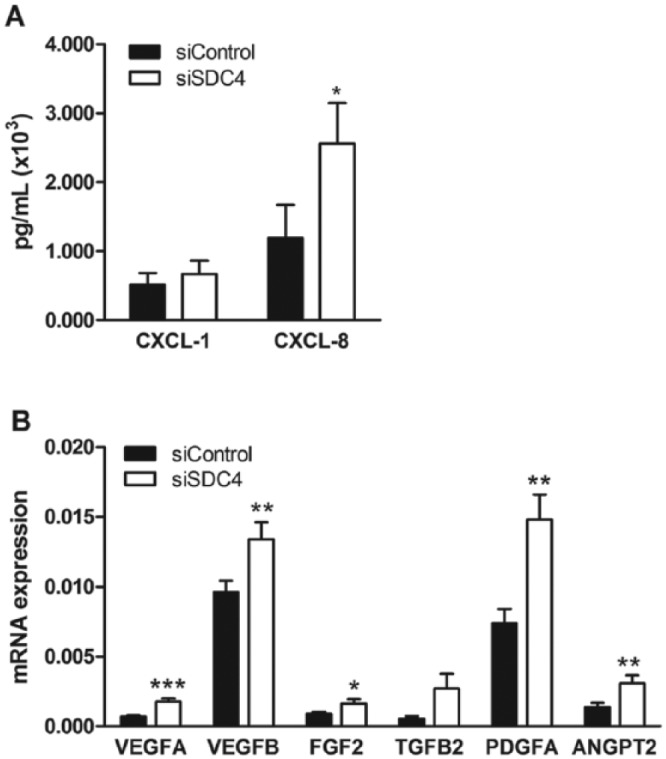
Chemokine (C-X-C motif) ligand 1 (CXCL1) and CXCL8 secretion and growth factor expression in syndecan-4 knocked down HUVECs. (A) ELISA analysis of CXCL1 (n=4) and CXCL8 (n=6) levels in the conditioned media from siControl- and siSDC4-treated cells. (B) qRT-PCR determination of various angiogenic growth factors in siControl- and siSDC4-treated cells (n=6–10). Results are presented as the mean ± SEM, and a value of p<0.05 is considered a statistically significant difference between the treatment and control. *p<0.05 and **p<0.01. Abbreviations: VEGF, vascular endothelial growth factor (A and B); FGF2, fibroblast growth factor 2; TGFB2, transforming growth factor beta-2; PDGFA, platelet-derived growth factor-A.
Discussion
In this study, we have made use of primary human umbilical cord endothelial cells and shown that syndecan-4 is expressed at higher levels in these cells than syndecan-1 and syndecan-2, whereas syndecan-3 was expressed at the highest levels. Focusing on syndecan-4 expression, we have demonstrated that the inflammatory mediators LPS, which mimics a bacterial infection, and IL-1β, which mimics a more generalized inflammatory condition, had different effects on the expression of syndecans. Only syndecan-4 expression increased after 4 h. Syndecan-3 expression was increased to a lesser extent but not until after 24 h. Syndecan-1 and -2, on the other hand, were decreased after both stimuli. Hence, there is a substantial difference in response to such stimuli between the different members of the syndecan family, suggesting different functions in human endothelial cells.
In HUVECs, syndecan-4 was shed into the culture medium in a process involving MMPs. The shedding cleavage sites in the protein cores of syndecan-1 and syndecan-4 have been thoroughly studied (Manon-Jensen et al. 2013) and MMPs and serine proteases have been shown to be of importance. Syndecan-4 shedding in human endothelial cells in vitro can be relevant for several biological processes, such as wound healing, angiogenesis and inflammation. Syndecan-4 has been thoroughly studied in fibroblasts and epithelial cells, and its increased expression has been documented in wounded skin in the epithelium; syndecan-1, by comparison, was more highly expressed in the endothelium in the same wound area (Gallo et al. 1996). However, using syndecan-4 knock-out mice, the authors observed delayed skin wound healing along with impaired angiogenesis. The latter observation is of relevance for the data generated here, suggesting functions for endothelial syndecan-4 in repair processes. In a previous study, we demonstrated increased concentrations of syndecan-1 in the circulation during heart surgery (Svennevig et al. 2008), and this finding was also reported by others (Rehm et al. 2007). A limited number of the same blood samples were later analyzed and shown to also contain elevated levels of syndecan-4 (Kolset SO; unpublished observations), confirming that such shedding is relevant in circulatory stress situations in vivo (Takahashi et al. 2011). Our data demonstrating syndecan-4 expression in HUVECs and increased expression after inflammatory stimuli (Gotte 2003) in vitro are, accordingly, relevant to in vivo situations where the endothelium is activated.
The relevance of our data on delayed endothelial wound healing in vitro after knockdown of syndecan-4 expression is supported by several studies. Skin wound repair studies in mice (Gallo et al. 1996) showed a transient increased expression of syndecan-4 in endothelial cells and fibroblasts in granulation tissue. Syndecan-4 expression peaked 3 days after wound induction and was down to basal levels after 10 days. Syndecan-2 and -3 were not detected, whereas syndecan-1 expression was also evident in endothelial cells and in granulation tissue with a different pattern than that expressed by syndecan-4.
Mice with a deleted syndecan-4 gene show a reduced accumulation of granulation tissue and lower vascular-ization in the wound area as compared to control mice (Echtermeyer et al. 2001). Furthermore, fibroblasts isolated from the knockdown mice show delayed wound closure time in vitro. The latter finding is in line with what we have reported here for human endothelial cells. Syndecan-4 may, therefore, have a general role in wound healing and angiogenesis, as well as a role in the corresponding cell proliferation, migration and tube formation processes necessary for their completion. The data presented here, pointing both to a high expression of syndecan-4 and a role in wound healing, suggest that syndecan-4 can have important functions in endothelial cells. However, syndecan-3 is highly expressed and increased in syndecan-4 knockdown cells (Fig. 5D). Therefore, we cannot exclude the possibility that our observations are due to this increase in syndecan-3 expression. This increase in syndecan-3 was only studied at the mRNA level, and further studies are thus needed to address the possible importance of syndecan-3 in endothelial cell wound healing.
Stimulation of the cells with LPS and IL-1β did not affect the time of wound closure; this is in contrast to what was seen when syndecan-4 was knocked down using siRNA. We observed increased syndecan-4 mRNA and cell-surface expression and secretion after inflammatory stimuli, suggesting that syndecan-4 has important roles in cellular defense reactions. This immediate response, only evident for syndecan-4, was observed already after 4 h (Fig. 1), whereas wound healing is a more time-demanding process. Syndecan-4 may have different functions in these two processes in human endothelial cells. The early increase in syndecan-4 expression is of particular interest and has also been demonstrated in a mouse pneumonia model where LPS was used as a stimulating agent (Tanino et al. 2012); in HUVECs, LPS has been demonstrated to mediate its stimulatory effects through Toll-like receptor-4 (Mako et al. 2010).
Syndecans anchored in the plasma membrane can participate in cellular signaling through many different pathways. Their associations with integrins through their core proteins (syndecan-1) or cytosolic portions (syndecan-4) can facilitate integrin-mediated angiogenesis (Beauvais et al. 2009) and cell attachment in focal adhesion through Rho-mediated increase in FAK phosphorylation, respectively (Saoncella et al. 1999; Wilcox-Adelman et al. 2002). These effects on FAK ultimately lead to changes in cell migration behavior (Llic et al. 1995). Furthermore, FGF-2 treatment of melanoma cells from FAK-deficient mice decreased syndecan-4 expression, and knockdown of syndecan-4 resulted in increased attachment and decreased cellular motility (Chalkiadaki et al. 2009). Hence, syndecan-4 can participate in the regulation of cellular behavior through the binding of growth factors or through its adhesion to factors in the extracellular matrix, both resulting in different types of downstream effects on intracellular signaling (Multhaupt et al. 2009; Xian et al. 2010; Elfenbein et al. 2012; Chaudhuri et al. 2005). Cell-surface syndecans can also modulate cellular behavior through shedding of the ectodomain through the actions of proteases; e.g., MMPS, as shown here (Manon-Jensen et al. 2013). The presence of syndecan-4 on the cell surface fully equipped with several HS chains has also been shown to be of importance in the control of cellular functions (Gopal et al. 2010). A novel aspect of syndecan biology is the possibility that it can participate in gene regulation through different types of interactions after reaching the nucleus (Stewart and Sanderson 2014).
Our data show that, after the syndecan-4 knockdown, HUVECs increased the release of CXCL8, a chemokine known to be important in inflammatory reactions but also as a potential angiogenic factor (Heidemann et al. 2003). Hence, syndecan-4 is involved in controlling the release of such factors from endothelial cells and, consequently, the availability of such factors in proximity to endothelial cells and their surroundings. The significant change in the secretion of CXCL8 observed after syndecan-4 knockdown may affect the autocrine regulation of angiogenesis, as can be hypothesized from the data presented on wound closure and tube formation. Our qRT-PCR data on the increased expression of several growth factors after knockdown also support the possible importance of syndecan-4 in regulating HUVEC proliferation.
In conclusion, our studies highlight the importance of syndecan-4 expression in human endothelial cells. Syndecan-2 has been regarded as the most important endothelial PG; but our studies on primary HUVECs show that syndecan-3 (De Rossi and Whiteford 2013) and syndecan-4 are expressed highest at the mRNA level. Studies from mice clearly demonstrate that syndecan-4 is important in preventing endotoxin shock in the vasculature (Ishiguro et al. 2001), as well as in regulating arterial blood pressure and endothelial cells size (Partovian et al. 2008). Endothelial cells are important in many physiological and pathological processes and the in vitro results presented here suggest that syndecan-4 can participate in such processes.
Acknowledgments
The excellent technical assistance of Van Thi Thu Pham is acknowledged, as is the support from Confocal Microscopy Core Facility Gaustad, Department of Pathology, Oslo University Hospital, Rikshospitalet.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from The South Eastern Norway Regional Health Authority, The Throne Holst foundation and The Norwegian Diabetes Association.
References
- Alexopoulou AN, Multhaupt HA, Couchman JR. (2007). Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol 39:505-28. [DOI] [PubMed] [Google Scholar]
- Beauvais DM, Ell BJ, Mcwhorter AR, Rapraeger AC. (2009). Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Cell Biol 206:691-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. (1999). Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729-77. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. (1992). Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol 8:365-93. [DOI] [PubMed] [Google Scholar]
- Chalkiadaki G, Nikitovic D, Berdiaki A, Sifaki M, Krasagakis K, Katonis P, Karamanos NK, Tzanakakis GN. (2009). Fibroblast growth factor-2 modulates melanoma adhesion and migration through a syndecan-4-dependent mechanism. Int J Biochem Cell Biol 41:1323-1331. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Colles SM, Fox PL, Graham LM. (2005). Protein kinase Cδ–dependent phosphorylation of syndecan-4 regulates cell migration. Circ Res 97:674-681. [DOI] [PubMed] [Google Scholar]
- Couchman JR. (2010). Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26:89-114. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Pataki CA. (2012). An introduction to proteoglycans and their localization. J Histochem Cytochem 60:885-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossi G, Whiteford JR. (2013). A novel role for syndecan-3 in angiogenesis. F1000Res 2:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovas A, Yoneda A, Couchman JR. (2006). PKCα-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci 119:2837-2846. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck PF. (2001). Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Inv 107:R9-R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein A, Lanahan A, Zhou TX, Yamasaki A, Tkachenko E, Matsuda M, Simons M. (2012). Syndecan 4 regulates FGFR1 signaling in endothelial cells by directing macropinocytosis. Sci Signal 5:ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster MM, Wang L. (2010). Endothelial heparan sulfate in angiogenesis. Prog Mol Biol Transl Sci 93:179-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux L, Ilan N, Sanderson RD, Vlodavsky I. (2009). Heparanase: busy at the cell surface. Trends Biochem Sci 34:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R, Kim C, Kokenyesi R, Adzick NS, Bernfield M. (1996). Syndecans-1 and -4 are induced during wound repair of neonatal but not fetal skin. J Investig Dermatol 107:676-683. [DOI] [PubMed] [Google Scholar]
- Gopal S, Bober A, Whiteford JR, Multhaupt HA, Yoneda A, Couchman JR. (2010). Heparan sulfate chain valency controls syndecan-4 function in cell adhesion. J Biol Chem 285:14247-14258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M. (2003). Syndecans in inflammation. FASEB J 17:575-591. [DOI] [PubMed] [Google Scholar]
- Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. (2003). Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem 278:8508-8515. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. (2005). Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol 6:646-656. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, Kusugami K, Saito H, Muramatsu T. (2001). Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem 276:47483-47488. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. (1973). Culture of human endothelial cells from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellén L, Claesson-Welsh L. (2006). Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell 10:625-634. [DOI] [PubMed] [Google Scholar]
- Lambaerts K, Wilcox-Adelman SA, Zimmermann P. (2009). The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opin Cell Biol 21:662-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HC, Couchman JR. (2014). Syndecan-2 regulation of morphology in breast carcinoma cells is dependent on RhoGTPases. Biochim Biophys Acta 1840:2482-2490. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- Ilić D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. (1995). Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- Mako V, Czucz J, Weiszhar Z, Herczenik E, Matko J, Prohaszka Z, Cervenak L. (2010). Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1beta, TNF-alpha, and LPS. Cytometry A 77:962-970. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y, Couchman JR. (2010). Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J 277:3876-3889. [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Multhaupt HA, Couchman JR. (2013). Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J 280:2320-2331. [DOI] [PubMed] [Google Scholar]
- Martin D, Galisteo R, Gutkind JS. (2009). CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem 284:6038-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo I, Kimura-Yoshida C. (2013). Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Curr Opin Genet Dev 23:399-407. [DOI] [PubMed] [Google Scholar]
- Meen AJ, Oynebraten I, Reine TM, Duelli A, Svennevig K, Pejler G, Jenssen T, Kolset SO. (2011). Serglycin is a major proteoglycan in polarized human endothelial cells and is implicated in the secretion of the chemokine GROalpha/CXCL1. J Biol Chem 286:2636-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M, Goodison S, Urquidi V, Gomes Giacoia E, Rosser CJ. (2013). Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab Invest 93:768-778. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. (2007). Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol 8:957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaupt HA, Yoneda A, Whiteford JR, Oh ES, Lee W, Couchman JR. (2009). Syndecan signaling: when, where and why? J Physiol Pharmacol 60 Suppl 4:31-38. [PubMed] [Google Scholar]
- Okina E, Grossi A, Gopal S, Multhaupt HA, Couchman JR. (2012). Alpha-actinin interactions with syndecan-4 are integral to fibroblast–matrix adhesion and regulate cytoskeletal architecture. Int J Biochem Cell Biol 44:2161-2174. [DOI] [PubMed] [Google Scholar]
- Okina E, Manon-Jensen T, Whiteford JR, Couchman JR. (2009). Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand J Med Sci Sports 19:479-489. [DOI] [PubMed] [Google Scholar]
- Partovian C, Ju R, Zhuang ZW, Martin KA, Simons M. (2008). Syndecan-4 regulates subcellular localization of mTOR complex2 and Akt activation in a PKCalpha-dependent manner in endothelial cells. Mol Cell 32:140-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnath R, Foster RR, Qiu Y, Cope G, Butler MJ, Salmon AH, Mathieson PW, Coward RJ, Welsh GI, Satchell SC. (2014). Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J 28:4686-4699. [DOI] [PubMed] [Google Scholar]
- Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. (2007). Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 116:1896-1906. [DOI] [PubMed] [Google Scholar]
- Salmon AHJ, Satchell SC. (2012). Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol 226:562-574. [DOI] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. (1999). Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A 96:2805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MD, Sanderson RD. (2014). Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol 35:56-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpio BE, Riley JT, Dardik A. (2002). Cells in focus: endothelial cell. Int J Biochem Cell Biol 34:1508-1512. [DOI] [PubMed] [Google Scholar]
- Svennevig K, Hoel TN, Thiara AS, Kolset SO, Castelheim A, Mollnes TE, Brosstad F, Fosse E, Svennevig JL. (2008). Syndecan-1 plasma levels during coronary artery bypass surgery with and without cardiopulmonary bypass. Perfusion 23:165-171. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Negishi K, Watanabe A, Arai M, Naganuma F, Ohyama Y, Kurabayashi M. (2011). Serum syndecan-4 is a novel biomarker for patients with chronic heart failure. J Cardiol 57:325-332. [DOI] [PubMed] [Google Scholar]
- Tanino Y, Chang MY, Wang X, Gill SE, Skerrett S, Mcguire JK, Sato S, Nikaido T, Kojima T, Munakata M, Mongovin S, Parks WC, Martin TR, Wight TN, Frevert CW. (2012). Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am J Respir Cell Mol Biol 47:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YH-F, Aquino RS, Park PW. (2012). Molecular functions of syndecan-1 in disease. Matrix Biol 31:3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, Werb Z, Rosen S. (2006). HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: effects on VEGF, FGF-1, and SDF-1. BMC Biochem 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox-Adelman SA, Denhez F, Goetinck PF. (2002). Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem 277:32970-32977. [DOI] [PubMed] [Google Scholar]
- Xian X, Gopal S, Couchman J. (2010). Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res 339:31-46. [DOI] [PubMed] [Google Scholar]