Abstract
Background:
Patients experience severe pain after craniotomy surgery that leads to discomfort. Our target in this study that performed in interventional method is an evaluation of sufentanil and paracetamol effect on postoperative pain control in patients undergone craniotomy surgery at Urmia Imam Khomeini Hospital.
Materials and Methods:
Totally, 45 patients between the ages 18 and 65 were studied. The effect of sufentanil and paracetamol medicines in pain management, hemodynamic stability, and side effects compared with control group that were receiving morphine (subcutaneous [SC]) in 3 groups of 15 people at time 0, 2, 4, 12 and 24-h were evaluated. Collected data were included and monitoring blood pressure, O2 Sat, heart rate (HR) and pain, nausea, vomiting and use of morphine.
Results:
According to the analysis of results, there was a significant difference between 3 groups on postoperative pain (P < 0.05). In patients that used sufentanil, pain score of visual analog scale (VAS) is lowest and in the paracetamol group the highest VAS score was seen. There was a significant difference in HR between 3 groups (P < 0.05). Maximum average of HR was observed in the paracetamol group. There was a significant difference in mean arterial pressure between 3 groups (P < 0.05). In paracetamol group, there was the highest value (99.3). There was no significant difference in Glasgow Coma scale and SPO2 between 3 groups (P > 0.05).
Conclusion:
Sufentanil compared to morphine (which is routinely used for patients pain control after craniotomy surgery) has better pain control, less nausea and vomiting, and better hemodynamic stability. Although paracetamol has the least nausea and vomiting, it has the lowest quality of pain relief.
Keywords: Craniotomy, paracetamol, postoperative pain management, sufentanil, visual analogue scale
INTRODUCTION
After craniotomy surgery, there is somatic pain with origin in the scalp, muscles, and soft tissues.[1] Use of opioids following intracranial surgery has limitations due to lack of proper estimation of pain intensity and affecting postoperative assessment. Evaluations show these patients suffer from moderate to severe pain due to inadequate analgesia.[2] Sedation and analgesia are used for patient especially when mechanical ventilation is applied. Certain diseases, surgery, trauma, invasive monitoring, intubation and nursing interventions are only a part of the disturbing factors for patients in Intensive Care Unit (ICU). In addition to the distressing issues mentioned above, inadequate treatment of pain can lead to stressful responses such as tachycardia, increased body oxygen consumption, increased coagulability property, suppression of the immune response, increased metabolic rate and internal activity of catecholamines.[3]
Analgesia and inadequate sedation increase intracranial pressure (ICP), high blood pressure, restlessness, and vomiting that may increase the risk of intracranial hemorrhage or other neurological complications.[4]
According to several studies, there is frequency dispersion in methods of pain control in these patients. Opioids relieve this pain well but may result in respiratory depression and hypercapnic leading to cerebrovascular vasodilatation and increased ICP. Nonsteroidal anti-inflammatory drugs are alternative to opioids but may lead to cerebral hemorrhage due to interfere with platelet function.[5,6]
The method of craniotomy and pain control should be selected in such a way that maintains patient's Glasgow Coma Scale (GCS) with a minimum of interference and neurologic examination as it is possible.[7]
Despite analgesic effects, opioids have side effects such as retentive neurologic symptoms, increase in PaCO2 due to hypoventilation followed by ICP increase, nausea, and vomiting.[8]. Controlling pain and anxiety in these patients reduces ICP, hemodynamic changes and the incidence of nausea and vomiting, which reduces the risk of cerebral hemorrhage and neurologic complications.[1] For detection and characterization of pain, patients should be constantly evaluated. One of the acceptable methods for pain assessment in ICU and is based on the patient's own statements. In addition to recording vital signs, information about pain, its location, quality and intensity of pain should be recorded and evaluated. Pain evaluation can be done through visual analog scale (VAS).[3]
In this study, it is attempted to propose medicine and method for pain control in these patients, which controls the pain and anxiety of the patients and has minimum side effects such as changes in blood pressure, heart rate (HR), ICP, consciousness level, nausea, and vomiting; and neurologic examination is also possible.
MATERIALS AND METHODS
This study was done as intervention and target population included patients undergoing craniotomy surgery in Imam Khomeini Hospital in Urmia. Assignment of individuals in the group was randomly, and participation in the study was completely voluntary.
Overall, 45 patients (19 females and 26 males) who met the inclusion criteria were selected; 57.8% were males, and 42.2% were females [Figure 1]. Tools of data collection included alternative examination and completion of prepared questionnaires by nurses distinct from nurse who prescribed the drug.
Figure 1.
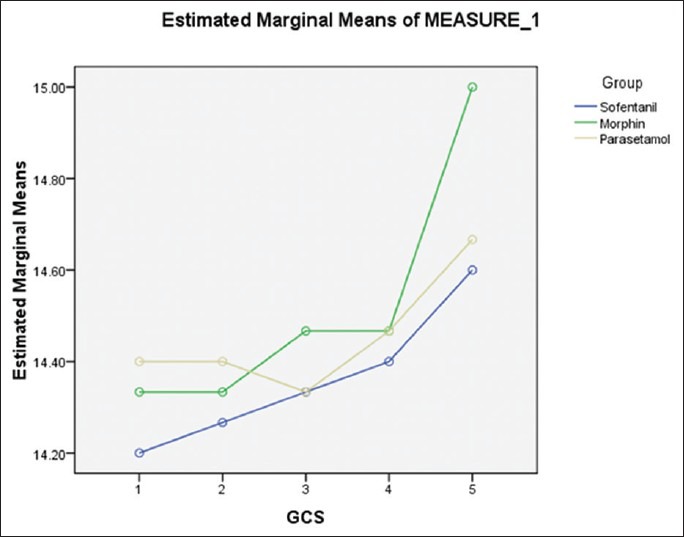
GCS changes in three groups
Patients with brain tumors with ASAI, II were between 18 and 65 years and free of any dependence on drugs. Patients had a preoperative GCS = 14–15. Exclusion criteria were GCS = 13 and lower on arrival at the ICU.
Anesthetic technique used in these patients was general anesthesia. Premedication in all patients was done as follows: With 0.05 mg/kg midazolam, 3 μg/kg fentanyl, anesthetic induction 2 mg/kg propofol, 0.5 mg/kg atracurium and 1.5 mg/kg lidocaine. Maintenance of anesthesia was in intravenous (IV) anesthesia (total IV anesthesia) including propofol infusion dose of 0.15 μg/kg/min and 0.1 μg/kg/min remifentanil, respectively. A volume of 0.07 mg/kg morphine was administrated early in operation, and 0.07 mg/kg was administered at the time of closing Dora. After transferrin patients from recovery to ICU, they were assigned to groups A, B, and C randomly. Group A received 0.0015 μ/kg/min sufentanil as continuous infusion and group B received 15 mg/kg/6 h paracetamol in 100 mL of normal saline within 15 min as intermittent infusion and Group C received subcutaneous (SC) morphine 5 mg every 4 h. On times 0, 2, 4, 12, 24-h, pain scores were recorded based on VAS, mean arterial pressure (MAP), HR, ICP, level of consciousness, arterial oxygenation (Sat O2) and presence or absence of nausea and vomiting, and 3 mg adjuvant IV morphine was administered in three groups if VAS ≥4.
RESULTS
Pain
According to the analysis, a significant difference was observed between the three groups in terms of pain intensity (P = 0.005). Lowest VAS score was seen in the sufentanil group and highest VAS score was seen in the paracetamol group. Pain in the morphine group was more than sufentanil group. In sufentanil group, only 1 patient and only 1 time showed VAS ≥4. In morphine group, 6 patients in a total of 8 controlling times showed VAS ≥4 (2 patients, 2 times showed VAS ≥ 4). A total of 11 patients in totally 16 control times showed VAS ≥4 in the paracetamol group.
Total mean of VAS in sufentanil group was 1.7 ± 0.1 (standard error), it was 2.3 ± 0.1 (standard error) in morphine group and 3.2 ± 0.1 in paracetamol group (standard error) [Table 1]. Highest VAS was seen in times 2 and 4.
Table 1.
Evaluated factors in three groups
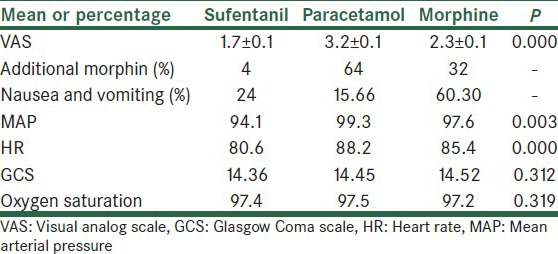
Adjunct morphine
In the sufentanil group, only 1 patient at a time needed adjunct morphine. In morphine SC group, total of 8 doses was used for 6 patients (2 patients at 2 times needed adjunct morphine). Most need to IV morphine was observed in the paracetamol group, and 16 doses were used for 11 patients [Table 1].
Nausea and vomiting
Most nausea and vomiting were observed in the morphine group and then in the sufentanil group, and ultimately least nausea and vomiting was observed in the paracetamol group. In morphine group, nausea and vomiting were observed in 7 patients in 10 h. In paracetamol group, 4 patients at 4 times suffered nausea and vomited. In sufentanil group, nausea and vomiting were observed in 4 patients at 5 controlling times [Table 1]. Metoclopramide was administered to patients with nausea and vomiting.
Mean arterial pressure
Heart rate was significantly different between the groups (P = 0.000). Maximum HR was observed in the paracetamol group; mean HR was about 80.6 in the sufentanil group. In morphine group, it was 85.4 and 88.2 in the paracetamol group [Table 1].
Significant difference was observed in three groups (P = 0.003). Highest 24-h average of MAP was seen in the paracetamol group (99.3), and it was lowest in the sufentanil group (94.1). Morphine group showed intermediate MAP (97.6) [Table 1, Figure 2].
Figure 2.
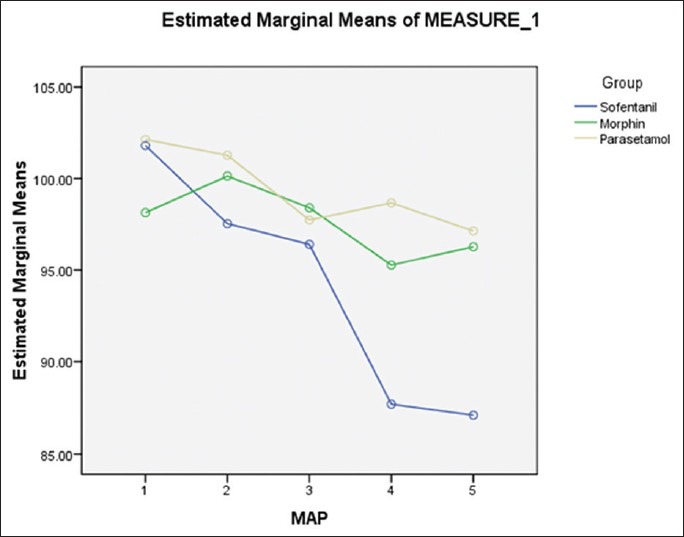
Mean arterial pressure in three groups
GCS differences among the three groups were not significant (P = 0.312). Mean GCS in the sufentanil group was 14.36, 14.45 in the paracetamol group and 14.52 in the morphine group [Figure 2].
No significant difference was observed in terms of arterial oxygen saturation (P = 0.319) [Table 1].
DISCUSSION
Many studies have been conducted for helping analgesia after head operations. Nair and Rajshekha conducted a study on 43 patients with GCS 15 and age above 16 years who undergone craniotomy and were accepted in ICU. Oral paracetamol was administered for postoperative analgesia that developed suitable analgesia in only 26% of patients. Remaining patients complained of moderate to severe pain.[4] In our study, paracetamol (IV) had the poorest quality of pain control. In the study by Rahimi et al., 27 patients were divided into two groups of 13 and 14 members. First group received only narcotic, while the second group also benefited from cyclooxygenase-2. Second group used less narcotics compared to the first group, and the first group received more narcotics and experienced more VAS and had longer hospitalization.[6] In our study, paracetamol alone is not adequate for pain control, while using narcotic improved quality of pain control that is consistent with the findings in this study. Verchère et al. conducted a study on 64 patients.[9] In the mentioned study, 8 patients were assigned in the paracetamol group, which received 30 mg/kg paracetamol 1 h before anesthesia and then every 6 h. The other group including 29 patients received paracetamol and tramadol. This group received 1.5 mg/kg tramadol before operation ends. Third group received paracetamol with nalbuphine. VAS was controlled in times of 1, 2, 4, 8, and 24-h. Analgesia was inadequate in the group receiving only paracetamol,[9] findings in the current work showed that it was no sufficient to use only paracetamol for pain control.
Findings in the current work show sufentanil, with maintaining optimal hemodynamics and analgesia with effects of sedation and lack of respiratory status drop and GCS of patients, can be a suitable drug for pain control after craniotomy operation. Results suggest a significant difference in VAS among the groups. Paracetamol group had the highest VAS. O2 Sat reduction was not observed among the groups. Only in 5 patients O2 Sat 90% was observed. Comparing HR, there was a significant and highest amount seen in the paracetamol group. Most percent of nausea and vomiting was seen in the morphine group. Lowest rate of nausea and vomiting was observed in the paracetamol group. GCS change between the groups was not significant.
CONCLUSION
Results of the current work showed sufentanil compared to morphine (which is routinely used for controlling pain after craniotomy operation) has better pain control, lower vomiting and nausea, and better hemodynamic stability. Although paracetamol has the lowest rate of vomiting and nausea, it has lowest pain control quality and the highest rate of using adjuvant drugs, which is not recommended for pain control after Craniotomy operation. Thus, sufentanil, with maintaining optimal hemodynamics and analgesia with effects of sedation and lack of respiratory status drop and GCS of patients, can be suitable drug for pain control after craniotomy operation and it is recommended to be investigated further.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cottrell J, Yong W. In: Cottrell and Young's neuroanesthesia. Philadelphia: Elsevier Health Sciences; 2010. Postoperative and intensive care including head injury and multisystem sequelae. Cottrell Young's Neuroanesthesia; pp. 410–1. [Google Scholar]
- 2.Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, et al. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: A prospective randomized controlled trial. Clinical article. J Neurosurg. 2009;111:343–50. doi: 10.3171/2008.11.JNS08797. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J, Abraham E. Philadelphia: Elsevier Saunders; 2011. Textbook of Critical Care; pp. 1492–3. [Google Scholar]
- 4.Nair S, Rajshekhar V. Evaluation of pain following supratentorial craniotomy. Br J Neurosurg. 2011;25:100–3. doi: 10.3109/02688697.2010.534199. [DOI] [PubMed] [Google Scholar]
- 5.Morad A, Winters B, Stevens R, White E, Weingart J, Yaster M, et al. The efficacy of intravenous patient-controlled analgesia after intracranial surgery of the posterior fossa: A prospective, randomized controlled trial. Anesth Analg. 2012;114:416–23. doi: 10.1213/ANE.0b013e31823f0c5a. [DOI] [PubMed] [Google Scholar]
- 6.Rahimi SY, Vender JR, Macomson SD, French A, Smith JR, Alleyne CH., Jr Postoperative pain management after craniotomy: Evaluation and cost analysis. Neurosurgery. 2006;59:852–7. doi: 10.1227/01.NEU.0000232646.35678.D8. [DOI] [PubMed] [Google Scholar]
- 7.Saha P, Chattopadhyay S, Rudra A, Roy S. Pain after craniotomy: A time for reappraisal? Indian J Pain. 2013;27:7–11. [Google Scholar]
- 8.Lai LT, Ortiz-Cardona JR, Bendo AA. Perioperative pain management in the neurosurgical patient. Anesthesiol Clin. 2012;30:347–67. doi: 10.1016/j.anclin.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Verchère E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14:96–101. doi: 10.1097/00008506-200204000-00002. [DOI] [PubMed] [Google Scholar]


