Abstract
Introduction
In most patients with type 2 diabetes mellitus (T2DM) and progressive beta-cell insufficiency, insulin therapy is required to achieve sufficient glycemic control. However, insulin therapy may lead to weight gain and increasing risk of hypoglycemia. Glucagon-like peptide-1 receptor agonists are being used as add-on therapy to insulin with favorable metabolic effects. Nonetheless, to date only few studies exist reporting on the combination of liraglutide and insulin with a short follow-up period. The aim of this study was to evaluate the efficacy and safety of liraglutide as add-on to insulin in patients with T2DM over a time period of up to 24–28 months.
Methods
Data of patients with T2DM, treated with insulin and liraglutide at an outpatient clinic in a tertiary referral hospital from October 2009 until December 2011 were retrospectively examined (n = 36). Glycated hemoglobin (HbA1c), weight, total daily insulin dose and side effects were assessed 5–8 months prior to liraglutide, at baseline and at follow-up visits after 3, 6, 12–16 and 24–28 months.
Results
Median HbA1c decreased significantly from 7.7% [interquartile range (IQR) 7.0–8.6] at baseline to 6.8% (IQR 6.5–7.7, p = 0.001) at 3 months and 6.9% (IQR 6.3–7.6, p = 0.0001) at 6 months, but re-increased thereafter (at 24–28 months, median 7.5%, IQR 7.1–8.2, p = 1.0). Median weight decreased significantly from 99.8 kg (IQR 81–110) at baseline to 97.7 kg (IQR 81.2–108.2, p = 0.027) at 3 months, but rose again thereafter. Insulin dosage did not change significantly over time. No severe hypoglycemia or major side effects occurred.
Conclusions
In this observational study, adding liraglutide to insulin in daily clinical practice reduced HbA1c significantly within 6 months, but there may be a non-sustainable effect during long-term treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-014-0093-8) contains supplementary material, which is available to authorized users.
Keywords: HbA1c, Insulin therapy, Liraglutide, Type 2 diabetes mellitus, Weight
Introduction
Lifestyle modification and oral antidiabetic drugs are first line treatment measures in patients with type 2 diabetes mellitus (T2DM). Over time, progressive beta cell dysfunction results in deteriorating glycemic control requiring insulin therapy. However, insulin is often associated with weight gain and increased risk of hypoglycemia [1]. Together with the need for (multiple) injections, insulin therapy frequently meets reluctance in both patients and physicians, and initiation of therapy is often delayed [2]. Several trials showed that Glucagon-like peptide-1 (GLP-1) receptor agonists as monotherapy or in combination with one or more oral antidiabetic agent have beneficial metabolic effects leading to improved glycemic control with a low risk of hypoglycemia, and weight loss [3]. They are now recommended as one of several two-drug combination therapies, if metformin monotherapy does not achieve/maintain the defined glycated hemoglobin (HbA1c) target over 3 months [4].
Several reports demonstrated favorable effects of a combination therapy of a GLP-1 receptor agonist with insulin [5]. However, only few studies included the use of liraglutide [6–10], and follow-up time was short. Despite these limited data, liraglutide is widely used (off-label) as add-on therapy in patients on various insulin regimens and oral antidiabetic drugs. In 2013, liraglutide was officially approved for use in combination with basal insulin in Europe (and the US). The aim of this observational study was to retrospectively assess the efficacy and safety of liraglutide as add-on therapy to insulin in a daily clinical practice setting for a duration up to 24–28 months.
Methods
Eligible participants were adults (18–80 years) with T2DM, BMI ≥ 28 kg/m2 and HbA1c values of 6.5–10% treated with metformin ± another oral antidiabetic drug and insulin. Exclusion criteria were pregnancy and intolerance for liraglutide. The authors reviewed the charts of all patients with T2DM with liraglutide being added-on to insulin from October 2009 until December 2011 at their outpatient clinic in a tertiary referral hospital. Weight, body mass index (BMI), HbA1c and insulin dose (units/day, units/kg/day) were assessed 5–8 months prior to liraglutide, at baseline, after 3, 6, 12–16 and 24–28 months.
Starting dose of liraglutide was 0.6 mg once daily, with an increase to 1.2 mg after 2 weeks. During the treatment period, no additional antidiabetic drug was initiated. Oral drugs and insulin dosage were individually reduced by the attending physician according to current glucose control. As the outpatient clinic is part of a teaching hospital, all physicians followed the same treatment strategies according to the guidelines of the Swiss Society of Endocrinology and Diabetes [11]. Hypoglycemic events were assessed by patient interview and patients’ blood glucose meter readings were imported into the DIABASS® Pro Software, Mediaspects GmbH, Konstanz, Germany. Severe hypoglycemia was defined as an event requiring assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions [12].
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. Informed consent was obtained from all patients for being included in the study.
For statistical analysis linear mixed models with random intercept were used to examine changes in endpoints over time from baseline, after 3, 6, 12–16 and 24–28 months. Such regression models do not require complete data (unlike in analysis of variance), so all available data was analyzed [13]. All analysis was performed in the R programming language (version 3.0.1, R Core Team 2013 Center for Statistics, Copenhagen, Denmark). The package lme4 was used to estimate the mixed models, while the multcomp [14] was used to compute the p values and confidence intervals.
Results
A total of 36 patients was on this combined therapy regimen. After the baseline visit, four patients preferred to be followed up by their family physician and were therefore not included in this follow-up. Three patients stopped liraglutide due to exanthema at the injection site, nausea and abdominal pain, respectively. Among the remaining 29 patients, median age was 59 years [interquartile range (IQR) 53–67], disease duration 11 years (IQR 5–13), HbA1c 7.7% (IQR 7.0–8.6), weight 99.8 kg (IQR 81–110) and BMI 33.5 kg/m2 (IQR 29.4–37.6). At baseline, 17 were on basal insulin, 2 on prandial insulin and 10 on multiple insulin injections. In addition, 24 patients were on metformin, 12 on sulfonylureas, and 6 on other oral antidiabetic agents.
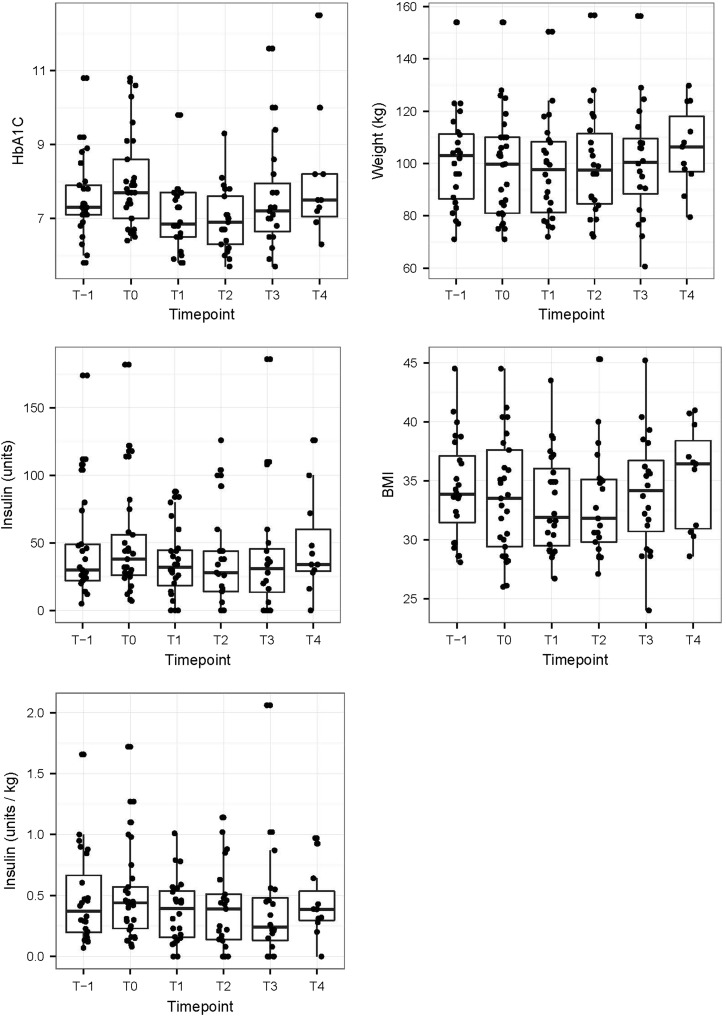
Median HbA1c decreased significantly from 7.7% (IQR 7.0–8.6) at baseline to 6.8% (IQR 6.5–7.7, p = 0.001) at 3 months and 6.9%, (IQR 6.3–7.6, p = 0.0001) at 6 months, but re-increased at 12–16 months (median 7.2%, IQR 6.7–7.9, p = 0.32) and 24–28 months (median 7.5%, IQR 7.1–8.2, p = 1.0). HbA1c prior to intervention was lower (median 7.3%, IQR 7.1–7.9) than at baseline. Weight and BMI decreased significantly from 99.8 kg and 33.5 kg/m2 (IQR 81–110 and 29.4–37.6) respectively at baseline to 97.7 kg and 31.9 kg/m2 (IQR 81.2–108.2 and 29.4–36), (p = 0.023 and 0.027) at 3 months. However weight and BMI increased again after 6 months (median 97.5 kg and 31.5 kg/m2, IQR 84.5.5–111.5, p = 0.16 and 29.4–35.1, p = 0.15), at 12–16 months (100.5 kg and 34.1 kg/m2, IQR 100.5–109.5, p = 0.17 and 30.7–36.7, p = 0.16) and at 24–28 months (106.4 kg and 36.4 kg/m2, IQR 96.9–118, p = 1.0 and 30.9–38.4, p = 1.0). Insulin dosage did not change significantly over time (Fig. 1). However, during follow-up, three patients (10.3%) were able to completely stop insulin therapy, and two patients (6.9%) could simplify their multiple daily insulin injection regimens to basal insulin at bedtime only. Furthermore, sulfonylureas were stopped in 5 of 12 patients (41.7%) and all other antidiabetic drugs beside metformin were stopped (e.g. glitazones, glinides). No severe hypoglycemia occurred over the entire period. Overall, 5 patients (15.6%) out of 32 followed-up patients discontinued liraglutide due to side effects. Three patients stopped liraglutide due to exanthema at the injection site, nausea or abdominal pain at the beginning and two patients discontinued liraglutide due to nausea/vomiting and pain at the injection site after 3.5 and 12 months.
Fig. 1.
Course of glycated hemoglobin (HbA1c), insulin (total units and units per kg body weight), weight and body mass index (BMI) prior (T-1), at baseline (T0) and during follow-up after 3 (T1), 6 (T2), 12–16 (T3) and 24–28 months (T4)
Discussion
This retrospective study shows that liraglutide as add-on therapy to insulin therapy decreases HbA1c and weight during the first 6 months with a fading effect thereafter. No serious hypoglycemic event occurred. The most frequently reported side effects were nausea/vomiting and pain/exanthema at the injection site, leading to discontinuation of liraglutide in 15.6% of the patients.
The short-term findings on HbA1c and weight reduction are in accordance with earlier randomized and observational studies over 12 weeks on liraglutide added to insulin, which found a reduction of HbA1c by 1.4–1.9% and body weight by 5.1–5.6 kg, respectively [7, 8].
A recent report showed a decrease in HbA1c levels of 0.77% and 4.5 kg weight reduction over 26 weeks [10]. A most recent meta-analysis of 15 studies on GLP-1 agonists in combination with basal insulin with a maximum observation time of 36 weeks (mean 24.8 weeks) yielded an HbA1c reduction of 0.44% and weight loss of 3.22 kg [15]. The longest follow-up was in a retrospective study until 38 weeks, where body weight also re-increased in 20 patients on liraglutide or exenatide in combination with insulin [6], similar to the patient population in the current study. Results with longer follow-up time are only available in the reverse situation of basal insulin added on to metformin and liraglutide in a prospective study, showing sustained HbA1c and weight reduction even after 52 weeks [16].
Several reasons may account for the unsustained effect of liraglutide on body weight and HbA1c beyond 6 months in the patients presented here. Firstly, reduction of insulin dose or termination of oral antidiabetic drugs may have led to the re-increase in HbA1c. Secondly, no antidiabetic treatment has been shown to prevent deterioration of β-cell function, which drives the natural history of the disease [1]. Thirdly, the authors often observe that patient adherence, especially regarding dietary restrictions, fades after a few months of starting liraglutide, which may be explained by the disappearing gastrointestinal side effects. Subjects willing to participate in a prospective study are supposedly more motivated and adherent to improve their glycemic control in general. Finally, subjects early achieving adequate glycemic control within a few weeks or months usually return to their referring physicians, whereas the more challenging patients remained in the tertiary clinic and were eligible for this study. The clinical setting reflects a real world situation and shows the difficulties which diabetologists face in their daily work.
In contrast to other studies with GLP-1 receptor agonists and insulin [17], there was no statistically significant reduction in daily insulin dose in the present study. However, a remarkable proportion of the patients either completely ceased insulin or at least were able to omit prandial insulin injections. Moreover, in many of the patients, oral antidiabetic drugs (beside metformin) could be stopped, which is especially beneficial for patients with sulfonylureas bearing an increased risk of hypoglycemia.
In accordance with earlier reports, liraglutide was well tolerated. Remarkably, no events of severe hypoglycemia were observed in the present cohort. The dropout rate of 15.6% due to side effects was comparable to other reports [18, 19].
Limitations of this study are the small patient number and potentially heterogeneous patient characteristics, including patients with long-standing diabetes as well as the retrospective study design. As this was an observational study, there was no control group. The fact that HbA1c was increasing before baseline may point to a regression towards the mean. The setting reflects the real life situation in an outpatient clinic of a tertiary referral hospital covering around 500,000 inhabitants. During the observational period, combination therapy of liraglutide and insulin was not yet approved by Swissmedic (Swiss agency for the authorisation and supervision of therapeutic products), which explains the small number of patients. However, a major strength of this study is the long observational period of up to 24–28 months, as compared to most earlier studies with a follow-up time of usually 24 weeks with a few lasting up to 38 weeks. In the meantime, add-on therapy of liraglutide to basal insulin was approved due to its unequivocal positive effect. The combination of liraglutide with multiple insulin injections, however, is still awaiting approval.
Conclusion
Adding liraglutide to pre-existing insulin therapy in T2DM reduces HbA1c and body weight significantly during the initial 6 months of treatment, but there may be a non-sustainable effect during long-term treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
No funding was received for this study or publication of this manuscript. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. The authors wish to thank Sarah Roberta Haile and Nicole Graf (Clinical Trials Unit Kantonsspital St. Gallen) for statistical evaluation of the data.
Conflict of interest
Christof Lipowsky received unrestricted grant support for clinical studies from Novo Nordisk. Lisa Sze, Ina Krull, Michael Brändle declare they have no conflicts of interest.
Compliance with ethics
All procedures followed were in accordance with the ethical standards of the committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 and 2008. The study was approved by the local ethics committee and informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed]
- 2.Jabbour S. Primary care physicians and insulin initiation: multiple barriers, lack of knowledge or both ? Int J Clin Pract. 2008;62:845–847. doi: 10.1111/j.1742-1241.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- 3.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344 (Review). [DOI] [PMC free article] [PubMed]
- 4.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglyceaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 5.Holst JJ, Vilsbøll T. Combining GLP-1 receptor agonists with insulin: therapeutic rationales and clinical findings. Diabetes Obes Metab. 2013;15:3–14. doi: 10.1111/j.1463-1326.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 6.Lind M, Jendle J, Torffvit O, Lager I. Glucagon-like peptide 1 (GLP-1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes. 2012;6:41–46. doi: 10.1016/j.pcd.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U-500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther. 2011;13:592–595. doi: 10.1089/dia.2010.0221. [DOI] [PubMed] [Google Scholar]
- 8.Li CJ, Li J, Zhang QM, et al. Efficacy and safety comparison between liraglutide as add-on therapy to insulin and insulin dose-increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol. 2012;11:142. doi: 10.1186/1475-2840-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON) Diabetes Obes Metab. 2014;16:636–644. doi: 10.1111/dom.12262. [DOI] [PubMed] [Google Scholar]
- 10.de Wit HM, Vervoort GM, Jansen HJ, de Grauw WJ, de Galan BE, Tack CJ. Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT) Diabetologia. 2014;57:1812–1819. doi: 10.1007/s00125-014-3302-0. [DOI] [PubMed] [Google Scholar]
- 11.Philippe J, Brändle M, Carrel J, et al. Massnahmen zur Blutzuckerkontrolle bei Patienten mit Typ-2 Diabetes mellitus. Consensus statement der Schweizerischen Gesellschaft für Endokrinologie und Diabetologie (SGED) Schweiz Med Forum. 2009;9(3):50–55. [Google Scholar]
- 12.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 14.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 15.Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the managment of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014. doi:10.1016/S0140-6736(14)61335-0. [DOI] [PubMed]
- 16.Rosenstock J, Rodbard HW, Bain SC, Liraglutide-Detemir Study Group et al. One-year sustained glycemic control and weight reduction in type 2 diabetes after addition of liraglutide to metformin followed by insulin detemir according to HbA1c target. J Diabetes Complicat. 2013;27:492–500. doi: 10.1016/j.jdiacomp.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Rosenstock J, Sesti G, LEAD-6 Study Group et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 18.DeVries JH, Bain SC, Rodbard HW, Liraglutide-Detemir Study Group et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35:1446–1454. doi: 10.2337/dc11-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponzani P. Long-term effectiveness and safety of liraglutide in clinical practice. Minerva Endocrinol. 2013;38:103–112. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.