Summary
Antibodies to receptors can block or mimic hormone action. Taking advantage of receptor isoforms, co-receptors and other receptor modulating proteins, antibodies and other designer ligands can enhance tissue specificity and provide new approaches to the therapy of diabetes and other diseases.
Keywords: Anti-receptor antibodies, FGF21, insulin receptor, co-receptors, receptor isoforms, brown adipose tissue
In this issue of Science Translational Medicine, Wu et al. report the development of monoclonal antibodies to fibroblast growth factor receptor-1 (FGFR1) which activate the receptor, mimicking the glucose lowering effects of FGF21 and producing a sustained anti-diabetic effect (1). Indeed, at the end of the 19th century, even before the first hormone was isolated, classical work by Ehrlich, Koch, and Behring led to the concept of antibodies as potential therapeutics for disease. But it was the discovery of stimulatory autoantibodies directed at the TSH receptor in Graves’ disease (originally termed long-acting thyroid stimulator or LATS and now termed anti-TSH receptor antibodies or TRAbs) which led to the recognition that antibodies to cell surface receptors could alter their function and mimic hormone action (2). Since then multiple surface proteins have been identified as targets of autoantibodies in endocrine and neurological diseases (3). In some cases, as with LATS, these antibodies produce stimulatory effects, but in most cases, these anti-receptor antibodies inhibit receptor binding and/or signaling, as first shown for antibodies to the acetylcholine receptor in myasthenia gravis (4). Autoantibodies to the insulin receptor can be either inhibitory, creating severe insulin-resistant diabetes, or stimulatory, resulting in hypoglycemia, in different individuals at different phases of the disease (3).
Based on these historical observations and the ability to create epitope-specific humanized antibodies, over 30 monoclonal antibodies have been developed and are in clinical use for treatment of cancer, autoimmune, inflammatory and infectious diseases. In most cases, these therapeutic antibodies are inhibitory, and most are used for severe or life-threatening conditions for which the lack of more effective alternatives justifies the potential side effects. Like LATS and some anti-insulin receptor antibodies, the therapeutic antibodies used in this report are stimulatory, activating the FGF receptor and mimicking the anti-diabetic effects of FGF21(1) (Figure 1, left panel). Any new treatment of diabetes, however, will not only have to lower glucose, but be superior to current treatments and without significant side effects, including hypoglycemia, thus setting a high bar for clinical use.
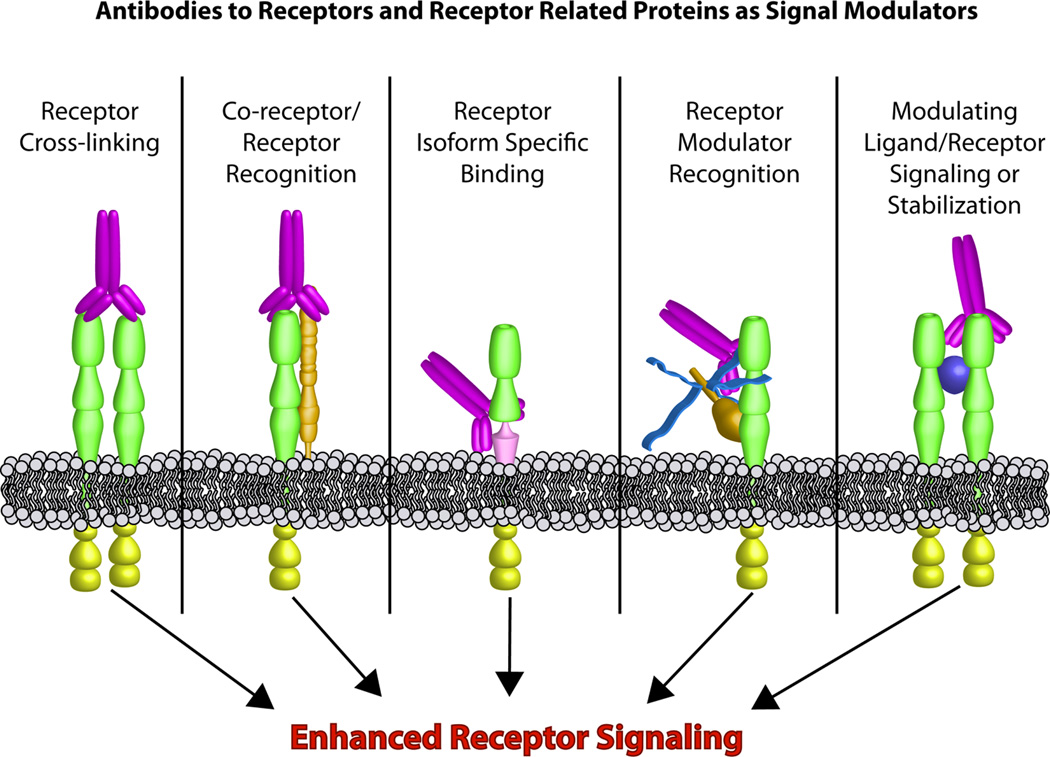
Figure 1. Mechanisms of stimulatory antibodies.
Antibodies can enhance receptor signaling in multiple ways. Antibodies can promote ligand independent receptor dimerization and activation and thereby circumvent limitations of ligand stability and production. Tissue specific receptor activation can be achieved by targeting receptor/ co-receptor complexes, or individual receptor splice variants. In addition, circulating modulatory protein/ receptor complexes provide a tempting target to incorporate paracrine signals into receptor activation. Finally ligand induced receptor activation can be fine tuned using allosteric antibodies.
FGF21 and brown fat as potential targets for therapy of diabetes
FGF21, along with FGF19 and FGF23, constitute a group of endocrine-like members of the fibroblast growth factor family (5). These FGFs lack a heparin binding domain allowing them to diffuse away from their tissue of origin into the circulation, where depending on the presence of appropriate receptors and co-receptors, they exert their effects. FGF21 is mainly produced by the liver and increased in response to fasting, where it acts upon the liver itself to increase lipid oxidation, ketogenesis and gluconeogenesis, as well as adipose tissue to increase utilization of fat as an energy source. Treatment of animal models of type 2 diabetes with recombinant FGF21 corrects hyperglycemia, decreases bodyweight, lowers triglycerides and cholesterol levels, and increases energy expenditure, making FGF21 a promising candidate for treatment of this disease (6). There are four fibroblast growth factor receptor (FGFR) genes, which with alternative splicing, result in over 48 different isoforms of FGFR. These vary in their ligand-binding properties, interaction partners and kinase domains (7). FGF21 binds to isoforms of FGFR1-3 in the presence of an essential co-receptor βKlotho (8). βKlotho is expressed primarily in white (WAT) and brown (BAT) adipose tissue, but also in liver and pancreas, giving FGF21 its specificity of action. Unlike FGF21 itself, the antibodies described by Wu et al. are specific to FGFR1 and function independent of βKlotho (1). As with agonistic anti-insulin receptor antibodies (3), these antibodies work by facilitating homo-dimerization and activation of these receptors (1). Since FGFR1 is most highly expressed in adipose tissue, especially brown adipose tissue, and minimally expressed in liver, stimulation by the anti-receptor antibody distinguishes the contribution of these tissues to the metabolic effects of FGF21/FGFR1 signaling.
Despite many similarities, there were some differences between FGF21 and FGFR1 antibody treatment. Thus, FGF21 acts on the liver to increase β-hydroxybutyrate production, while the antibody does not have this effect. On the other hand, the antibody lowered inorganic phosphate levels suggesting that it also activates FGFR1 in kidney mimicking the effect of FGF23. While such side effects of FGFR1 activation by antibody may limit its use, the current study demonstrates the potential of receptor isoform specific reagents to modify kinetics and tissue specificity of the hormones and growth factors they mimic, selecting desirable actions and limiting some undesirable ones. FGF21 action on the brain has been shown to be important in some of its metabolic effects (9), however it is unlikely that antibodies to FGFR1 will cross the blood brain barrier. Whether the antibodies would overcome the FGF21 resistance present in obesity, which usually accompanies type 2 diabetes, also remains to be determined (10).
One of the important targets of FGF21 and the FGFR1 antibody appears to be brown adipose tissue (BAT), which is activated by these treatments. Other growth factors and their cell surface receptors on BAT have also emerged as tempting targets for modulating energy expenditure and glucose homeostasis. BMP7, for example, has been shown to promote brown fat differentiation, and this is accompanied by increased energy expenditure and improvement in glucose homeostasis (11). Like FGF21, BMP7 acts through a family of receptors and associated co-receptors and has effects on other tissues and cell types, including precursors of bone. Identification of ligands or antibodies that would selectively activate the BMP7 receptor/co-receptor pair on BAT could increase tissue specificity. Similarly, targeting β3-adrenergic receptors on brown fat cells by antibodies might mimic the sympathetic nervous system stimulation essential for full activation of BAT function.
Targeting receptor isoforms, co-receptors and receptor modulators to increase specificity
While specificity of signal transduction is controlled by binding of a ligand to its receptor, over the past few years it has become clear that many receptors exist in families with multiple related species created from related genes, as well as by alternative splicing and different post-translational modifications. In addition, some receptors collaborate with co-receptors which act as modulators of receptor affinity and specificity. To date, at least eight different families of co-receptors, each with multiple members, have been recognized, including proteoglycans like syndecans, glypicans, and LRPs (12). These transmembrane or membrane associated proteins, with few exceptions, do not signal on their own, but rather refine or enhance receptor affinity or specificity. Like βKlotho in FGF21 signaling, the combination of differential expression of the receptors and co-receptors allows for a vastly increased tissue or cell type specificity of the ligand-generated signal. Thus, identifying ways to target specific co-receptor/receptor complexes could increase specificity and reduce potential adverse side effects of hormone and growth factor signaling (Figure 1, middle panels).
In addition to classical co-receptors, proteins may act as inhibitors of receptor binding/function. Insulin receptor signaling, for example, is known to be inhibited by the transmembrane glycoprotein PC-1/ENPP-1 and circulating alpha 2-HS glycoprotein, both of which interact with the extracellular domain of the receptor and inhibit insulin binding and activation. These classes of inhibitory proteins provide additional targets to modulate signal transduction.
Another approach to increase tissue specificity is to take advantage of the extensive modifications most cell surface proteins undergo, such as alternative splicing or glycosylation. Of the approximately 30,000 human genes, up to 60% are alternatively spliced. Many receptors, including the FGF receptor, insulin receptor and hepatocyte growth factor receptor undergo alternative splicing. Posttranslational modifications like glycosylation of the IGF-1, EGF, and FGF receptors modulate receptor cycling and thereby receptor signaling (13). These receptor isoforms often have differing tissue distribution, and thus an isoform specific antibody or isoform-specific ligand could be used to create tissue specific hormonal effects (Figure 1, middle panels). For example, insulin is normally secreted into the portal vein producing a concentration acting on the liver that is about three times that acting on peripheral tissues. Thus in treating diabetes, if one could enhance the effects of insulin on liver versus muscle by three-fold, one might better mimic the physiological profile of this hormone. In addition, a tissue- or isoform-specific antibody could also be used to fine tune receptor stimulation, avoiding hypoglycemia which occurs in some patients with stimulatory anti-insulin receptor antibodies.
Conclusion
Hormone deficiency and hormone resistance are cause and consequence of many diseases and their treatments. In type 2 diabetes, insulin resistance is central in the development of disease. Current therapies to overcome this resistance are very limited. Recently discovered peptides, such as FGF21, BMP7, as well as the adipokines leptin and adiponectin, can potentially circumvent the insulin resistance and improve glucose and lipid homeostasis. Wu et al. demonstrate that antibodies to FGFR1 may also be effective (1). Building on the observed insulin-like effects of insulin receptor autoantibodies, others are now considering using allosteric activating antibodies to potentiate endogenous insulin action, potentially producing more physiological regulation (14)(Figure 1, right panel). While antibodies have the advantage over most hormones and growth factors of long action and thus need for infrequent injection, one needs to keep in mind that most hormones and growth factors are highly regulated throughout the day, and thus mimicking their action chronically may induce desensitization or other unanticipated side effects. Nonetheless, targeting receptors, co-receptors and other signal modulating factors through the use of antibodies and other “designer” ligands can provide increased target cell specificity and therefore potentially less toxicity making these attractive candidates for treatment for many diseases.
Reference List
- 1.Wu A, Kolumam G. Amelioration of Type 2 Diabetes by Antibody-mediated Activation of Fibroblast Growth Factor Receptor 1. 2011 doi: 10.1126/scitranslmed.3002669. (to be completed by editor) [DOI] [PubMed] [Google Scholar]
- 2.Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun.Rev. 2009;9:113–116. doi: 10.1016/j.autrev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flier JS, Kahn CR, Roth J. Receptors, antireceptor antibodies and the mechanism of insulin resistance. N.Engl.J.Med. 1979;300:413–419. doi: 10.1056/NEJM197902223000808. [DOI] [PubMed] [Google Scholar]
- 4.Lang B, Vincent A. Autoimmune disorders of the neuromuscular junction. Curr.Opin.Pharmacol. 2009;9:336–340. doi: 10.1016/j.coph.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am.J.Clin.Nutr. 2010;91:254S–257S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharitonenkov A, Shanafelt AB. FGF21: a novel prospect for the treatment of metabolic diseases. Curr.Opin.Investig.Drugs. 2009;10:359–364. [PubMed] [Google Scholar]
- 7.Polanska UM, Fernig DG, Kinnunen T. Extracellular interactome of the FGF receptor-ligand system: complexities and the relative simplicity of the worm. Dev.Dyn. 2009;238:277–293. doi: 10.1002/dvdy.21757. [DOI] [PubMed] [Google Scholar]
- 8.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J.Biol.Chem. 2007 Sep 14;282:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat.Rev.Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkbride KC, Ray BN, Blobe GC. Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem.Sci. 2005;30:611–621. doi: 10.1016/j.tibs.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Partridge EA, Le RC, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004 Oct 1;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 14.Corbin JA, Bhaskar V, Goldfine ID, Bedinger DH, Kuan HF, Gross L, Lau A, Handa M, Watson SR, Webster L, Levy R, Maddux BA, Wijesuriya S, Liu N, Wu X, Chemla-Vogel D, Narasimha AJ, Lee SR, Tran C, White ML. High Affinity Insulin Receptor Antibodies Sensitize the Insulin Receptor to Insulin and Restore Glycemic Control in Murine Models of Diabetes. Diabetes. 2011;60:A276–A276. [Google Scholar]