Abstract
Objectives:
The aim was to evaluate pathologic diagnosis, treatment and prognosis of 125 patients with nontransitional cell carcinoma of the urinary bladder.
Materials and Methods:
A total of 3590 patients with bladder tumors operated in our clinic between September 1998 and May 2013 were retrospectively evaluated. A total of 125 patients (107 men and 18 women) with nontransitional cell bladder cancer, confirmed by histopathology, were included in this study. The patients’ characteristics, including age, gender, smoking history, tumor size, and localization, histological types, pathological tumor stages, treatment modalities, and survival rates were all recorded.
Results:
Of these tumors, 47 (37.6%) were adenocarcinoma (AC), 42 (33.6%) were squamous cell carcinoma (SCC), 23 (18.4%) were undifferentiated carcinoma (UC), 13 (10.4%) were other types of bladder carcinoma. Sixty-three (50.4%) patients had undergone radical cystectomy and pelvic lymphadenectomy ± adjuvant treatment (chemotherapy [CT]/radiotherapy) and 52 (41.6%) patients received radiotherapy ± CT. The median survival time of patients with AC and SCC were significantly higher than patients with UC (AC vs UC, P = 0.001; SCC vs UC, P = 0.000; AC vs. SCC, P = 0.219). Median survival time was significantly higher in radical cystectomy ± adjuvant treatment group (P < 0.05) in all histological types.
Conclusion:
Prognosis of urinary bladder tumors was directly related to histological type and stage of the tumor. CT or radiotherapy has limited response rates. Early radical cystectomy should be performed to improve prognosis.
Keywords: Adenocarcinoma, cystectomy, squamous cell carcinoma
INTRODUCTION
Bladder cancer is the most common malignancy of the urinary tract and the 7th most common cancer in men and the 17th in women.[1] The most (more than 90%) of malignant tumors arising from the urinary tract are transitional cell carcinoma (TCC).[2] Nontransitional cell carcinoma (non-TCC) of the bladder accounts for only 5-7% of all bladder tumors, comprising about 3% of squamous cell carcinomas (SCCs), 2% of adenocarcinomas (ACs), 1% of undifferentiated carcinomas (UCs) and a smaller percentage of minor histologies such as small-cell carcinomas and lymphomas.[3] It is necessary to distinguish histopathological type of tumors because squamous or glandular differentiation can be found with TCC. Bladder cancer is related with and known to be caused by several important risk factors, most notably smoking, occupational, and environmental exposure to carcinogens, urinary tract infection, kidney, and bladder stones, parasitic infections, and the causes of chronic bladder irritation.[4]
Squamous cell carcinoma can emerge in both nonbilharzial and bilharzial bladders; the two subtypes differ in epidemiology, pathogenesis, and clinicopathological characteristics. The nonbilharzial type occurs most often in the seventh decade, and main predisposing factor is chronically infected urine and the presence of chronic indwelling Foley catheter in patients with neuropathic bladder and spinal cord injury. Bilharzial SCC is diagnosed in the fifth decade and frequently occurs in some areas of East Africa and the Middle East, where bilharzia is endemic.[5] Primary bladder AC is more frequent in males and commonly classified as nonurachal and urachal AC.[6] Risk factors include extrophied bladders, Schistosoma infection, villous adenoma, and cystocele. Primary bladder AC is an aggressive malignancy with a tendency to present at a higher stage and is associated with a worse overall survival.[7,8,9] Primary small cell carcinoma of the urinary bladder affects mostly elderly male patients who present with gross hematuria, and the prognosis is very poor.[10]
This study was conducted to evaluate pathologic diagnosis, treatment, and prognosis of 125 patients with nonTCC.
MATERIALS AND METHODS
A total of 3590 patients with bladder tumors operated in our clinic between September 1998 and May 2013 were retrospectively evaluated. All patients who were diagnosed bladder cancer by cystoscopy had undergone transurethral resection for staging and histological diagnosis. After the histological diagnosis of the tumor, radiological examination (abdominal and chest chemotherapy [CT], radionuclide bone scan) were performed for staging in the patients with invasive disease. In histopathological evaluation; development pattern, depth of invasion, angiogenesis, necrosis, mitosis, immunological staining, differentiation patterns, and superficial epithelium changes, neural and vascular invasion were evaluated. Pancytokeratin, cytokeratin (CK) 7, CK 20, carcinoembryonic antigen and epithelial membrane antigen for all cases; beta human chorionic gonadotropin and alpha-fetoprotein for cases including giant cells; vimentin, desmin, and actin for cases including sarcomatoid areas; periodic acid-Schiff/alcian blue those with AC morphology; neuron-specific enolase and chromagranin were obtained for cases including small cell areas. A 125 patients (107 men and 18 women) with nontransitional cell bladder cancer, confirmed by histopathology, were included in this study. The patients’ characteristics including age, gender, smoking history, tumor size, and localization, histological types, pathological tumor stages, treatment modalities, and survival rates were all recorded.
Statistical analysis
Data were entered and analyzed using SPSS (SPSS version 17.0, Chicago, IL, USA) statistical software package. Descriptive statistics was reported as percentage and medians. Categorical variables were analyzed using the Chi-square or Fisher exact test, and continuous variables were analyzed using Kruskal–Wallis test. Overall survival was calculated as the difference between date of death or the last follow-up date and the beginning of treatment. Survival distributions were analyzed using the Kaplan–Meier method and compared using the log rank test. Comparative differences were considered as statistically significant when the P < 0.05.
RESULTS
Patients’ characteristics were summarized in Table 1. The median age of the patients at diagnosis was 62-year (range; 19-85) and the male to female ratio was 5.9:1. Of these tumors, 47 (37.6%) were AC, 42 (33.6%) were SCC, 23 (18.4%) were UC, 3 (2.4%) were small cell carcinoma, 3 (2.4%) were sarcomatous carcinoma, 2 (1.6%) were lymphepithelioma-like carcinoma, 1 (0.8%) was clear cell carcinoma, 1 (0.8%) was choriocarcinoma, 1 (0.8%) was malign fibrous histiocytoma, 1 (0.8%) was Langerhans cell sarcoma and 1 (0.8%) was diffuse large B-cell lymphoma. Basoloid type was present as a histological variant in two of the 42 patients with SCC. Tumor growth pattern was polypoid-infiltrative in 30 (24.0%), diffuse-infiltrative in 43 (34.4%), solid-nodular in 18 (14.4%), and tubulovillous in 2 (1.6%) cases. Simultaneously, multiple growth pattern types were observed in 32 (25.6%) cases.
Table 1.
Patients’ characteristics
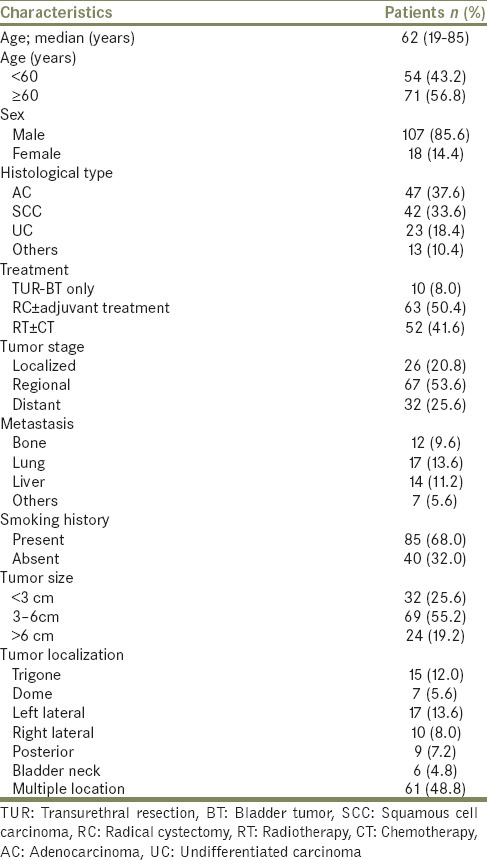
The most common localization of tumor was left lateral, trigone, right lateral, posterior, dome, and bladder neck, respectively. Widespread intravesical distribution was detected in 61 (48.8%) patients. Sixty-three (50.4%) patients had undergone radical cystectomy and pelvic lymphadenectomy ± adjuvant treatment (CT/radiotherapy) and 52 (41.6%) patients received systemic radiotherapy ± CT. Much as different CT regimens were administered, among the patients who received CT, MVAC and gemcitabine + cisplatin were the most frequent therapy. 10 (8.0%) patients had undergone only transurethral resection without any adjuvant therapy; 6 patients had T1 tumor, 2 patients had died postoperatively and 2 patients had refused additional treatment.
In the comparison of patients with AC, SCC, and UC, there was no difference between three groups according to age, gender, smoking history, tumor size, tumor stage, multicentricity, and treatment modalities [Table 2]. The median survival time of patients with AC and SCC were significantly higher than patients with UC (AC vs. UC, P = 0.001; SCC vs. UC, P = 0.000; AC vs. SCC, P = 0.219) [Table 3 and Figure 1]. Similarly, there were significant differences between tumor stage groups in terms of survival (localized vs. regional, P = 0.001; localized vs. distant, P = 0.000; Regional vs. Distant, P = 0.000) [Table 3]. Median survival time was significantly higher in radical cystectomy ± adjuvant treatment group (P < 0.05) in all histological types [Table 3 and Figures 2–4].
Table 2.
Comparison of histological types in urinary bladder cancer
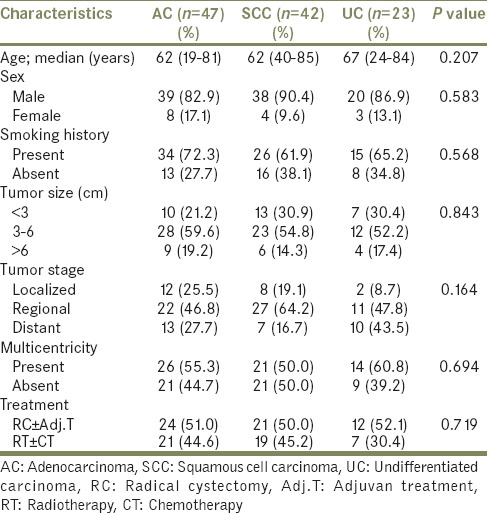
Table 3.
Analysis of factors affecting overall survival rates
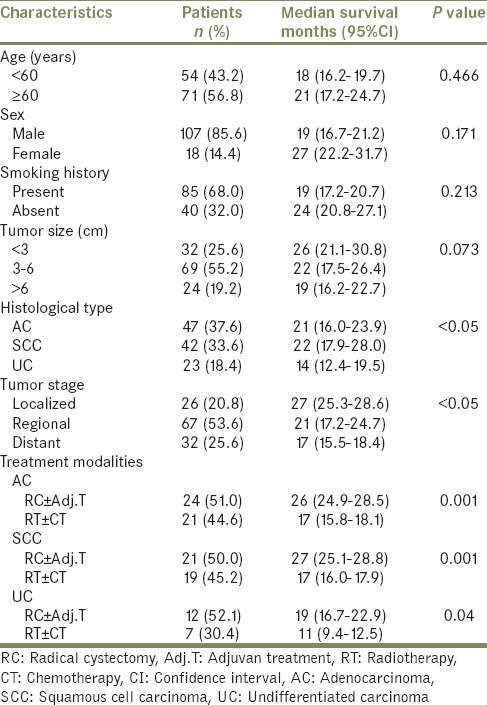
Figure 1.
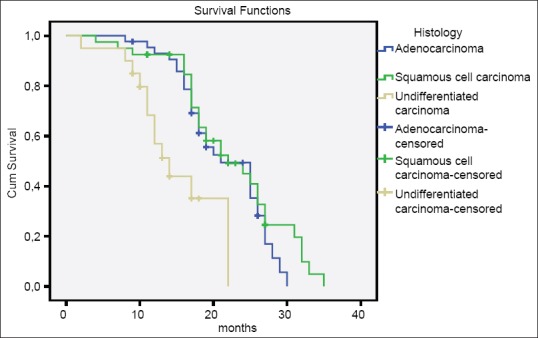
Overall survival according to the histological types
Figure 2.
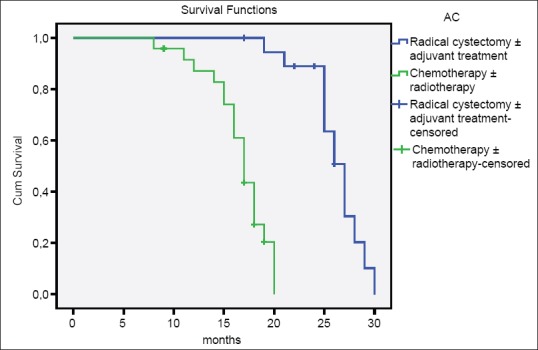
Overall survival of patients with adenocarcinoma
Figure 4.
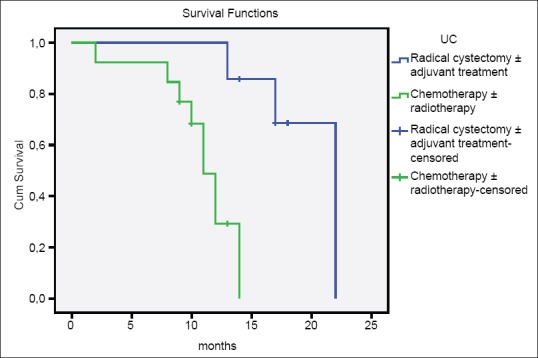
Overall survival of patients with undifferentiated carcinoma
Figure 3.
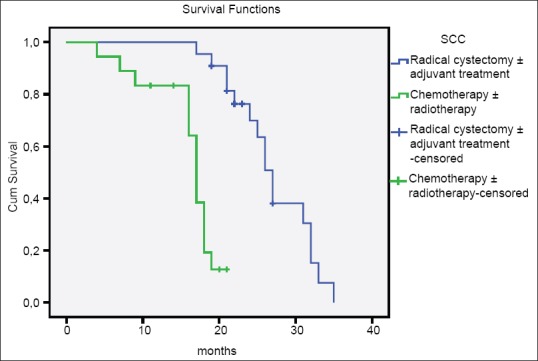
Overall survival of patients with squamous cell carcinoma
DISCUSSION
Nontransitional cell urothelial tumors are uncommon, and the origin of these tumors is not completely clear. Due to these tumors are rarely seen, the clinical course and treatment outcome of non-TCCs are still under debate. Many published studies are revealed that non-TCCs of the urothelial tract have a different biological attitude from TCC.[11]
Squamous cell carcinoma of the urinary bladder constitutes 2-7% of urothelial cancers and arise through a process of squamous metaplasia.[12] The incidence of bilharzial SCC of the bladder may reach up to 58.8-80.7% especially in African countries.[13] It accounts 26.3% of all malignancies and more than 75% of bladder tumors in Egypt, and about 80% of these cancers are related with chronic infection with Schistosoma haematobium.[14] Nonbilharzial SCC of the bladder represents <5% of bladder tumors diagnosed in the Western world and mostly associated with inflammation secondary to chronic irritation from urinary tract infections, stones, foreign bodies or chronic bladder outlet obstruction.[15] The reported male-to-female ratio varies from 4 to 5:1 for bilharzial SCC and 1.3-1.8:1 for nonbilharzial SCC of urinary bladder.[16] In the present study, none of the SCC cases were related with S. haematobium but high incidence of smoking (62.9%) and urinary stones may be liable for the etiology of SCC. In addition, the male-to-female ratio was significantly higher (9.5:1) for nonbilharzial SCC. Several studies confirm that most of the patients with SCC had advanced stage disease at the time of diagnosis, with T3-T4 accounts for 78.4% of cases.[16] These findings were consistent with our advance stage (T3-T4) rates (80.9%) when compared. In a study of 114 patients with nonbilharzial SCC, Rundle et al. have reported a slight predilection for trigone and lateral walls in 56 and 99 patients, respectively. However, the tumor was occupied ≥3 bladder walls in 22 patients.[17] Among the 42 patients with SCC in our study, 21 (50.0%) cases had involvement of two or more bladder walls.
Adenocarcinoma of the urinary bladder is the third most common bladder cancer, after urothelial cancer and squamous cell cancer, comprising no more than 2% of all primary bladder malignancies.[8] It is more frequent in males in their sixth decade of life.[6] In our study bladder, AC is male predominant with the median age of 62 (19-81) years and 34 (72.3%) of the 47 patients had a history of smoking. It can be broadly classified according to its origin as primary, urachal and metastatic type and histologically classified as enteric, signet-ring cell, mucinous, clear cell, hepatoid, mixed or AC not otherwise specified (NOS).[18] In series reported by Grignon et al.; 24 of 72 patients were considered to be an urachal origin and histologically; AC NOS category accounted for the most cases (27.8%), followed by mucinous (23.6%), enteric (19.4%), signet-ring cell (16.7%), and the mixed type (12.5%).[8] In our study; 2 of the 47 patients had urachal origin, and the tumors were classified as AC NOS (44.6%), mucinous (29.8%), signet-ring cell (12.8%), clear cell (4.3%) and mixed (8.5%). AC of the urinary bladder has an aggressive biological behavior, and the vast majority is muscle-infiltrating at diagnosis. In two large cohort studies el-Mekresh et al. and Anderström et al. reported that 68.0% of the patients with bladder AC were classified as having regional or distant stage.[19,20] Similarly, Grignon et al. stated that T3 and T4 stage tumors account for 66.6% of cases in their studies.[8] Our study revealed that, when the diagnosis was confirmed, most of the patients with bladder AC (35 of 47 patients; 74.5%) were staged as advanced disease.
Although patients with SCC and AC had advanced stage disease at the time of diagnosis, many published data revealed different survival rates according to treatment modalities.
In a single center study, Girgin et al. reported that the median survival time of patients who had undergone radical cycstectomy for AC, SCC, and UC were 6, 19 and 12 months, respectively.[21] In another study Ghoneim et al. presented their radical cystectomy series and 5-year overall survival for AC, SCC and UC were 50.3%, 46.4%, and 35.8%, respectively.[22] Radiotherapy ± CT is associated with poor results in most series; 5-year overall survival for AC and SCC was 20% and 5-18% in two large studies.[20,23]
The results of the present study demonstrate that patients with UC had a worse median survival time of 14 months compared with 21 and 22 months in patients with AC and SCC (P = 0.001). 2-year overall survival for AC, SCC and UC were 48%, 50%, and 0% in cystectomy ± adjuvant treatment group and 10%, 13%, and 0% in radiotherapy ± CT group. The survival times of the patients with AC and SCC were similar, but UC cases had worse survival time when compared with previous studies reported in the literature.
Primary small cell carcinoma and sarcomatoid carcinoma of the urinary bladder are rare neoplasms (<1%). Approximately, 250 small cell carcinoma and 70 sarcomatoid carcinoma cases have been reported in the literature.[24,25] Cheng et al. reviewed the data of 64 patients and reported that, 65% of patients with small cell carcinoma had a smoking history, and overall survival rate was 56%. In our study, there were 3 patients with pure small cell carcinoma, and none of them had a previous smoking history. These results suggest that genetic risk factors may contribute to this disease. Two of our patients had undergone radical cystectomy, and both died in 6 months after surgery. One patient who received CT died 15 months after the end of therapy. Wang et al. analyze 221 patients with sarcomatoid carcinoma and stated that, the median overall survival was 14 months in their study.[26] In our study, survival times of 3 patients who had undergone radical cystectomy and received radiotherapy after surgery were 23 months, 19 months, 18 months, respectively.
A limitation of our study is that it is a retrospective study, and the number of patients is relatively low.
CONCLUSION
Prognosis of urinary bladder tumors was directly related to histological type and stage of the tumor. Non-TCCs are typically deep invasive and advanced tumors indicating that they are highly aggressive neoplasms and have poor survival rates. CT or radiotherapy has limited response rates. Early radical cystectomy should be performed to improve prognosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Galsky MD, Iasonos A, Mironov S, Scattergood J, Donat SM, Bochner BH, et al. Prospective trial of ifosfamide, paclitaxel, and cisplatin in patients with advanced non-transitional cell carcinoma of the urothelial tract. Urology. 2007;69:255–9. doi: 10.1016/j.urology.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Fortuny J, Kogevinas M, Chang-Claude J, González CA, Hours M, Jöckel KH, et al. Tobacco, occupation and non-transitional-cell carcinoma of the bladder: An international case-control study. Int J Cancer. 1999;80:44–6. doi: 10.1002/(sici)1097-0215(19990105)80:1<44::aid-ijc9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Bachir BG, Kassouf W. Cause-effect? Understanding the risk factors associated with bladder cancer. Expert Rev Anticancer Ther. 2012;12:1499–502. doi: 10.1586/era.12.140. [DOI] [PubMed] [Google Scholar]
- 5.Shokeir AA. Squamous cell carcinoma of the bladder: Pathology, diagnosis and treatment. BJU Int. 2004;93:216–20. doi: 10.1111/j.1464-410x.2004.04588.x. [DOI] [PubMed] [Google Scholar]
- 6.Roy S, Smith MA, Cieply KM, Acquafondata MB, Parwani AV. Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: A persisting diagnostic challenge. Diagn Pathol. 2012;7:151. doi: 10.1186/1746-1596-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas AA, Stephenson AJ, Campbell SC, Jones JS, Hansel DE. Clinicopathologic features and utility of immunohistochemical markers in signet-ring cell adenocarcinoma of the bladder. Hum Pathol. 2009;40:108–16. doi: 10.1016/j.humpath.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Grignon DJ, Ro JY, Ayala AG, Johnson DE, Ordóñez NG. Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer. 1991;67:2165–72. doi: 10.1002/1097-0142(19910415)67:8<2165::aid-cncr2820670827>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Sung MT, Lopez-Beltran A, Eble JN, MacLennan GT, Tan PH, Montironi R, et al. Divergent pathway of intestinal metaplasia and cystitis glandularis of the urinary bladder. Mod Pathol. 2006;19:1395–401. doi: 10.1038/modpathol.3800670. [DOI] [PubMed] [Google Scholar]
- 10.Hou CP, Lin YH, Chen CL, Chang PL, Tsui KH. Clinical outcome of primary small cell carcinoma of the urinary bladder. Onco Targets Ther. 2013;6:1179–85. doi: 10.2147/OTT.S49879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manunta A, Vincendeau S, Kiriakou G, Lobel B, Guillé F. Non-transitional cell bladder carcinomas. BJU Int. 2005;95:497–502. doi: 10.1111/j.1464-410X.2005.05327.x. [DOI] [PubMed] [Google Scholar]
- 12.Vakar-López F, Abrams J. Basaloid squamous cell carcinoma occurring in the urinary bladder. Arch Pathol Lab Med. 2000;124:455–9. doi: 10.5858/2000-124-0455-BSCCOI. [DOI] [PubMed] [Google Scholar]
- 13.Khaled HM. Bladder cancer and bilharziazis today. Cancer J. 1993;6:65–71. [Google Scholar]
- 14.El-Bolkainy MN, Mokhtar NM, Ghoneim MA, Hussein MH. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer. 1981;48:2643–8. doi: 10.1002/1097-0142(19811215)48:12<2643::aid-cncr2820481216>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Serretta V, Pomara G, Piazza F, Gange E. Pure squamous cell carcinoma of the bladder in western countries. Report on 19 consecutive cases. Eur Urol. 2000;37:85–9. doi: 10.1159/000020105. [DOI] [PubMed] [Google Scholar]
- 16.El-Sebaie M, Zaghloul MS, Howard G, Mokhtar A. Squamous cell carcinoma of the bilharzial and non-bilharzial urinary bladder: A review of etiological features, natural history, and management. Int J Clin Oncol. 2005;10:20–5. doi: 10.1007/s10147-004-0457-6. [DOI] [PubMed] [Google Scholar]
- 17.Rundle JS, Hart AJ, McGeorge A, Smith JS, Malcolm AJ, Smith PM. Squamous cell carcinoma of bladder. A review of 114 patients. Br J Urol. 1982;54:522–6. doi: 10.1111/j.1464-410x.1982.tb13580.x. [DOI] [PubMed] [Google Scholar]
- 18.Ayala AG, Tamboli P, El-Bolkainy MN, editors. Lyon: IARC Press; 2004. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; p. 359. [Google Scholar]
- 19.el-Mekresh MM, el-Baz MA, Abol-Enein H, Ghoneim MA. Primary adenocarcinoma of the urinary bladder: A report of 185 cases. Br J Urol. 1998;82:206–12. doi: 10.1046/j.1464-410x.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderström C, Johansson SL, von Schultz L. Primary adenocarcinoma of the urinary bladder. A clinicopathologic and prognostic study. Cancer. 1983;52:1273–80. doi: 10.1002/1097-0142(19831001)52:7<1273::aid-cncr2820520724>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Girgin C, Sezer A, Uc R, Ermete M, Ozkan U, Gurel G. Outcome of the treatment of invasive non-transitional cell carcinoma. Int J Urol. 2003;10:525–9. doi: 10.1046/j.1442-2042.2003.00679.x. [DOI] [PubMed] [Google Scholar]
- 22.Ghoneim MA, el-Mekresh MM, el-Baz MA, el-Attar IA, Ashamallah A. Radical cystectomy for carcinoma of the bladder: Critical evaluation of the results in 1,026 cases. J Urol. 1997;158:393–9. [PubMed] [Google Scholar]
- 23.Johnson DE, Schoenwald MB, Ayala AG, Miller LS. Squamous cell carcinoma of the bladder. J Urol. 1976;115:542–4. doi: 10.1016/s0022-5347(17)59272-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, MacLennan GT, Lopez-Beltran A, Cheng L. Small cell carcinoma of the urinary bladder- histogenesis, genetics, diagnosis, biomarkers, treatment, and prognosis. Appl Immunohistochem Mol Morphol. 2007;15:8–18. doi: 10.1097/01.pai.0000213106.12731.d7. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, et al. Carcinosarcoma and sarcomatoid carcinoma of the bladder: Clinicopathological study of 41 cases. J Urol. 1998;159:1497–503. doi: 10.1097/00005392-199805000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Wang FW, Lagrange CA, Hemstreet Iii GP, Kessinger A. Clinical features of sarcomatoid carcinoma (carcinosarcoma) of the urinary bladder: Analysis of 221 cases. Sarcoma 2010. 2010 doi: 10.1155/2010/454792. ID- 454792. [DOI] [PMC free article] [PubMed] [Google Scholar]


