Abstract
Objectives:
The objective of this study is to assess the dose-related effects of tramadol on a group of patients with premature ejaculation (PE).
Subjects and Methods:
During the period of months between June 2010 and July 2012, 180 PE patients presented to outpatient clinic of our hospital. Patients were randomized in a 1:1:1 fashion to receive different sequences of the three medications: placebo, 50 mg of tramadol and 100 mg of tramadol. Every patient received 10 doses of each medication for 2 months. Intra-vaginal ejaculatory latency time (IELT) was recorded in seconds initially and for each arm. Successful treatment of PE is defined if IELT exceeded 120 s. Side-effects of medications were reported.
Results:
Of patients enrolled, 125 (69.4%) continued the study. Patients’ age range was 20-55 years with PE complaint of 1 to 10 years duration. Mean IELT was 72 at presentation, 82 for placebo, 150 for tramadol 50 mg, and 272 for tramadol 100 mg (P < 0.001 for all comparisons). PE was successfully treated in only 2.4% of patients with placebo, in contrast to 53.6% and 85.6% with 50 and 100 mg tramadol, respectively (P < 0.001 for all comparisons). On multivariate logistic regression analysis, baseline IELT was the only predictor of successful treatment of PE with both tramadol 50 mg (odds ratio [OR]: 1.05, 95% confidence interval [CI]: 1.03-1.07, P < 0.001) and tramadol 100 mg (OR: 1.07, 95% CI: 1.04-1.11, P < 0.001). Postmicturition dribble annoyed 12.8% of those who received 50 mg tramadol and 33.6% of those who received 100 mg tramadol (P < 0.001). Weak scanty ejaculation was the main complaint in 7.2% versus 21.6% of those using 50 and 100 mg tramadol, respectively (P = 0.002). Two patients discontinued tramadol 100 mg due to side-effects.
Conclusion:
Tramadol hydrochloride exhibits a significant dose-related efficacy and side-effects over placebo for treatment of PE.
Keywords: Intra-vaginal ejaculatory latency time, premature ejaculation, tramadol hydrochloride
INTRODUCTION
Premature ejaculation (PE) is the most common type of ejaculatory dysfunction with an unclear etiology.[1] However, its estimated prevalence has been controversial for decades due to the lack of a strict globalized definition, being reported to reach as high as 20-30% of adult males.[2,3]
The International Society for Sexual Medicine (ISSM) currently defines PE as “ejaculation which always or nearly always occurs prior to or within 1 min of vaginal penetration; together with the inability to delay ejaculation on all/nearly all vaginal penetrations; and negative personal consequences, e.g. distress, bother, frustration and/or the avoidance of sexual intimacy”.[4] The condition may be primary or acquired.[1] The problem doesn’t only affect the male partner, but it has also a major impact on quality of life of their sexual partners. Several studies revealed that PE can lead to poor sexual satisfaction and increased ejaculation-related distress and inter-personal difficulties.[5,6,7,8]
Until recently, PE has been often attributed to a psychological rather than a physiological problem, and was managed by behavioral modification and psychotherapy. However, these lines are rarely successful on the long-term.[6,9] Several studies suggest that PE may be related partly to decreased serotonergic activity in the brain.[1] There are several lines of treatment for PE which include: topical agents, sprays, and systemic drugs, e.g. selective serotonin reuptake inhibitors (SSRIs), α-blockers, and phosphodiesterase-5 inhibitors.[1,10,11]
Dapoxetine, a short acting SSRIs, is the only currently approved drug for treatment of PE in some countries,[6,11] but it has not been yet available in our country at the time of the study. Several studies have shown that tramadol HCl oral therapy has yielded promising results in the treatment of PE.[1,11,12,13,14,15,16,17,18,19,20,21,22,23,24] It is still not clearly understood how tramadol delays ejaculation; however, several pharmacological studies have proposed some actions as; acting as μ-opioid agonist by the drug or its M1 metabolite,[25] 5-hydroxytryptamine type 2C receptor antagonist,[26] 5-nicotinic acetylcholine receptor antagonist,[27] N-methyl-D-aspartate receptor antagonist,[28] M1/M3 muscarinic receptor antagonist[29,30] and an inhibitor of serotonin/norepinephrine uptake.[31] Thus, the exact mechanism remains speculative, and it might be a combination of the fore mentioned mechanisms.
The objectives of this work are to assess the dose-related effects of tramadol on the intra-vaginal ejaculatory latency time (IELT), ability to delay ejaculation and enjoyment of sexual intimacy, as well as to evaluate the side-effects and their frequency and tolerability in a group of patients with PE.
SUBJECTS AND METHODS
This is a prospective double-blinded placebo-controlled crossover study that was conducted in the period from June 2010 to July 2012. A total of 180 patients (age range: 20-55 years) with PE for >1 year attending the outpatient clinic in our hospital were enrolled. Sample size calculation was carried out using Epi-info™, version 3.5 (CDC, 2008; Atlanta, GA, USA); a calculated sample of 105 or more was needed, with a P < 0.05 and 80% power to detect a difference of 90 s between mean IELT before and after therapy.
The study was reviewed and approved by our Institutional Ethical Review Board. All of the consented participants were potent with no history of erectile dysfunction, and had a stable, regular (≥1 sexual attempts per week), single-partner heterosexual relationship for ≥ 1 year. Patients were instructed to stop any relevant medications 1 month before and throughout the study. A detailed history taking (including detailed medical, surgical, and sexual history) and a thorough clinical examination were done for all. Those with diabetes mellitus, hepatic/renal insufficiency, neurological/psychiatric disorder, or drug abuse were excluded. IELT was recorded in seconds by patients initially (for a month before starting the medications); those with mean baseline IELT >120 were excluded.
Patients were coded and randomized in a 1:1:1 fashion to receive three different sequences of three medications; the first sequence was: 50 mg tramadol, placebo (multi vitamin tablets) and 100 mg tramadol; the second sequence was 100 mg tramadol, placebo and 50 mg tramadol; and the third sequence was: 100 mg tramadol, 50 mg tramadol and placebo. Every patient received 10 doses of each medication for 2 months. IELT was recorded throughout the 2 months treatment for each arm. The mean IELT at the end of each treatment arm was then calculated. Successful treatment of PE was defined by IELT >120 s together with the ability to control ejaculation and enjoying the sexual intimacy. Side-effects of medications were reported.
Patients were instructed to take the medication orally, 2-3 h before intercourse for at least six successive intercourses. Patients used a stop-watch to record the baseline IELT (in seconds) before treatment and in every intercourse during the period of intake of the medications. Patients were instructed to record any encountered side-effects during the period of the study. The following data were collected prospectively and analyzed: patient age, educational level, history of premarital practice of masturbation, duration of marriage in years, frequency of intercourse, duration of PE in years, type of PE (primary or lifelong/secondary or acquired), IELT pre-/post-treatment and side-effects of medications.
Statistical analysis and graphical illustrations were performed using IBM® SPSS© statistics V19.0, (SPSS 19 for Windows [SPSS, Inc., Chicago, IL]). Analysis included; the Chi-square test or Fisher's exact test for comparison of the categorical data and the Student's paired-samples t-test (values expressed as mean and standard deviation) or the Mann-Whitney U-test (values expressed as median, inter-quartile range) to compare the noncategorical data. A multivariate logistic regression analysis of all factors associated with PE was done to detect the predictors of successful tramadol therapy. P value was considered as significant when ≤0.05.
RESULTS
Of the 180 initially enrolled patients, 125 (69.4%) continued the study, while 65 were lost to follow-up. The basic patient characteristics are listed in Table 1.
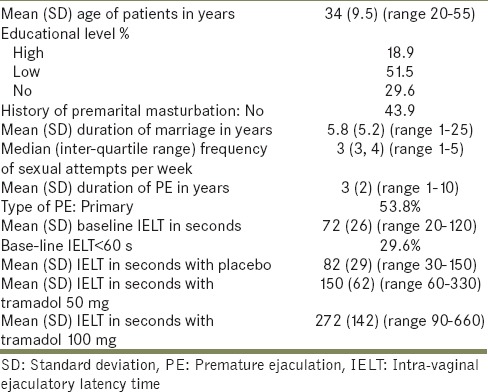
Table 1.
Demographic and characteristics of 125 patients with PE before and after therapy
Mean IELT was 72 at baseline, 82 for placebo, 150 for tramadol 50 mg and 272 for tramadol 100 mg (P < 0.001 for all comparisons). PE was successfully treated in only 2.4% of patients with placebo, in contrast to 53.6% and 85.6% with 50 and 100 mg tramadol, respectively (P < 0.001 for all comparisons). Tramadol therapy was significantly more effective for those with baseline IELT 60-120 [Figure 1].
Figure 1.
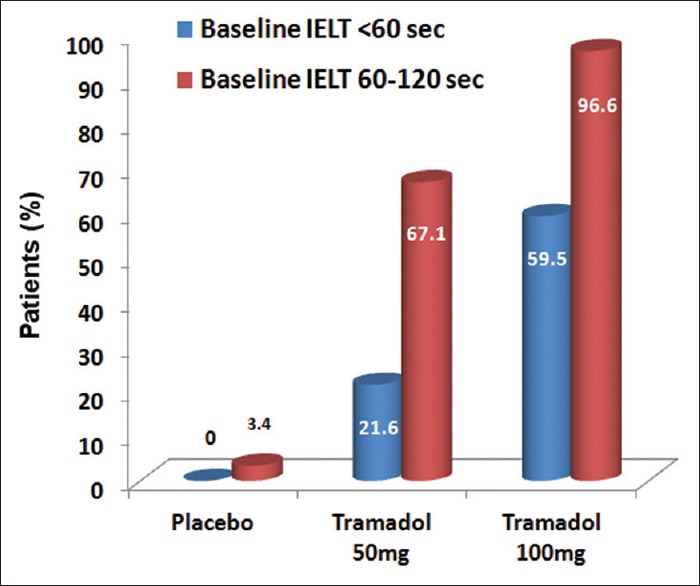
Success rate of premature ejaculation treatment in relation to baseline intra-vaginal ejaculatory latency time among the three therapeutic arms; placebo (P=0.554), tramadol 50 mg (P<0.001) and tramadol 100 mg (P<0.001)
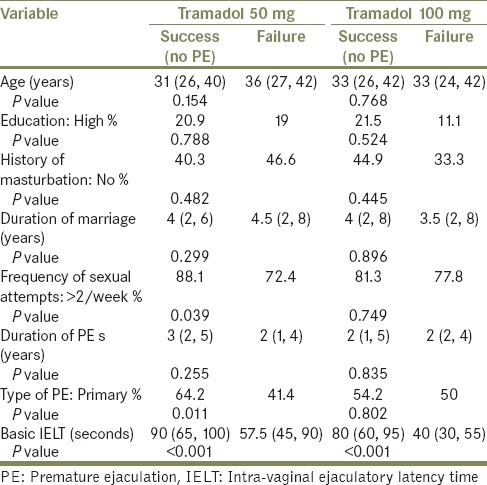
On univariate analysis; factors significantly associated with successful tramadol 50 mg treatment of PE were: baseline IELT, primary PE and sexual intercourse more than twice per week. Baseline IELT was the only factor significantly associated with successful resolution of PE using tramadol 100 mg [Table 2]. On multivariate logistic regression analysis, baseline IELT was the only predictor of successful treatment of PE with both tramadol 50 mg (odds ratio [OR]: 1.05, 95% confidence interval [CI]: 1.03-1.07, P < 0.001) and tramadol 100 mg (OR: 1.07, 95% CI: 1.04-1.11, P < 0.001).
Table 2.
Factors associated with successful treatment of 125 patients with PE using 50 and 100 mg tramadol doses
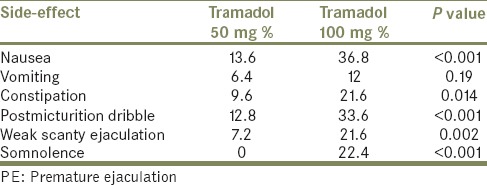
Among the 125 patients included in the study, adverse events were noted in 3.2% with placebo, 21.6% with tramadol 50 mg and 45.6% with tramadol 100 mg (P < 0.001). A summary of the patient-reported reversible side-effects with tramadol 50 mg and tramadol 100 mg doses are shown in Table 3. Two patients discontinued tramadol 100 mg temporarily after the third and fifth dose, respectively due to severe nausea and vomiting. No withdrawal symptoms were noted in any.
Table 3.
Side-effects noted with tramadol 50 mg and tramadol 100 mg doses in 125 patients with PE
DISCUSSION
Premature ejaculation is a prevalent male sexual dysfunction, which has a significant influence on male sexual practice. Historically, the first report on this condition dates back to 1887 when gross first described a patient complaint of rapid ejaculation. Since then, several treatment lines have been proposed for PE, but none has been the ultimate remedy.[5] In addition to the recent definition of PE by ISSM, another definition of PE that is frequently utilized in literature is that of the Diagnostic and Statistical Manual of Mental Disorders-IV-TR; which did not use IELT.[32] The criteria we used for successful treatment of PE depended on both the fore mentioned definitions and included the IELT as well as ejaculation control and personal satisfaction.
Tramadol is a potent centrally acting synthetic opioid analgesic. It was approved by the Food and Drug Administration (FDA) as an analgesic for the United States market with established safety over 30 years of human use.[19] It is suitable for on-demand use as it is completely absorbed after oral intake with peak concentration achieved within 95-110 min and a mean elimination half-life of about 6 h.[24] Its bioavailability is 70% after a single dose and approaching 100% with multiple dosing. It is metabolized in the liver and eliminated mainly in urine (90%) and in feces (10%).[33] Being an opioid, tramadol has a potential risk of drug dependence. Such drug abuse risk is lower with the on-demand use and required continuous administration of 400 mg for >3 months. The rate of tramadol addiction was reported by US FDA as low and almost seen in patients with previous substance abuses.[34,35,36]
There has been an increasing trend toward the use of tramadol to treat PE with promising results.[1,11,12,13,14,15,16,17,18,19,20,21,22,23,24] The main possible two modes of action for treatment of PE are; binding to μ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.[19] In the current study, the aim was to evaluate both the effects and side-effects of oral tramadol HCl in two different (50 and 100 mg) doses versus placebo. All the patients included in this work have reported an evident ejaculation-related bother as well as a significant intercourse-related stress, and lack of sexual intimacy with a baseline IELT of <2 min. This cut-off limit was used by seven studies addressing the role of tramadol in PE, including ours [Table 4]. There is only one report using IELT cut-off of 1 min (as stated in the PE definition of ISSM).[18] Regardless of the IELT, PE is dependent on 2 other important criteria, namely the ejaculatory control and patient satisfaction, which should be targeted as well.
Table 4.
Outcomes of tramadol therapy for of PE in the literature
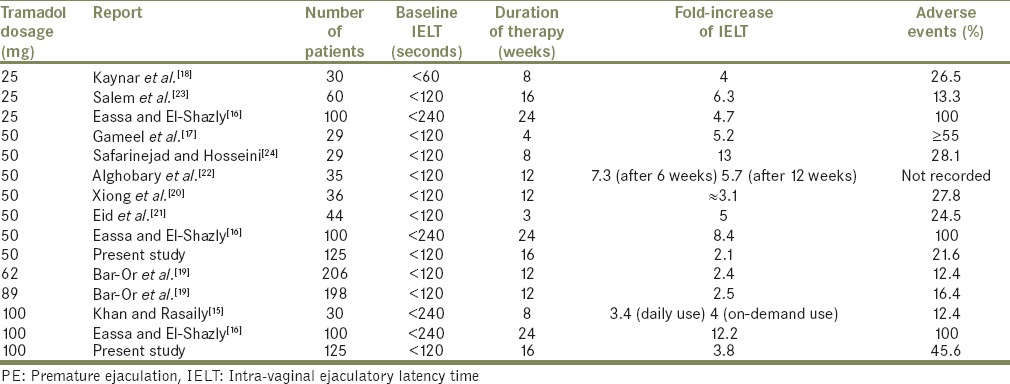
Wu et al. conducted a comprehensive meta-analysis of literature (including seven publications) on this topic and reported a 3 min increase of IELT with tramadol HCl over placebo (mean difference 2.77 min; 95% CI: 1.12-4.47; P < 0.001).[14] Such finding is similar to our results with 100 mg tramadol doses.
The reported outcome of tramadol therapy in PE is summarized in Table 4. This table includes 10 previously published studies in addition to ours. Among the seven studies of on-demand tramadol versus placebo; one study had similar results of on-demand tramadol for PE treatment when compared with continuous use.[15] Two studies reported tramadol versus paroxetine; showing no differences between the tramadol and paroxetine groups in IELT (mean difference –0.44; 95% CI: –5.07-4.18; P = 0.85).[14,21,22] Another study compared on-demand tramadol with three other on-demand PE potential therapies (sildenafil, paroxetine, and local lidocaine gel); tramadol significantly prolonged IELT values, while sildenafil was significantly associated with better sexual satisfaction than the other drugs.[17] No study compared dapoxetine with tramadol. A reported clinical trial of on-demand dapoxetine showed promising results with moderate efficacy in the treatment of PE with relatively few side-effects (when compared to tramadol and other SSRIs); 1.7% (for 30 mg dose) and 5.1% (for 60 mg dose).[6,11]
The number of participants in our study was relatively small, as they represent only the patients who accepted to participate and were fitting to the inclusion criteria of the study. However, it is still comparable or even larger than patient numbers reported in several single-center studies that discussed the same point.[18,22,23] We made sure that all patients involved did not have any diseases nor were taking any medications that would modify the effect of therapy. Moreover, we tested both placebo and tramadol HCl (50/100 mg) on the same group of patients in a double blind way to avoid inter-personal as well as subjective variations. In both doses of tramadol (available in or country), it has proven its quantitative and subjective efficacy in the treatment of PE that is not attributed to a placebo-effect.
We did not use a validated questionnaire neither for enrollment nor for assessment of the degree of satisfaction with treatment results as most of our patients were illiterate/of low education level. Many questionnaires for assessment of patient-reported outcome were used in the literature including; ejaculation control (AEC) and sexual satisfaction scores, Premature Ejaculation Profile, International Index of Erectile Function scores and Arabic Index of Premature Ejaculation score.[15,16,17,18,19,20,21,22,23,24] We believe that our definition of PE is a reliable objective as well as subjective measure of outcome impressions. The regression analysis has shown that the therapeutic effect was stable with no confounding effect noticed with any of age, duration of marriage, premarital masturbation history, occupational nature, educational level, and duration of PE. For those with base-line IELT <60 s, tramadol seems to be less effective.
Despite the high frequency of some side-effects of tramadol (up to 37% in our study), the adverse events were minor, reversible and generally well-tolerated by the patients. This was indicated by the small percentage (1.6%) of patients who discontinued tramadol 100 mg temporarily due to side-effects. In a meta-analysis, tramadol had a significant more adverse events when compared with placebo (OR: 2.89; 95% CI: 1.88-4.43; P < 0.0001).[14] The reported side-effects of tramadol include; ED (1%), vertigo, dizziness, headache, drowsiness, and the common cold (<1% for each).[19] Other studies showed the predominance of nausea, sleepiness, and headache.[18,23,24] Adverse events affected a wide range of patient ranging from 12.4% to 100%; mainly related to higher doses (>50 mg) and longer duration of therapy (>12 weeks) as well as personal/racial variations [Table 4]. The more palatable; tramadol orally disintegrating tablet had generally a lower rate of side-effects despite high doses (62 mg and 89 mg). No withdrawal symptoms were noted with on-demand tramadol use.[19]
Both the therapeutic effects and side-effects of tramadol for treatment of PE were dose/duration dependent as obvious from previous results [Table 4]. Although in our study, we did a logistic regression model to predict successful therapy of PE, we have some limitations including; relatively small number of cases and lack of a validated questionnaire for assessment of patient/partner satisfaction.
In conclusion, our study revealed that tramadol hydrochloride exhibits a significant dose-related advantage over placebo in the treatment of PE. Tramadol increased IELT as well as improve ejaculatory control and personal satisfaction. The only predictor of successful treatment of PE was the baseline IELT. On-demand tramadol 50 mg significantly improved PE with minor side-effects. For those with baseline IELT <60 s, tramadol 100 mg is usually needed.
Despite the diversity of the side-effects, their impact on the patients can be fairly referred to as minor, tolerable, and reversible. The potential risk of drug dependence is the limiting factor for routine use of tramadol in PE therapy. Accordingly, larger-scale and longer-term studies are required to investigate the tolerability and safety of tramadol. In addition, future studies are needed to compare the safety and efficacy of tramadol HCl against other therapeutic lines namely; dapoxetine.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Xin ZC, Zhu YC, Yuan YM, Cui WS, Jin Z, Li WR, et al. Current therapeutic strategies for premature ejaculation and future perspectives. Asian J Androl. 2011;13:550–7. doi: 10.1038/aja.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson C, Gunn K. Premature ejaculation: Definition and prevalence. Int J Impot Res. 2006;18(Suppl 1):S5–13. doi: 10.1038/sj.ijir.3901507. [DOI] [PubMed] [Google Scholar]
- 3.Bejma JP, Hellstorm WJG. Premature ejaculation. Am Urol Assoc Update Ser. 2007;26:365–71. [Google Scholar]
- 4.McMahon CG, Althof SE, Waldinger MD, Porst H, Dean J, Sharlip ID, et al. An evidence-based definition of lifelong premature ejaculation: Report of the International Society for Sexual Medicine (ISSM) ad hoc committee for the definition of premature ejaculation. J Sex Med. 2008;5:1590–606. doi: 10.1111/j.1743-6109.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 5.Waldinger MD. The neurobiological approach to premature ejaculation. J Urol. 2002;168:2359–67. doi: 10.1016/S0022-5347(05)64146-8. [DOI] [PubMed] [Google Scholar]
- 6.McMahon CG, Althof SE, Kaufman JM, Buvat J, Levine SB, Aquilina JW, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: Integrated analysis of results from five phase 3 trials. J Sex Med. 2011;8:524–39. doi: 10.1111/j.1743-6109.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano F, Patrick DL, Porst H, La Pera G, Kokoszka A, Merchant S, et al. Premature ejaculation: Results from a five-country European observational study. Eur Urol. 2008;53:1048–57. doi: 10.1016/j.eururo.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Patrick DL, Althof SE, Pryor JL, Rosen R, Rowland DL, Ho KF, et al. Premature ejaculation: An observational study of men and their partners. J Sex Med. 2005;2:358–67. doi: 10.1111/j.1743-6109.2005.20353.x. [DOI] [PubMed] [Google Scholar]
- 9.St Lawrence JS, Madakasira S. Evaluation and treatment of premature ejaculation: A critical review. Int J Psychiatry Med. 1992;22:77–97. doi: 10.2190/UWP1-CNHH-L0NK-YQY9. [DOI] [PubMed] [Google Scholar]
- 10.Linton KD, Wylie KR. Recent advances in the treatment of premature ejaculation. Drug Des Devel Ther. 2010;4:1–6. doi: 10.2147/dddt.s6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon CG, Porst H. Oral agents for the treatment of premature ejaculation: Review of efficacy and safety in the context of the recent International Society for Sexual Medicine criteria for lifelong premature ejaculation. J Sex Med. 2011;8:2707–25. doi: 10.1111/j.1743-6109.2011.02386.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Qian S, Liu H, Liu L, Pu C, Han P, et al. Role of tramadol in premature ejaculation: A systematic review and meta-analysis. Urol Int. 2013;91:197–205. doi: 10.1159/000348826. [DOI] [PubMed] [Google Scholar]
- 13.Wong BL, Malde S. The use of tramadol “on-demand” for premature ejaculation: A systematic review. Urology. 2013;81:98–103. doi: 10.1016/j.urology.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Yue X, Duan X, Luo D, Cheng Y, Tian Y, et al. Efficacy and safety of tramadol for premature ejaculation: A systematic review and meta-analysis. Urology. 2012;80:618–24. doi: 10.1016/j.urology.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Khan AH, Rasaily D. Tramadol use in premature ejaculation: Daily versus sporadic treatment. Indian J Psychol Med. 2013;35:256–9. doi: 10.4103/0253-7176.119477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eassa BI, El-Shazly MA. Safety and efficacy of tramadol hydrochloride on treatment of premature ejaculation. Asian J Androl. 2013;15:138–42. doi: 10.1038/aja.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gameel TA, Tawfik AM, Abou-Farha MO, Bastawisy MG, El-Bendary MA, El-Gamasy A. On-demand use of tramadol, sildenafil, paroxetine and local anaesthetics for the management of premature ejaculation: A randomised placebo-controlled clinical trial. Arab J Urol. 2013;11:392–7. doi: 10.1016/j.aju.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaynar M, Kilic O, Yurdakul T. On-demand tramadol hydrochloride use in premature ejaculation treatment. Urology. 2012;79:145–9. doi: 10.1016/j.urology.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 19.Bar-Or D, Salottolo KM, Orlando A, Winkler JV Tramadol ODT Study Group. A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol. 2012;61:736–43. doi: 10.1016/j.eururo.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 20.Xiong GG, Wu FH, Chen SH, Yao WL. Safety and efficacy of tramadol hydrochloride with behavioral modification in the treatment of premature ejaculation. Zhonghua Nan Ke Xue. 2011;17:538–41. [PubMed] [Google Scholar]
- 21.Eid MA, Ahmed HH, Ismail NN, Shehada SY. Comparative study between tramadol (50 mg) on demand and paroxitine HCl (20 mg) on demand in the treatment of premature ejaculation. Hum Androl. 2011;1:69–73. [Google Scholar]
- 22.Alghobary M, El-Bayoumy Y, Mostafa Y, Mahmoud el-HM, Amr M. Evaluation of tramadol on demand vs. daily paroxetine as a long-term treatment of lifelong premature ejaculation. J Sex Med. 2010;7:2860–7. doi: 10.1111/j.1743-6109.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 23.Salem EA, Wilson SK, Bissada NK, Delk JR, Hellstrom WJ, Cleves MA. Tramadol HCL has promise in on-demand use to treat premature ejaculation. J Sex Med. 2008;5:188–93. doi: 10.1111/j.1743-6109.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 24.Safarinejad MR, Hosseini SY. Safety and efficacy of tramadol in the treatment of premature ejaculation: A double-blind, placebo-controlled, fixed-dose, randomized study. J Clin Psychopharmacol. 2006;26:27–31. doi: 10.1097/01.jcp.0000195110.79027.3f. [DOI] [PubMed] [Google Scholar]
- 25.Hennies HH, Friderichs E, Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988;38:877–80. [PubMed] [Google Scholar]
- 26.Ogata J, Minami K, Uezono Y, Okamoto T, Shiraishi M, Shigematsu A, et al. The inhibitory effects of tramadol on 5-hydroxytryptamine type 2C receptors expressed in Xenopus oocytes. Anesth Analg. 2004;98:1401–6. doi: 10.1213/01.ane.0000108963.77623.a4. [DOI] [PubMed] [Google Scholar]
- 27.Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A, Shibuya I. Inhibitory effects of tramadol on nicotinic acetylcholine receptors in adrenal chromaffin cells and in Xenopus oocytes expressing alpha 7 receptors. Br J Pharmacol. 2002;136:207–16. doi: 10.1038/sj.bjp.0704703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara K, Minami K, Sata T. The effects of tramadol and its metabolite on glycine, gamma-aminobutyric acidA, and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Anesth Analg. 2005;100:1400–5. doi: 10.1213/01.ANE.0000150961.24747.98. [DOI] [PubMed] [Google Scholar]
- 29.Shiraishi M, Minami K, Uezono Y, Yanagihara N, Shigematsu A. Inhibition by tramadol of muscarinic receptor-induced responses in cultured adrenal medullary cells and in Xenopus laevis oocytes expressing cloned M1 receptors. J Pharmacol Exp Ther. 2001;299:255–60. [PubMed] [Google Scholar]
- 30.Shiga Y, Minami K, Shiraishi M, Uezono Y, Murasaki O, Kaibara M, et al. The inhibitory effects of tramadol on muscarinic receptor-induced responses in Xenopus oocytes expressing cloned M (3) receptors. Anesth Analg. 2002;95:1269–73. doi: 10.1097/00000539-200211000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Frink MC, Hennies HH, Englberger W, Haurand M, Wilffert B. Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittelforschung. 1996;46:1029–36. [PubMed] [Google Scholar]
- 32.Text Revision. (DSM-IVTR) 4th ed. Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; p. 554. [Google Scholar]
- 33.Lintz W, Erlaçin S, Frankus E, Uragg H. Biotransformation of tramadol in man and animal (author's transl) Arzneimittelforschung. 1981;31:1932–43. [PubMed] [Google Scholar]
- 34.Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol Psychol. 2006;73:90–9. doi: 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cicero TJ, Adams EH, Geller A, Inciardi JA, Muñoz A, Schnoll SH, et al. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 36.Manchikanti L, Ailinani H, Koyyalagunta D, Datta S, Singh V, Eriator I, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011;14:91–121. [PubMed] [Google Scholar]


