Abstract
Lipoprotein associated phospholipase A2 (Lp-PLA2) - an enzyme with several pro-inflammatory properties - has been hypothesized to be involved in the pathogenesis of atherosclerosis and plaque vulnerability. Lp-PLA2 activity has been demonstrated to be an independent predictor of coronary heart disease (CHD) and ischemic stroke. However, it has been recently reported that carriers of loss of function variants in PLA2G7 gene (encoding Lp-PLA2) had no lower CHD risk than non-carriers. The Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY), and the Stabilization of Plaque Using Darapladib - Thrombolysis in Myocardial Infarction 52 (SOLID-TIMI 52) studies are two phase III, randomized, placebo-controlled, clinical trials that were conducted to evaluate the clinical efficacy and safety of the Lp-PLA2 inhibitor (darapladib), against a background of optimal medical therapy, in patients with stable CHD and acute coronary syndrome, respectively. In both studies, darapladib failed to reduce the risk of major coronary events as compared to placebo. In addition, darapladib was associated with significantly higher rates of drug discontinuation, and adverse side effects such as diahrrea and malodorous feces, urine, and skin, as compared to placebo. These data suggest that Lp-PLA2 may be a biomarker of vascular inflammation rather than a causal pathway of cardiovascular (CV) diseases. It also challenges the notion that inhibition of Lp-PLA2 is a worthwhile approach in patients with CHD. Alternate therapies that target inflammation are awaited to reduce residual risk in patients with CV diseases.
Introduction
Inflammation plays a crucial role in the pathogenesis, progression, and rupture of atherosclerotic plaques. Lipoprotein-associated phospholipase A2 (Lp-PLA2) - also known as platelet-activating-factor acetylhydrolase (PAF-AH) - is an enzyme synthesized in macrophages and activated platelets, which co-travels with circulating low density lipoprotein (LDL) particles, and is expressed abundantly in atherosclerotic plaques.
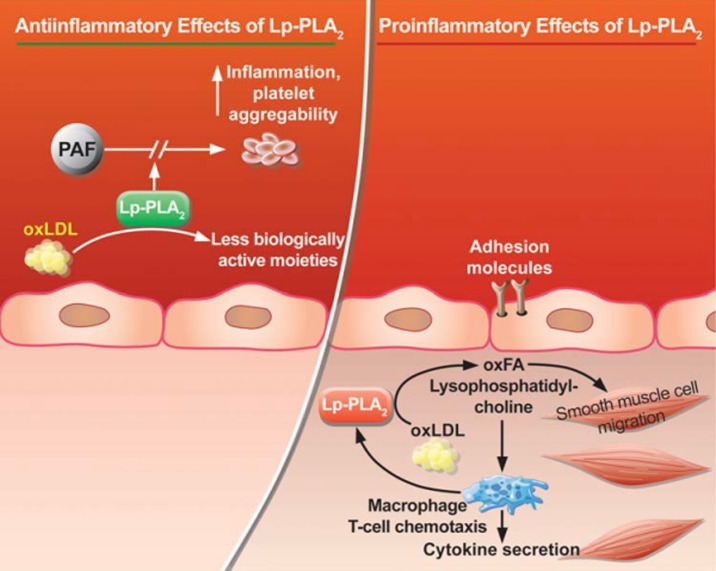
The biological role of Lp-PLA2 has been controversial (Figure 1) with initial reports demonstrating atheroprotective effects thought to be a consequence of the enzymatic catabolism of the pro-inflammatory mediator PAF and its analogues formed upon oxidation of LDL.1,2 However, recent findings have ascribed several pro-inflammatory properties for this enzyme that could be pro-atherogenic; Lp-PLA2 hydrolyzes oxidized phospholipids generated during LDL oxidation and leads to the formation of lysophosphatidyl-choline and oxidized non-esterified fatty acids, which up-regulate monocyte adhesion molecules and monocyte chemotactic protein-1 that elicit several inflammatory responses.2,3 Lp-PLA2 acts preferentially on oxidized phospholipids with no effect on naturally occurring phospholipids in cellular membranes.
Figure 1.
The controversial role of Lp-PLA2 (From Munzel et al. European Heart Journal 2009; 30: 2829–2831).4
In a recent meta-analysis including 79,036 participants in 32 prospective studies, circulating Lp-PLA2 mass and activity showed consistent and significant association with increased risk of coronary heart disease (CHD).5 Moreover, Lp-PLA2 activity has been demonstrated to be an independent predictor of CHD and ischemic stroke – even after adjustment for traditional cardiovascular (CV) risk factors and C-reactive protein.6–8
These findings have prompted the development of inhibitors to this enzyme as a potential therapeutic tool in patients with CV diseases. Darapladib is a selective reversible potent oral inhibitor of Lp-PLA2 activity that has been initially reported to prevent necrotic core expansion - a key determinant of plaque vulnerability - in 330 patients with angiographically documented coronary artery disease evaluated by intravascular coronary ultrasound.9 This effect was achieved without alteration in serum lipoprotein levels.9
In line with the clinical utility of genetic studies to predict the success of novel drugs to reduce the risk of CV diseases - such as targeting proprotein convertase subtilisin kexin 9, and Niemann–Pick C1 like 1 protein -10–12, PLA2G7 genetic variants (encoding Lp-PLA2) were analyzed in order to evaluate whether its relation to CV diseases is causal. In addition, several randomized studies have been recently conducted and their results are reviewed here to demonstrate the clinical utility versus futility of the Lp-PLA2 inhibitor (darapladip) as a novel therapeutic approach in CV management.
PLA2G7 variants and CHD risk
The exomes of 6325 participants in the Atherosclerosis Risk in Communities (ARIC) study were sequenced in the Human Genome Sequencing Center at Baylor College of Medicine in order to find out PLA2G7 variants that lower Lp-PLA2 activity. The results were recently published in a research letter in the New England Journal of Medicine in January 2015.13
After an average of 25 years of follow up, loss of function variants had no significant effect on levels of CV risk factors. Moreover, carriers of loss of function variants had no lower CHD risk than non-carriers in the Americans of either European [hazard ratio (HR) 1.06; 95% confidence interval (CI) = − 0.33 to 2.45; p = 0.93] or African [HR 0.92; 95% CI = 0.35 to 1.49; p = 0.78] ancestry.13
Stability Study
The Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial was a phase III, multicenter, randomized, double-blind, placebo-controlled trial that was published in the New England Journal of Medicine in May 2014.14 The study aimed to investigate the clinical efficacy and safety of darapladib -when added to standard of care- in patients with stable CHD [prior myocardial infarction (MI), prior percutaneous coronary intervention (PCI) or coronary-artery bypass grafting (CABG), or multivessel coronary artery disease]. A total of 15,828 patients were randomly assigned to receive either a once-daily oral dose of darapladib (160 mg) or matching placebo to be taken with food. The assigned dose of darapladib was expected to lower plasma levels of Lp-PLA2 by approximately 60%.9 The primary end-point was the composite of CV death, MI, or stroke. Secondary end-points included major coronary events (a composite of death from CHD, MI, or urgent coronary revascularization for myocardial ischemia); total coronary events (a composite of death from
CHD, MI, hospitalization for unstable angina, or any coronary revascularization procedure); the individual components of the primary end point; a composite of all-cause mortality, MI, or stroke; and all-cause mortality.
Over a median follow up period of 3.7 years, the primary end-point occurred in 9.7% of patients in the darapladib group compared to 10.4% in the placebo group (HR 0.94; 95% CI = 0.85 to 1.03; p = 0.20). No significant difference in primary end points was detected in almost all pre-specified subgroups. There was no significant difference in the rates of the individual components of the primary end point or in all-cause mortality between both study groups. Darapladib, as compared to placebo, reduced the rate of major coronary events (9.3% vs. 10.3%; HR 0.90; 95%CI = 0.82 to 1.00; p = 0.045) and total coronary events (14.6% vs. 16.1%; HR 0.91; 95% CI = 0.84 to 0.98; p = 0.02).
Darapladib was discontinued more frequently than placebo (32.7% vs. 26.8%; HR 1.29; 95% CI = 1.22 to 1.37). Patients in the darapladib group discontinued the study drug more frequently than in the placebo group because of diarrhea (3.2% vs. 0.8%), feces odor (2.2% vs. 0.1%), urine odor (1.4% vs. < 0.1%), and skin odor (2.2% vs. 0.1%). There were more serious adverse events of renal failure in the darapladib group than in the placebo group (1.5% vs. 1.1%; HR 1.35, 95% CI 1.03 to 1.78). No significant difference in the number of overall cancers or gastrointestinal cancers was observed between both study groups.
Solid-timi 52 Study
The Stabilization of Plaque Using Darapladib - Thrombolysis in Myocardial Infarction 52 (SOLID-TIMI 52) trial was a phase III, multicenter, randomized, double blind, placebo-controlled trial that has been conducted at 868 sites at 36 countries and was published in the Journal of American Medical Association in September 2014.15 The study was designed to evaluate the efficacy and safety of darapladib - on a background of guideline directed therapy- in patients after an acute coronary syndrome (ACS) event. A total of 13,026 patients within 30 days of hospitalization with an ACS (non–ST-elevation or ST-elevation MI) were randomized to either once-daily oral darapladib (160 mg) or placebo. The primary end point (major coronary events) was the composite of CHD death, MI, or urgent coronary revascularization for myocardial ischemia. Secondary endpoints included the composite of CV death, MI, or stroke; Individual components of primary endpoint; and all cause mortality.
After a median follow up period of 2.5 years, the primary end point occurred in 16.3% of the darapladib group compared to 15.6% of the placebo group (HR 1.00, 95% CI = 0.91–1.09; P = 0.93). There was no significant difference in the composite of CV death, MI, or stroke between darapladib and placebo groups (15.0% vs. 15.0% at 3 years; HR 0.99; 95% CI = 0.90–1.09; P = 0.78). There were no differences between the treatment groups for additional secondary end points, or in all-cause mortality (7.3% vs. 7.1% at 3 years; HR 0.94; 95% CI = 0.82–1.08; P = 0.40). These results were consistent across all key subgroups, including those stratified by baseline LDL-cholesterol concentration and baseline Lp-PLA2 activity level. The rates of discontinuation due to any adverse event were 17% in darapladib group and 12% in the placebo group. Patients were more likely to report an odor-related concern in the darapladib group vs. the placebo group (11.5% vs. 2.5% respectively) and also more likely to report diarrhea (10.6% vs. 5.6% respectively).
Discussion
Lp-PLA2 has been hypothesized to be involved in the pathogenesis of atherosclerosis through pathways related to inflammation.3 However, the negative results came from genetic studies8,13,16 suggest that Lp-PLA2 may be a biomarker that is related to lipoprotein metabolism, vascular inflammation, and plaque vulnerability rather than a causal pathway of CV diseases. In agreement with a meta-analysis by Casas et al,8 PLA2G7 loss of function variants were not associated with increase in the risk of CHD or CV risk factors. This is supported by the recent disappointing results of Lp-PLA2 inhibitor (darapladib) which failed to reduce the risk of major coronary events when added to optimal medical therapy in both SOLID-TIMI 52 and STABILITY studies. There was only a small benefit in the secondary end points - a 10% reduction in the risk of major coronary events and a 9% reduction in total coronary events - in the STABILITY study, but the findings were deemed exploratory only, and of uncertain significance in the absence of effect on the primary endpoint.
These data do not argue against the role of inflammation in atherosclerosis but highlight the challenges that are faced during the development of anti-inflammatory agents for secondary prevention of CV diseases. Inflammation is known to be mediated through complex pathways. If a specific mediator is targeted, perhaps there's compensation along others. On the other hand, it may be unfair to demonstrate an incremental benefit for Lp-PLA2 inhibition beyond existing high level of standard of care for secondary prevention. In both SATBILLITY and SOLID TIMI 52 studies, patients were strictly treated according to international guidelines for secondary prevention of CHD. Moreover, metrics of standard of care were monitored every 6 months, and the importance of adherence to medications was reinforced over the duration of the trial. Statins have been also shown to reduce levels of Lp-PLA2 by up to 35%17, and the extent of the reduction can't be predicted based on LDL-cholesterol levels alone.
It is possible that the coronary risk among patients in STABILITY and SOLID-TIMI 52 may already have been minimized by concurrent therapy, however darapladip failed to reduce the residual risk of adverse CV events that reached up to 16% in study patients.
Regarding the safety profile, the most commonly reported side effects with darapladib were diarrhea and malodorous feces, urine, and skin. There was an increase in the rate of diarrhea among patients receiving darapladib, along with increases in the rates of malodorous feces, urine, and skin; an effect that is thought to be related to the sulfhydryl group in the darapladib molecule. The rate of drug discontinuation in the daraplacib group was significantly higher than in the placebo group in both STABILITY and SOLID-TIMI 52. In the STABILITY study, one in five patients stopped the drug because of adverse events. In addition, there were more serious adverse events of renal failure in the darapladib group than in the placebo group, however its mechanism and clinical significance remains uncertain.
What have we learned?
The disappointing results of the genetic, STABILITY, and SOLID-TIMI 52 studies challenge the notion that inhibition of Lp-PLA2 is a worthwhile approach in patients with CHD. Alternate therapies that target inflammation are awaited to improve outcomes and reduce residual risk in patients with CV diseases.
References
- 1.Watson AD, Navab M, Hama SY, Sevanian A, Prescott SM, Stafforini DM, McIntyre TM, Du BN, Fogelman AM, Berliner JA. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J. Clin. Invest. 1995;95(2):774–782. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karabina S-A, Ninio E. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim. Biophys. Acta. 2006;1761(11):1351–1358. doi: 10.1016/j.bbalip.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler. Thromb. Vasc. Biol. 2005;25(5):923–931. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 4.Münzel T, Gori T. Lipoprotein-associated phospholipase A(2), a marker of vascular inflammation and systemic vulnerability. Eur. Heart J. 2009;30(23):2829–2831. doi: 10.1093/eurheartj/ehp311. [DOI] [PubMed] [Google Scholar]
- 5.Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109(7):837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 7.Oei H-HS, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation. 2005;111(5):570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 8.Casas JP, Ninio E, Panayiotou A, Palmen J, Cooper JA, Ricketts SL, Sofat R, Nicolaides AN, Corsetti JP, Fowkes FG, Tzoulaki I, Kumari M, Brunner EJ, Kivimaki M, Marmot MG, Hoffmann MM, Winkler K, März W, Ye S, Stirnadel HA, Boekholdt SM, Khaw KT, Humphries SE, Sandhu MS, Hingorani AD, Talmud PJ. Genetics Activity, and Coronary Heart Disease Risk in 10 494 Cases and 15 624 Controls of European Ancestry. Circulation. 2010;121:2284–2293. doi: 10.1161/CIRCULATIONAHA.109.923383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serruys PW, García-García HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Bøtker HE, von Birgelen C, D'Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118(11):1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 10.Hassan M. Niemann-Pick C1-Like 1 protein: Another target for treatment of dyslipidemia? Evidence from the Myocardial Infarction Genetic Consortium and IMPROVE-IT trials. Glob. Cardiol. Sci. Pract. 2014;4:1–10. doi: 10.5339/gcsp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan M, Yacoub M. DESCARTES, and TESLA Part B: PCSK9 inhibitors gain momentum DESCARTES, and TESLA Part B: PCSK9 inhibitors gain momentum. Glob. Cardiol. Sci. Pract. 2014;4:11–25. doi: 10.5339/gcsp.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elguindy A, Yacoub MH. The discovery of PCSK9 inhibitors: A tale of creativity and multifaceted translational research. Glob. Cardiol. Sci. Pract. 2013;4:1–5. doi: 10.5339/gcsp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polfus Linda M, Gibbs Richard A, Boerwinkle Eric. Coronary Heart Disease and Genetic Variants with Low Phospholipase A 2 Activity. NEJM. 2015;372:295–296. doi: 10.1056/NEJMc1409673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.STABILITY. investigators. Darapladib for Preventing Ischemic Events in Stable Coronary Heart Disease. N Engl J Med. 2014;370(18):1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 15.O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman S, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312(10):1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 16.Song K, Nelson MR, Aponte J, Manas ES, Bacanu SA, Yuan X, Kong X, Cardon L, Mooser VE, Whittaker JC, Waterworth DM. Sequencing of Lp-PLA2-encoding PLA2G7 gene in 2000 Europeans reveals several rare loss-of-function mutations. Pharmacogenomics J. 2012;12(5):425–431. doi: 10.1038/tpj.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, MacFadyen JG, Wolfert RL, Koenig W. Relationship of lipoprotein-associated phospholipase A2 mass and activity with incident vascular events among primary prevention patients allocated to placebo or to statin therapy: an analysis from the JUPITER trial. Clin. Chem. 2012;58(5):877–886. doi: 10.1373/clinchem.2011.180281. [DOI] [PubMed] [Google Scholar]