Abstract
Background:
Central venous cannulation (CVC) is frequently required during the management of patients with liver disease with deranged conventional coagulation parameters (CCP). Since CVC is known to be associated with vascular complications, it is standard practice to transfuse Fresh-Frozen Plasma or platelets to correct CCP. These CCP may not reflect true coagulopathy in liver disease. Additionally CVC when performed under ultrasound guidance (USG-CVC) in itself reduces the incidence of complications.
Aim:
To assess the safety of USG-CVC and to evaluate the incidence of complications among liver disease patients with coagulopathy.
Setting and Design:
An audit of all USG-CVCs was performed among adult patients with liver disease in a tertiary care center.
Materials and Methods:
Data was collected for all the adult patients (18-60 years) of either gender suffering from liver disease who had required USG-CVC. Univariate and multivariate regression analysis was done to identify possible risk factors for complications.
Results:
The mean age of the patients was 42.1 ± 11.6 years. Mean international normalized ratio was 2.17 ± 1.16 whereas median platelet count was 149.5 (range, 12-683) × 109/L. No major vascular or non-vascular complications were recorded in our patients. Overall incidence of minor vascular complications was 18.6%, of which 13% had significant ooze, 10.3% had hematoma formation and 4.7% had both hematoma and ooze. Arterial puncture and multiple attempts were independent risk factors for superficial hematoma formation whereas low platelet count and presence of ascites were independent risk factors for significant oozing.
Conclusion:
Ultrasound guidance -CVC in liver disease patients with deranged coagulation is a safe and highly successful modality.
Keywords: Chronic liver disease, convention coagulation parameters, ultrasound guided central vein cannulation
INTRODUCTION
Central venous cannulation (CVC) is frequently required during the management of critically ill patients. In patients with liver disease, CVC is an important intervention for fluid management, administration of vasoactive drugs and concentrated glucose solution as well as for performing specialized procedures such as transjugular liver biopsies and measuring hepatic venous pressure gradient, apart from its other common indications.[1]
Insertion of CVC has its known complications, both vascular and non-vascular. Patients with deranged coagulation are considered to be at higher risk of vascular complications and it is commonly assumed that transfusion of Fresh-Frozen Plasma (FFP) and/or platelets would lower this risk.[2] It is a standard practice to correct coagulopathy by transfusing FFP/platelets prior to attempting CVC.[3] However, this practice has little scientific basis,[2,3] especially in patients with liver disease. There is ample evidence in literature to suggest that deranged coagulation parameters are not predictive of bleeding complications and preprocedural correction of coagulation abnormalities is not always warranted.[1,3,4,5,6] The practice of empirical correction of coagulopathy by transfusing FFP/platelets in patients with liver disease besides adding to the cost of care also causes volume overload and exposes patients to inherent risks of transfusion of blood products.
The incidence of complications of CVC has further declined in recent years with the introduction of ultrasound guidance (USG) cannulation. The rate of major and minor vascular complications with landmark technique ranges from 6% to 25% in the literature whereas the same for USG-CVC ranges from 0.4% to 1.4% in patients with normal coagulation profile.[7,8,9,10] Many professional societies have recommended USG-CVC as a norm if USG is readily available.[10,11,12]
Patients with chronic liver disease (CLD) have a rebalanced coagulation as there is reduction in both procoagulants and anticoagulants.[13] This implies that there is a restored balance of hemostasis due to concomitant reduction in procoagulant and anticoagulant factors. Contrary to earlier belief CLD patients are not considered an epitome of bleeding disorders.[14] Of the many studies conducted to evaluate the safety of CVC in patients with altered coagulation only a few have been conducted in patients with liver disease.[4]
In patients with liver failure, prothrombin time (PT) serves as a marker of disease progression and forms an important criteria for deciding treatment options.[15] Since administration of FFP could confound the decision making process, it is an institutional policy to avoid transfusions for correction of PT unless indicated. Transfusions are avoided prior to minimally invasive procedures such as CVC which can be performed under USG imaging. The internal jugular vein (IJV) is usually preferred over the subclavian and femoral veins for CVC under USG due to its ease of visualization and amenability to external compression in case of vascular puncture and hematoma formation.
In patients with liver disease with deranged coagulation, who require a liver biopsy, transjugular liver biopsy (TJLB) is considered a safer alternative to the traditional method of percutaneous liver biopsy. Using a transvenous approach, the biopsy needle is inserted into the liver via the hepatic vein, avoiding the peritoneum and the liver capsule. Thus, if there is any bleeding related to the procedure, it bleeds back into the venous system.[16] The transjugular route is preferred over the transfemoral for taking the liver biopsy at our centre and hence in all patients scheduled for TJLB, it is a standard practice to insert 10F sheath in the right IJV under USG.
We performed an audit to evaluate the incidence of major and minor vascular complications after USG-CVC in patients with liver disease and to observe if these complications correlated with conventional coagulation parameters (CCP) measured in these patients.
MATERIALS AND METHODS
An audit of all USG-CVC was performed in adult patients with liver disease between September 2011 and August 2012. After approval from institutional ethics committee, data was collected for all adult patients (age >18 years) of both gender, suffering from liver disease (acute or chronic) with coagulopathy, who underwent USG-CVC. Only those USG-CVC which were carried out in elective setting by trained anesthesiologists with 5 years of work experience in the specialty along with an experience of at least 50 USG-CVC were included in the study. Coagulopathy was defined as having international normalized ratio (INR) >1.5 and/or platelet count <100,000/cu mm.[4,17] Laboratory reports were consulted and data was retrieved from hospital data base. Patients who had received platelet and/or FFP transfusion for correction of coagulopathy were excluded from the study. Patients with active bleeding, unstable hemodynamic parameters, those on anticoagulation therapy, and with other known blood dyscrasias like hemophilias and Von Willebrand's disease were excluded.
All CVCs in the department were conducted under real time US guidance (LOGIQ e GE Healthcare 9900 Innovation Drive, Wauwatosa, WI 53226, U.S.A with 12L linear array probe) and internal vein was cannulated using either a 7Fr Triple lumen catheter (Arrow-Howes™ Multilumen catheter, Arrow International, 2400 Bernville Road, PA 19605, U.S.A) or 10Fr sheath for TJLB (INPUT™ Introducer Sheath, Medtronic Inc., 710 Medtronic pathway, MN 55432, U.S.A). As per protocol, check bedside chest radiograph in sitting posture was done after cannulation and all patients were followed up for 24 h for development of any complication. Data regarding patient's age, gender, diagnosis, etiology, child status, decompensation of liver disease namely presence of jaundice, ascites, hepatic encephalopathy was noted, along with laboratory reports of PT/INR, Platelet count, site of cannulation, number of attempts, arterial puncture and complications were recorded from hospital database for all procedures. The complications were classified as vascular (arterial puncture, superficial hematoma, ooze and hemothorax) and non-vascular (pneumothorax, nerve injury, malpositioning). The vascular complications were further divided into minor (hematoma, ooze) and major (for which active medical intervention was required like blood transfusion, chest drain insertion). Significant ooze was defined by persistence of ooze even after 15 min of digital compression or requiring change in dressing for >3 times in 24 h. Hematoma was defined as development of swelling of size >2 cm, at site of skin puncture.
Statistical analysis
Data was entered in statistical software package (SPSS, version 22.0, Chicago, IL, USA) and analyzed. Descriptive statistics are presented as proportions and mean ± standard Deviation. For categorical variables Chi-square test was used while student t-test was used for comparison of continuous variables. The conditional univariate logistic regression analysis was used to calculate the significance level (unadjusted odds ratio [OR] and confidence intervals) of each variable in the study. A multivariate forward stepwise logistic regression analysis taking inclusion and exclusion criterion of 0.05 and 0.10, respectively, was also performed. Receiver operator curve (ROC) was plotted for significant variables and youden's index was calculated to determine significant cut-offs.
RESULTS
A total of 421 patients were included in the study and data of 699 USG-CVC was recorded. Table 1 provides the basic demographic profile of our patients. The mean age of the patients was 42.1 ± 11.6 years. Most of our patients had CLD (69.3%) followed by acute on chronic liver failure (21.4%) and acute liver failure (9.3%). Mean INR was 2.17 ± 1.16 whereas median platelet count was 149.5 (range, 12-683) × 109/L. About 40% of our patients had severe liver disease with a Child C status. Of all the USG-CVC (699), right IJV was cannulated in 85.6% cases and left IJV was cannulated in 14.4% cases. 140 cannulations (20.02%) were done using 10 Fr sheath for TJLB whereas the rest were done using 7 Fr triple lumen line (79.97%).
Table 1.
Demographic and baseline characteristics of patient population (n = 421)
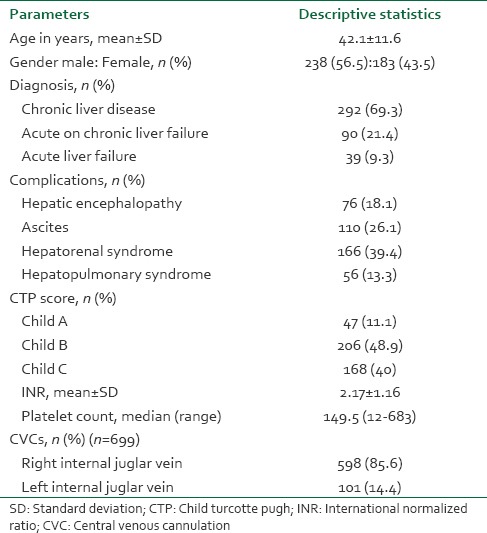
Technical success was achieved in 100% of the cases. 86.3% of the cannulations were successful in single attempt with average number of attempt being 1.6 ± 0.51. Overall incidence of arterial puncture was 1.3%. There was no major vascular or non-vascular complication recorded in our patients. Overall incidence of minor vascular complication was 18.6% of which 13% had significant ooze, 10.3% had hematoma formation and 4.7% had both hematoma and ooze. Table 2 describes minor vascular complications according to site and other possible risk factors. Multivariate logistic regression analysis showed that arterial puncture and multiple attempts were independent risk factors for superficial hematoma formation whereas low platelet count and presence of ascites came out as independent risk factors for significant oozing. 3.3% of the central lines were found to be misplaced on the check chest X-ray. Figure 1 provides the ROC predicting hematoma formation and ooze for INR and platelet count, respectively. Calculating youden's index for each ROC provides INR of >3 and platelet count <30,000/μL as cut-offs. Table 3 describes the coagulation indices of patients with and without vascular complications.
Table 2.
Incidence of minor vascular complication along with associated risk factors
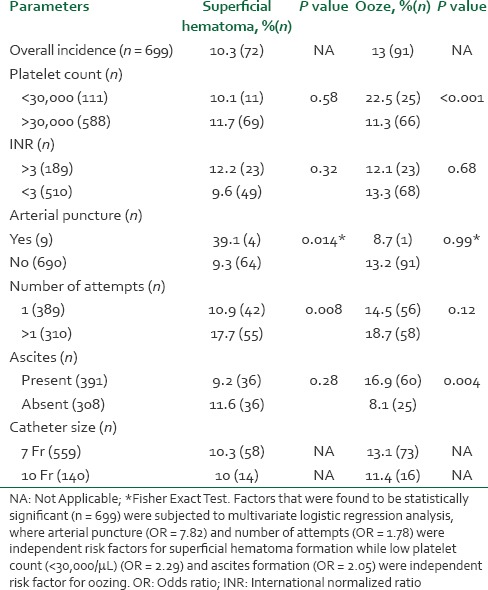
Figure 1.

Receiver operating curves for international normalized ratio to predict hematoma formation and for platelet counts to predict oozing
Table 3.
Correlation of age, CTP and coagulation indices with minor vascular complications (hematoma, ooze or both)

DISCUSSION
Safety of CVCs in patients with coagulation abnormalities has been a subject of active research in the past. Despite presence of various studies on its safety there are no clear cut guidelines.[18] The American Society of Anesthesiology task force guidelines on perioperative blood transfusion and adjuvant therapies in 2006 provides thresholds for management of coagulation in perioperative period but does not address specific procedures.[19] It is known that indication for preprocedural correction of coagulopathy depends on invasiveness of the procedure, easy detection and controllability of bleeding complication.
Guidelines specific to USG-CVC are given by cardiovascular and intervention radiological society of Europe also endorsed by Agency for Healthcare Research and Quality. These guidelines mandate correction of coagulation to an INR of <1.5 and platelet count of >50,000/μL.[20] However the two systematic reviews on which these guidelines are based remains inconclusive. Stanworth et al. reported a review of 57 randomized controlled trials investigating the efficacy of FFP to prevent hemorrhagic complications over a wide variety of indications and clinical settings, including cardiac surgery. They found the data insufficient to recommend or refute the prophylactic use of FFP. Because of the lack of data, percutaneous procedures were not included in this comprehensive review. They concluded that there is a clear need for additional investigation of the use of FFP with image-guided procedures.[21] In the other review, Segal et al.[2] concluded that elevated coagulation parameters provide little to no predictive value for bleeding complications from image-guided interventions. They assert that, in the absence of randomized controlled studies, mild to moderate elevation of coagulation times should neither be assumed to represent an increased risk for peri-procedural bleeding nor be used as an indication for transfusion of FFP or clotting factor concentrates.[2]
Of all the studies conducted in patients with deranged coagulation only a few address patients with liver disease. Fisher and Mutimer in the largest prospective study in 580 patients with liver disease used the landmark technique for CVC. Majority of his patients (68.84%) had both a platelet count <150,000/μL and an INR of ≥1.5. Only one patient had major bleeding (0.2%) as a result of inadvertent puncture of the carotid artery. The authors concluded that CVC can be performed safely by experienced physicians in the presence of abnormal coagulation parameters.[4]
In our study we collected data on USG-CVC for a similar subset of patients and observed them for the next 24 h to document the incidence of major and minor vascular complications. We found that USG CVC is highly successful and relatively safe in patients with liver disease. There were no major vascular and non-vascular complications noted. The overall incidence of minor vascular complications was 17.7% (Ooze, 13% and Hematoma, 10.3%). This was higher when compared with USG-CVC in patients with normal coagulation where these complications ranged between 0.4% and 1.4% for ooze and 0.4-1% for hematoma.[9,10] A significant number of patients in our study had previous history of recent cannulation and partial thrombus at the time of cannulation. This might explain a relatively high attempt rate (1.6 ± 0.51) in an USG technique during which although the needle would puncture the vein in a single attempt but there is no blood flow on aspiration. The exact incidence of thrombus formation was not recorded but it was realized in hindsight that this is fairly common in our set of patients. We thus recommend routine use of color Doppler while performing USG-CVC. Also there is high incidence of double wall puncture during USG-CVC which may have led to higher incidence of ooze and hematoma. However these minor vascular complications could be easily managed and did not require special medical intervention.
Right IJV cannulation was attempted in these patients first. In case of failure or contraindication to right IJV cannulation, left IJV cannulation was done. The site of cannulation was decided keeping in view lower incidence of complications in the presence of coagulopathy and amenability to USG.
Patients with liver disease are no longer considered to be hypocoagulopathic. Instead a newer concept of rebalanced coagulation is finding greater acceptance among the fraternity.[22] Clinically, this rebalanced hemostatic system is reflected by the large proportion of patients with liver disease who can undergo major surgery without any requirement for intraoperative blood products.[23] Despite this understanding, it is common practice in many centers to prophylactically correct a prolonged PT and activated partial thromboplastin time before invasive procedures by administration of FFP. Blood product use in patients with liver disease is substantial with a significant proportion being used prophylactically, although exact figures have not been reported.[24] A recent survey on the use of blood products indicated that hepatobiliary disorders were one of the primary indications for FFP, platelet concentrate and packed red cells transfusion.[25] Indeed, studies show that plasma from patients with cirrhosis generates as much thrombin (the final enzyme of coagulation) as plasma from healthy subjects, provided that thrombin is measured by methods that reflect the action of both procoagulants and anticoagulants.[22,26] Thrombin generation in vivo and in vitro is down-regulated by thrombomodulin, a transmembrane protein situated on vascular endothelial cells that acts as the main physiologic activator of protein C.[27] Plasma and reagents that are used to measure the PT do not contain thrombomodulin. Accordingly, this test measures the amount of thrombin generated in plasma as a function of the procoagulant drivers, but not the thrombin inhibited by the anticoagulant drivers, especially protein C, which is not fully activated in the absence of thrombomodulin. This might explain why the prothrombin-time test and related tests do not truly represent the balance of coagulation in vivo and are inadequate for assessing the risk of hemorrhage in those acquired conditions, such as the coagulopathies of liver disease.
The policy of preprocedural correction of coagulopathy based on deranged numbers may not be effective and may in fact be counterproductive for a number of reasons. It has been shown in several studies that abnormal preoperative coagulation tests are a very poor predictor of intra-operative bleeding.[23,28,29,30] The CCP namely INR was developed only for patients on oral anticoagulants and its use in other patient populations is not always appropriate. Moreover correction of a prolonged PT with FFP does not result in complete normalization, and the duration of the reversal is relatively short.[31] Thirdly it leads to fluid overload and a subsequent increase of the central and portal venous pressure, which is already elevated in many cirrhotic patients. The increase in portal pressure is shown to result in increased bleeding during abdominal surgical dissection. On the other hand transfusion of blood products is associated with significant side-effects both infectious and non-infectious. Lastly, the use of blood products during liver transplantation has been shown to be a predictor of increased morbidity and mortality.[32]
Our study further supports the finding that USG-CVC in patients with liver disease is safe and highly successful. On the basis of our study, the blind practice of preprocedural correction of coagulation can be questioned and further studies can be designed to see if there are any better predictors of bleeding complications in the form of viscoelastic test of coagulation instead of conventional coagulation assays.
CONCLUSION
Our study concludes that the incidence of major vascular and non-vascular complications of USG CVC in patients of liver disease with deranged coagulation profile is low even in the absence of preprocedural correction of coagulopathy. The incidence of minor vascular complications is comparatively higher but acceptable and easily manageable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Doerfler ME, Kaufman B, Goldenberg AS. Central venous catheter placement in patients with disorders of hemostasis. Chest. 1996;110:185–8. doi: 10.1378/chest.110.1.185. [DOI] [PubMed] [Google Scholar]
- 2.Segal JB, Dzik WH. Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Della Vigna P, Monfardini L, Bonomo G, Curigliano G, Agazzi A, Bellomi M, et al. Coagulation disorders in patients with cancer: Nontunneled central venous catheter placement with US guidance — A single-institution retrospective analysis. Radiology. 2009;253:249–52. doi: 10.1148/radiol.2531081963. [DOI] [PubMed] [Google Scholar]
- 4.Fisher NC, Mutimer DJ. Central venous cannulation in patients with liver disease and coagulopathy — A prospective audit. Intensive Care Med. 1999;25:481–5. doi: 10.1007/s001340050884. [DOI] [PubMed] [Google Scholar]
- 5.Foster PF, Moore LR, Sankary HN, Hart ME, Ashmann MK, Williams JW. Central venous catheterization in patients with coagulopathy. Arch Surg. 1992;127:273–5. doi: 10.1001/archsurg.1992.01420030035006. [DOI] [PubMed] [Google Scholar]
- 6.Weigand K, Encke J, Meyer FJ, Hinkel UP, Munder M, Stremmel W, et al. Low levels of prothrombin time (INR) and platelets do not increase the risk of significant bleeding when placing central venous catheters. Med Klin (Munich) 2009;104:331–5. doi: 10.1007/s00063-009-1070-2. [DOI] [PubMed] [Google Scholar]
- 7.Denys BG, Uretsky BF, Reddy PS. Ultrasound-assisted cannulation of the internal jugular vein. A prospective comparison to the external landmark-guided technique. Circulation. 1993;87:1557–62. doi: 10.1161/01.cir.87.5.1557. [DOI] [PubMed] [Google Scholar]
- 8.Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146:259–61. doi: 10.1001/archinte.146.2.259. [DOI] [PubMed] [Google Scholar]
- 9.Oguzkurt L, Tercan F, Kara G, Torun D, Kizilkilic O, Yildirim T. US-guided placement of temporary internal jugular vein catheters: Immediate technical success and complications in normal and high-risk patients. Eur J Radiol. 2005;55:125–9. doi: 10.1016/j.ejrad.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Tercan F, Ozkan U, Oguzkurt L. US-guided placement of central vein catheters in patients with disorders of hemostasis. Eur J Radiol. 2008;65:253–6. doi: 10.1016/j.ejrad.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Central venous catheters-ultrasound locating devices: Guidance, National Institute for Health and Clinical Excellence Website. Published 2002. [Updated 2005 and 2010 Dec 28; Last accessed on 2012 Oct 21]. Available from: http://www.guidance.nice.org.uk/TA49/Guidance/pdf/English .
- 12.Rothschild JM. Rockville, MD: AHRQ Publications; 2001. [Last accessed on 2012 Oct 21]. Ultrasound guidance of central vein catheterization, on making health care safer: A critical analysis of patient safety practices; pp. 245–55. Available from: http://www.ahrq.gov/clinic/ptsafety/chap21.htm . [Google Scholar]
- 13.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: Evidence and clinical consequences. Blood. 2010;12(116):878–85. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 14.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–56. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 15.Schilsky ML, Honiden S, Arnott L, Emre S. ICU management of acute liver failure. Clin Chest Med. 2009;30:71–87. doi: 10.1016/j.ccm.2008.10.001. viii. [DOI] [PubMed] [Google Scholar]
- 16.Keshava SN, Mammen T, Moses V. Transjugular liver biopsy: What to do and what not to do. Indian J Radiol Imaging. 2008;18:245–8. doi: 10.4103/0971-3026.41839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stéphan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999;115:1363–70. doi: 10.1378/chest.115.5.1363. [DOI] [PubMed] [Google Scholar]
- 18.Baron RM. Point: Should coagulopathy be repaired prior to central venous line insertion? Yes: Why take chances? Chest. 2012;141:1139–42. doi: 10.1378/chest.11-3225. [DOI] [PubMed] [Google Scholar]
- 19.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–36. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Stanworth SJ, Brunskill SJ, Hyde CJ, McClelland DB, Murphy MF. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–52. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 22.Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553–8. doi: 10.1002/hep.20569. [DOI] [PubMed] [Google Scholar]
- 23.Steib A, Freys G, Lehmann C, Meyer C, Mahoudeau G. Intraoperative blood losses and transfusion requirements during adult liver transplantation remain difficult to predict. Can J Anesth. 2001;48:1075–9. doi: 10.1007/BF03020372. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–46. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 25.Wells AW, Llewelyn CA, Casbard A, Johnson AJ, Amin M, Ballard S, et al. The EASTR Study: Indications for transfusion and estimates of transfusion recipient numbers in hospitals supplied by the National Blood Service. Transfus Med. 2009;19:315–28. doi: 10.1111/j.1365-3148.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 26.Tripodi A, Primignani M, Chantarangkul V, Clerici M, Dell’Era A, Fabris F, et al. Thrombin generation in patients with cirrhosis: The role of platelets. Hepatology. 2006;44:440–5. doi: 10.1002/hep.21266. [DOI] [PubMed] [Google Scholar]
- 27.Dahlbäck B. Progress in the understanding of the protein C anticoagulant pathway. Int J Hematol. 2004;79:109–16. doi: 10.1532/ijh97.03149. [DOI] [PubMed] [Google Scholar]
- 28.Mannucci PM. Abnormal hemostasis tests and bleeding in chronic liver disease: Are they related? No. J Thromb Haemost. 2006;4:721–3. doi: 10.1111/j.1538-7836.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- 29.Findlay JY, Rettke SR. Poor prediction of blood transfusion requirements in adult liver transplantations from preoperative variables. J Clin Anesth. 2000;12:319–23. doi: 10.1016/s0952-8180(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 30.Massicotte L, Beaulieu D, Thibeault L, Roy JD, Marleau D, Lapointe R, et al. Coagulation defects do not predict blood product requirements during liver transplantation. Transplantation. 2008;85:956–62. doi: 10.1097/TP.0b013e318168fcd4. [DOI] [PubMed] [Google Scholar]
- 31.Youssef WI, Salazar F, Dasarathy S, Beddow T, Mullen KD. Role of fresh frozen plasma infusion in correction of coagulopathy of chronic liver disease: A dual phase study. Am J Gastroenterol. 2003;98:1391–4. doi: 10.1111/j.1572-0241.2003.07467.x. [DOI] [PubMed] [Google Scholar]
- 32.Pereboom IT, de Boer MT, Haagsma EB, Hendriks HG, Lisman T, Porte RJ. Platelet transfusion during liver transplantation is associated with increased postoperative mortality due to acute lung injury. Anesth Analg. 2009;108:1083–91. doi: 10.1213/ane.0b013e3181948a59. [DOI] [PubMed] [Google Scholar]


