Abstract
Context:
Dexmedetomidine as an adjuvant to local anesthetics in peripheral nerve blocks has been used in only a few studies.
Aims:
We aimed at assessing the effect of dexmedetomidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block.
Settings and Design:
Random, controlled, and triple blind.
Materials and Methods:
Sixty American Society of Anesthesiologist grade I and II patients of either sex scheduled for elective upper limb surgery under supraclavicular brachial plexus block were divided into three equal groups in a prospective randomized double-blind controlled manner. For block patients in Group C received 0.5% ropivacaine (30cc), 0.5% ropivacaine with 50 μg dexmedetomidine (30cc) in Group D and 0.5% ropivacaine (30cc) in Group D-IV along with intravenous infusion of 50 μg dexmedetomidine in normal saline.
Statistical Analysis Used:
IBM-SPSS software version 17, Chi-square test, Mann-Whitney U-test.
Results:
Demographic profile and surgical characteristics were similar in all the three groups. Sensory block and motor block onset was earlier in group D than in group D-IV and group C. The sensory block and motor block duration was also prolonged in group D when compared with group D-IV and group C. The duration of analgesia was significantly longer in group D and D-IV when compared to group C.
Conclusions:
Dexmedetomidine as an adjuvant to 0.5%ropivacaine in ultrasound guided brachial plexus block shortens the sensory as well as motor block onset time, prolongs sensory and motor block duration and also increases the duration of analgesia. The action of dexmedetomidine most probably is local rather than centrally mediated.
Keywords: Dexmedetomidine, ropivacaine, supraclavicular brachial plexus block
INTRODUCTION
Brachial plexus block has evolved as an important tool in the anesthesiologist's armamentarium as a safe alternative to general anesthesia for upper limb surgery and for relief of perioperative pain. Its increased popularity is because of advancements in regional anesthesia techniques in terms of local anesthetic drugs, newer adjuvant drugs and use of ultrasound for safe and successful conduct of block. It helps in reduced hospital stay, less financial burden and also leads to avoidance of undesirable side-effects of general anesthesia.
Since the introduction of first brachial plexus block using cocaine by Halstead (1884) the technique of brachial plexus block has evolved from classical blind technique to use of nerve stimulators and ultrasound guidance for supraclavicular brachial plexus block.[1] Many additives to local anesthetics such as opioids, clonidine, neostigmine and tramadol etc. have been used to increase the duration of the block, to improve postoperative pain management[2] and to avoid the need for placing catheter for continuous local anesthetic drug infusion. Dexmedetomidine a newer α2-adrenoreceptor agonist is currently in focus for its sedative, anxiolytic and analgesic properties. Pre- and intra-operative intravenous dexmedetomidine administration has shown to prolong the duration of sensory block with local anesthetics during peripheral nerve blocks.[3]
Animal studies have shown that dexmedetomidine enhances onset of sensory and motor blockade along with increased duration of analgesia.[4,5] In human beings, dexmedetomidine has also shown to prolong the duration of block and postoperative analgesia when added to local anesthetic in various regional blocks.[6,7,8] Most human studies of dexmedetomidine as an adjuvant to local anesthetics involved combinations with bupivacaine or levobupivacaine.[9,10] Due to unique pharmacologic properties and fewer side effects, ropivacaine is being preferred by an increasing number of anesthesiologists for peripheral nerve blocks. However, there are very few published studies on dexmedetomidine in combination with ropivacaine.[6,11] The current study was designed with aim to evaluate the effect of adding dexmedetomidine to ropivacaine 0.5% in supraclavicular brachial plexus block in terms of onset and duration of sensory and motor block, quality of block, duration of postoperative analgesia and to test the hypothesis whether the effect of dexmedetomidine, is due to local action on nerve plexus or is centrally mediated.
MATERIALS AND METHODS
After ethical committee approval and written informed consent, 60 American Society of Anesthesiologist (ASA) grade I or II patients, scheduled for elective upper limb surgery below mid-humerus level under supraclavicular brachial plexus block were enrolled in this prospective, randomized, double-blind controlled trial. Preanesthetic assessment of all the patients was done the day before scheduled surgery. Patients were premedicated with tablet alprazolam 0.25 mg and tablet ranitidine 150 mg on night before surgery and also in the morning of surgery with a sip of water.
Patients with preexisting peripheral neuropathy of upper limb, bleeding disorders, infection at injection site, untreated pneumothorax, patients on adrenoreceptor agonist or antagonist therapy, history of severe cardiac, respiratory, hepatic or renal disease, pregnancy and known hypersensitivity to the study drugs, were excluded from the study.
Using a computer generated randomization, patients were randomized into three groups of 20 patients each as:
Group C: Ultrasound-guided supraclavicular brachial plexus block given with 30 ml Ropivacaine 0.5% (study drug [I]) and 50 ml normal saline (study drug [II]) administered as IV infusion over 15 min.
Group D: Ultrasound-guided supraclavicular brachial plexus block given with 30 ml Ropivacaine 0.5% containing 50 μg dexmedetomidine (study drug [I]) and 50 ml normal saline (0.9%) (study drug [II]) administered as IV infusion over 15 min.
Group D-IV: Ultrasound-guided supraclavicular brachial plexus block given with 30 ml Ropivacaine 0.5% (study drug [I]) and 50 ml normal saline containing 50 μg dexmedetomidine (study drug [II]) administered as IV infusion over 15 min.
Coded study drug solutions were prepared by an anesthesiologist not involved in further study and handed over to concerned anesthesiologist for administration.
After shifting the patient to operating table, standard anesthesia monitoring in the form of the baseline measurement of heart rate, noninvasive arterial blood pressure, and peripheral oxygen saturation (SpO2) was started. Intravenous access was achieved using 20 G cannula in the nonoperative arm.
After aseptic preparation of the area, surpraclavicular brachial plexus block was performed under ultrasound guidance (Sonosite, Micromaxx machine with high frequency (13 MHz) linear probe) with 30 ml of study drug (I) by an anesthesiologist who was unaware of the nature of study drug solution. The spread of injected drug was observed sonologically in real time to achieve a satisfactory spread of the drug around the brachial plexus. Intravenous infusion of 50 ml study drug (II) was also started at the time of starting the block.
Sensory and motor block evaluation was done every 3 min after giving block until complete sensory and motor block or 30 min, whichever was earlier. Sensory block was assessed by pinprick test with a blunt 23 G hypodermic needle in the distribution of all four nerves (ulnar, median, radial and musculocutaneous nerves) using a 3-point scale as: 0 = Normal sensation, 1 = Loss of sensation of prick (analgesia), 2 = Loss of sensation of touch (anesthesia). Motor block was evaluated by thumb abduction (radial nerve), thumb adduction (ulnar nerve), thumb opposition (median nerve), and flexion at elbow (musculocutaneous nerve) on a 3-point scale as: 0 = Normal motor function, 1 = Reduced motor strength (but able to move fingers), 2 = Complete motor block.
Onset time for sensory or motor block was defined as the time interval between the end of total local anesthetic administration and complete sensory or motor block. Complete sensory block was defined by anesthetic block (score 2) on all nerve territories. Complete motor block was defined as the absence of voluntary movement on hand and forearm (score 2).
At the end of the operation, quality of anesthesia was graded by the anesthesiologist as: Excellent (4): No complaint from the patient, Good (3): Minor complaint with no need for supplemental analgesics, Moderate (2): Complaint that required supplemental analgesics, and Unsuccessful (1): Patient required general anesthesia.
The observations in the recovery room were made by anesthesiologist who was unaware of the nature of drugs administered. On arrival in recovery room patients were asked to rate their pain on 11 point visual analog scale (VAS) and thereafter pain was assessed regularly every 30 min for first 2 h and then every 1 hourly till 24 h. Testing for sensory and motor block regression was done every 15 min until complete resolution. Duration of sensory block was defined as the time interval between the end of study drug (1) administration and complete resolution of sensation on all nerves. Duration of motor block was defined as the time interval between the end of study drug (1) administration and the recovery of complete motor power of the hand and forearm. Injection diclofenac sodium 75 mg intramuscular was administered when VAS score was ≥4. The time between the end of local anesthetic administration and first rescue analgesic administration was recorded as the duration of analgesia. Total amount of diclofenac sodium used in first 24 h period postoperatively was noted.
Patients were questioned for nausea, vomiting, skin rash and observed for tachycardia (>20% above baseline value), bradycardia (<50 beats per minute), hypotension (>20% below baseline value), hypertension (>20% above baseline value), hypoxemia (SpO2 <90%), sedation or any other side effect if any, during 24 h postoperative period. Sedation was assessed using 5 Point sedation score 1 = Alert and wide awake, 2 = Arousable to verbal command, 3 = Arousable with gentle tactile stimulation, 4 = Arousable with vigorous shaking, 5 = Unarousable.
Statistical analysis
Data were analyzed using IBM-SPSS software (Produced by SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc.). Age, height, weight, body mass index (BMI), sensory and motor block onset time, duration of surgery were analyzed by using independent Student's t-test. Sex ratio, ASA grade and quality of anesthesia were compared using the Chi-square test. VAS was expressed as median and interquartile range. VAS and sedation score were compared using Mann-Whitney U-test for pairwise comparison.
Power analysis
A post-hoc power analysis was conducted using the software package; GPower (Faul and Erdfelder 1992). The alpha level used for this analysis was P < 0.05. The post-hoc analyses revealed the statistical power for this study was 0.40 for detecting a small effect, whereas the power exceeded 0.99 for the detection of a moderate to large effect size. Thus, there was more than adequate power (i.e., power ×0.80) at a moderate to large effect size level, but less than adequate statistical power at the small effect size level. A sample size of 60 was used; the statistical power analyses and calculated effect size come out to be 1.91 for analgesic duration and gave the power of 1.
RESULTS
There was no statistically significant difference among the patients in the three groups with respect to age, height, weight, BMI, sex ratio, duration of surgery, type of surgery and the ASA physical status [Table 1].
Table 1.
Demographic data
The sensory and motor block onset was significantly quicker in group D than in group D-IV and group C. The mean sensory block onset time was 9.75 ± 4.23 min in group D as compared to 22.20 ± 8.62 min and 14.55 ± 8.39 min in group C and D-IV, respectively. The mean motor block onset time was 18.75 ± 6.37 min in group D when compared to 39.05 ± 16.38 min and 30.15 ± 14.52 min in group C and D-IV, respectively [Table 2].
Table 2.
Block characteristics
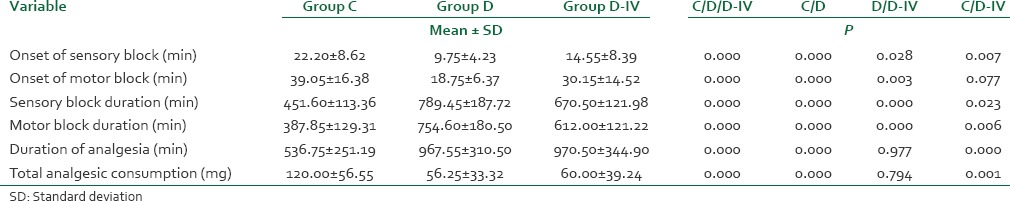
The duration of sensory as well as motor block was significantly prolonged in group D and group D-IV as compared to group C. The duration of sensory block was maximum in group D (789.45 ± 187.72 min) followed by group D-IV (667.50 ± 121.98 min) and group C (451.60 ± 113.36 min). The duration of motor block was also maximum in group D (754.60 ± 180.50 min), followed by group D-IV (612.00 ± 121.22 min) and group C (387.85 ± 129.31 min).
The duration of analgesia was significantly prolonged in group D (967.55 ± 310.50 min) and D-IV (970.50 ± 344.90 min) when compared with group C (536.75 ± 251.19 min). The duration of analgesia was comparable between groups D and D-IV.
The total analgesic consumption in 24 h postoperatively was significantly higher in group C than group D and D-IV. However, the difference in total analgesic consumption between group D and D-IV was not statistically significant.
No episode of hypoxemia or respiratory depression during 24 h period postoperatively was seen in any patient. Patients in group D and D-IV were more sedated compared to group C. Most of the patients in our study had sedation grade ≤3, except one patient in group D who had grade 4 sedation.
Bradycardia was observed in one patient belonging to group D-IV intraoperatively that was treated with injection atropine sulfate 0.6 mg IV. Hypotension was observed in two patients each belonging to group D and group D-IV, which was effectively treated with incremental 3 mg IV boluses of injection mephentermine. Skin rash was observed in one patient belonging to group D-IV that was treated with injection pheniramine maleate 45.5 mg IV. No episode of nausea, vomiting, or any other side-effect was observed.
DISCUSSION
Apart from sedative, analgesic, hemodynamic-stabilizing properties, and sympatholytic pharmacologic effects, the alpha (α)-2-adrenergic receptor (α2 -AR) agonists have been used to increase the duration of thermal anti-nociception and analgesia in some animal studies.[4,5] Animal studies have proven the combination of dexmedetomidine with ropivacaine to be safe and neuro-protective. The use of dexmedetomidine decreases inflammation around peripheral nerves, thereby decreasing the potential for peripheral nerve injury.[12] In human beings, the beneficial effects of adding dexmedetomidine to local anesthetics during regional anesthesia and some peripheral nerve blockade procedures have proved to be efficacious for the surgical patients.[6,7,11] To best of our knowledge, this is probably the first human study showing that the addition of dexmedetomidine to ropivacaine in ultrasound-guided supraclavicular brachial plexus block shortens the sensory and motor block onset time, prolongs sensory and motor block duration and also prolongs the duration of analgesia.
We used 0.5% ropivacaine for supraclavicular block. The rationale for choosing this concentration is supported by the study done by Klein et al. in 1998, who found that for interscalene brachial plexus block, increasing the concentration of ropivacaine from 0.5% to 0.75% failed to improve onset or duration of block, suggesting that the risk of increased total dose of local anesthetic may be avoided.[13] Hickey and coworkers have shown that 0.25% ropivacaine when used for subclavian perivascular brachial plexus block for upper limb surgery required frequent analgesia supplementation due to the low concentration of local anesthetic used.[14]
In our study, we have found that addition of dexmedetomidine (50 μg) to 30 ml ropivacaine 0.5% in ultrasound-guided supraclavicular brachial plexus block resulted in a quick onset of sensory and motor block [Figure 1], prolonged duration of both sensory and motor block [Figure 2], delayed time to first request for analgesia supplementation, that is, prolonged duration of analgesia, and significantly decreased 24 h analgesic consumption [Figure 3] and a good quality of analgesia when compared with control group (ropivacaine 0.5% alone in block).
Figure 1.
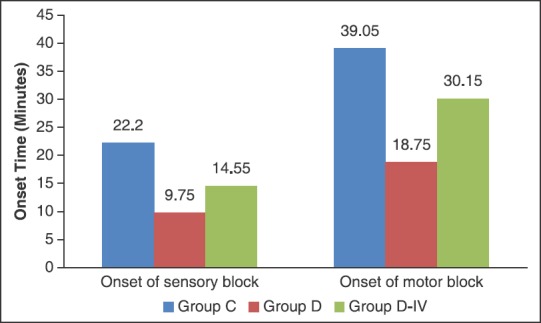
Sensory and motor block onset time
Figure 2.
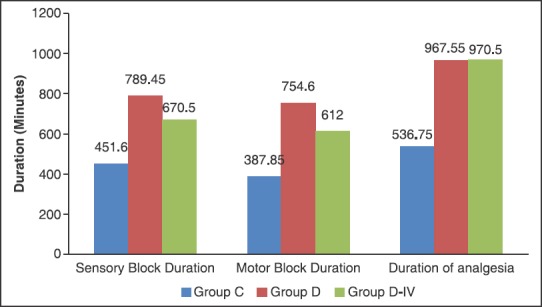
Duration of block
Figure 3.
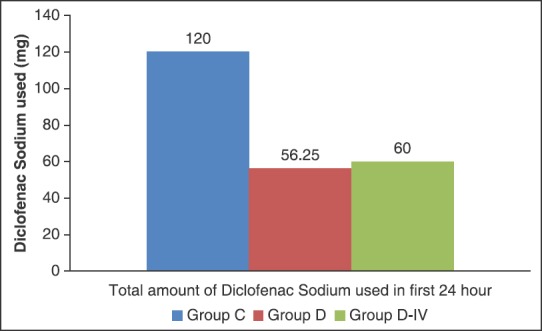
Total analgesic consumption in 24 h
Esmaoglu et al. in 2010 have concluded that dexmedetomidine (100 μg) when used as an additive to 40 ml of 0.5% levobupivacaine prolongs axillary brachial plexus block duration. They have shown that dexmedetomidine shortened the sensory block onset time (9.03 ± 1.15 min in dexmedetomidine group vs. 10.46 ± 1.30 min in control group), the motor block onset time (9.50 ± 1.04 min in dexmedetomidine group vs 11.10 ± 1.24 min in control group) and prolonged the duration of the sensory block (887 ± 66.23 min in dexmedetomidine group and 673 ± 73.77 min in control group), duration of the motor block (773 ± 67.62 min in dexmedetomidine group and 575 ± 65 min in control group) and postoperative analgesia (1008.69 ± 164.04 min in dexmedetomidine group and 887.14 ± 260.82 min in control group).[10] Esmaoglu et al. used nerve stimulation as the guidance method and 40 ml local anesthetic plus 100 mg dexmedetomidine was administered for axillary brachial plexus block. Nowadays, ultrasound guidance is described as the “golden standard” in peripheral regional anesthesia which enables reduction in local anesthetic dose.
Swami et al. in 2012 concluded that dexmedetomidine (1 μg/kg) when added to local anesthetic (35cc, bupivacaine 0.25%) in supraclavicular brachial plexus block enhanced the duration of sensory and motor block and also the duration of analgesia.[11] The time for rescue analgesia was prolonged in patients receiving dexmedetomidine. It also enhanced the quality of block as compared with clonidine (1 μg/kg).
Zhang et al. in 2014 also reported prolonged sensory and motor blockade duration in patients who received dexmedetomidine (50 μg) in 40 ml of 0.33% ropivacaine when compared to control group for axillary brachial plexus blockade.[6] However, dexmedetomidine was also associated with an increased incidence of side effects such as bradycardia, hypertension, and hypotension.
In the accordance with study by Swami et al.[11] and Esmaoglu et al.[10] in our study no significant serious side effects were reported in any group except for lower pulse rates and blood pressures observed in dexmedetomidine groups that were managed conservatively.
In a study on sciatic nerve block in rats, addition of dexmedetomidine to ropivacaine resulted in increased duration of sensory and motor block and showed no evidence of neurotoxicity.[5] In addition, use of dexmedetomidine decreases inflammation around peripheral nerves, thereby decreasing the potential for peripheral nerve injury.[12] Thus, use of dexmedetomidine is safe in peripheral nerve blocks. Supporting the animal study data no neurological deficit was observed in any of our patients. No neurological deficit was reported in the study by Swami et al.[11] and Esmaoglu et al.[10] also.
Besides studying the effect of adding dexmedetomidine to ropivacaine when administered through brachial plexus blockade, we also studied the effect of intravenous supplementation of dexmedetomidine (50 μg) in patients who received 30 ml ropivacaine 0.5% in ultrasound-guided supraclavicular brachial plexus block and found that it also resulted in quick onset of sensory and motor block, prolonged duration of both sensory and motor block, delayed time to first request for analgesia supplementation, and significantly decreased 24 h analgesic consumption and a good quality of analgesia when compared with control group (ropivacaine 0.5% alone in block). However, the sensory and motor block onset was found to be significantly quicker when dexmedetomidine was given in block when compared to when it was administered intravenously suggesting the presence of α2 -ARs in brachial plexus and hence a faster local action. The duration of sensory and motor block was also significantly more prolonged when dexmedetomidine was given in block than when it was administered intravenously. However, the increased duration of analgesia and decreased total analgesic consumption in 24 h postoperative period was comparable in both the dexmedetomidine groups.
Marhofer et al.[7] also reported a profound prolongation of ulnar nerve block (UNB) of 60% with perineural dexmedetomidine when added to 0.75% ropivacaine. Whereas, systemic administration of 20 mg dexmedetomidine resulted in a prolongation of only 10% during UNB with 0.75% ropivacaine.
The mechanism of the analgesic actions of α2 agonists has not been fully elucidated and is probably multifactorial. A number of supraspinal and spinal sites modulate the transmission of nociceptive signals in the CNS. Peripheral α2 adrenoceptors may also mediate the antinociception.[15] α2 blockers by acting at any of these sites reduce nociceptive transmission, leading to analgesia. The activation of inwardly rectifying G1 -protein-gated potassium channels resulting in membrane hyperpolarization and decreasing the firing rate of excitable cells in the CNS is considered to be a significant mechanism of the inhibitory neuronal action of α2 -adrenoceptor agonists.[16] Reduction of calcium conductance into cells, thus inhibiting neurotransmitter release is other prominent physiologic action ascribed to α2 adrenoceptors. This effect involves direct regulation of entry of calcium through N-type voltage-gated calcium channels and is independent of cAMP and protein phosphorylation and is mediated by G0 proteins. These mechanisms represent 2 very different ways of effecting analgesia, that is, the nerve is prevented from firing, and it also prevents propagation of signals to the neighbors.
Hence, we hypothesize that it is mainly the direct peripheral action of dexmedetomidine on nerves in block, which is responsible for these improvements rather than due to central action of dexmedetomidine after absorption through block site into systemic circulation resulting in its systemic effects. However, the central effects of dexmedetomidine also seems to play some role in prolongation of sensory and motor block duration, as 50 μg of dexmedetomidine intravenous infusion significantly prolonged brachial plexus block duration when compared to control group in our study. However, further detailed studies are warranted to investigate the mechanisms of how α2 agonists, and especially dexmedetomidine, prolong the action of LA in peripheral nerve blocks.
LIMITATIONS OF THE STUDY
The major limitation of this study was that we did not measure the levels of dexmedetomidine in the plasma that could have further supported the hypothesis that dexmedetomidine has a peripheral action rather than centrally mediated.
CONCLUSION
Thus, we conclude that in supraclavicular brachial plexus block addition of dexmedetomidine as adjuvant to 0.5% ropivacaine shortens the sensory and motor block onset time, prolongs both sensory and motor block duration. It also significantly delays the first demand for analgesia supplementation, decreases 24 h analgesic consumption and is not associated with any major side-effect. The action of dexmedetomidine is most probably peripheral than centrally mediated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Halstead C. In: A Practice of Anesthesia. 7th ed. London, UK: Lioyd-Luke; 2003. Great moments in the history of anaesthesiology; p. 5. [Google Scholar]
- 2.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: a systematic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowska K, Knapik P, Misiolek H. The effect of dexmedetomidine sedation on brachial plexus block in patients with end-stage renal disease. Eur J Anaesthesiol. 2009;26:851–5. doi: 10.1097/EJA.0b013e32832a2244. [DOI] [PubMed] [Google Scholar]
- 4.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–11. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111:1111–9. doi: 10.1097/ALN.0b013e3181bbcc26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Wang CS, Shi JH, Sun B, Liu SJ, Li P, et al. Perineural administration of dexmedetomidine in combination with ropivacaine prolongs axillary brachial plexus block. Int J Clin Exp Med. 2014;7:680–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 8.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S, Aggarwal R, Gupta P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2014;30:36–40. doi: 10.4103/0970-9185.125701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 11.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (a2 agonist drugs) as an adjuvant to local Anesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummett CM, Amodeo FS, Janda AM, Padda AK, Lydic R. Perineural dexmedetomidine provides an increased duration of analgesia to a thermal stimulus when compared with a systemic control in a rat sciatic nerve block. Reg Anesth Pain Med. 2010;35:427–31. doi: 10.1097/AAP.0b013e3181ef4cf0. [DOI] [PubMed] [Google Scholar]
- 13.Klein SM, Greengrass RA, Steele SM, D’Ercole FJ, Speer KP, Gleason DH, et al. A comparison of 0.5% bupivacaine, 0.5% ropivacaine, and 0.75% ropivacaine for interscalene brachial plexus block. Anesth Analg. 1998;87:1316–9. doi: 10.1097/00000539-199812000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Hickey R, Rowley CL, Candido KD, Hoffman J, Ramamurthy S, Winnie AP. A comparative study of 0.25% ropivacaine and 0.25% bupivacaine for brachial plexus block. Anesth Analg. 1992;75:602–6. doi: 10.1213/00000539-199210000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: Mediation by release of endogenous enkephalin-like substances. Eur J Pharmacol. 1988;146:223–8. doi: 10.1016/0014-2999(88)90296-8. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]


