Most plants form mutually beneficial relationships with microorganisms in their roots, especially fungi. Invasive plants can release substances toxic to other species known as allelochemicals. Allelochemicals from the invasive species garlic mustard can inhibit beneficial soil fungi, thereby disrupting the plant-fungal mutualism. We demonstrate that treatment with garlic mustard leaves reduces the ability of a native plant, False Solomon's seal, to store carbohydrates. Additionally, we demonstrate that weeding garlic mustard from forest plots allows this native to grow larger, flower more frequently, and enter long-term dormancy less often relative to plots where garlic mustard occurs.
Keywords: Allelochemicals, Alliaria petiolata, carbon stress/carbon starvation, Maianthemum racemosum, mutualism disruption, root fungal symbiont, species invasion, vital rates
Abstract
Invasive plants can negatively affect belowground processes and alter soil microbial communities. For native plants that depend on soil resources from root fungal symbionts (RFS), invasion could compromise their resource status and subsequent ability to manufacture and store carbohydrates. Herbaceous perennials that depend on RFS-derived resources dominate eastern North American forest understories. Therefore, we predict that forest invasion by Alliaria petiolata, an allelopathic species that produces chemicals that are toxic to RFS, will diminish plant carbon storage and fitness. Over a single growing season, the loss of RFS could reduce a plant's photosynthetic physiology and carbon storage. If maintained over multiple growing seasons, this could create a condition of carbon stress and declines in plant vital rates. Here we characterize the signals of carbon stress over a short timeframe and explore the long-term consequence of Alliaria invasion using Maianthemum racemosum, an RFS-dependent forest understory perennial. First, in a greenhouse experiment, we treated the soil of potted Maianthemum with fresh leaf tissue from either Alliaria or Hesperis matronalis (control) for a single growing season. Alliaria-treated plants exhibit significant overall reductions in total non-structural carbohydrates and have 17 % less storage carbohydrates relative to controls. Second, we monitored Maianthemum vital rates in paired experimental plots where we either removed emerging Alliaria seedlings each spring or left Alliaria at ambient levels for 7 years. Where Alliaria is removed, Maianthemum size and vital rates improve significantly: flowering probability increases, while the probability of plants regressing to non-flowering stages or entering prolonged dormancy are reduced. Together, our results are consistent with the hypothesis that disruption of a ubiquitous mutualism following species invasion creates symptoms of carbon stress for species dependent on RFS. Disruption of plant–fungal mutualisms may generally contribute to the common, large-scale declines in forest biodiversity observed in the wake of allelopathic invaders.
Introduction
The majority of flowering plant species form mutualisms with root fungal symbionts (RFS) such as arbuscular mycorrhizal fungi (AMF; 74 % of angiosperms; Brundrett 2009) and dark septate endophytes (DSE; ≥600 species; Jumpponen and Trappe 1998). Arbuscular mycorrhizal fungi and DSE live inside plant roots and deploy hyphae outside the root that increase water, nitrogen, phosphorus and other soil nutrients' availability to their plant partner (Smith and Read 2008; Newsham 2011). The RFS receive a substantial fraction of the plant partner's fixed carbon (for AMF up to 20 %; Smith and Read 2008).
Recent work highlights how anthropogenic changes in the environment, such as invasion, can negatively affect mutualisms (Tylianakis et al. 2008; Kiers et al. 2010). Invasive species can impact belowground processes and directly or indirectly alter soil microbial communities, including RFS. Mechanisms through which belowground impacts can occur (summarized in part by Wolfe and Klironomos 2005) include alterations in the quality, quantity and timing of litter inputs and subsequent changes in soil nutrient status (reviewed by Ehrenfeld 2003), direct changes to soil nutrient status through novel nutrient fixation strategies by the invader (e.g. Vitousek and Walker 1989), mutualist degradation (Vogelsang and Bever 2009) and allelopathy (e.g. Callaway et al. 2008; Grove et al. 2012). Specifically, allelochemicals can act as novel weapons that are directly toxic to plants or act indirectly on their associated microbes (Callaway and Ridenour 2004; Weir et al. 2004).
The invasion of North American forests by Alliaria petiolata (Brassicaceae, garlic mustard) is an emerging model system for investigations of allelopathic effects on belowground processes (Rodgers et al. 2008a). This species produces a suite of allelochemicals (Vaughn and Berhow 1999; Cipollini and Gruner 2007) that are toxic to RFS (Roberts and Anderson 2001; Stinson et al. 2006; Koch et al. 2011) even at low concentrations (Callaway et al. 2008; Cantor et al. 2011). Field studies document that areas infested with Alliaria exhibit shifts in soil fungal community composition with frequent reductions in AMF species richness (Burke et al. 2011; Lankau 2011a; Lankau et al. 2014), declines in total soil hyphal abundances (Cantor et al. 2011; Koch et al. 2011) and changes in the within-root community of AMF-dependent plants (Burke 2008; Bongard et al. 2013). Together, these studies suggest that within Alliaria-invaded ecosystems the function of the mutualistic fungal community can be compromised and that these changes contribute to Alliaria's invasive success.
Herbaceous perennials dominate the temperate forest understories that Alliaria invades and these species as a group are typically highly- to obligately-dependent on RFS (Brundrett and Kendrick 1988; Whigham 2004). The fact that temperate forest soils are strongly resource limited (Whigham 2004; Gilliam 2014) likely drives the obligate nature of the relationship for many understory herbaceous perennials. Typically these species are slow growing (Gilliam 2014), exhibit high rates of RFS colonization (e.g. Brundrett and Kendrick 1988; Boerner 1990; Burke 2008) and have long-lived arbuscules (Brundrett and Kendrick 1990). Many also lack fine roots or root hairs (e.g. LaFrankie 1985) perhaps because their associated RFS hyphae fulfil this soil resource-gathering role. Since resources supplied by RFS are intimately tied to many plant metabolic functions (Schweiger et al. 2014), disruption of soil mutualisms is expected to severely limit the physiological rates of forest species (Hale et al. 2011). In the absence of RFS, plants generally exhibit reduced photosynthetic rates (Allen et al. 1981; Wright et al. 1998; Zhu et al. 2011) and subsequent carbon stress can curb their ability to carry out carbon-demanding functions such as growth (Lu and Koide 1994) and flowering (Koide et al. 1994).
Carbon stress is the reduction of a plant's pool of total non-structural carbohydrates (NSCs) (sensu Anderegg et al. 2012). In herbaceous perennials, chronic carbon stress can alter key vital rates including survival (Gremer and Sala 2013), flowering (Crone et al. 2009) and prolonged dormancy (Gremer et al. 2010). Invaders like Alliaria that alter the soil environment and essential RFS functions could induce carbon stress or ‘carbon starvation’ (sensu McDowell et al. 2008), ultimately diminishing the stability of populations of RFS-dependent native species.
Our prior experiments on the RFS-dependent understory perennial, Maianthemum racemosum (Ruscaceae, false Solomon's seal) confirm the dramatic physiological consequences of short-term RFS disruption by Alliaria's allelochemicals. Key physiological traits including stomatal conductance, which is known to be highly dependent on RFS colonization (Augé et al. 2014), and photosynthetic rate both significantly declined in plants exposed to fresh Alliaria leaf litter (Hale et al. 2011). Soil respiration, to which fungi are the primary contributors (Anderson and Domsch 1975), was also reduced with Alliaria treatment. Importantly, in field plots invaded by Alliaria and in pot experiments with an Alliaria litter treatment, we demonstrated significant declines in the abundance of soil fungal hyphae relative to controls (37 % decline, Cantor et al. 2011; 29–38 % decline, A. N. Hale et al., submitted for publication). Together these data strongly support the idea that the observed physiological declines are driven by the inhibition of the RFS hyphal network in the soil (Hale et al. 2011).
Here we explore how the physiological stress of RFS-mutualism disruption in Alliaria-invaded forests could result in performance declines in an RFS-dependent forest perennial across two time scales. First, we ask: Given that Alliaria's allelochemicals cause detectable shifts in the soil fungal community and alternative plant physiological rates, do they also cause declines in carbon storage in plants within a single growing season? In a greenhouse experiment we show that Alliaria-treated Maianthemum store significantly less carbon in their rhizome over one growing season relative to controls. Second, to determine the potential for short-term effects to scale up over time and affect population processes, we conducted a 7-year field experiment in an Alliaria-invaded forest in which Alliaria was weeded or left at ambient levels. We test whether Maianthemum exhibit lower growth rates consistent with carbon stress in the Alliaria-ambient plots. We also ask if Alliaria reduces size-based vital rates of Maianthemum and if so, how quickly these changes occur. We show that where Alliaria is present, Maianthemum have suppressed growth and vital rates relative to adjacent plot where Alliaria is removed.
Methods
Greenhouse study: assessing potential for carbon stress
The greenhouse study was conducted during the summer of 2010 in the greenhouse facilities at the University of Pittsburgh. In May, we obtained bare-root adult Maianthemum plants (N = 42) from a native plant nursery (Prairie Moon Nursery, Winona, MN, USA). Rhizomes ranged in size from 6.7 to 39.7 g fresh weight. We potted each rhizome in a 3 : 1 mixture of autoclaved Fafard potting soil and Turface. We inoculated plants with RFS by adding 150 g of field soil collected from areas adjacent to Maianthemum plants at our experimental field site (see details below). Pots were then placed in the greenhouse and watered every 2–3 days for 1 month, allowing the plants to complete stem elongation and establish the RFS mutualism.
In June, we assigned each plant to either an Alliaria treatment or a control treatment. To control for potential differences in initial carbohydrate status due to differences in plant age and/or size (e.g. Olano et al. 2006), we stratified the randomized assignment of rhizomes into the treatments to ensure that mean rhizome mass was the same in the Alliaria and control treatments. Plants in the Alliaria treatment were then exposed to Alliaria allelochemicals by placing 25 g of fresh Alliaria leaf tissue collected from a population with a recent history of invasion (<20 years) on top of the soil. When these plants were watered, the glucosinolates leached out of the Alliaria leaves and into the soil (A. N. Hale et al., submitted for publication). As in previous experiments (Hale et al. 2011), plants in the control treatment received 25 g of fresh Hesperis matronalis (dame's rocket; Brassicaceae) leaf tissue. Like Alliaria, Hesperis is an invasive mustard in eastern North America (Leicht-Young et al. 2012). While Hesperis produces some glucosinolates (Larsen et al. 1992), RFS hyphae and vesicles have been observed within its root system (DeMars and Boerner 1995), indicating that Hesperis chemicals are less toxic to RFS than Alliaria. In the field, the high mortality rates of Alliaria seedlings and rosettes throughout the year (Davis et al. 2006) and the mortality of adults in the summer (Anderson et al. 1996) likely result in a sustained supply of allelochemicals into the soil. Thus, we re-applied fresh leaf tissue in both treatments every 2 weeks until the end of August to simulate a season-long supply of Alliaria allelochemicals.
We destructively harvested plants three times during the growing season (9 July, 6 August and at senescence) to assess the effect of the treatments on the carbohydrate status. For the last time point, we classified plants as being senesced when 40 % of the leaf tissue had yellowed and photosynthetic rates were <1.0 μmol m−2 s−1. Details of the leaf gas exchange protocol for Maianthemum can be found in Hale et al. (2011). To harvest the plants, we carefully clipped the shoot and roots away from the rhizome. We also stained the roots of a subset of plants per treatment following Brundrett et al. (1984) to confirm RFS colonization. We then weighed the rhizome and immediately flash-froze it in liquid nitrogen. We stored samples at −80 °C until they could be lyophilized and ground. We followed the protocol of Zuleta and Sambucetti (2001) to analyse rhizome inulin (storage carbohydrate) and sucrose (mobile carbohydrate) content via high-performance liquid chromatography (HPLC). [Note: Starch is not present in the rhizome of Maianthemum (A. N. Hale et al., submitted for publication).] In brief, a 0.03 g dried sample for each plant is boiled while stirring with a magnetic stir bar. Once samples cool to room temperature, they are filtered through a 0.20 μm filter, and run on HPLC (Aminex HPX-87C anion-exchange column, deionized water at 85 °C was set as the mobile phase with a flux rate of 0.6 mL min−1). Standards are used (inulin from dahlia tubers, Sigma-Aldrich; sucrose, Sigma-Aldrich) to confirm the identity of the sample peaks and to create standard curves to determine inulin and sucrose concentrations. Here, we express inulin and sucrose concentrations as a percentage of the HPLC dry sample mass. We also sum each plant's inulin and sucrose content to determine total NSC concentration (%).
To explore the effect of our treatments on rhizome carbohydrate status, we use a multivariate analysis of covariance (MANCOVA). Following a significant MANCOVA, individual ANCOVA tests are conducted for inulin, sucrose and total NSC. For all models, we include harvest date as a main effect because rhizome carbohydrate concentration varies over the growing season in perennial herbs (e.g. Lapointe 1998; Wyka 1999; Kleijn et al. 2005). We also include initial plant mass as a covariate to account for differences in carbohydrate storage that are related to plant size/age (MANCOVA model: total NSC + inulin + sucrose = treatment + harvest date + initial plant mass; ANCOVA models: carbohydrate = treatment + harvest date + initial plant mass). We calculate least squares means and standard errors for all ANCOVA models with a significant (P < 0.05) treatment effect. All analyses were conducted in SAS (v. 9.3, SAS Institute, Cary, NC, USA).
Field study: measuring impacts on vital rates of native plant populations
Study site
Our experimental plots are located in a beech-maple forest in southwest Pennsylvania [Trillium Trail Nature Reserve (hereafter TT), Allegheny County, PA, USA: 40°52′01.40″N; 79°90′10.75″W] with a rich herbaceous perennial understory flora (Knight et al. 2009). Based on previous work at TT (Burke 2008) and other temperate deciduous forests (e.g. Brundrett and Kendrick 1988), we estimate that 73 % of TT herbaceous perennials are AMF-dependent (Hale et al. 2011). We detected Alliaria allelochemicals in the soil of TT in concentrations that are toxic to AMF spores in lab assays (Cantor et al. 2011). Additionally, we showed that in soils where Alliaria occurs at TT, the density of fungal hyphae is lower (Cantor et al. 2011) and the fungal community composition shifts (Burke et al. 2011) relative to paired, non-invaded areas. Maianthemum plants collected at TT are heavily colonized by RFS, but their intra-root AMF community is significantly altered where Alliaria is present (Burke 2008). These results motivate further investigation of mutualism disruption by Alliaria in understanding mechanisms driving native plant performance declines.
Field experiment
We collected data on naturally occurring individuals of M. racemosum within six 14 × 14 m plots in TT from 2003 through 2013. Our six plots are split in half longitudinally so that each contains two experimental treatments: Alliaria removal (= low or no allelochemicals) or Alliaria present at ambient levels (= allelochemicals present). Annual removal of Alliaria from half of each plot (i.e. a 14 × 7 m area) began in spring 2006, ∼15 years after Alliaria became established at this site (L. Smith, pers. comm.) This time frame for TT invasion coincides with the estimated Alliaria invasion history in the region that indicates that this invader has been present locally for <25 years (Lankau et al. 2009). We remove Alliaria concurrent with the onset of emergence of the perennial herb community. Alliaria individuals are removed as tiny seedlings, minimizing disturbance to the soil and other plants. Removed plants are discarded off site. In June of each year prior to Alliaria seed dispersal we erect a barrier at the border of the two treatments to block seed dispersal from the ambient into the Alliaria removal treatment. All Maianthemum plants emerging in the plots are permanently tagged and have annually been scored for individual size, stage (i.e. seedling, non-flowering, flowering and dormant) and deer browse status. Prior to initiation of the Alliaria removal treatment in 2006 there was no difference in Alliaria per cent cover between the plots (χ2 = 0.11, P = 0.74) or total per cent cover of all species (χ2 = 0.038, P = 0.85).
Plant vital rates
We assess the effect of Alliaria removal on Maianthemum growth and three vital rates: annual flowering frequency, retrogression of flowering plants to non-flowering the following year and the frequency of prolonged vegetative dormancy (Shefferson 2009). We test for differences using data collected prior to the implementation of the removal treatment (2003–06) and after the removal treatment began (2007–13). All models have the general form: response variable = treatment + year + treatment × year. To estimate differences in growth rate, we investigate the differences in average size between treatments for the initial cohort of plants first observed when the experiment began in 2003. The mean size of this cohort is estimated with a linear mixed model for each year since 2006 (Zuur et al. 2009). We model log(plant size) to improve normality of the residuals.
Annual flowering frequencies are modelled using a logistic mixed model. Retrogression frequencies were modelled without random effects for the years 2008–13 because of limited sample size. Our retrogression model, stated in terms of probability, is
Our sample for retrogression was therefore set by the number of plants that flowered the previous year (time t − 1) that emerged as either flowering or non-flowering the next year (time t).
Growth and vital rate analyses are conducted in R 3.1.0 (R Development Core Team 2014) using the lme4 package (Bates et al. 2014). To account for repeated measures and blocking effects, we include random intercepts for individual plants and pairs of treatments within a plot. For each response variable we test for significant differences between annual means using the multcomp package in R (Bretz et al. 2010). We test for the presence of a long-term trend since 2006 in each treatment mean by specifying a trend contrast (Rosenthal and Rosnow 1985; Gurevitch and Chester 1986). All tests are planned contrasts so we do not correct for multiple comparisons. To further investigate trends in flowering frequencies, we also analyse these data using a two-level hierarchical model with time as a continuously varying main effect and year as a random effect.
Results of flowering and retrogression analyses are reported as effect sizes using odds ratios (OR) (Rita and Komonen 2008). Odds ratios have a lower bound of zero and no upper bound. Odds ratios of 1 indicate no difference between two treatments in the odds of an event happening. Statistical tests for OR therefore test whether they are different from 1. Odds ratios and their 95 % confidence intervals (CIs) are given in the text on their normal scale but graphed on a log scale to improve interpretation (sensu Galbraith 1988).
Mark-recapture models
We use mark-recapture models, a modified logistic regression approach (Kéry et al. 2005), to estimate the probability of prolonged vegetative dormancy. To test for pre-existing differences in dormancy rates, we conduct separate mark-recapture analyses of the 3 years prior to implementation of the removal treatment (2003–05) and the 7 years after the treatment began (2007–13). Mark-recapture results are assessed using the small sample size corrected information criteria AICc (AICc = AIC + 2k(k+1)/(n−k−1), where k = the number of parameters and n = sample size) to rank the explanatory ability of different models (Anderson 2010). To summarize the data we also analyse the entire data set (2003–13) and calculate the mean difference in dormancy rates between treatments. We first calculate dormancy rates for each treatment in each year, calculate the difference between these means and average the differences for the pre- and post-treatment time periods. We use the delta method (Powell 2007) in the R package msm to combine multiple standard errors and construct 95 % CIs around our final effect size estimates. Mark-recapture models are run in the R package marked (Laake et al. 2013).
Missing data due to herbivory
Deer browse compromised our ability to gain information on some individuals. Deer preferentially browse flowering Maianthemum and flowering individuals are of larger size than non-flowering individuals (N. L. Brouwer and S. Kalisz, unpubl. data). Accordingly, in the cases where an individual was browsed before its reproductive status was determined during the 10 annual censuses (n = 103 instances across 10 years), we assumed the browsed individual was flowering. Further, if browse occurred before an individual's size data was collected or size was otherwise unavailable, we used linear imputation (Gelman and Hill 2006) to estimate its size (412 instances of size imputation out of 1481 total size records). Including imputed size data for the browsed plants prevents biasing our results against detecting a treatment effect (Hadfield 2008; Nakagawa and Freckleton 2008).
We imputed missing size data using estimates generated from multiple rounds of linear regression based on observed size data from the years prior to and after the missing data. We averaged these multiple estimates to arrive at a final imputed size estimate for each browsed individual. Linear regression models included all available covariates, including previous size, current status, treatment and reproductive output for flowering plants. We validated our imputations by comparing mean plant size and the overall size distribution in the population with and without imputed data [see Supporting Information—Table S1].
Results
Greenhouse study: assessing potential for carbon stress
All M. racemosum plants examined exhibit colonization by internal RFS structures. However, Maianthemum's rhizome carbohydrates were significantly affected by the Alliaria treatment (MANCOVA; Roy's greatest root = 7.57, P = 0.002), with plants in the Alliaria treatment experiencing a significant reduction in total NSC (Fig. 1; ANCOVA F1,36 = 7.31, P = 0.01). Specifically, plants treated with Alliaria stored, on average, 17 % less inulin relative to plants in the Hesperis treatment (Fig. 1; ANCOVA F1,36 = 9.28, P = 0.004). While plants in the Alliaria treatment had fewer stored sugars, they had higher sucrose concentrations in their rhizomes compared with plants in the Hesperis treatment (Fig. 1; ANCOVA F1,36 = 12.88, P = 0.001). The increase in mobile sugars did not compensate for the dramatic difference in stored sugars between treatments as total NSC in the Alliaria-treated plants was 13 % lower than that of Hesperis-treated plants. Harvest date was not a significant predictor of total NSC, inulin or sucrose.
Figure 1.
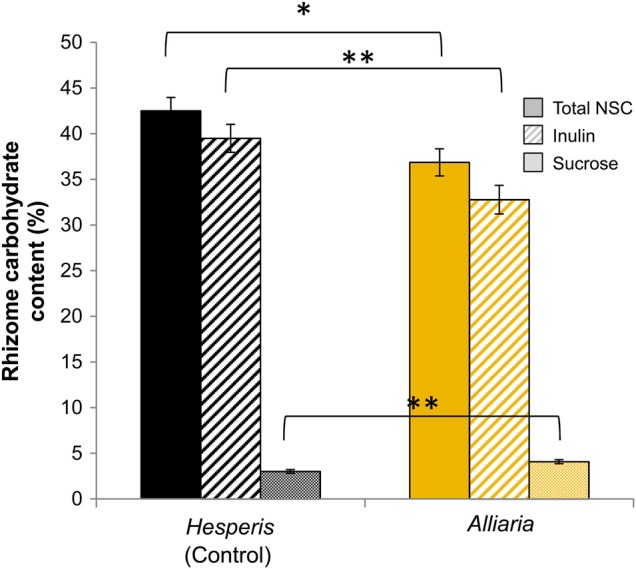
Maianthemum racemosum rhizome carbohydrate content (%) from Alliaria (yellow) and Hesperis (control; black) treatments in the greenhouse experiment. Total NSC content is shown in solid-coloured bars. Total NSC is a composite measure of stored sugars (inulin; bars with diagonal shading) and mobile sugars (sucrose; stippled bars). Values are least squares means from ANCOVAs ±1 standard error. *P < 0.05; **P < 0.005.
Field study: impact on vital rates
Growth
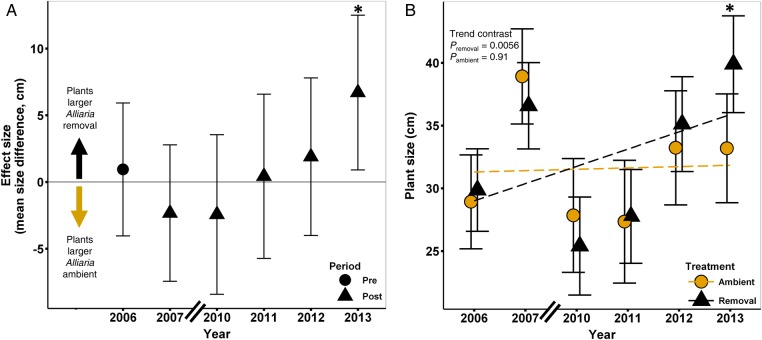
Prior to implementation of the removal treatment, there was no difference in the mean size of plants in the initial 2003 cohort (Fig. 2; P = 0.55). By 2013 plants in the removal treatment are significantly larger than those in the ambient Alliaria treatment (mean difference = 6.70 cm, SE = 2.96; P = 0.02). There is a significant positive linear trend in size from 2006 to 2013 (trend contrast P = 0.0056) in the Alliaria removal plots but no trend in the ambient plots (P = 0.91).
Figure 2.
Effect of Alliaria on plant size of Maianthemum marked in the initial 2003 survey of the field experiment. (A) Mean difference (effect size) in plant size between Alliaria in ambient and removal treatments. (B) Annual mean plant sizes in both treatments and ANOVA trend contrasts. Error bars represent ±95 % CIs. Asterisk indicates a significant difference in plant size between the two treatments (P < 0.05). Size data were not available for 2008 and 2009.
Flowering
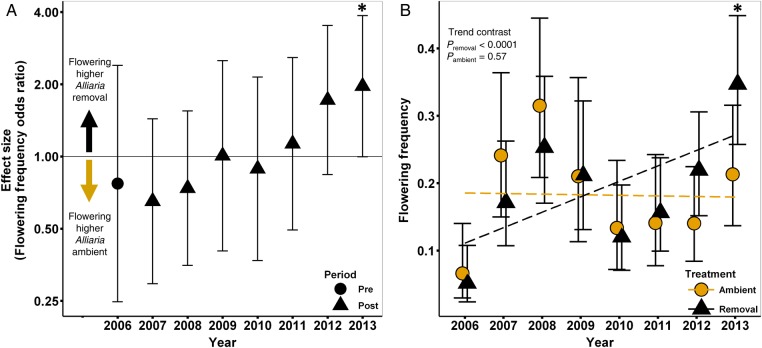
There is no significant difference in flowering probability across treatments for the first 6 years of the Alliaria removal (e.g. Fig. 3; P2006 = 0.65, P2007 = 0.29, P2008 = 0.42). However, by 2012 the flowering probability is ‘leaning’ (sensu Tukey 1991) in the predicted direction (OR = 1.72, CI95% = 0.84–3.52, P = 0.14) and by 2013 is significantly higher (OR = 1.96 CI95% = 1.0–3.87, P = 0.051) in the removal treatment. Across all years (2006–13) there is an increasing trend in flowering probability in the removal treatment (trend contrast P = 0.00008) but no increase in the ambient treatment (Ptrend = 0.57).
Figure 3.
Effect of Alliaria on Maianthemum flowering frequency. (A) Mean difference (effect size, ES) in flowering frequency in Alliaria-ambient and removal plots. Effect size is expressed as an OR and plotted on the log scale. (B) Annual mean flowering frequencies for both treatments and ANOVA trend contrasts. Error bars represent ±95 % CIs. Asterisk indicates a significant effect of Alliaria removal (P < 0.05).
Analyses using time as a continuous variable and year as a random effect confirmed that flowering frequencies diverged between the treatments (treatment × time χ2 = 6.81, P = 0.009) with a significant positive linear trend in the removal treatment (βremoval × time = 0.18, SE = 0.069) contrasted with evidence of a decrease in flowering probability in Alliaria-ambient plots (βtime = −0.10, SE = 0.072).
Retrogression
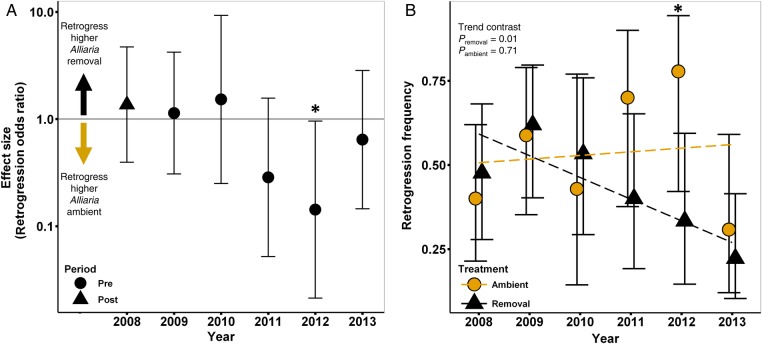
The number of flowering individuals was too low in 2005 and 2006 to accurately estimate retrogression of flowering plants in 2006 and 2007. By 2011, there was evidence that removal-treatment plants were less likely to retrogress (OR = 0.28 CI95% = 0.052–1.57, P = 0.15) and in 2012 they were significantly less likely to retrogress (OR = 0.14 CI95% = 0.021–0.96, P = 0.045). There was a significant decreasing trend in retrogression in the removal treatment from 2008 until 2013 (Ptrend = 0.011) but no trend in the ambient treatment (Ptrend = 0.90).
Dormancy
Dormancy rates were highly variable between years, ranging from <10 to >30 %, but estimated to be lower in the Alliaria removal treatment in six out of 7 years [see Supporting Information—Table S2]. For years prior to the implementation of the Alliaria removal treatment (2003–06) the best-ranked model contains only a year effect (Table 1) while for models of post-treatment years (2007–13) and the entire dataset (2003–13) the best models contain an effect of Alliaria removal, indicating that dormancy rates were typically lower in this treatment. There was an initially large difference in dormancy rates between plots that would be allocated to the two treatments in the first year of the study [see Supporting Information—Table S2], potentially resulting in the model of the pre-treatment years containing an Alliaria removal effect (AICc = 454.6) ranked almost as high as a year-only model (AICc = 452.8). However, since the year-only model has a lower AICc and fewer parameters, the larger model is not considered competitive (Arnold 2010). Moreover, in the other two pre-treatment years (2004 and 2005), there is no difference between dormancy estimates [see Supporting Information—Table S2]. The results of model selection are reinforced by the calculation of average effect sizes for the period prior to Alliaria removal and after removal (Fig. 5). Prior to removal there is no significant difference between dormancy rates (ES = −0.05, CI95% = −0.13–0.03) but after removal dormancy rates are ∼7 % lower than in the Alliaria-ambient treatments (ES = −0.069, CI95% = −0.12 to −0.2).
Table 1.
Ranking of mark-recapture models testing the effects of Alliaria removal on prolonged vegetative dormancy. Three sets of models were run over different time periods during the study: Set 1: years before Alliaria removal began (Pre-treatment); Set 2: years after the annual weeding treatment was initiated (post-treatment) and Set 3: all years. N, number of plants tracked over each time period; K, number of parameters in a model; Ln(lik), log likelihood. To calculate the mean pre-treatment and post-treatment effect size (Fig. 5) we used the parameters from the ‘Removal × Year’ model in the ‘All years’ model Set 3.
| Set | Period | Model | N | K | AICc | ΔAICc | Ln(lik) |
|---|---|---|---|---|---|---|---|
| 1 | Pre-Alliaria removal (2003–06) | Year | 158 | 5 | 452.8 | 0.00 | −216.21 |
| Removal + Year | 6 | 454.6 | 1.74 | −215.00 | |||
| Removal × Year | 9 | 466.2 | 11.59 | −214.47 | |||
| 2 | Post-Alliaria removal (2007–13) | Removal + Year | 210 | 9 | 1166.4 | 0.00 | −564.73 |
| Year | 8 | 1172.4 | 6.03 | −569.84 | |||
| Removal × Year | 15 | 1187.2 | 14.76 | −562.34 | |||
| 3 | All years (2003–13) | Removal + Year | 236 | 12 | 1646.3 | 0.00 | −798.46 |
| Year | 11 | 1652.5 | 6.23 | −803.68 | |||
| Removal × Year | 21 | 1680.3 | 27.74 | −795.98 |
Figure 5.
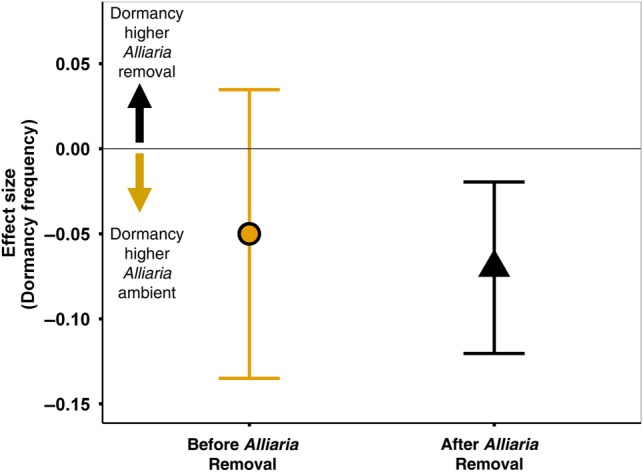
Effect size of Alliaria removal on the frequency of prolonged vegetative dormancy in Maianthemum before (2003–06; yellow) and after the treatment began (2007–13; black). Calculated with mark-recapture models; error bars represent ± 95 % CIs. Asterisk indicates a significant effect of Alliaria removal (P < 0.05).
Discussion
To our knowledge this is the first study to explore the connections between an allelopathic invasive species' impacts on the soil biotic environment and changes in individual plants' carbon status and vital rates. The results presented here in conjunction with prior studies substantiate multiple steps in a physiologically based causal pathway between invasion and population-level impacts on native plants. Our prior work demonstrates that Alliaria treatment of soil around Maianthemum reduces the density of soil fungal hyphae (A. N. Hale et al., submitted for publication) and plant photosynthetic rates (Hale et al. 2011). Here, our results demonstrate that treatment with Alliaria across the entire growing season results in negative effects on season-long carbon storage (Fig. 1). Relative to control plants, Maianthemum exposed to Alliaria stored 17 % less inulin in their rhizomes and experienced an overall reduction in total NSCs at the end of the season. Stomatal conductance modulates carbon fixation and is a key physiological rate affected by Alliaria exposure (Hale et al. 2011). Interestingly, a recent meta-analysis (Augé et al. 2014) comparing the effects of AMF inoculation on stomatal conductance (gs) in field vs. greenhouse studies indicates that greenhouse experiments have smaller effect sizes than field studies. Thus, our carbon storage results are likely conservative estimates of the carbon impacts of mutualism disruption in the field.
Over time, chronic exposure to Alliaria was predicted to compound this carbon deficit and affect plant growth and vital rates. Results from our long-term field study of Alliaria removal are consistent with this prediction. Individual aboveground plant size (Fig. 2) and multiple carbon-intensive and size-dependent vital rates (Figs 3–5) are positively affected in Alliaria removal relative to Alliaria-ambient plots.
Figure 4.
Effect of Alliaria on Maianthemum retrogression from flowering to non-flowering. (A) Annual mean difference in retrogression frequency (ES) in Alliaria-ambient and removal plot. Effect size is expressed as an OR and plotted on the log scale. (B) Mean retrogression frequencies in both treatments and ANOVA trend contrasts. Error bars represent ±95 % CIs. Asterisk indicates a significant effect of Alliaria removal (P < 0.05). Retrogression is calculated conditional on a plant being observed above-ground and not dormant. Sample sizes for 2006 and 2007 were insufficient for vital rate calculation.
Other experimental studies where Alliaria and native plants are grown together in pots (Meekins and McCarthy 1999; Wixted and McGraw 2010; Lankau 2012; Smith and Reynolds 2014) or in the field (McCarthy 1997; Carlson and Gorchov 2004; Cipollini et al. 2008; Lankau 2011b) also find negative effects of Alliaria on native species. Competition, direct allelopathic phytotoxicity and allelopathic RFS-mutualism disruption are all mechanisms that could contribute to these results. Our greenhouse experiment adds support to the idea that it is Alliaria's disruption of key belowground mutualists (RFS) rather than competition or direct phytotoxicity that accounts for its success as an invader. Below we discuss the general support or lack thereof for the likelihood of all three mechanisms.
Competition
We are aware of only two studies that have attempted to quantify reciprocal competition between Alliaria and focal plants. These pot studies found that Alliaria was equal to or weaker in competitive ability than three of four species tested (Meekins and McCarthy 1999; Leicht-Young et al. 2012). However, these studies are problematic in that they cannot separate competition from phytotoxicity or mutualism disruption. Bossdorf et al. (2004) found that Alliaria individuals from the native range outcompete Alliaria plants from the invaded range, supporting the hypothesis that invasive Alliaria express a different trade-off relative to their source populations. Invasive Alliaria are armed with novel allelochemical weapons but have evolved to be less competitive (Bossdorf et al. 2004). Further, field experiments demonstrate that native competitors can suppress Alliaria performance and abundance when the natives are not experiencing overabundant herbivore pressure (Eschtruth and Battles 2009a), as deer preferentially consume native plants and facilitate the high population growth and spread of Alliaria (Kalisz et al. 2014). In experimental studies that exclude deer from invaded sites, Alliaria abundance rapidly declines (Eschtruth and Battles 2009b; Knight et al. 2009; Kalisz et al. 2014). In total, these results underscore the widely held view that Alliaria is a relatively poor competitor (Rodgers et al. 2008a).
Direct phytotoxicity
Glucosinolates are known antimicrobial chemicals produced by members of the mustard family as defences against pathogens (Tierens et al. 2001). While Alliaria's allelochemicals can be inhibitory to germinating seeds and inhibit new seedling root growth (lettuce and radish seed experiments: Vaughn and Berhow 1999; Roberts and Anderson 2001; Pisula and Meiners 2010; Impatiens and Viola seed experiments: Prati and Bossdorf 2004; Barto et al. 2010; Cipollini and Flint 2013), to our knowledge direct toxicity of Alliaria on mature plant tissues has never been demonstrated. Alliaria invades forest understories dominated by adult perennial plants dependent on RFS. The direct effect of allelochemicals is inversely proportional to target plant density or biomass (Weidenhamer 2006). Single-celled fungal spores and thin fungal hyphae should be much more susceptible to Alliaria allelochemicals than mature plant tissues. Thus, while we cannot rule out direct phytotoxic effects of Alliaria on adult Maianthemum performance in our field or greenhouse experiments, a direct allelochemical effect is likely of small magnitude relative to indirect effects on RFS.
RFS-mutualism disruption
Mounting evidence shows that Alliaria can exert potent indirect effects on plants by suppressing RFS. Glucosinolates, like those produced by Alliaria, have a short half-life in the soil (<15 h; Gimsing et al. 2006). Yet, native plants grown in soils conditioned by Alliaria, treated with Alliaria tissue extracts, or collected from Alliaria-invaded sites all express reduced growth (Stinson et al. 2006; Callaway et al. 2008; Wolfe et al. 2008) despite the fact that the volatile allelochemicals were likely no longer present. Importantly, these studies demonstrate that Alliaria impacts are similar in magnitude to soil sterilization and that experimental soils result in lower colonization of roots by mycorrhizae (Stinson et al. 2006; Callaway et al. 2008; Wolfe et al. 2008). Finally, Maianthemum plants treated with Alliaria retain RFS structures internal to their roots, while exhibiting significant declines in soil hyphae (A. N. Hale et al., submitted for publication). Together these experiments provide strong support for RFS-mutualism disruption and that its effects are of large magnitude relative to competition or direct phytotoxicity.
Mechanistically, our working model linking RFS-mutualism disruption to carbon stress is based on the following premises: If Alliaria's allelochemicals destroy the hyphal network, yet the normally long-lived internal structures (Brundrett and Kendrick 1990) remain intact, then we would predict that the plant would increase carbon allocation to its RFS to provision the regrowth of the soil hyphal network, resulting in significant carbon stress for the plant. Loss of the hyphal network severely limits available soil nutrients and water to the plant (Newsham 2011; Augé et al. 2014). As a result, the plants photosynthesize less (Hale et al. 2011) and fix less carbon (NSC; Fig. 1). With this limited carbon pool, we suggest that plants may maintain concentrations of mobile sugars in the rhizome and roots to re-establish a functional RFS hyphal network that is repeatedly destroyed by our application of fresh Alliaria tissue. While our results are consistent with this working model (e.g. we observe greater sucrose concentrations in the rhizome of Alliaria vs. Hesperis-treated plants (Fig. 1)), additional experiments are needed to fully explore this hypothesis.
We note that the effects of allelopathic mutualism disruption by Alliaria could be amplified by additional factors. Like other invasive species of deciduous forests (Ehrenfeld et al. 2001; Poulette and Arthur 2012; Smith and Reynolds 2012; Kuebbing et al. 2014; Schuster and Dukes 2014), Alliaria can affect multiple components of the soil environment. Alliaria increases soil nutrient availability (Rodgers et al. 2008b), litter decomposition rates and nitrogen loss (Ashton et al. 2005). Since the RFS community in general (Van Diepen et al. 2011) and specific RFS–plant interactions (e.g. Klironomos 2002) are sensitive to soil conditions, multiple invader-mediated changes to the soil environment could magnify the impacts of allelopathic RFS-mutualism disruption. These diverse and widespread consequences of invasive species for soil environments and RFS communities are alarming given the potentially central role RFS and other microbes play in the diversity, productivity and functioning of plant communities (van der Heijden et al. 2008).
Our greenhouse study indicates that Maianthemum carbon storage declines significantly in response to Alliaria treatment in just one growing season. In contrast, we observe a relatively slow recovery of individual size, growth and vital rates following Alliaria removal in our field study. The predicted significant trends indicative of recovery (Figs 2–4) emerged after a few years of Alliaria removal while significant differences within the single-year comparisons were not seen until ∼6–7 years post removal (2012 or 2013). Two, non-mutually exclusive mechanisms could underlie this lag. First, the lag could be due to Maianthemum's habit (LaFrankie 1985). In general, forest understory herbaceous perennials are light-limited, slow-growing, long-lived species (Whigham 2004) with slow responses to perturbation (Morris et al. 2008). Our data are consistent with the idea that following Alliaria removal, Maianthemum may take multiple years to re-gain sufficient carbon stores to allow size growth, sustain flowering and maintain low dormancy rates. Second, the observed lag in Maianthemum vital rate responses may be due to slow recovery of the RFS soil community following Alliaria removal, a phenomenon observed by Anderson et al. (2010) and Lankau et al. (2014). If populations of beneficial RFS have gone locally extinct and low dispersal distance limits RFS re-colonization (Rout and Callaway 2012), then the observed time lag of Maianthemum could be due to the low abundance of effective fungal partners. Given the reciprocal obligate dependence of AMF and forest herbaceous perennial plants, declines in the native understory community may drive reciprocal declines in the RFS soil community (Lankau et al. 2014).
Conclusions
Increases in invasive species are generally correlated with declines in native biodiversity (e.g. Butchart et al. 2010). However, the mechanistic underpinnings leading to native population collapse are rarely understood yet are the subject of numerous studies and invasion hypotheses (Levine et al. 2003; Hulme et al. 2013). The disruption of plant soil feedbacks and root fungal symbioses are common aspects of plant invasions (i.e. Grove et al. 2012; Meinhardt and Gehring 2012; Ruckli et al. 2014; Shannon et al. 2014). As suggested by Hale and Kalisz (2012), chronic RFS-mutualism disruption could act as the first step in native plant biodiversity loss. In our system, the disruption of RFS by an allelopathic invader appears to begin a downward spiral in the physiological function (Hale et al. 2011), carbon status (Fig. 1) and ultimately vital rates (Figs 2–5) of a common native forest plant. Loss of these critical belowground mutualisms may be the proximate cause of plant mortality that is instead attributed to second-order effects (e.g. drought or herbivory) that are easier to observe (sensu McDowell 2011). Additional studies in invaded communities that explore the links between plant physiology, carbon allocation and population demographic performance are needed to determine the generality of these results. Mutualism disruption may be a widespread mechanism that helps explain how invasive species can cause large-scale changes to forest biodiversity observed in the wake of invasion (e.g. Rodgers et al. 2008a).
Sources of Funding
Funding was supplied by a United States National Science Foundation award DEB-0958676 to S.K., a NSF pre-doctoral fellowship to N.L.B. and a Phipps Conservatory Botany-in-Action award and an Andrew K. Mellon pre-doctoral fellowship to A.N.H.
Contributions by the Authors
S.K. conceived, designed, implemented and led data collection of the field experiment and assisted with the conception, design and implementation of the greenhouse experiment. A.N.H. designed, implemented and analysed data from the greenhouse experiment and assisted in data collected for the field experiment from 2008 to 2011. N.L.B. managed and analysed data from the field experiment and assisted with data collection for the field experiment since 2010. All three authors collaborated on the conception and writing of this article.
Conflicts of Interest Statement
None declared.
Supporting Information
The following additional information is available in the online version of this article –
Table S1. Validation of imputed Maianthemum size data from field experiment. Original and imputed size data are compared using t-tests and Kolmogorov–Smirnov tests.
Table S2. Estimated frequency of prolonged vegetative dormancy of Maianthemum from field experiment using a Mark-Recapture model.
Acknowledgements
We thank the members of the Kalisz lab for field, lab and greenhouse assistance, and two anonymous reviewers whose detailed suggestions improved the manuscript.
Literature Cited
- Allen MF, Smith WK, Moore TS, Jr, Christensen M. 1981. Comparative water relations and photosynthesis of mycorrhizal and non-mycorrhizal Bouteloua gracilis H.B.K. Lag ex steud. New Phytologist 88:683–693. 10.1111/j.1469-8137.1981.tb01745.x [DOI] [Google Scholar]
- Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB. 2012. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences of the USA 109:233–237. 10.1073/pnas.1107891109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR. 2010. Model based inference in the life sciences: a primer on evidence. New York: Springer. [Google Scholar]
- Anderson JPE, Domsch KH. 1975. Measurement of bacterial and fungal contributions to respiration of selected agricultural and forest soils. Canadian Journal of Microbiology 21:314–322. 10.1139/m75-045 [DOI] [PubMed] [Google Scholar]
- Anderson RC, Dhillion SS, Kelley TM. 1996. Aspects of the ecology of an invasive plant, garlic mustard (Alliaria petiolata), in central Illinois. Restoration Ecology 4:181–191. 10.1111/j.1526-100X.1996.tb00118.x [DOI] [Google Scholar]
- Anderson RC, Anderson MR, Bauer JT, Slater M, Herold J, Baumhardt P, Borowicz V. 2010. Effect of removal of garlic mustard (Alliaria petiolata, Brassicaeae) on arbuscular mycorrhizal fungi inoculum potential in forest soils. The Open Ecology Journal 3:41–47. 10.2174/1874213001003010041 [DOI] [Google Scholar]
- Arnold TW. 2010. Uninformative parameters and model selection using Akaike's Information Criterion. The Journal of Wildlife Management 74:1175–1178. 10.1111/j.1937-2817.2010.tb01236.x [DOI] [Google Scholar]
- Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT. 2005. Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecological Applications 15:1263–1272. 10.1890/04-0741 [DOI] [Google Scholar]
- Augé R, Toler HD, Saxton AM. 2014. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24. [DOI] [PubMed] [Google Scholar]
- Barto EK, Friese C, Cipollini D. 2010. Arbuscular mycorrhizal fungi protect a native plant from allelopathic effects of an invader. Journal of Chemical Ecology 36:351–360. 10.1007/s10886-010-9768-4 [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effect models using Eigen and S4. R package version 1.1-7. http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Boerner REJ. 1990. Role of mycorrhizal fungus origin in growth and nutrient uptake by Geranium robertianum. American Journal of Botany 77:483–489. 10.2307/2444382 [DOI] [PubMed] [Google Scholar]
- Bongard CL, Fulthorpe RR, Bongard CL, Fulthorpe RR. 2013. Invasion by two plant species affects fungal root colonizers. Ecological Restoration 31:253–263. 10.3368/er.31.3.253 [DOI] [Google Scholar]
- Bossdorf O, Prati D, Auge H, Schmid B. 2004. Reduced competitive ability in an invasive plant. Ecology Letters 7:346–353. 10.1111/j.1461-0248.2004.00583.x [DOI] [Google Scholar]
- Bretz F, Hothorn T, Westfall P. 2010. Multiple comparisons using R. London: CRC Press. [Google Scholar]
- Brundrett MC. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320:37–77. 10.1007/s11104-008-9877-9 [DOI] [Google Scholar]
- Brundrett MC, Kendrick B. 1988. The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Canadian Journal of Botany 66:1153–1173. 10.1139/b88-166 [DOI] [Google Scholar]
- Brundrett MC, Kendrick B. 1990. The roots and mycorrhizas of herbaceous woodland plants. I. Quantitative aspects of morphology. New Phytologist 114:457–468. 10.1111/j.1469-8137.1990.tb00414.x [DOI] [PubMed] [Google Scholar]
- Brundrett MC, Piché Y, Peterson RL. 1984. A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Canadian Journal of Botany 62:2128–2134. 10.1139/b84-290 [DOI] [Google Scholar]
- Burke DJ. 2008. Effects of Alliaria petiolata (garlic mustard; Brassicaceae) on mycorrhizal colonization and community structure in three herbaceous plants in a mixed deciduous forest. American Journal of Botany 95:1416–1425. 10.3732/ajb.0800184 [DOI] [PubMed] [Google Scholar]
- Burke DJ, Weintraub MN, Hewins CR, Kalisz S. 2011. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biology and Biochemistry 43:795–803. 10.1016/j.soilbio.2010.12.014 [DOI] [Google Scholar]
- Butchart SHM, Walpole M, Collen B, van Strien A, Scharlemann JPW, Almond REA, Baillie JEM, Bomhard B, Brown C, Bruno J, Carpenter KE, Carr GM, Chanson J, Chenery AM, Csirke J, Davidson NC, Dentener F, Foster M, Galli A, Galloway JN, Genovesi P, Gregory RD, Hockings M, Kapos V, Lamarque JF, Leverington F, Loh J, McGeoch MA, McRae L, Minasyan A, Morcillo MH, Oldfield TEE, Pauly D, Quader S, Revenga C, Sauer JR, Skolnik B, Spear D, Stanwell-Smith D, Stuart SN, Symes A, Tierney M, Tyrrell TD, Vié JC, Watson R. 2010. Global biodiversity: indicators of recent declines. Science 328:1164–1168. 10.1126/science.1187512 [DOI] [PubMed] [Google Scholar]
- Callaway RM, Ridenour WM. 2004. Novel weapons: invasive success and the evolution of increased competitive ability. Frontiers in Ecology and the Environment 2:436–443. 10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2 [DOI] [Google Scholar]
- Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J. 2008. Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043–1055. 10.1890/07-0370.1 [DOI] [PubMed] [Google Scholar]
- Cantor A, Hale A, Aaron J, Traw MB, Kalisz S. 2011. Low allelochemical concentrations detected in garlic mustard-invaded forest soils inhibit fungal growth and AMF spore germination. Biological Invasions 13:3015–3025. 10.1007/s10530-011-9986-x [DOI] [Google Scholar]
- Carlson AM, Gorchov DL. 2004. Effects of herbicide on the invasive biennial Alliaria petiolata (Garlic Mustard) and initial responses of native plants in a southwestern Ohio forest. Restoration Ecology 12:559–567. 10.1111/j.1061-2971.2004.00373.x [DOI] [Google Scholar]
- Cipollini D, Gruner B. 2007. Cyanide in the chemical arsenal of garlic mustard, Alliaria petiolata. Journal of Chemical Ecology 33:85–94. 10.1007/s10886-006-9205-x [DOI] [PubMed] [Google Scholar]
- Cipollini KA, Flint WN. 2013. Comparing allelopathic effects of root and leaf extracts of invasive Alliaria petiolata, Lonicera maackii, and Ranunculus ficaria on germination of three native woodland plants. Ohio Journal of Science 112:37–43. [Google Scholar]
- Cipollini KA, McClain GY, Cipollini D. 2008. Separating above- and belowground effects of Alliaria petiolata and Lonicera maackii on the performance of Impatiens capensis. The American Midland Naturalist 160:117–128. 10.1674/0003-0031(2008)160[117:SAABEO]2.0.CO;2 [DOI] [Google Scholar]
- Crone EE, Miller E, Sala A. 2009. How do plants know when other plants are flowering? Resource depletion, pollen limitation and mast-seeding in a perennial wildflower. Ecology Letters 12:1119–1126. 10.1111/j.1461-0248.2009.01365.x [DOI] [PubMed] [Google Scholar]
- Davis AS, Landis DA, Nuzzo V, Blossey B, Gerber E, Hinz HL. 2006. Demographic models inform selection of biocontrol agents for garlic mustard (Alliaria petiolata). Ecological Applications 16:2399–2410. 10.1890/1051-0761(2006)016[2399:DMISOB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- DeMars BG, Boerner REJ. 1995. Arbuscular mycorrhizal development in three crucifers. Mycorrhiza 5:405–408. 10.1007/BF00213440 [DOI] [Google Scholar]
- Ehrenfeld JG. 2003. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6:503–523. 10.1007/s10021-002-0151-3 [DOI] [Google Scholar]
- Ehrenfeld JG, Kourtev P, Huang W. 2001. Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecological Applications 11:1287–1300. 10.1890/1051-0761(2001)011[1287:CISFFI]2.0.CO;2 [DOI] [Google Scholar]
- Eschtruth AK, Battles JJ. 2009a. Assessing the relative importance of disturbance, herbivory, diversity, and propagule pressure in exotic plant invasion. Ecological Monographs 79:265–280. 10.1890/08-0221.1 [DOI] [Google Scholar]
- Eschtruth AK, Battles JJ. 2009b. Acceleration of exotic plant invasion in a forested ecosystem by a generalist herbivore. Conservation Biology 23:388–399. 10.1111/j.1523-1739.2008.01122.x [DOI] [PubMed] [Google Scholar]
- Galbraith RF. 1988. A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in Medicine 7:889–894. 10.1002/sim.4780070807 [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. 2006. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press. [Google Scholar]
- Gilliam F. 2014. The herbaceous layer in forests of eastern North America. Oxford: Oxford University Press. [Google Scholar]
- Gimsing AL, Sorensen JC, Tovgaard L, Jorgensen AMF, Hansen HCB. 2006. Degradation kinetics of glucosinolates in soil. Environmental Toxicology and Chemistry 25:2038–2044. 10.1897/05-610R.1 [DOI] [PubMed] [Google Scholar]
- Gremer JR, Sala A. 2013. It is risky out there: the costs of emergence and the benefits of prolonged dormancy. Oecologia 172:937–947. 10.1007/s00442-012-2557-8 [DOI] [PubMed] [Google Scholar]
- Gremer JR, Sala A, Crone EE. 2010. Disappearing plants: why they hide and how they return. Ecology 91:3407–3413. 10.1890/09-1864.1 [DOI] [PubMed] [Google Scholar]
- Grove S, Haubensak KA, Parker IM. 2012. Direct and indirect effects of allelopathy in the soil legacy of an exotic plant invasion. Plant Ecology 213:1869–1882. 10.1007/s11258-012-0079-4 [DOI] [Google Scholar]
- Gurevitch J, Chester ST., Jr 1986. Analysis of repeated measures experiments. Ecology 67:251–255. 10.2307/1938525 [DOI] [Google Scholar]
- Hadfield JD. 2008. Estimating evolutionary parameters when viability selection is operating. Proceedings of the Royal Society B: Biological Sciences 275:723–734. 10.1098/rspb.2007.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale AN, Kalisz S. 2012. Perspectives on allelopathic disruption of plant mutualisms: a framework for individual- and population-level fitness consequences. Plant Ecology 213:1991–2006. 10.1007/s11258-012-0128-z [DOI] [Google Scholar]
- Hale AN, Tonsor SJ, Kalisz S. 2011. Testing the mutualism disruption hypothesis: physiological mechanisms for invasion of intact perennial plant communities. Ecosphere 2:art110. [Google Scholar]
- Hulme PE, Pysěk P, Jarošík V, Pergl J, Schaffner U, Vilà M. 2013. Bias and error in understanding plant invasion impacts. Trends in Ecology and Evolution 28:212–218. 10.1016/j.tree.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Jumpponen A, Trappe JM. 1998. Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytologist 140:295–310. 10.1046/j.1469-8137.1998.00265.x [DOI] [PubMed] [Google Scholar]
- Kalisz S, Spigler RB, Horvitz CC. 2014. In a long-term experimental demography study, excluding ungulates reversed invader's explosive population growth rate and restored natives. Proceedings of the National Academy of Sciences of the USA 111:4501–4506. 10.1073/pnas.1310121111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéry M, Gregg KB, Schaub M. 2005. Demographic estimation methods for plants with unobservable life-states. Oikos 108:307–320. 10.1111/j.0030-1299.2005.13589.x [DOI] [Google Scholar]
- Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL. 2010. Mutualisms in a changing world: an evolutionary perspective. Ecology Letters 13:1459–1474. 10.1111/j.1461-0248.2010.01538.x [DOI] [PubMed] [Google Scholar]
- Kleijn D, Treier UA, Müller-Schärer H. 2005. The importance of nitrogen and carbohydrate storage for plant growth of the alpine herb Veratrum album. New Phytologist 166:565–575. 10.1111/j.1469-8137.2005.01321.x [DOI] [PubMed] [Google Scholar]
- Klironomos JN. 2002. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70. 10.1038/417067a [DOI] [PubMed] [Google Scholar]
- Knight TM, Dunn JL, Smith LA, Davis J, Kalisz S. 2009. Deer facilitate invasive plant success in a Pennsylvania forest understory. Natural Areas Journal 29:110–116. 10.3375/043.029.0202 [DOI] [Google Scholar]
- Koch AM, Antunes PM, Barto EK, Cipollini D, Mummey DL, Klironomos JN. 2011. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biological Invasions 13:1627–1639. 10.1007/s10530-010-9920-7 [DOI] [Google Scholar]
- Koide RT, Shumway DL, Mabon SA. 1994. Mycorrhizal fungi and reproduction of field populations of Abutilon theophrasti Medic. (Malvaceae). New Phytologist 126:123–130. 10.1111/j.1469-8137.1994.tb07537.x [DOI] [Google Scholar]
- Kuebbing SE, Classen AT, Simberloff D. 2014. Two co-occurring invasive woody shrubs alter soil properties and promote subdominant invasive species. Journal of Applied Ecology 51:124–133. 10.1111/1365-2664.12161 [DOI] [Google Scholar]
- Laake JL, Johnson DS, Conn PB. 2013. marked: an R package for maximum likelihood and Markov Chain Monte Carlo analysis of capture–recapture data. Methods in Ecology and Evolution 4:885–890. 10.1111/2041-210X.12065 [DOI] [Google Scholar]
- LaFrankie JV., Jr 1985. Morphology, growth, and vasculature of the sympodial rhizome of Smilacina racemosa (Liliaceae). Botanical Gazette 146:534–554. 10.1086/337559 [DOI] [Google Scholar]
- Lankau RA. 2011a. Resistance and recovery of soil microbial communities in the face of Alliaria petiolata invasions. New Phytologist 189:536–548. 10.1111/j.1469-8137.2010.03481.x [DOI] [PubMed] [Google Scholar]
- Lankau RA. 2011b. Interpopulation variation in allelopathic traits informs restoration of invaded landscapes. Evolutionary Applications 5:270–282. 10.1111/j.1752-4571.2011.00218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau RA. 2012. Coevolution between invasive and native plants driven by chemical competition and soil biota. Proceedings of the National Academy of Sciences of the USA 109:11240–11245. 10.1073/pnas.1201343109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau RA, Nuzzo V, Spyreas G, Davis AS. 2009. Evolutionary limits ameliorate the negative impact of an invasive plant. Proceedings of the National Academy of Sciences of the USA 106:15362–15367. 10.1073/pnas.0905446106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankau RA, Bauer JT, Anderson MR, Anderson RC. 2014. Long-term legacies and partial recovery of mycorrhizal communities after invasive plant removal. Biological Invasions 16:1979–1990. [Google Scholar]
- Lapointe L. 1998. Fruit development in Trillium: dependence on stem carbohydrate reserves. Plant Physiology 117:183–188. 10.1104/pp.117.1.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen LM, Nielsen JK, Sørensen H. 1992. Host plant recognition in monophagous weevils: specialization of Ceutorhynchus inaffectatus to glucosinolates from its host plant Hesperis matronalis. Entomologia Experimentalis et Applicata 64:49–55. 10.1111/j.1570-7458.1992.tb01593.x [DOI] [Google Scholar]
- Leicht-Young SA, Pavlovic NB, Adams JV. 2012. Competitive interactions of garlic mustard (Alliaria petiolata) and damesrocket (Hesperis matronalis). Invasive Plant Science and Management 5:27–36. 10.1614/IPSM-D-11-00025.1 [DOI] [Google Scholar]
- Levine JM, Vilà M, Antonio CMD, Dukes JS, Grigulis K, Lavorel S. 2003. Mechanisms underlying the impacts of exotic plant invasions. Proceedings of the Royal Society B: Biological Sciences 270:775–781. 10.1098/rspb.2003.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Koide RT. 1994. The effects of mycorrhizal infection on components of plant growth and reproduction. New Phytologist 128:211–218. 10.1111/j.1469-8137.1994.tb04004.x [DOI] [PubMed] [Google Scholar]
- McCarthy BC. 1997. Response of a forest understory community to experimental removal of an invasive nonindigenous plant (Alliaria petiolta, Brassicaceae). In: Luken JO, Thieret JW, eds. Assessment and management of plant invasions. New York: Springer, 117–130. [Google Scholar]
- McDowell NG. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology 155:1051–1059. 10.1104/pp.110.170704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist 178:719–739. 10.1111/j.1469-8137.2008.02436.x [DOI] [PubMed] [Google Scholar]
- Meekins JF, McCarthy BC. 1999. Competitive ability of Alliaria petiolata (Garlic Mustard, Brassicaceae), an invasive, nonindigenous forest herb. International Journal of Plant Sciences 160:743–752. 10.1086/314156 [DOI] [Google Scholar]
- Meinhardt KA, Gehring CA. 2012. Disrupting mycorrhizal mutualisms: a potential mechanism by which exotic tamarisk outcompetes native cottonwoods. Ecological Applications 22:532–549. 10.1890/11-1247.1 [DOI] [PubMed] [Google Scholar]
- Morris WF, Pfister CA, Tuljapurkar S, Haridas CV, Boggs CL, Boyce MS, Bruna EM, Church DR, Coulson T, Doak DF, Forsyth S, Gaillard J-M, Horvitz CC, Kalisz S, Kendall BE, Knight TM, Lee CT, Menges ES. 2008. Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89:19–25. 10.1890/07-0774.1 [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Freckleton RP. 2008. Missing inaction: the dangers of ignoring missing data. Trends in Ecology and Evolution 23:592–596. 10.1016/j.tree.2008.06.014 [DOI] [PubMed] [Google Scholar]
- Newsham KK. 2011. A meta-analysis of plant responses to dark septate root endophytes. New Phytologist 190:783–793. 10.1111/j.1469-8137.2010.03611.x [DOI] [PubMed] [Google Scholar]
- Olano JM, Menges ES, Martínez E. 2006. Carbohydrate storage in five resprouting Florida scrub plants across a fire chronosequence. New Phytologist 170:99–106. 10.1111/j.1469-8137.2005.01634.x [DOI] [PubMed] [Google Scholar]
- Pisula NL, Meiners SJ. 2010. Relative allelopathic potential of invasive plant species in a young disturbed woodland. The Journal of the Torrey Botanical Society 137:81–87. 10.3159/09-RA-040.1 [DOI] [Google Scholar]
- Poulette MM, Arthur MA. 2012. The impact of the invasive shrub Lonicera maackii on the decomposition dynamics of a native plant community. Ecological Applications 22:412–424. 10.1890/11-1105.1 [DOI] [PubMed] [Google Scholar]
- Powell LA. 2007. Approximating variance of demographic parameters using the delta method: a reference for avian biologists. The Condor 109:949–954. 10.1650/0010-5422(2007)109[949:AVODPU]2.0.CO;2 [DOI] [Google Scholar]
- Prati D, Bossdorf O. 2004. Allelopathic inhabitation of germination by Alliaria petiolate (Brassicaceae). American Journal of Botany 91:285–288. 10.1890/11-1105.1 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- Rita H, Komonen A. 2008. Odds ratio: an ecologically sound tool to compare proportions. Annales Zoologici Fennici 45:66–72. 10.5735/086.045.0106 [DOI] [Google Scholar]
- Roberts KJ, Anderson RC. 2001. Effect of garlic mustard [Alliaria petiolata (Beib. Cavara & Grande)] extracts on plants and arbuscular mycorrhizal (AM) fungi. The American Midland Naturalist 146:146–152. 10.1674/0003-0031(2001)146[0146:EOGMAP]2.0.CO;2 [DOI] [Google Scholar]
- Rodgers VL, Stinson KA, Finzi AC. 2008a. Ready or not, garlic mustard is moving in: Alliaria petiolata as a member of Eastern North American forests. BioScience 58:426–436. 10.1641/B580510 [DOI] [Google Scholar]
- Rodgers VL, Wolfe BE, Werden LK, Finzi AC. 2008b. The invasive species Alliaria petiolata (garlic mustard) increases soil nutrient availability in northern hardwood-conifer forests. Oecologia 157:459–471. 10.1007/s00442-008-1089-8 [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow R. 1985. Contrast analysis: focused comparisons in the analysis of variance. Cambridge: Cambridge University Press. [Google Scholar]
- Rout ME, Callaway RM. 2012. Interactions between exotic invasive plants and soil microbes in the rhizosphere suggest that ‘everything is not everywhere’. Annals of Botany 110:213–222. 10.1093/aob/mcs061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckli R, Rusterholz HP, Baur B. 2014. Invasion of an annual exotic plant into deciduous forests suppresses arbuscular mycorrhiza symbiosis and reduces performance of sycamore maple saplings. Forest Ecology and Management 318:285–293. 10.1016/j.foreco.2014.01.015 [DOI] [Google Scholar]
- Schuster MJ, Dukes JS. 2014. Non-additive effects of invasive tree litter shift seasonal N release: a potential invasion feedback. Oikos 123:1101–1111. [Google Scholar]
- Schweiger R, Baier MC, Persicke M, Müller C. 2014. High specificity in plant leaf metabolic responses to arbuscular mycorrhiza. Nature Communications 5:Article 3886 10.1038/ncomms4886 [DOI] [PubMed] [Google Scholar]
- Shannon SM, Bauer JT, Anderson WE, Reynolds HL. 2014. Plant-soil feedbacks between invasive shrubs and native forest understory species lead to shifts in the abundance of mycorrhizal fungi. Plant and Soil 382:317–328. 10.1007/s11104-014-2158-x [DOI] [Google Scholar]
- Shefferson RP. 2009. The evolutionary ecology of vegetative dormancy in mature herbaceous perennial plants. Journal of Ecology 97:1000–1009. 10.1111/j.1365-2745.2009.01525.x [DOI] [Google Scholar]
- Smith LM, Reynolds HL. 2012. Positive plant-soil feedback may drive dominance of a woodland invader, Euonymus fortunei. Plant Ecology 213:853–860. 10.1007/s11258-012-0047-z [DOI] [Google Scholar]
- Smith LM, Reynolds HL. 2014. Light, allelopathy, and post-mortem invasive impact on native forest understory species. Biological Invasions 16:1131–1144. 10.1007/s10530-013-0567-z [DOI] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis, 3rd edn London: Academic Press. [Google Scholar]
- Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN. 2006. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biology 4:e140 10.1371/journal.pbio.0040140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierens K, Thomma BPH, Brouwer M, Schmidt J, Kistner K, Porzel A, Mauch-Mani B, Cammue BPA, Broekaert WF. 2001. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiology 125:1688–1699. 10.1104/pp.125.4.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. 1991. The philosophy of multiple comparisons. Statistical Science 6:100–116. 10.1214/ss/1177011945 [DOI] [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecology Letters 11:1351–1363. 10.1111/j.1461-0248.2008.01250.x [DOI] [PubMed] [Google Scholar]
- van der Heijden MGA, Bardgett RD, van Straalen NM. 2008. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11:296–310. 10.1111/j.1461-0248.2007.01139.x [DOI] [PubMed] [Google Scholar]
- Van Diepen LTA, Lilleskov EA, Pregitzer KS. 2011. Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. Molecular Ecology 20:799–811. 10.1111/j.1365-294X.2010.04969.x [DOI] [PubMed] [Google Scholar]
- Vaughn SF, Berhow MA. 1999. Allelochemicals isolated from tissues of the invasive weed garlic mustard (Alliaria petiolata). Journal of Chemical Ecology 25:2495–2504. 10.1023/A:1020874124645 [DOI] [Google Scholar]
- Vitousek PM, Walker LR. 1989. Biological invasion by Myrica Faya in Hawai'i: plant demography, nitrogen fixation, ecosystem effects. Ecological Monographs 59:247–265. 10.2307/1942601 [DOI] [Google Scholar]
- Vogelsang KM, Bever JD. 2009. Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 90:399–407. 10.1890/07-2144.1 [DOI] [PubMed] [Google Scholar]
- Weidenhamer JD. 2006. Distinguishing allelopathy from resource competition: the role of density. In: Reigosa MJ, Pedrol N, Gonzalez L, eds. Allelopathy: a physiological process with ecological implications. The Netherlands: Springer, 85–103. [Google Scholar]
- Weir TL, Park SW, Vivanco JM. 2004. Biochemical and physiological mechanisms mediated by allelochemicals. Current Opinion in Plant Biology 7:472–479. 10.1016/j.pbi.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Whigham DF. 2004. Ecology of woodland herbs in temperate deciduous forests. Annual Review of Ecology, Evolution, and Systematics 35:583–621. 10.1146/annurev.ecolsys.35.021103.105708 [DOI] [Google Scholar]
- Wixted KL, McGraw JB. 2010. Competitive and allelopathic effects of garlic mustard (Alliaria petiolata) on American ginseng (Panax quinquefolius). Plant Ecology 208:347–357. 10.1007/s11258-009-9711-3 [DOI] [Google Scholar]
- Wolfe BE, Klironomos JN. 2005. Breaking new ground: soil communities and exotic plant invasion. BioScience 55:477–487. 10.1641/0006-3568(2005)055[0477:BNGSCA]2.0.CO;2 [DOI] [Google Scholar]
- Wolfe BE, Rodger VL, Stinson KA, Pringle A. 2008. The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. Journal of Ecology 96:777–783. 10.1046/j.1365-3040.1998.00351.x [DOI] [Google Scholar]
- Wright DP, Read DJ, Scholes JD. 1998. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant, Cell and Environment 21:881–891. 10.1046/j.1365-3040.1998.00351.x [DOI] [Google Scholar]
- Wyka T. 1999. Carbohydrate storage and use in an alpine population of the perennial herb, Oxytropis sericea. Oecologia 120:198–208. 10.1007/s004420050849 [DOI] [PubMed] [Google Scholar]
- Zhu X-C, Song F-B, Liu S-Q, Liu T-D. 2011. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant and Soil 346:189–199. 10.1007/s11104-011-0809-8 [DOI] [Google Scholar]
- Zuleta A, Sambucetti ME. 2001. Inulin determination for food labeling. Journal of Agricultural and Food Chemistry 49:4570–4572. 10.1021/jf010505o [DOI] [PubMed] [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer Science & Business Media. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.