Graphical abstract
α-Glucosyl ceramides 4 and 5 have been synthesised and their ability to stimulate the activation and expansion of iNKT cells evaluated.
Keywords: CD1d, iNKT, Antigen, Ceramide, Lipid
Abstract
α-Glucosyl ceramides 4 and 5 have been synthesised and evaluated for their ability to stimulate the activation and expansion of human iNKT cells. The key challenge in the synthesis of both target molecules was the stereoselective synthesis of the α-glycosidic linkage. Of the methods examined, glycosylation using per-TMS-protected glucosyl iodide 16 was completely α-selective and provided gram quantities of amine 11, from which α-glucosyl ceramides 4 and 5 were obtained by N-acylation. α-GlcCer 4, containing a C24 saturated acyl chain, stimulated a marked proliferation and expansion of human circulating iNKT cells in short-term cultures. α-GlcCer 5, which contains a C20 11,14-cis-diene acyl chain (C20:2), induced extremely similar levels of iNKT cell activation and expansion.
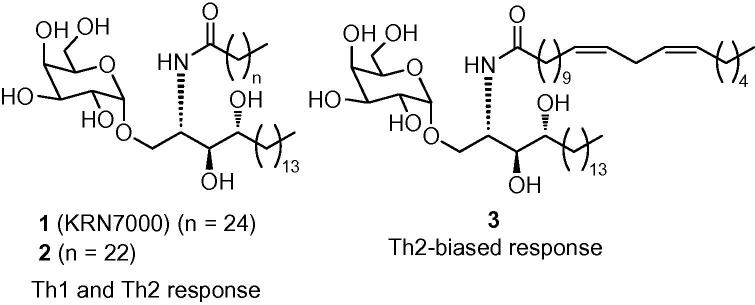
CD1d is a non-polymorphic glycoprotein expressed on the surface of antigen-presenting cells (APCs). It is specifically associated with presenting lipid antigens that activate the distinctive class of T cells known as invariant Natural Killer T (iNKT) cells. iNKT cells display characteristics of both T cells and NK cells and play a crucial role in diverse immune responses and other pathologic conditions.1, 2, 3, 4 When the synthetic glycolipid α-galactosyl ceramide (α-GalCer),5 also known as KRN7000 (1, Fig. 1), is bound to CD1d and presented to T cell receptors (TCRs) on the surface of iNKT cells, the latter are activated to release diverse cytokines, including both Th1 and Th2 cytokines.6, 7, 8 Similar results are obtained with the more readily obtained C24:0 analogue (2, Fig. 1).9, 10 It is believed that the release of Th1 cytokines may contribute to antitumour and antimicrobial functions, whilst the secretion of Th2 cytokines may help alleviate autoimmune diseases11, 12, 13 such as multiple sclerosis14 and arthritis.15 The opposing effects induced by Th1 and Th2 cytokines have complicated efforts to develop KRN7000 as a therapeutic agent, since it induces high levels of both types of cytokine and therefore may induce mixed and unpredictable biological effects.16 Switching the C26:0 acyl chain of KRN7000 for a C20 11,14-cis-diene acyl chain modifies the outcome of iNKT cell activation and potently induces a Th2-biased cytokine response.9 This C20:2 analogue (3, Fig. 1) also exhibits less stringent requirements for loading on to CD1d.10
Figure 1.
α-Galactosyl ceramides 1 (C26:0), 2 (C24:0) and 3 (C20:2).
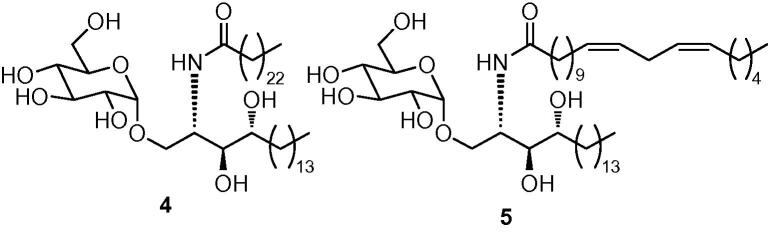
Although extensive studies have examined the impact on the iNKT cell-stimulating activities of modifications to the fatty acyl and sphingosine structures of α-GalCer, there has been less analysis of the effects of structural modifications of the carbohydrate head group.17 Subtle changes in this part of the glycolipid are likely to have significant effects on iNKT cell recognition since the monosaccharide group is exposed and makes direct contacts with the TCR in complexes formed by the binding of α-GalCer to CD1d.18 To this end, we now report the synthesis and preliminary biological activity of α-glucosyl ceramide analogues 4 and 5 (Fig. 2).
Figure 2.
Target α-glucosyl ceramides 4 (C24) and 5 (C20:2).
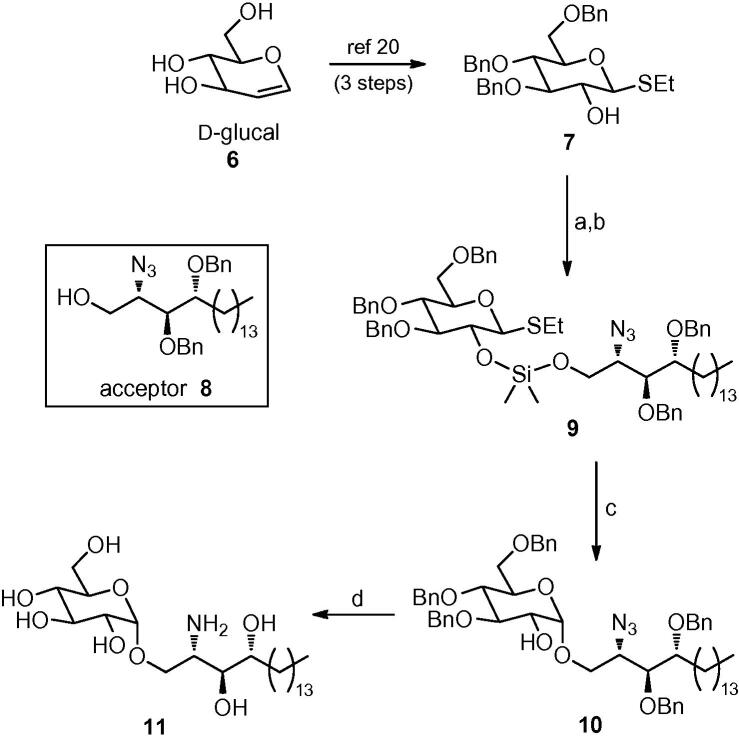
Since targets 4 and 5 differ only in their acyl chain substitution, we elected to pursue a synthetic strategy that would allow the introduction of this point of diversity in the final step. We therefore examined several routes to amine 11 from which both glucosyl ceramide targets would then be accessed through chemoselective acylation of the amino residue. The key challenge in a synthesis of amine 11 is to form the glycosidic linkage with high α-selectivity. To this end, we first opted to employ a stereospecific glucosylation method developed by Bols (Scheme 1).19 This method involves the use of a silyl tether to attach the acceptor temporarily to the 2-position of the glucosyl donor prior to the key glycosylation step. Glycosylation proceeds with 1,2-syn specificity, owing to the formation of a five-membered silylacetal intermediate, which in the case of glucosyl donors, ensures the formation of the α-glycoside product. Thioglucoside 7, synthesised in three steps from d-glucal 6,20 was reacted with a fivefold excess of dichlorodimethylsilane. This reaction afforded a silyl chloride intermediate, which, after removal of the excess dichlorosilane reagent under reduced pressure, reacted with known alcohol 821 to form mixed silyl acetal 9, our glucosylation precursor, in modest yield. Treatment of silyl acetal 9 with N-iodosuccinimide (NIS) furnished the desired glucoside 10 as a single diastereoisomer, albeit in modest yield. Hydrogenolysis of the benzyl groups and reduction of the azide in 10 using Pd(OH)2 as the catalyst,22 provided our acylation precursor, amine 11 in 57% yield (Scheme 1).
Scheme 1.
Reagents: (a) Me2SiCl2, pyridine, toluene; (b) acceptor 8, pyridine, toluene, 38% over two steps; (c) NIS, MeNO2, 47%; (d) H2, Pd(OH)2, CHCl3/MeOH (1:1), 57%.
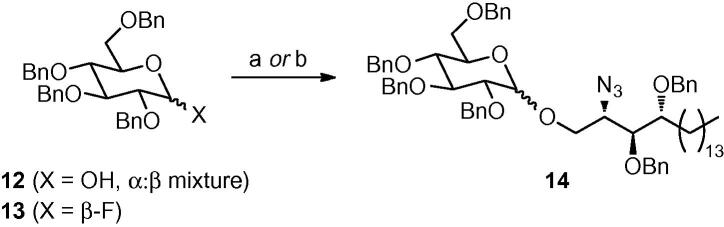
Although this synthetic approach allowed a completely stereoselective route to our target amine 11, a number of steps in the sequence suffered from poor yields, which hindered access to significant quantities of material. We therefore examined other glycosylation methods. Kobayashi has described a stereoselective α-glycosylation using a galactosyl bromide generated in situ from 2,3,4,6-tetra-O-benzyl galactose.23 Unfortunately, we found that glycosylation using the corresponding glucosyl bromide derived from 12 afforded significant amounts of the unwanted β-anomer (Scheme 2). The use of perbenzylated glucosyl fluoride 1324 also provided a mixture of α- and β-glycosides 14, which proved difficult to separate (Scheme 2).
Scheme 2.
Reagents: (a) From 12: CBr4, PPh3, CH2Cl2, then 8, nBu4NBr, tetramethyl urea, 67% (α:β ratio: 1:1); (b) from 13: 8, SnCl4, AgIO4, THF, 41% (α:β ratio: 5:1).
We therefore turned our attention to the use of glucosyl iodides,25 specifically per-TMS-protected glucosyl iodide 16, as an alternative donor. Du et al. have shown that the corresponding galactosyl iodide provides excellent levels of α-selectivity with a variety of alcohol acceptors.26 The reaction conditions for this glycosylation are also extremely mild and the silyl protecting groups are easily removed using an acid work-up. We reasoned that the use of phytosphingosine acceptor 17,27 in which the internal 1,2-diol is protected as an acetal, would deliver the completely O-deprotected glucoside 18 upon acid work-up. To this end, 1,2,3,4,6-penta-O-trimethylsilyl glucose 15, which is commercially available or can be readily synthesised on large scale by treating glucose with a mixture of TMSCl and hexamethyldisilazane (HMDS) in pyridine,28 was converted to glycosyl iodide 16 by treatment with TMSI in CH2Cl2 (Scheme 3). Adding a solution of crude 16 to a solution of alcohol 17, nBu4NI, Hünig’s base and 4 Å molecular sieves in CH2Cl2 successfully effected glycosylation. Treating the initially formed glycoside product with p-toluenesulfonic acid (pTSA) in methanol provided the fully O-deprotected glycoside 18 as a single anomer. Although the yield for this three-step process was a modest 45%, we now had very rapid access to our target molecules. A final Staudinger reduction of azide 18 delivered our requisite amine 11 in quantitative yield (Scheme 3).29 This reaction sequence is short and scalable and proved to be particularly effective for accessing multigram quantities of amine 11. The final acylation reactions were accomplished by adding either tetracosanoyl chloride or 11,14-eicosadienoyl chloride (formed from the corresponding carboxylic acids using oxalyl chloride) in THF to amine 11 in a vigorously stirred biphasic mixture of THF and 8 M NaOAc solution. Both reactions provided the desired amide products 4 and 5 in good yields (Scheme 3).30, 31
Scheme 3.
Reagents: (a) TMSI, CH2Cl2; (b) 17, nBu4NI, iPr2NEt, 4 Å molecular sieves, CH2Cl2; then pTSA, MeOH, 45% from 15; (c) PMe3, wet THF, quant.; (d) tetracosanoyl chloride, THF/8 M NaOAc, 68%; (e) 11,14-eicosadienoyl chloride, THF/8 M NaOAc, 66%.
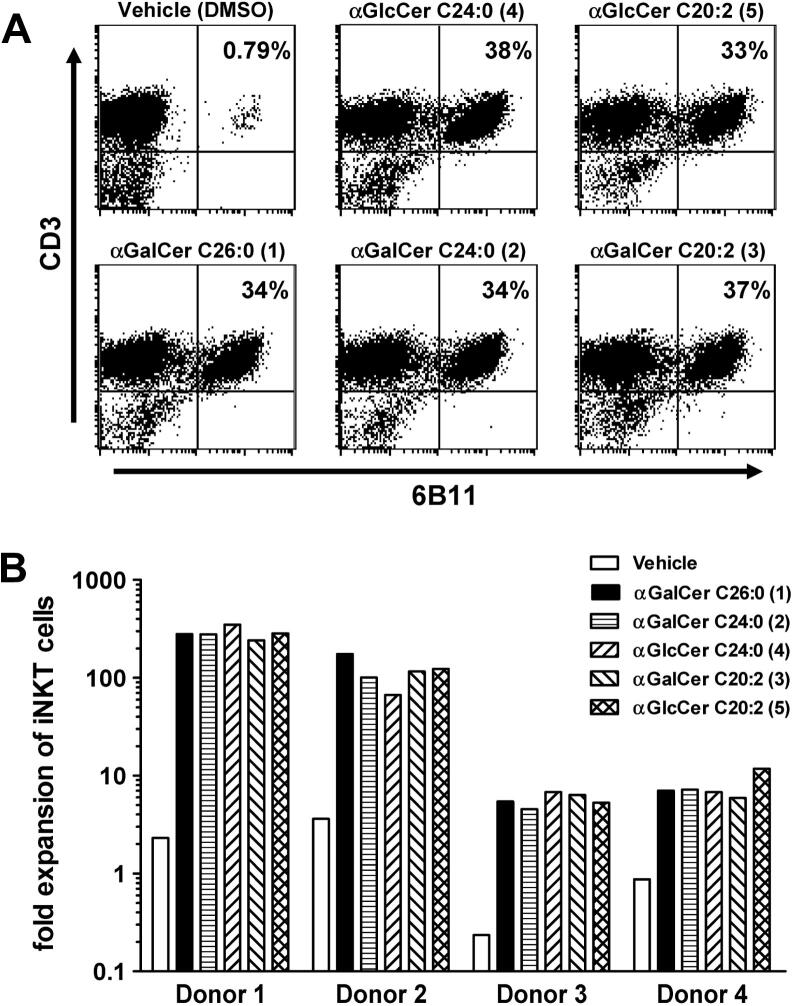
To assess the biological activity of the α-glucosyl ceramides 4 and 5 and compare these to KRN7000 1 and the α-galactosyl ceramide analogues 2 (C24:0) and 3 (C20:2), we assessed the ability of each compound to induce the expansion of iNKT cells in samples of human peripheral blood mononuclear cells (PBMC) during an eight-day in vitro culture.32 The results showed that both the percentages and absolute numbers of iNKT cells in the cultures were markedly increased to similar levels by stimulation with both of the α-GlcCer analogues 4 and 5 (Fig. 3). The level of iNKT cell expansion, at least with a relatively high concentration of the glycolipids (250 nM), was comparable for both of the N-acyl variants of α-GlcCer and very similar to levels obtained with the related α-GalCer analogues (2 (C24:0) and 3 (C20:2)) and with the prototypical iNKT cell activator KRN7000 (1 (C26:0)). Representative profiles obtained by flow cytometry of cultures from one normal blood donor are shown in Figure 3A. This analysis was carried out with PBMC from four separate donors (Fig. 3B). Although differences were observed for the levels of iNKT cell expansion between different donors, all donors responded well to the two α-GlcCer analogues. In all cases, these responses were similar to those generated by the analogous α-GalCer compounds.
Figure 3.
Ex vivo expansion of human iNKT cells by α-GlcCer and α-GalCer analogues. Peripheral blood mononuclear cells (PBMC) from four different donors were stimulated with the indicated glycolipids at a concentration of 250 nM in the presence of low levels of exogenous IL-2 and IL-7. At day 8, cultures were harvested and analysed by flow cytometry using monoclonal antibodies specific for CD3 and for the invariant TCRα chain expressed by iNKT cells (6B11). (A) Dot plots showing relative levels of CD3+ 6B11+iNKT cells are shown for one representative donor. Numbers in upper right quadrant indicate percentages of total lymphocytes that are iNKT cells. (B) Absolute numbers of iNKT cells in the cultures were determined by flow cytometry using fluorescent counting beads, and the values of iNKT cell fold expansion were determined by dividing by the input number of iNKT cells.
The strong biological activity of the α-GlcCer compounds was consistent with findings from the initial study that described the reactivity of CD1d-restricted iNKT cells to synthetic glycosylceramides.5 This showed an α-GlcCer with a C26 saturated acyl group to be stimulatory for mouse iNKT cells, with a level of activity only slightly less than that of KRN7000 (1). Our analysis confirms the activity of α-GlcCer compounds as ligands for human iNKT cells. It is also notable that we observed human iNKT cell activation and expansion for an α-GlcCer with a shorter acyl chain containing unsaturations (5). Previous work with analogues of α-GalCer containing C20:2 or other unsaturated fatty acyl groups revealed a marked tendency for these to bias iNKT cell-dependent cytokine responses in mice to give preferential secretion of Th2 cytokines such as IL-4 and IL-13.9 This Th2 cytokine bias has been associated with therapeutic benefits in a variety of mouse models of autoimmune and inflammatory diseases, indicating potential therapeutic applications for such glycolipids in human diseases.17 It will thus be important to determine whether compound 5 or other α-GlcCer analogues bearing an unsaturated acyl chain also show an ability to induce Th2-biased cytokine responses, which is a focus for future studies.
In summary, we have developed an efficient route to α-glucosyl ceramides that provided two biologically active ligands 4 and 5 for stimulation of human iNKT cell responses. Of the range of glycosylation methods that were investigated for accessing the target molecules with high levels of stereoselectivity, the use of per-TMS-protected glucosyl iodide 16 as the donor is the most attractive, reacting with acceptor 17 to provide a single α-glycoside product. This glycosylation reaction is also scalable and with an acidic work-up effecting global deprotection, followed by Staudinger reduction of the azide, allows rapid access to advanced intermediate 11, which can now be used to provide a broad range of α-GlcCer compounds with different acyl chains. Compounds produced using this approach will assist in expanding the current understanding of the structure–activity relationships for glycolipid activators of iNKT cells, which is of central importance to the further development of this class of compounds as clinically useful immunomodulators.
Acknowledgements
G.S.B. acknowledges support from a Personal Research Chair from Mr. James Bardrick, a Royal Society Wolfson Research Merit Award, a former Lister Institute-Jenner Research Fellowship, the Medical Research Council and The Wellcome Trust (084923/B/08/Z). S.A.P. and G.B. were supported by NIH/NIAID Grant AI45889. Core resources that facilitated flow cytometry were supported by the Einstein Center for AIDS Research (AI 051519) and the Einstein Cancer Center (CA 13330). The NMR spectrometers used in this research were funded in part through Birmingham Science City: Innovative Uses for Advanced Materials in the Modern World (West Midlands Centre for Advanced Materials Project 2), with support from Advantage West Midlands (AWM) and part-funded by the European Regional Development Fund (ERDF).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2010.05.010.
Supplementary data
Experimental procedures.
References and notes
- 1.Kronenberg M. Annu. Rev. Immunol. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 2.Brutkiewicz R.R. J. Immunol. 2006;177:769. doi: 10.4049/jimmunol.177.2.769. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac R., Rivera M.N., Park S.H., Roark J.H. Annu. Rev. Immunol. 1997;15:535. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 4.Hong S., Scherer D.C., Mendiratta S.K., Serizawa I., Koezuka Y., Van Kaer L. Immunol. Rev. 1997;169:31. doi: 10.1111/j.1600-065x.1999.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E., Koseki H., Taniguchi M. Science. 1997;278:1626. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Crowe N., Uldrich A.P., Kyparissoudis K., Hammond K.J.L., Hayakawa Y., Sidobre S., Keating R., Kronenberg M., Smyth M.J., Godfrey D.I. J. Immunol. 2003;171:4020. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 7.Burdin N., Brossay L., Kronenberg M. Eur. J. Immunol. 1999;29:2014. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Carnaud C., Lee D., Donnars O., Park S.H., Beavis A., Koezuka Y., Bendelac A. J. Immunol. 1999;163:4647. [PubMed] [Google Scholar]
- 9.Yu K.O.A., Im J.S., Molano A., Dutronc Y., Illarionov P.A., Forestier C., Fujiwara N., Arias I., Miyake S., Yamamura T., Chang Y.T., Besra G.S., Porcelli S.A. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3383. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Im J.S., Arora P., Bricard G., Molano A., Venkataswamy M.M., Baine I., Jerud E.S., Goldberg M.F., Baena A., Yu K.O.A., Ndonye R.M., Howell A.R., Yuan W.M., Cresswell P., Chang Y.T., Illarionov P.A., Besra G.S., Porcelli S.A. Immunity. 2009;30:888. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi M., Harada M., Kojo S., Nakayama T., Wakao H. Annu. Rev. Immunol. 2003;21:483. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey D.I., MacDonald H.R., Kronenberg M., Smyth M.J., Van Kaer L. Nat. Rev. Immunol. 2004;4:231. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Aseguinolaza G., Van Kaer L., Bergmann C.C., Wilson J.M., Schmieg J., Kronenberg M., Nakayama T., Taniguchi M., Koezuka Y., Tsuji M. J. Exp. Med. 2002;195:617. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto K., Miyake S., Yamamura T. Nature. 2001;413:513. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 15.Chiba A., Oki S., Miyamoto K., Hashimoto H., Yamamura T., Miyake S. Arthritis Rheum. 2004;50:305. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- 16.(a) Oki S., Chiba A., Yamamura T., Miyake S. J. Clin. Invest. 2004;113:1631. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu K.O.A., Porcelli S.A. Immunol. Lett. 2005;100:42. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Venkataswamy M.M., Porcelli S.A. Semin. Immunol. 2010:22. doi: 10.1016/j.smim.2009.10.003. 68 and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borg N.A., Wun K.S., Kjer-Nielsen L., Wilce M.C., Pellicci D.G., Koh R., Besra G.S., Bharadwaj M., Godfrey D.I., McCluskey J., Rossjohn J. Nature. 2007;448:44. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 19.(a) Bols M. Tetrahedron. 1993;49:10049. [Google Scholar]; (b) Bols M. J. Chem. Soc., Chem. Commun. 1992:913. [Google Scholar]
- 20.(a) Cheshev P., Marra A., Dondoni A. Carbohydr. Res. 2006;341:2714. doi: 10.1016/j.carres.2006.09.003. [DOI] [PubMed] [Google Scholar]; (b) Seeberger P.A., Eckhardt M., Gutteridge C.E., Danishefsky S.J. J. Am. Chem. Soc. 1997;119:10064. [Google Scholar]
- 21.(a) Kratzer B., Mayer T.G., Schmidt R.R. Eur. J. Org. Chem. 1998:291. [Google Scholar]; (b) Xia C., Schümann J., Emmanuel R., Zhang Y., Chen W., Zhang W., De Libero G., Wang P.G. J. Med. Chem. 2007;50:3489. doi: 10.1021/jm0701066. [DOI] [PubMed] [Google Scholar]
- 22.Lu X., Bittman R. Tetrahedron Lett. 2005;46:3165. [Google Scholar]
- 23.(a) Shingu Y., Nishida Y., Dohi H., Kobayashi K. Org. Biomol. Chem. 2003;1:2518. doi: 10.1039/b303984f. [DOI] [PubMed] [Google Scholar]; (b) Nishida Y., Shingu Y., Dohi H., Kobayashi K. Org. Lett. 2003;5:2377. doi: 10.1021/ol034269+. [DOI] [PubMed] [Google Scholar]
- 24.Toshima K. Carbohydr. Res. 2000;327:15. doi: 10.1016/s0008-6215(99)00325-0. [DOI] [PubMed] [Google Scholar]
- 25.Meloncelli P.J., Martin A.D., Lowary T.L. Carbohydr. Res. 2009;344:1110. doi: 10.1016/j.carres.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 26.(a) Du W., Kulkarni S.S., Gervay-Hague J. Chem. Commun. 2007:2336. doi: 10.1039/b702551c. [DOI] [PubMed] [Google Scholar]; (b) Du W., Gervay-Hague J. Org. Lett. 2005;7:2063. doi: 10.1021/ol050659f. [DOI] [PubMed] [Google Scholar]; (c) Schombs, M.; Park, F. E.; Du, W.; Kulkarni, S. S.; Gervay-Hague, J. J. Org. Chem. 2010.doi:10.1021/jo100366v. [DOI] [PMC free article] [PubMed]
- 27.(a) Fan Q.-H., Ni N.-T., Li Q., Zhang L.H., Ye X.-S. Org. Lett. 2006;8:1007. doi: 10.1021/ol0601996. [DOI] [PubMed] [Google Scholar]; (b) Garcia Diaz Y.R., Wojno J., Cox L.R., Besra G.S. Tetrahedron: Asymmetry. 2009;20:747. [Google Scholar]
- 28.Toubiana R., Das B.C., Defaye J., Mompon B., Toubiana M.J. Carbohydr. Res. 1975;44:308. doi: 10.1016/s0008-6215(00)84175-0. [DOI] [PubMed] [Google Scholar]
- 29.Worthington R.J., Bell N.M., Wong R., Micklefield J. Org. Biomol. Chem. 2008;6:92. doi: 10.1039/b714580m. [DOI] [PubMed] [Google Scholar]
- 30.Data for α-GlcCer 4: Mp 151–152 °C; Rf = 0.21 (10% MeOH in CHCl3); +47.2 (c 0.50, CHCl3/MeOH (1:1)); νmax(film)/cm−1 3336 br s (OH, NH2), 2917 s, 2850 s, 1623 m (C O), 1463 m, 1350 s, 1143 s, 1071 m, 1033 m, 798 s, 718 m; δH (500 MHz, CDCl3/CD3OD (2:1)) 0.88 (6H, t, J = 6.7, 2× terminal CH3), 1.20–1.44 (64H, stack, alkyl chain), 1.51–1.69 (4H, stack, alkyl chain), 2.19 (2H, app. t, J = 7.8, CH2CONH), 3.31–3.37 (1H, m, 4-H), 3.45 (1H, dd, J = 9.8, 3.8, 2-H), 3.52–3.59 (3H, stack, 5-H, 3′-H, 4′-H), 3.62 (1H, app. t, J = 9.5, 3-H), 3.65 (2H, stack, 6-Ha, 1′-Ha), 3.79 (1H, dd, J = 12.0, 2.5, 6-Hb), 3.86 (1H, dd, J = 10.5, 4.5, 1′-Hb), 4.17 (1H, app. q, J = 4.5, 2′-H), 4.85 (1H, d, J = 3.5, 1-H), CONH resonance not observed; δC (125 MHz, CHCl3/CD3OD (3:1)) 13.5 (CH3, 2× terminal CH3), [22.2, 25.5, 28.9, 29.0, 29.2, 29.27, 29.30, 29.4, 31.5, 32.0, 36.0 (CH2, alkyl chain, some overlapping resonances)], 50.1 (CH, C-2′), 61.2 (CH2, C-6), 66.8 (CH2, C-1′), 69.9 (CH, C-4), [71.6 (CH), 71.6 (CH, C-2), overlapping resonances], 71.8 (CH), 73.5 (CH, C-3), 74.2 (CH, C-3′), 98.9 (CH, anomeric-C), 174.2 (quat. C, CONH); m/z (TOF ES+) 852.8 ([M+Na]+, 100%); HRMS m/z (TOF ES+) 852.6912. C48H95NO9Na requires 852.6905.
- 31.Data for α-GlcCer 5: Mp 83–85 °C; Rf = 0.23 (10% MeOH in CHCl3); +41.3 (c 0.50, CHCl3/MeOH (1:1)); νmax(film)/cm−1 3300 br s (OH), 3009 m, 2922 s, 2851 s, 1697 m (C O), 1633 s (C C), 1540 m, 1466 m, 1378 w, 1282 w, 1215 w, 1044 m, 760 m; δH (400 MHz, CDCl3/CD3OD (2:1)) 0.86 (3H, t, J = 6.6, terminal CH3), 0.87 (3H, t, J = 7.0, terminal CH3), 1.17–1.43 (42H, stack, alkyl chain), 1.47–1.64 (4H, stack, alkyl chain), 2.00–2.08 (4H, stack, CH2CH CHCH2CH CHCH2), 2.19 (2H, app. t, J = 7.6, CH2CONH), 2.75 (2H, app. t, J = 6.4, CH CHCH2CH CH), 3.31–3.38 (1H, m, 4-H), 3.44 (1H, dd, J = 9.8, 3.7, 2-H), 3.51–3.58 (2H, stack, 5-H, 4′-H), 3.58–3.73 (4H, stack, 1′-Ha, 3′-H, 3-H, 6-Ha), 3.78 (1H, dd, J = 11.8, 2.6, 6-Hb), 3.84 (1H, dd, J = 10.6, 4.2, 1′-Hb), 4.11–4.18 (1H, m, 2′-H), 4.84 (1H, d, J = 3.7, 1-H), 5.25–5.40 (4H, stack, 2× CH CH), CONH resonance not observed; δC (100 MHz, CDCl3) [15.2, 15.3 (CH3, 2× terminal CH3)], [23.9, 24.0, 26.9, 27.2, 28.5, 28.6, 30.76, 30.81, 30.9, 31.1, 32.9, 33.3, 33.4, 37.7 (CH2, alkyl chain, some resonance overlap)], 51.7 (CH, C-2′), 62.8 (CH2, C-6), 68.6 (CH2, C-1′), 71.5 (CH, C-2), 73.26 (CH), 73.32 (CH), 73.6 (CH), 75.1 (CH, C-3), 75.6 (CH, C-3′), 100.6 (CH, anomeric-C), 129.26 (CH, CH CH), 129.30 (CH, CH CH), 131.38 (CH, CH CH), 131.45 (CH, CH CH), 175.8 (quat. C, CONH); m/z (TOF ES+) 792.7 ([M+Na]+, 100%); HRMS m/z (TOF ES+) 792.5963. C44H83NO9Na requires 792.5966.
- 32.Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll–Hypaque centrifugation of leukocyte concentrates obtained from healthy volunteer blood donors. For ex vivo expansion of human iNKT cells, 4 million PBMC were cultured in wells of 24-well tissue culture plates in RPMI-1640 medium with 10% foetal calf serum and recombinant IL-2 (60 IU/mL) and IL-7 (5 ng/mL) (Peprotech). Glycolipids were solubilised in 100% DMSO and added directly to culture media to achieve a final concentration of 250 nM. Control wells received an amount of DMSO vehicle identical to that added with the glycolipids (0.00125%). Cultures were incubated for 8 days at 37 °C in a 5% CO2 humidified incubator. Cultures were harvested and the cells stained with fluorochrome labelled monoclonal antibodies specific for CD3 and the iNKT cell TCR (6B11, eBiosciences). Samples were also stained with propidium iodide to exclude dead cells, and a known number of Caltag fluorescent counting beads (Invitrogen) were added to the samples to allow quantitation of absolute cell numbers. The total number of iNKT cells (CD3+, 6B11+, PI negative lymphocytes) was calculated by normalising according to counting beads and the fold expansion values were calculated based on the initial number of iNKT cells. Data were collected on a FACSCalibur flow cytometer using CellQuest software (BD Biosciences).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures.