Abstract
Objectives:
The objective of the following study is to clarify a suitable group whereby a bone scan could be spared at the initial staging of prostate cancer, we wished to identify the possible relationship between bone metastasis and clinical and pathological parameters including serum total prostate specific antigen (PSA) concentration, alkaline phosphatase (ALP), biopsy Gleason Score (GS), and percentage of pathological cores.
Materials and Methods:
We reviewed the results of 220 bone scintigraphies, which were done between January 1, 2011 and June 30, 2013 in patients with newly diagnosed prostate cancer. These parameters were evaluated together with standard clinicopathological data to determine the prediction ability of the bone scan by univariate and multivariate analyses.
Results:
Bone metastases were seen in 44 patients of all 220 patients (20%, 95% confidence interval, 17-24%). In univariate analysis, PSA and biopsy GS were useful in predicting the bone scan result, but ALP and percentage of pathological cores was not. In multivariate analysis, the single most useful parameter in predicting the bone scan result was PSA (P < 0.001).
Conclusions:
A bone scan seems to be impractical in newly diagnosed prostate cancer patients with serum PSA level <20 ng/ml and GS up to seven and pre-treatment PSA is the best predictor of the need for the bone scan according to results of this study.
Keywords: Alkaline phosphatase, bone scan, Gleason, pathological cores, prostate cancer, prostate specific antigen
INTRODUCTION
Prostate cancer is one of the most common cancers in Asia as all over the world. Bone is the most common site of metastasis and this metastasis presents over %80 of patients who die from prostate cancer.[1] Although, there is no consensus on the pre-treatment of this cancer, current recommendations point out bone scanning as the most sensitive modality for the detection of bone metastasis.[2] The extent of bone metastases was shown to predict the outcome in metastatic prostate cancer. The percentage of the positive areas in bone scan was reported as a novel parameter for predicting the prognosis in advanced and metastatic prostate cancer.[3,4]
Bone scanning is an expensive, nonspecific staging modality sometimes necessitate further investigations that may delay therapy.[5,6] Furthermore, the utilization of bone scans seems to depend on conflicting data.[7] There have been many debates regarding whether all patients with newly diagnosed prostate carcinoma should undergo radionuclide bone imaging. Patients with the lowest risk may continue to undergo unnecessary testing, while those at highest risk may not be appropriately evaluated. From this point of view, for this population new data from different regions of the world, about the investigation strategy is an important issue.
In this study, it was aimed to see the relationship between bone metastasis and some clinical or pathological variables including biopsy Gleason score (GS), percentage of pathological cores, serum prostate specific antigen (PSA) and alkaline phosphatase (ALP) concentration. We undertook retrospective analysis of newly diagnosed prostate cancer patients. With this, patients with a low probability of bone metastasis can be determined leading shorter waiting times for bone scans, financial savings. That would also give an idea of whether a combination of those can be used more effectively than each alone in predicting the likelihood of positive bone scans in newly diagnosed prostate cancer patients.
MATERIALS AND METHODS
The cross-sectional retrospective study included 220 consecutive patients who were referred to the Nuclear Medicine Division of Ankara Dişkapi Education and Research Hospital, Ankara, Turkey for the evaluation of bone metastasis of prostate cancer between January 2011 and June 2013, who had not yet undergone definitive treatment for the newly diagnosed prostate cancer. They all had serum PSA levels, transrectal ultrasound guided prostate biopsies, and bone scans within maximum 3 weeks. Patients were referred to prostate biopsy for evaluation of abnormal digital rectal examination, elevated serum PSA, or both. There was no strict limit regarding age, patients with advanced age and/or significant comorbidities were counseled regarding the risks and benefits of undergoing prostate biopsy. The serum PSA level was determined by the Hybritech, (Beckman Coulter Inc., Fullerton, CA) assay with the normal range set between 0 and 4.0 ng/mL. The serum ALP level was determined by the Olympus AU2700 (NY, USA).
All prostate biopsies were performed using a standard 18-gauge biopsy gun, and the number of biopsy sites ranged between 10 and 16 with additional targeted biopsies for any hypoechoic or suspicious lesion. For each needle biopsy, certain variables were assessed, including GS, the percentage of tumor as a function of all the biopsy tissues, the number of cancer-positive cores, and the total number of cores from all biopsy sites. All biopsy specimens were reviewed by experienced pathologists in accordance with the standard Gleason grading criteria. The percentages of biopsy cores positive were grouped as ≤33%, 34-67%, and >67%. The assigned percentage of biopsy core-positive subgroups along with pre-treatment PSA and GS were used to develop probability for pathologic bone scan.
At least 2 h after the intravenous injection of 740 MBq (20 mCi) 99mTc-MDP, whole-body bone scan images were obtained on the anterior and posterior projections, if necessary together with oblique or lateral static images for areas of interest, using a large-field of view, dual-head gamma camera (ECAM; Dual Head Variable Systems, Siemens, Illinois, USA) equipped with high-resolution collimator. All scans were reported by experienced nuclear medicine specialists with knowledge of clinical and laboratory findings, regardless of the PSA level at diagnosis, GS, clinical T stage and symptom.
The relationship between the serum PSA, ALP, GS, percentage of positive biopsy cores and the result of the bone scan was examined by calculating series of crude, stratified, and adjusted odd ratios (OR) (three of four factors aforementioned held constant when calculating the OR in the multivariate analysis) with corresponding 95% confidence intervals (95% CI). The groupings were based on previous papers on this subject published on journals with high impact factors.[8,9] Multivariate analysis was performed to the variables with P < 0.2 in univariate analysis. The association between the exposure and the outcome was considered statistically significant when the P < 0.05. All analyses were performed using SPSS 15 for Windows (LEAD Technologies, Chicago, IL) and SAS 8.02 for Windows (SAS Institute, Cary, NC) statistical software packages.
The proportion of positive bone metastases were evaluated by PSA level at diagnosis, GS and percent of positive cores (PPC) of biopsy. Two-sided t-tests were used to compare the continuous parameters. Univariate and multivariate logistic regression analyses were performed to assess the predictors of patients with positive bone metastasis. In addition, for PSA values, a receiver operating characteristic (ROC) analysis was performed and data are given as area under the curve (AUC) with 95% CI and significance levels.
RESULTS
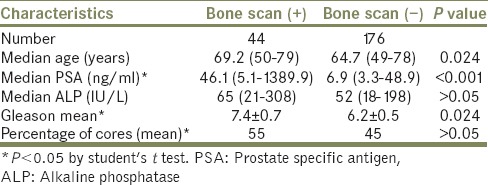
Two hundred twenty consecutive newly diagnosed prostate cancer patients were included in the analysis. Forty-four patients had a positive scan indicative of metastatic disease (20%, 95% CI, 17-24%). Median age of patients with and without bone metastasis was 69.2 and 64.7 years old, respectively (P = 0.024). Among patients with bone metastasis diagnosis, positive mean PSA concentration and biopsy GS were significantly higher compared with those without bone metastasis; 46.1 ng/mL versus 6.9 ng/mL and 7.4 versus 6.2 respectively [Table 1] (P < 0.001 and P = 0.024).
Table 1.
Characteristics of 220 patients
When we used PSA serum concentration <10 ng/mL, 10 to ≤20 ng/mL and >20 ng/mL as a cut-off point, of these 44, patients presented bone metastasis in scintigraphy four patients (9%) had PSA serum concentration <10 ng/mL, 5 (%11.3) had PSA serum concentration ≥10 to ≤20 ng/mL and 35 (79.5%) had PSA concentration >20 ng/mL. According to ROC analysis, a cut-off value of 19.8 was found with a sensitivity of 79.5% and specificity of 94.3%. OR for this cut-off value was 14.0. AUC of the ROC analysis was found as 0.902 (P < 0.0001) [Figure 1]. The same cutting points were used for 176 patients presented without bone metastasis in scintigraphy; 120 (68.1%) patients had PSA value <10 ng/mL, 47 (26.7%) had PSA serum concentration ≥10 to ≤20 ng/mL and 9 (5.1%) had PSA value >20 ng/mL, as given in Table 2.
Figure 1.
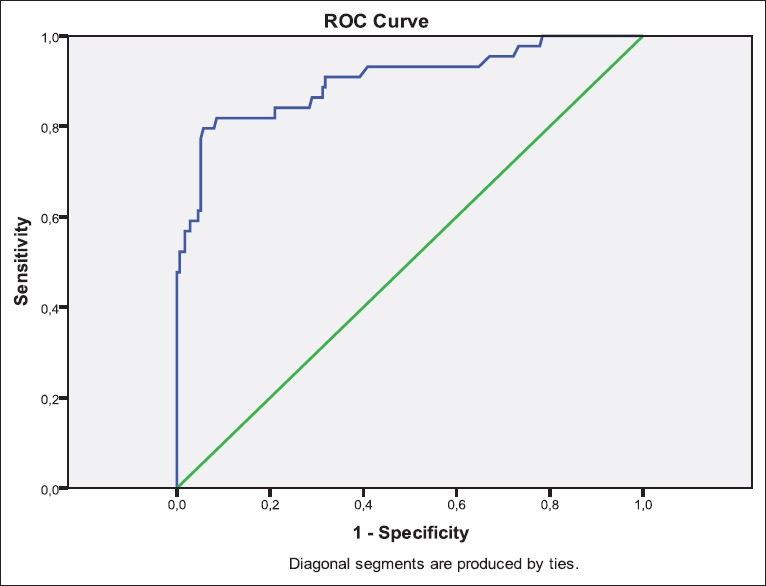
Receiver operating characteristics-analysis for prostate specific antigen values (area under the curve: 0,902, [95% confidence interval: 0,855; 0,938] P < 0.001)
Table 2.
Association between PSA, ALP, Gleason score, % of biopsy cores positive for cancer and bone metastasis
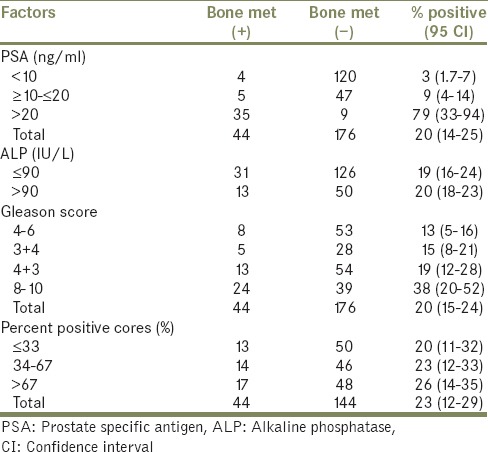
By using a PSA >20 ng/mL as the only criterion for not taking a staging bone scan nine positive scans (20% of all positive scans) would have been missed in this cohort.
When the ALP value was used ≤90 IU/L and >90 IU/L as a cut-off point of the 220 patients 63 (28.3%) had ALP serum concentration >90 IU/L. Of the 44 patients with bone metastasis 13 (%29.5) had ALP serum concentration >90 IU/L.
Of the 44 patients with the bone metastasis who had GS 4, 5, 6 were 2 (4.5%), 3 (6.8%), 3 (6.8%), respectively. There were 15 patients (34%) with GS 7, of whom 10 (67%) had a major Gleason pattern of 4 and 5 (33%) of 3. In cases where different histological patterns were present in different biopsy cores from the same pathological specimen, the pattern in the most cores or greater percentage of the specimen was designated as that of the patient. There was a statistically significantly greater proportion of positive bone scans in patients with a major pattern of Gleason grade 4 or 5 (31/44) than in those with better differentiated major patterns (13/44) on Chi-squared analysis (P < 0.001) [Table 2].
PPC information was available for 188 patients. The percentages of biopsy cores positive were grouped as <33%, 33-67%, and >67% [Table 2]. Increasing risk features including T stage, PSA, GS, were directly correlated with increasing PPC (data not shown).
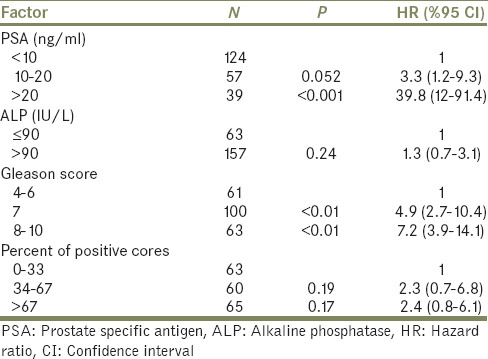
On univariate analysis, positive bone scans, were not associated with PPC, with higher metastasis as PPC increased. On multivariate analysis, all factors apart from serum ALP and PPC remained independently predictive of a positive bone scan. The strongest correlation with a positive scan was a serum PSA of > 20 ng/mL (P < 0.001; hazard ratio [HR], 39.8 [95% CI, 10.1-59.5]). The next strongest predictor was the presence of a GS ≥ 8 (P = 0.001; HR, 7.2 [95% CI, 3.0-14.6]). Serum PSA levels of 10-19.9 ng/mL did not independently predict for a positive scan on multivariate analysis [Table 3].
Table 3.
Multivariate analysis
DISCUSSION
The purpose of this study was to explore of predicting factors of positive scan in prostate cancer patients. These factors which were introduced in previous reports are presented in Table 4.
Table 4.
Predicting factors of a positive scan introduced in previous reports
Following the introduction of PSA, the test soon became the most commonly utilized prognostic parameter in prostate cancer. Using PSA cut-offs alone, we found the negative predictive value of a serum PSA <10 ng/mL for the absence of skeletal metastasis to be 53.7% and serum PSA 10.0-20.0 ng/mL to be 76.7%. The results of this study confirm those of previous research, which showed the close relationship between PSA level and the risk of bone scan positivity.[10,11,12] The incidence of bone metastases in the subgroup with a PSA level of <10 ng/mL ranged from 0.0% to 8.3% and our result of 3.2% was also included within this range.[7,13] Using the PSA cut-off value of 20 ng/mL, the incidence of bone metastases sharply increased to a very high level; 79.5%.
It is known that the bone metastases of different cancers can be indirectly ascertained through a measurement of ALP. In our study, no statistically significant difference was noted in the ALP levels of patients with and without bone metastasis (P = 0.24; HR, 1.3 [95% CI, 0.7-3.1]).
The current results revealed that bone metastasis is common in Turkish patients with newly diagnosed, untreated prostate carcinoma, with an overall positive rate of %20 (44/220) on bone scans. The positive rate is approximately double that reported recently reported in the United States (8.9%) and same in Japan.[13,14] The greater metastasis rate compared to these studies may be due to different sampling methods.
Recent studies demonstrated Gleason grade to be a predictor of bone scans in prostate cancer like pre-treatment PSA values.[15,16] The overall proportion of positive scans was significantly higher in patients with GS ≥7 compared to those with GS <7 (22.6% vs. 13.1%; P < 0.001). Coincidently with international literature findings, patients having well-differentiated tumors and low PSA levels (PSA <10) were found to have low positivity rate for bone metastasis. Many report pointed out that routine bone scanning should not be mandatory in all newly diagnosed prostate cancer patients.[13,14,15,16] Therefore, our study demonstrates that a staging radionuclide bone scan in a patient with untreated prostate cancer and a presenting serum PSA <20.0 ng/mL, GS up to seven in the absence of symptoms suggestive of bone metastasis is unlikely to be informative, and should be omitted. Guidelines from Europe and the USA advocate that the bone scan may be spared in the expected low-risk patients. For example, the EAU guideline describes that the bone scan may not be needed in the asymptomatic patients if the serum PSA is <20 ng/ml in the presence of well- or moderately differentiated tumors.[17] The National Comprehensive Cancer Network guideline recommends that for symptomatic patients and/or those with life expectancy of greater than 5 years, a bone scan is appropriate for patients with T1-T2 stage, as well as with a PSA level >20 ng/ml or GS of ≥8.[18] Indeed, the proportion of the bone metastases of the present study cohort showed a low prevalence of bone metastases (in patients with the PSA level <20 ng/ml [5.3%] and GS up to 7 [13.1%]).
Although many studies of prostatectomy specimens and needle biopsies comparing Gleason 4 + 3 with Gleason 3 + 4 adenocarcinoma show a significantly worse prognosis for the former, in our study there was a statistically insignificant association between scan positivity and a GS of 4 + 3, compared with 3 + 4.[14,19,20]
The PPC is an independent and powerful predictor of clinical outcomes of prostate cancer. A risk model replacing T stage with the PPC to reduce subjectivity demonstrated potentially improved stratification.[9,21] Grossklaus et al.[22] showed that the overall percentage of tumor per biopsy set was a good predictor of final pathological stage after radical prostatectomy. Furthermore, it was previously reported by Kestin et al. that percent of positive pre-treatment biopsy cores is a powerful predictor of biochemical and clinical outcome for prostate cancer, independent of other known prognostic factors.[23] It was reported several times that PPC was associated with stage, GS, pre-treatment serum PSA level. Combining the PSA level, biopsy GS and percentage of cores positive from the dominant side of the prostate resulted in a model that provided a high degree of prediction for PSA failure after definitive treatment.[24,25] PPC should be seriously considered as a primary factor in risk group stratification for prostate. In our study, the percentages of biopsy cores positive were grouped as ≤33%, 34-67%, and >67%. This selection was based on the presumption that more than two-third of the cores being positive versus one-third to two-third or fewer was a clinically meaningful difference. The percentage of positive biopsies, when analyzed in this manner, was not found to be an independent predictor of positive scans as other known prognostic factors.
The present study has a number of limitations that should be mentioned. The data collection was made in a retrospective fashion and this has limited the power of the results. A large number of medical records were incomplete and consequently a great number of patients were excluded from the study. Second, given the fact that we could not confirm the histology of the bone metastasis detected by bone scans. Even though, bone scintigraphy is accurate and highly sensitive, there might be a margin of error due to specificity. Third there was not a standard of number of biopsy sites; therefore, the results of this study may not be applicable to patients with large gland volumes who had fewer cores obtained, although larger samples were generally performed in patients with larger prostate gland volumes, possibly in an effort to decrease sampling error. Needless to say, the accurate diagnosis of the GS is essential and gives important information if available, to spare a bone scan at initial staging.
CONCLUSION
Bone scan remains the gold standard for the evaluation of skeletal metastases. Every year tens of thousands of men newly diagnosed with prostate cancer are given a bone scan as part of their “normal” diagnostic workup. In newly diagnosed prostate cancer, detection rate is influenced by the prognostic factors; PSA and biopsy GS. PSA level correlates with the bone scan result, although all staging bone scan studies in patients with newly diagnosed, untreated prostate cancer and low serum PSA level are retrospective and consequently may be prone to a possible selection bias. Subsequently, clinicians may have been biased against requesting bone scans in patients with lower PSA level, resulting in the majority of bone scan investigations being omitted in patients presenting with PSA <10 ng/mL. Based on the considerations that bone metastases are apt to develop in a considerable proportion of men with prostate carcinoma, and because a “positive” radionuclide bone scan is sensitive but not high specific, a scan at the time of prostate carcinoma diagnosis may be a useful baseline study with which to compare with later scans, most authors recommend bone scans for men with newly diagnosed prostate carcinoma. In our study, the bone scan is of limited clinical value if PSA less than 20 ng/ml, GS up to seven, but ALP levels and PPC were not significantly related to positive bone scans although the last two parameters are good predictors of clinical outcomes in several studies. Considering the retrosspective design of our study, further prospective investigations may help to confirm our results. Clearly, individual judgment is also helpful on patients on the margins in order to give the best algorithms that may be at a higher risk for bony disease and no strict cut point should be used for all patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.McArthur C, McLaughlin G, Meddings RN. Changing the referral criteria for bone scan in newly diagnosed prostate cancer patients. Br J Radiol. 2012;85:390–4. doi: 10.1259/bjr/79184355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber G, Chodak GW. Assessment of value of routine bone scans in patients with newly diagnosed prostate cancer. Urology. 1991;37:418–22. doi: 10.1016/0090-4295(91)80101-c. [DOI] [PubMed] [Google Scholar]
- 3.Bantis A, Zissimopoulos A, Kalaitzis C, Giannakopoulos S, Sountoulides P, Parmenopoulou V, et al. Four prognostic indices in advanced prostate cancer patients, under palliative androgen deprivation treatment. Hell J Nucl Med. 2008;11:21–5. [PubMed] [Google Scholar]
- 4.Kiper A, Yigitbasi O, Imamoglu A, Tuygun C, Turan C. The prognostic importance of prostate-specific antigen in monitoring patients undergoing maximum androgen blockade for metastatic prostate cancer. Urologia. 2005;72:325–30. [Google Scholar]
- 5.Hur J, Yoon CS, Ryu YH, Yun MJ, Suh JS. Accuracy of fluorodeoxyglucose- positron emission tomography for diagnosis of single bone metastasis: Comparison with bone scintigraphy. J Comput Assist Tomogr. 2007;31:812–9. doi: 10.1097/rct.0b013e318031cc4d. [DOI] [PubMed] [Google Scholar]
- 6.Gomez P, Manoharan M, Kim SS, Soloway MS. Radionuclide bone scintigraphy in patients with biochemical recurrence after radical prostatectomy: When is it indicated? BJU Int. 2004;94:299–302. doi: 10.1111/j.1464-410X.2004.04927.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Lubeck DP, Grossfeld GD, Mehta SS, Carroll PR. Contemporary trends in imaging test utilization for prostate cancer staging: Data from the cancer of the prostate strategic urologic research endeavor. J Urol. 2002;168:491–5. [PubMed] [Google Scholar]
- 8.Haukaas S, Roervik J, Halvorsen OJ, Foelling M. When is bone scintigraphy necessary in the assessment of newly diagnosed, untreated prostate cancer? Br J Urol. 1997;79:770–6. doi: 10.1046/j.1464-410x.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Vicini FA, Williams SG, Ye H, McGrath S, Ghilezan M, et al. Percentage of positive biopsy cores: A better risk stratification model for prostate cancer? Int J Radiat Oncol Biol Phys. 2012;83:1141–8. doi: 10.1016/j.ijrobp.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Lee SH, Chung MS, Park KK, Yom CD, Lee DH, Chung BH. Is it suitable to eliminate bone scan for prostate cancer patients with PSA ≤20 ng/mL? World J Urol. 2012;30:265–9. doi: 10.1007/s00345-011-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal RP, Thiruudaian T, Khan MA. When is a bone scan study appropriate in asymptomatic men diagnosed with prostate cancer? Asian J Androl. 2008;10:890–5. doi: 10.1111/j.1745-7262.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin K, Szabo Z, Chin BB, Civelek AC. The value of a baseline bone scan in patients with newly diagnosed prostate cancer. Clin Nucl Med. 1999;24:579–82. doi: 10.1097/00003072-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gleave ME, Coupland D, Drachenberg D, Cohen L, Kwong S, Goldenberg SL, et al. Ability of serum prostate-specific antigen levels to predict normal bone scans in patients with newly diagnosed prostate cancer. Urology. 1996;47:708–12. doi: 10.1016/s0090-4295(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 14.O’sullivan JM, Norman AR, Cook GJ, Fisher C, Dearnaley DP. Broadening the criteria for avoiding staging bone scans in prostate cancer: A retrospective study of patients at the Royal Marsden Hospital. BJU Int. 2003;92:685–9. doi: 10.1046/j.1464-410x.2003.04480.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee N, Fawaaz R, Olsson CA, Benson MC, Petrylak DP, Schiff PB, et al. Which patients with newly diagnosed prostate cancer need a radionuclide bone scan? An analysis based on 631 patients. Int J Radiat Oncol Biol Phys. 2000;48:1443–6. doi: 10.1016/s0360-3016(00)00785-9. [DOI] [PubMed] [Google Scholar]
- 16.Bruwer G, Heyns CF, Allen FJ. Influence of local tumour stage and grade on reliability of serum prostate-specific antigen in predicting skeletal metastases in patients with adenocarcinoma of the prostate. Eur Urol. 1999;35:223–7. doi: 10.1159/000019850. [DOI] [PubMed] [Google Scholar]
- 17.EAU Guidelines. 2010. Available from: http://www.uroweb.org/guidelines/
- 18.NCCN Clinical Practice Guidelines in OncologyTM, Prostate Cancer Early Detection V.2. 2010. Available from: http://www.nccn.org . [DOI] [PubMed]
- 19.Chan TY, Partin AW, Walsh PC, Epstein JI. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823–7. doi: 10.1016/s0090-4295(00)00753-6. [DOI] [PubMed] [Google Scholar]
- 20.Lau WK, Blute ML, Bostwick DG, Weaver AL, Sebo TJ, Zincke H. Prognostic factors for survival of patients with pathological Gleason score 7 prostate cancer: Differences in outcome between primary Gleason grades 3 and 4. J Urol. 2001;166:1692–7. [PubMed] [Google Scholar]
- 21.Spalding AC, Daignault S, Sandler HM, Shah RB, Pan CC, Ray ME. Percent positive biopsy cores as a prognostic factor for prostate cancer treated with external beam radiation. Urology. 2007;69:936–40. doi: 10.1016/j.urology.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 22.Grossklaus DJ, Coffey CS, Shappell SB, Jack GS, Chang SS, Cookson MS. Percent of cancer in the biopsy set predicts pathological findings after prostatectomy. J Urol. 2002;167:2032–5. doi: 10.1016/s0022-5347(05)65077-x. [DOI] [PubMed] [Google Scholar]
- 23.Kestin LL, Goldstein NS, Vicini FA, Martinez AA. Percentage of positive biopsy cores as predictor of clinical outcome in prostate cancer treated with radiotherapy. J Urol. 2002;168:1994–9. doi: 10.1016/S0022-5347(05)64280-2. [DOI] [PubMed] [Google Scholar]
- 24.Stackhouse DA, Sun L, Schroeck FR, Jayachandran J, Caire AA, Acholo CO, et al. Factors predicting prostatic biopsy Gleason sum under grading. J Urol. 2009;182:118–22. doi: 10.1016/j.juro.2009.02.127. [DOI] [PubMed] [Google Scholar]
- 25.Freedland SJ, Aronson WJ, Terris MK, Kane CJ, Amling CL, Dorey F, et al. The percentage of prostate needle biopsy cores with carcinoma from the more involved side of the biopsy as a predictor of prostate specific antigen recurrence after radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer. 2003;98:2344–50. doi: 10.1002/cncr.11809. [DOI] [PubMed] [Google Scholar]


