Abstract
Previous research has shown that the right hemisphere processes low spatial frequencies more efficiently than the left hemisphere, which preferentially processes high spatial frequencies. These studies have typically measured RTs to single, briefly flashed gratings and/or have directed observers to attend to a particular spatial frequency immediately before making a judgment about a subsequently presented stimulus. Thus, it is unclear whether the hemispheres differ in perceptual selection from multiple spatial frequencies that are simultaneously present in the environment, without bias from selective attention. Moreover, the time course of hemispheric asymmetry in spatial frequency processing is unknown. We addressed both of these questions with binocular rivalry, a measure of perceptual selection from competing alternatives over time. Participants viewed a pair of rivalrous orthogonal gratings with different spatial frequencies, presented either to the left or right of central fixation, and continuously reported which grating they perceived. At the beginning of a trial, the low spatial frequency grating was perceptually selected more often when presented in the left hemifield (right hemisphere) than in the right hemifield (left hemisphere), whereas the high spatial frequency grating showed the opposite pattern of results. This hemispheric asymmetry in perceptual selection persisted for the entire 30-sec stimulus presentation, continuing long after stimulus onset. These results indicate stable differences in the resolution of ambiguity across spatial locations and demonstrate the importance of considering sustained differences in perceptual selection across space when characterizing conscious representations of complex scenes.
INTRODUCTION
When visual input is consistent with multiple perceptual interpretations, the brain constructs a coherent perceptual interpretation of this ambiguous sensory information to make sense of the surrounding environment. Bistable figures such as the Necker cube and Rubin’s face/vase illusion generate competing perceptual interpretations that alternate over time. The study of perceptual selection—the process of determining which of multiple possible percepts will be dominant at a given time—provides important insights into the bases of conscious awareness. Binocular rivalry is a particularly intriguing bistable phenomenon that occurs when two incompatible images are presented separately to the two eyes at overlapping retinal locations, resulting in perceptual alternation between the images, although the visual stimuli remain constant. Binocular rivalry has been used extensively to study the stimulus and cognitive factors that regulate perceptual selection and its neural substrates (reviewed in Bressler, Denison, & Silver, 2013; Blake & Wilson, 2011). Here, we tested whether a well-known asymmetry in spatial frequency processing between the brain’s two hemispheres influences visual perceptual selection.
Hemispheric asymmetries in spatial frequency processing result from differences in perceptual specialization between the two hemispheres (Ivry & Robertson, 1998; Kitterle, Christman, & Hellige, 1990; Sergent, 1982). Within a stimulus set, identification and discrimination of low spatial frequencies (LSFs) tend to be faster and more accurate for stimuli presented in the left visual field (LVF), whereas high spatial frequencies (HSFs) are more quickly and accurately processed in the right visual field (RVF). This asymmetry has been observed for both sinusoidal gratings (Christman, 1997; Hellige, 1993; Christman, Kitterle, & Hellige, 1991) and spatial frequency-filtered natural scenes (Peyrin, Chauvin, Chokron, & Marendaz, 2003). Moreover, fMRI studies indicate that areas in the left hemisphere respond preferentially to HSF compared with LSF stimuli, whereas the opposite pattern was observed in the right hemisphere (Musel et al., 2013; Peyrin, Baciu, Segebarth, & Marendaz, 2004), and EEG responses are larger in the left compared with the right hemisphere for HSF stimuli and larger in the right than in the left hemisphere for LSF stimuli (Martínez, Di Russo, Anllo-Vento, & Hillyard, 2001).
Hemispheric asymmetry of spatial frequency processing is also known to be task dependent. For example, there are clear interactions between spatial frequency and hemisphere for spatial frequency discrimination (Proverbio, Zani, & Avella, 1997; Kitterle & Selig, 1991) but not for simple detection (Kitterle et al., 1990). However, the effects of hemispheric asymmetry on perceptual selection and conscious representations are unknown.
Ivry and Robertson (1998) introduced the Double Filtering by Frequency (DFF) theory to account for hemispheric asymmetries in spatial frequency processing. The DFF theory is based on a two-stage model of spatial frequency filtering. In the first stage, a range of task-relevant frequencies is selected from the environment. In the second stage, frequencies within the selected range are asymmetrically filtered, with the right hemisphere processing relatively lower frequencies more efficiently and the left hemisphere preferentially processing relatively higher frequencies.
Previous behavioral studies of hemispheric asymmetries in spatial frequency processing have relied primarily upon measures of RTs to single stimuli briefly flashed in either the LVF or RVF. For example, Kitterle and Selig (1991) found that RTs for spatial frequency discrimination of two successively presented sinusoidal gratings were faster for lower spatial frequency gratings (1–2 cpd) in the LVF and for higher spatial frequency gratings (4–12 cpd) in the RVF. Although one of the primary predictions of the DFF theory involves selection from multiple spatial frequencies that are simultaneously present in the environment, there is little direct evidence for this aspect of the theory. One study (Kitterle, Hellige, & Christman, 1992) used gratings with multiple spatial frequency components (a low fundamental and higher harmonics) to compare selective processing of these components in the LVF versus RVF. However, in this study, participants were required to make a single perceptual judgment (either “Are the bars wide or narrow?” or “Are the bars sharp or fuzzy?”) based on a particular frequency component, so simultaneous perceptual processing of both low- and high-frequency components was never assessed within a given trial. Similarly, directing attention to one of two spatial frequency components in a grating while preparing to perform a local- or global-level discrimination of a subsequently presented Navon stimulus differentially modulated the amplitude of alpha band EEG signals in the two hemispheres (Flevaris, Bentin, & Robertson, 2011). In contrast to this previous work, our binocular rivalry study of competition between spatial frequencies provides a direct measure of perceptual selection without bias from selective attention to one of the spatial frequencies.
The DFF model (Ivry & Robertson, 1998) proposes two sequential stages of spatial frequency processing, but little is known regarding the temporal properties of the asymmetric processing that comprises the second stage. For example, it is unclear whether these hemispheric differences persist throughout the entire duration of a continuously presented stimulus or occur only during initial selection of that stimulus from the environment. Peyrin, Mermillod, Chokron, and Marendaz (2006) varied the presentation time of spatial frequency-filtered natural images (preceded by unfiltered natural images) and found the typical pattern of hemispheric asymmetry for brief (30 msec) stimulus durations as well as a right hemisphere advantage for longer (150 msec) exposure durations that was independent of spatial frequency. Although this study suggests that stimulus duration can influence asymmetric processing, it did not investigate the temporal dynamics of hemispheric processing throughout continuous stimulus presentation or for multiple simultaneously presented stimuli containing different spatial frequencies. In this study, we used binocular rivalry to continuously track perceptual selection of multiple spatial frequencies over time, thereby providing a direct measure of the time course of asymmetric processing in the two hemispheres. Our approach allows us to address two novel questions: first, does hemispheric asymmetry in spatial frequency filtering apply to perceptual selection, and second, how long does hemispheric asymmetry in spatial frequency processing persist after stimulus onset?
METHODS
Participants
Fourteen right-handed participants (aged 18–30 years, 11 women), including one of the authors (E.P.), completed this study. All participants provided informed consent, and all experimental protocols were approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley. Each participant completed a 1-hr session composed of two blocks. We collected data from 18 participants but excluded four participants’ data sets from analysis. Of the excluded participants, one initially claimed to be right-handed but later notified the experimenter that he was born left-handed, one was unable to align the stereoscope to position the two monocular stimuli at corresponding retinal locations, and two were missing data from an entire condition because of incorrect response key mapping.
Visual Stimuli
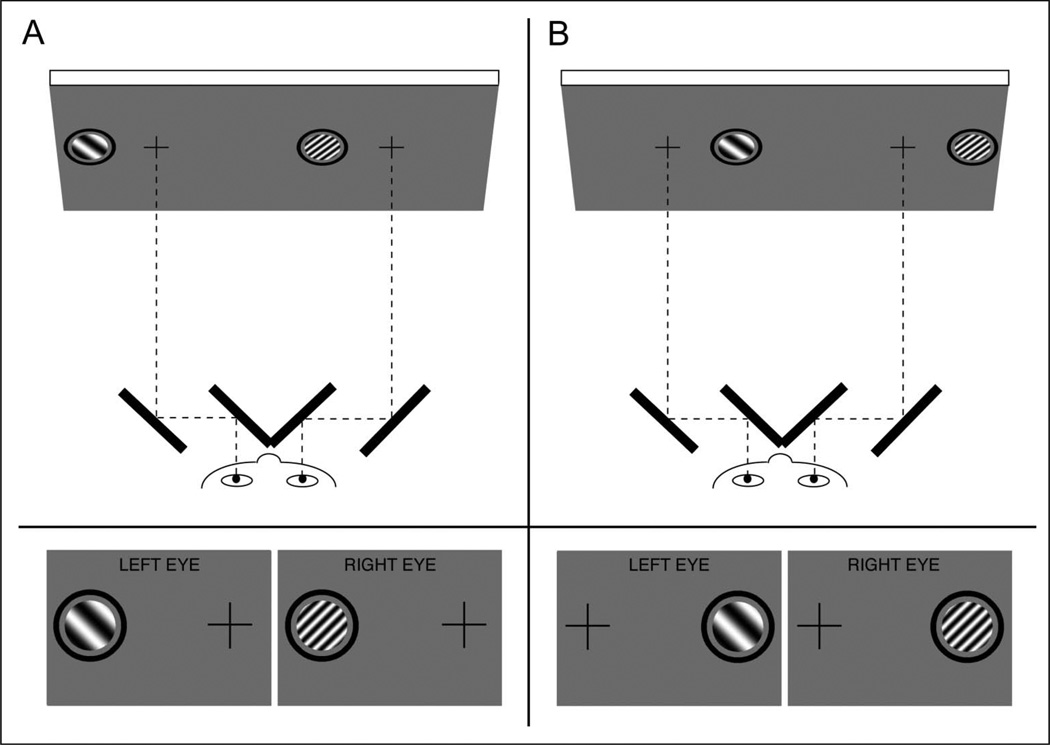
Binocular rivalry displays were generated on a Macintosh PowerPC using MATLAB (The MathWorks, Natick, MA) and Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) and were displayed on a gamma-corrected NEC Multi- Sync FE992 CRT monitor with a refresh rate of 60 Hz at a viewing distance of 100 cm. Participants viewed all stimuli through a mirror stereoscope with their heads stabilized by a chin rest. Stimuli were monochromatic circular patches of sine-wave grating 1.8° in diameter that were surrounded by a black annulus with a diameter of 2.6° and a thickness of 0.2° (Figure 1). Binocular presentation of this annulus allowed it to serve as a vergence cue to stabilize eye position. The stimuli were presented on the horizontal meridian, centered at 3.5° eccentricity either to the left or right of a black central fixation cross. Because the fixation crosses were in the same location on the screen in both hemifield conditions (Figure 1, top), participants’ eye position, relative to the head, was the same in both conditions. All gratings were presented at 100% contrast and had the same mean luminance as the neutral gray background (59 cd/m2). In each trial, one of the two sinusoidal gratings had a spatial frequency of 1 cycle per degree (LSF), and the other had a spatial frequency of 3 cycles per degree (HSF; Figure 1). The two gratings were orthogonal, with ±45° orientations. The spatial frequency and orientation of the grating presented to each eye were fully counterbalanced and randomly selected across trials.
Figure 1.
Top: Schematic of an example visual display and mirror stereoscope in (A) the left visual hemifield condition and (B) the right visual hemifield condition. Bottom: Example images presented dichoptically to the two eyes. Participants maintained fixation on a cross while continuously reporting their perception of rivalrous gratings that were presented to either the left (A) or the right (B) of the fixation cross
Procedure
Before starting the experiment, each participant adjusted the stereoscope by rotating its mirrors until the two eyes’ images (Figure 1, bottom, with the orthogonal gratings replaced by identical figures in both eyes for this adjustment phase) were fused and the participant could see only one cross and one annulus with binocular viewing. All participants completed five practice trials in each hemifield condition before starting the experiment to ensure that they were using the correct response keys and that the stereoscope was properly aligned.
In each trial, the static gratings, fixation cross, and annuli (Figure 1) were presented continuously for 30 sec with a 1500-msec blank interval (consisting of only the fixation cross and annuli) between trials. A brief (250 msec) pure tone auditory cue was presented immediately before the onset of the grating stimuli to signal the beginning of each trial. Throughout each trial, participants used two keys to indicate their percept: grating tilted to the left or grating tilted to the right. Participants were instructed to continuously press a key with their right hand for as long as the corresponding percept was dominant and to not press any key for ambiguous percepts. The experiment was separated into two blocked conditions: left hemifield and right hemifield, the order of which was counterbalanced across participants. Twelve participants completed 28 trials per condition, and two participants completed 32 trials per condition.
RESULTS
Initial Response
At the beginning of a binocular rivalry trial, there is often a period in which participants experience an ambiguous percept (consisting of a patchwork or mixture of the two images), followed by a perceptual alternation between two distinct images. We defined the initial response as the first key press of a trial, as this indicates the participant ’s first percept that clearly corresponded to one of the two gratings. In our study, the average initial response latency (the time between stimulus presentation and the initial response) was 925 msec. Only 15% of the total trials across participants contained any response in the first 500 msec, indicating that participants typically did not have an unambiguous percept until several hundred milliseconds after stimulus onset.
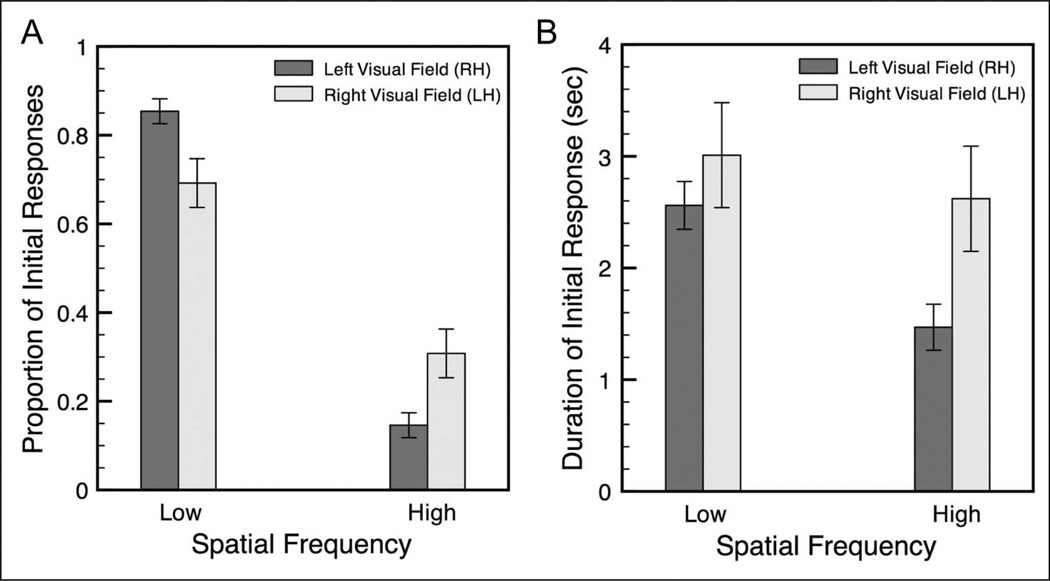
We measured the proportion of initial responses on each trial corresponding to either the LSF or HSF grating and found a highly significant Hemifield × Spatial Frequency interaction across participants (ANOVA, F(1, 13) = 8.23, p < .02, η2p = .39; Figure 2A). More specifically, simple contrasts revealed that participants were more likely to initially perceive the lower spatial frequency in the LVF-RH (left visual field/right hemisphere condition) than in the RVF-LH, F(1, 13) = 8.23, p < .02, and they were more likely to initially see the higher spatial frequency in the RVF-LH than the LVF-RH, F(1, 13) = 8.23, p < .02 (Figure 2A). The results of these two contrasts are identical because the comparisons are complementary; all responses were either LSF or HSF, so the proportion of initial LSF responses = (1 − proportion of initial HSF responses).
Figure 2.
Interaction of spatial frequency and visual hemifield in (A) the proportion and (B) the duration of initial responses to rivalrous stimuli. N = 14. Error bars are SEM across participants.
There was also a highly significant main effect of Spatial Frequency, such that the LSF (1 cpd) grating was initially selected more often than the HSF (3 cpd) grating in both visual hemifields, F(1, 13) = 66.11, p < .001, η2p = .84 (Figure 2A). This main effect is not surprising, given existing knowledge regarding visual processing of different spatial frequencies. Specifically, LSF channels have shorter latencies and integration times than HSF channels (Breitmeyer, 1975), and LSF gratings interfere with orientation discrimination of HSF gratings more than HSF gratings interfere with discrimination of LSF gratings (Hughes, 1986). In addition, LSF stimuli evoke larger neural responses than HSF stimuli (Peyrin et al., 2004). These behavioral and physiological findings are consistent with our observation that initial perceptual selection was generally biased toward the LSF grating. However, this main effect of spatial frequency is orthogonal to our finding of an interaction of hemisphere and spatial frequency in perceptual selection.
Initial Response Duration
Because initial perceptual selection and maintenance of a binocular rivalry percept have been shown to result from separate mechanisms (de Weert, Snoeren,& Koning, 2005), we also measured the duration of the initial response in each trial and again observed a significant Hemifield × Spatial Frequency interaction, F(1, 13) = 7.24, p < .02, η2p = .36 (Figure 2B). Specifically, simple contrasts showed that initial responses corresponding to the HSF grating were significantly longer in the RVF-LH than in the LVF-RH, F(1, 13) = 5.33, p < .05. However, initial response durations for responses corresponding to the LSF were not significantly different between the two hemifield conditions, F(1, 13) = 1.67, p = .22. In addition, initial responses in the LVF-RH condition were significantly longer for LSF than HSF gratings, F(1, 13) = 15.02, p < .01, and there was a trend for initial responses in the RVF-LH condition to be longer for the LSF than HSF, F(1, 13) = 4.48, p = .054. As we found for the initial response type, there was a significant main effect of Spatial Frequency on initial response duration, F(1, 13) = 13.92, p < .01, η2p = .52, with longer durations for LSF than HSF percepts. There was also a trend toward a main effect of Hemifield on initial response duration, F(1, 13) = 3.82, p = .07, η2p = .23.
Time Course of Asymmetric Perceptual Selection
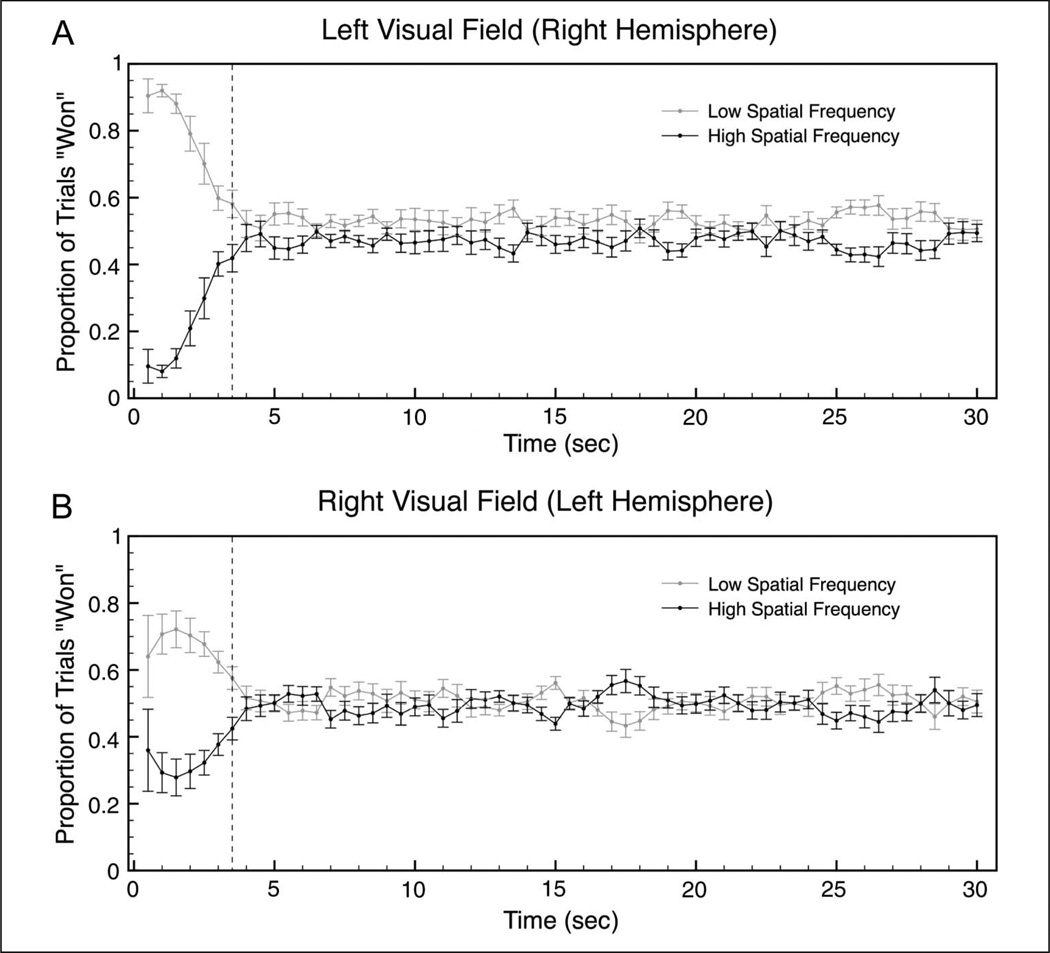
To characterize the persistence of hemispheric asymmetry in perceptual selection following stimulus onset, data from the 30-sec trials were divided into 60 time bins of 500 msec each (1–500 msec, 501–1000 msec, etc.). For each trial, we recorded the total amount of time in each bin that a participant responded either LSF or HSF. Each bin was then classified as “LSF” or “HSF” for that trial, based on a winner-take-all procedure. For each bin, we then computed the proportion of “LSF” trials (of those trials in which there was a response to at least one of the two gratings within that bin) for each participant and then averaged these proportion values across participants (Figure 3). Because all responses were either 1 cpd or 3 cpd, the proportion of “LSF” trials and “HSF” trials for each bin always added to 1, explaining the symmetric pattern of results within each hemifield condition (Figure 3).
Figure 3.
Time course of asymmetric selection of spatial frequencies. Each trial was divided into 500-msec bins; abscissa values correspond to the time at the end of each bin. Using a winner-take-all procedure (see Results), we determined which grating (LSF or HSF) was more dominant in each bin for each trial and plotted the proportion of “won” trials for each spatial frequency in each bin in the (A) LVF and (B) RVF conditions. Because all responses were either 1 cpd or 3 cpd, the two proportion values for each bin always add to 1, thereby explaining the symmetric pattern of results within each hemifield condition. N = 14. The dotted lines indicate the bin in which the initial response terminated, on average. Error bars are SEM across participants.
The strong hemispheric asymmetry present in the first few time bins (Figure 3) (i.e., the relatively stronger bias toward the LSF in the right hemisphere than in the left hemisphere) was primarily due to the initial response (Figure 2A). On average, the termination of the initial response occurred in Bin 7 (3300 msec after stimulus onset). We therefore examined the persistence of hemispheric asymmetry in perceptual selection by separately analyzing the data for initial selection (proportion values collapsed across Bins 1–7; to the left of and including the dotted line in Figure 3) and sustained selection (collapsed across Bins 8–60; to the right of the dotted line in Figure 3).
For Bins 1–7, we found a significant Hemifield × Spatial Frequency interaction, F(1, 13) = 9.60, p < .01, η2p = .43, and a significant main effect of Spatial Frequency, F(1, 13) = 62.43, p < .001, η2p = .83, confirming the pattern of results found for the initial response (Figure 2A). More specifically, simple contrasts again showed that participants were more likely to perceive the lower spatial frequency in the LVF-RH than in the RVF-LH, F(1, 13) = 9.60, p < .01, and they were more likely to perceive the higher spatial frequency in the RVF-LH than in the LVF-RH, F(1, 13) = 9.60, p < .01. Again, the results of these two contrasts are identical because the two proportion values for each bin always add to 1.
For Bins 8–60, we also found a significant Hemifield × Spatial Frequency interaction, F(1, 13) = 5.47, p < .05, η2p = .30, demonstrating hemispheric asymmetry in the perceptual selection of spatial frequencies that persisted well beyond the initial response. Specifically, simple contrasts revealed that participants were more likely to perceive the lower spatial frequency in the LVF-RH than in the RVF-LH, F(1, 13) = 5.47, p < .05, and they were more likely to perceive the higher spatial frequency in the RVF-LH than in the LVF-RH, F(1, 13) = 5.47, p < .05. There was also a significant main effect of Spatial Frequency in Bins 8–60, F(1, 13) = 6.66, p < .05, η2p = .34, but this was once again orthogonal to the Hemifield × Spatial Frequency interaction. These results demonstrate that spatial frequency selection differs between the two hemispheres both during the initial response and throughout the remainder of the stimulus presentation.
DISCUSSION
Our study provides the first evidence of hemispheric differences in perceptual selection of spatial frequencies. Specifically, we found a significant interaction between hemifield and spatial frequency that was in the direction predicted by the DFF theory, such that participants initially perceived the LSF more often in the LVF (right hemisphere) than the RVF (left hemisphere) and initially perceived the HSF more often in the RVF (left hemisphere) than the LVF (right hemisphere). In addition, we have shown that visual hemifield differences in perceptual selection persist throughout the entire duration of stimulus presentation.
An interaction between spatial frequency and visual hemifield has previously been reported for spatial frequency discrimination (Proverbio et al., 1997; Kitterle & Selig, 1991) but was not found for contrast sensitivity or visible persistence of gratings (Peterzell, Harvey, & Hardyck, 1989) or for spatial frequency-dependent detection of gratings (Kitterle et al., 1990). Our findings indicate that the two hemispheres differ in their perceptual selection of spatial frequencies from ambiguous stimuli, each exhibiting a bias toward selecting a visual interpretation that is most consistent with its relative perceptual specialization. This result has important implications regarding the factors that influence conscious perception, as it suggests that distinct types of information are selected at different locations within a visual scene.
Our finding that hemispheric differences persist throughout the entire 30-sec duration of stimulus presentation is the first demonstration that hemispheric asymmetry in spatial frequency processing is not simply a transient mechanism for filtering spatial frequency information. On the basis of previous findings using briefly presented gratings, it was unknown whether differences in preferential processing of frequencies between the two hemispheres occurred only for initial exposure to an image or whether they persisted for the entire duration of presentation. Our results clearly demonstrate that the right hemisphere has a significantly stronger bias toward LSF stimuli than the left hemisphere does and that this asymmetry persists throughout the entire 30-sec trial. This suggests that the right hemisphere plays an important role in continually selecting LSFs (which provide crucial information about global structure and coarse features) from the visual environment, whereas the left hemisphere divides its resources relatively more equally among multiple spatial frequencies.
Robertson (1996) reported spatial frequency-based, sequential priming effects, in the context of an “attentional print” model, that may be related to the persistent biases we report for perceptual selection of spatial frequency. In this previous work, participants judged whether one of two letter targets was present in a Navon stimulus, with the target appearing randomly at either the global (lower spatial frequencies) or local (higher spatial frequencies) level. Level-specific priming (i.e., faster RTs when the local/global level of the target was the same as in the previous trial) occurred regardless of changes across consecutive trials in features that were independent of spatial frequency (e.g., target letter identity, stimulus location, color) and persisted up to the longest intertrial interval that was tested (3 sec). However, when spatial frequency information was altered from one trial to the next, level-based priming was eliminated. This pattern of results was explained by an attentional print that contains information about recently relevant spatial frequencies (i.e., the previous trial). In our study, the initial period of dominance could reflect intrinsic hemispheric biases in spatial frequency selection, resulting in the generation of an attentional print that is maintained throughout the remainder of the trial and influences ongoing perceptual selection.
The hemispheric asymmetry in perceptual selection that we have characterized using binocular rivalry should be distinguished from the interhemispheric switch framework (Pettigrew, 2001; Miller et al., 2000), which postulates that perceptual alternation during binocular rivalry is controlled by midbrain structures. This model is based on results from experimental manipulation of activity in one hemisphere, either through caloric vestibular stimulation or TMS, and proposes that perceptual switches in rivalry are driven by a bistable oscillator circuit that alternately activates each hemisphere. In contrast, our results reflect stable hemispheric differences in processing LSFs versus HSFs.
The neural substrates of binocular rivalry have been the subject of much debate (Tong, Meng, & Blake, 2006; Blake & Logothetis, 2002; Logothetis, Leopold, & Sheinberg, 1996). Physiological correlates of perceptual alternations in binocular rivalry have been observed in areas as early as V1 (Tong & Engel, 2001; Polonsky, Blake, Braun, & Heeger, 2000) and the LGN (Haynes, Deichmann, & Rees, 2005; Wunderlich, Schneider, & Kastner, 2005). However, perceptual (interocular) grouping (Kovács, Papathomas, Yang, & Fehér, 1996) and selective attention (Chong, Tadin, & Blake, 2005) have been shown to influence rivalry, suggesting a contribution of top–down feedback from higher visual areas. Hemispheric asymmetries in spatial frequency processing can reflect relative, not absolute, differences in selected spatial frequencies (Hellige, 1993; Christman et al., 1991), and the same spatial frequency can be preferentially processed by either the left or right hemisphere, depending on the range of task-relevant spatial frequencies (Ivry & Robertson, 1998). This relative nature of hemispheric asymmetries in spatial frequency processing indicates critical roles for context and top–down processing. Although our data do not currently address the role of feedback in hemispheric differences in perceptual selection, future work could employ a broader range of spatial frequencies as stimuli to assess whether relative—not absolute—spatial frequency processing is responsible for the asymmetry we have reported here. Such studies could be combined with neurophysiological measures to elucidate the neural substrates of hemispheric differences in perceptual selection of spatial frequencies.
Acknowledgments
This research was supported by the Department of Defense (through a National Defense Science and Engineering Graduate Fellowship awarded to Elise A. Piazza) and NEI Core Grant EY003176. The authors thank Lynn Robertson and Martin Banks for helpful comments.
REFERENCES
- Blake R, Logothetis NK. Visual competition. Nature Reviews Neuroscience. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R, Wilson H. Binocular vision. Vision Research. 2011;51:754–770. doi: 10.1016/j.visres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Breitmeyer BG. Simple reaction time as a measure of the temporal response properties of transient and sustained channels. Vision Research. 1975;15:1411–1412. doi: 10.1016/0042-6989(75)90200-x. [DOI] [PubMed] [Google Scholar]
- Bressler DW, Denison RN, Silver MA. High-level modulations of binocular rivalry: Effects of stimulus configuration, spatial and temporal context, and observer state. In: Miller SM, editor. The constitution of visual consciousness: Lessons from binocular rivalry. Amsterdam: John Benjamins; 2013. pp. 253–280. [Google Scholar]
- Chong SC, Tadin D, Blake R. Endogenous attention prolongs dominance durations in binocular rivalry. Journal of Vision. 2005;5:1004–1012. doi: 10.1167/5.11.6. [DOI] [PubMed] [Google Scholar]
- Christman S. Hemispheric asymmetry in the processing of spatial frequency: Experiments using gratings and bandpass filtering. In: Christman S, editor. Cerebral asymmetries in sensory and perceptual processing. New York: Elsevier; 1997. pp. 3–30. [Google Scholar]
- Christman S, Kitterle FL, Hellige J. Hemispheric asymmetry in the processing of absolute versus relative spatial frequency. Brain and Cognition. 1991;16:62–73. doi: 10.1016/0278-2626(91)90085-m. [DOI] [PubMed] [Google Scholar]
- de Weert CMM, Snoeren PR, Koning A. Interactions between binocular rivalry and Gestalt formation. Vision Research. 2005;45:2571–2579. doi: 10.1016/j.visres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Flevaris AV, Bentin S, Robertson LC. Attentional selection of relative SF mediates global versus local processing: Evidence from EEG. Journal of Vision. 2011;11(7):1–12. doi: 10.1167/11.7.11. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J-D, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438:496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellige JB. Hemispheric asymmetry: What ’s right and what’s left. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Hughes HC. Asymmetric interference between components of suprathreshold compound gratings. Perception & Psychophysics. 1986;40:241–250. doi: 10.3758/bf03211503. [DOI] [PubMed] [Google Scholar]
- Ivry R, Robertson L. The two sides of perception. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Kitterle FL, Christman S, Hellige JB. Hemispheric differences are found in the identification, but not the detection, of low versus high spatial frequencies. Perception & Psychophysics. 1990;48:297–306. doi: 10.3758/bf03206680. [DOI] [PubMed] [Google Scholar]
- Kitterle FL, Hellige JB, Christman S. Visual hemispheric asymmetries depend on which spatial frequencies are task relevant. Brain and Cognition. 1992;20:308–314. doi: 10.1016/0278-2626(92)90023-f. [DOI] [PubMed] [Google Scholar]
- Kitterle FL, Selig LM. Visual field effects in the discrimination of sine-wave gratings. Perception & Psychophysics. 1991;50:15–18. doi: 10.3758/bf03212201. [DOI] [PubMed] [Google Scholar]
- Kovács I, Papathomas TV, Yang M, Fehér Á. When the brain changes its mind: Interocular grouping during binocular rivalry. Proceedings of the National Academy of Sciences, U.S.A. 1996;93:15508–15511. doi: 10.1073/pnas.93.26.15508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Leopold DA, Sheinberg DL. What is rivalling during binocular rivalry? Nature. 1996;380:621–624. doi: 10.1038/380621a0. [DOI] [PubMed] [Google Scholar]
- Martínez A, Di Russo F, Anllo-Vento L, Hillyard SA. Electrophysiological analysis of cortical mechanisms of selective attention to high and low spatial frequencies. Clinical Neurophysiology. 2001;112:1980–1998. doi: 10.1016/s1388-2457(01)00660-5. [DOI] [PubMed] [Google Scholar]
- Miller SM, Liu GB, Ngo TT, Hooper G, Riek S, Carson RG, et al. Interhemispheric switching mediates perceptual rivalry. Current Biology. 2000;10:383–392. doi: 10.1016/s0960-9822(00)00416-4. [DOI] [PubMed] [Google Scholar]
- Musel B, Bordier C, Dojat M, Pichat C, Chokron S, Le Bas J-F, et al. Retinotopic and lateralized processing of spatial frequencies in human visual cortex during scene categorization. Journal of Cognitive Neuroscience. 2013;25:1315–1331. doi: 10.1162/jocn_a_00397. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Peterzell DH, Harvey LO, Hardyck CD. Spatial frequencies and the cerebral hemispheres: Contrast sensitivity, visible persistence, and letter classification. Perception & Psychophysics. 1989;46:443–455. doi: 10.3758/bf03210859. [DOI] [PubMed] [Google Scholar]
- Pettigrew JD. Searching for the switch: Neural bases for perceptual rivalry alternations. Brain and Mind. 2001;2:85–118. [Google Scholar]
- Peyrin C, Baciu M, Segebarth C, Marendaz C. Cerebral regions and hemispheric specialization for processing spatial frequencies during natural scene recognition. An event-related fMRI study. Neuroimage. 2004;23:698–707. doi: 10.1016/j.neuroimage.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Chauvin A, Chokron S, Marendaz C. Hemispheric specialization for spatial frequency processing in the analysis of natural scenes. Brain and Cognition. 2003;53:278–282. doi: 10.1016/s0278-2626(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Mermillod M, Chokron S, Marendaz C. Effect of temporal constraints on hemispheric asymmetries during spatial frequency processing. Brain and Cognition. 2006;62:214–220. doi: 10.1016/j.bandc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nature Neuroscience. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, Avella C. Hemispheric asymmetries for spatial frequency discrimination in a selective attention task. Brain and Cognition. 1997;34:311–320. doi: 10.1006/brcg.1997.0901. [DOI] [PubMed] [Google Scholar]
- Robertson LC. Attentional persistence for features of hierarchical patterns. Journal of Experimental Psychology: General. 1996;125:227–249. doi: 10.1037//0096-3445.125.3.227. [DOI] [PubMed] [Google Scholar]
- Sergent J. The cerebral balance of power: Confrontation or cooperation? Journal of Experimental Psychology: Human Perception and Performance. 1982;8:253–272. doi: 10.1037//0096-1523.8.2.253. [DOI] [PubMed] [Google Scholar]
- Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411:195–199. doi: 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends in Cognitive Sciences. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Schneider KA, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nature Neuroscience. 2005;8:1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]