Abstract
Urethral diverticulum is a localized saccular or fusiform out-pouching of the urethra. It may occur at any point along the urethra in both male and females. Male urethral diverticulum is rare, and could be either congenital or acquired, anterior or posterior. The mainstay treatment of posterior urethral diverticulum (PUD) is the open surgical approach. Here we discuss our minimally invasive surgical approach (MIS) in managing posterior urethral diverticulum.
Keywords: Imperforate anus, laparoscopy, mullerian cyst, posterior urethral diverticulum, robotic
INTRODUCTION
Posterior urethral diverticulum (PUD) is very rare entity that's described in the literature.[1,2,3] There are few reports of congenital PUD.[1,3] Most of cases reported are acquired[2] and mostly follow surgical reconstruction of imperforate anus.[4,5,6,7,8] The mainstay treatment of PUD is open surgical approach.[1,3] Here, we discuss our minimally invasive surgical approach to treat both congenital and acquired PUD.
CASE REPORTS
Case 1
A 2-year-old boy, born at 29 weeks of gestation, found to have grade I left hydronephrosis, retrovesical cyst and active urinary tract infection (UTI) in the neonatal period. Voiding cystourethrogram (VCUG) showed smooth bladder wall, absence of vesico-ureteric reflux and PUD with insignificant post-void residual [Figure 1]. He was initially managed conservatively. Because of recurrent UTIs and retention episodes, endoscopic marsupialization of the diverticulum using a resectoscope was carried out, which failed to improve the patient's condition. Laparoscopic excision was planned. The patient was placed in dorsal lithotomy position initially. Cystoscopy showed huge PUD with a narrow neck opening proximal and lateral to verumontanum on the right side. 8F Foley's catheter was placed in the diverticulum over a guide wire after bladder evacuation. He was then shifted to supine position. 10 mm port was placed supraumbilically using open technique. After Insufflating peritoneal cavity with CO2-15 mm Hg, 2 other 5 mm ports were placed lateral to the rectus muscle around 1 cm below camera port at mid clavicular line A plane between the bladder and rectum was developed. Distending the diverticulum with saline through the fore placed catheter helped in its identification [Figure 2]. Stay suture was placed through the diverticulum and brought out through the abdominal wall as hitching suture to facilitate its dissection. After completely mobilizing the diverticulum it was opened and its wall was completely excised. Urethral edges were approximated using 5/0 vicryl sutures. Estimated blood loss (EBL) was around 20 cc. The patient was shifted to the floor on IV acetaminophen and cefuroxime. He was discharged home the 2nd day on oral acetaminophen and trimethoprim/sulfamethoxazole prophylaxis. Stent was left in place for 3 weeks. Follow-up VCUG 3 months later showed an area of suspicious stricture, which was confirmed by cystoscopy [Figure 3]. It was thin, passable and managed by simple dilatation [Figure 4]. The patient has been having good stream with no significant residual urine since then.
Figure 1.
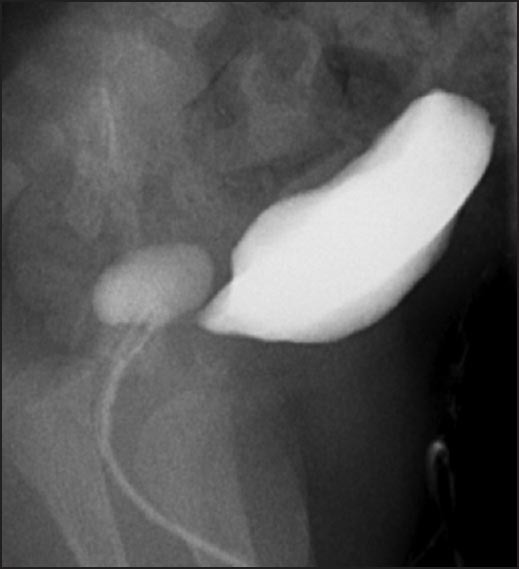
Voiding cystourethrogram showing diverticulum with communication to the prostatic urethra
Figure 2.
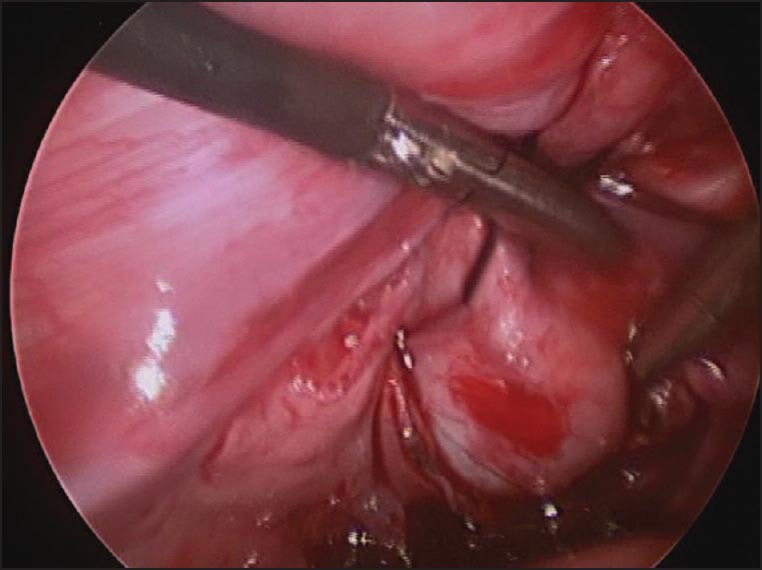
Laparoscopic posterior urethral diverticulectomy showing the hitching suture that's facilitate diverticular dissection
Figure 3.
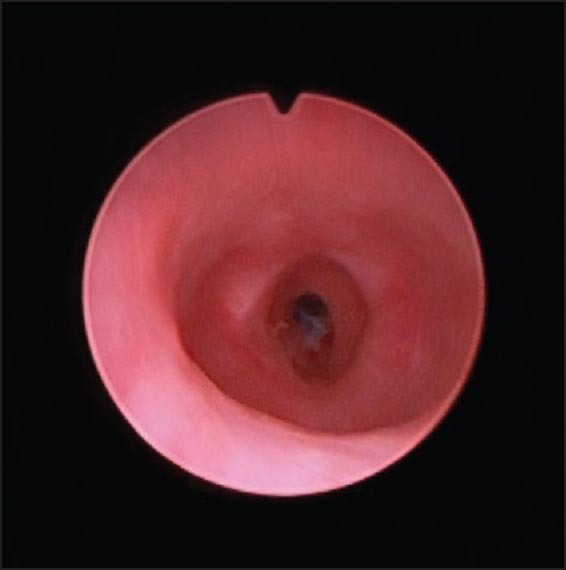
Cystoscopy showing thin stricture
Figure 4.
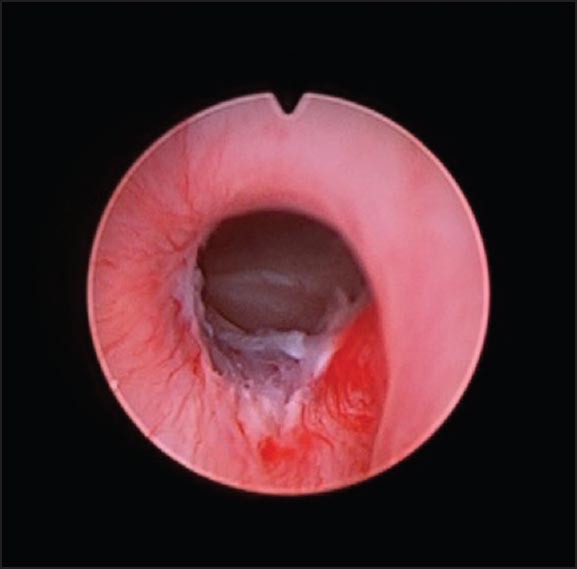
Cystoscopy showing stricture area post-dilatation
Case 2
4-year-old boy known case of imperforate anus post perineal pull-thru at neonatal period presented with history of weak stream and recurrent retention episodes. Ultrasound showed smooth bladder wall with normal upper tract. Huge PUD causing intermittent bladder outlet obstruction was detected by VCUG [Figure 5]. The patient was taken to operative room for cystoscopy and robotic assisted laparoscopic diverticulectomy. Cystoscopy showed huge PUD opening at the level of verumontanum on the left side. 8F Foley's catheter was placed into the diverticulum. He was then shifted to supine position. Ports were placed in a similar manner as discussed before in addition to an assistant 5 mm port. Da Vinci robotic surgical system was docked to the side of the patient. A plane between the bladder and rectum was developed where the PUD could be identified. Hitching suture was used to keep the diverticulum under tension. The diverticulum was completely mobilized and its wall excised [Figure 6]. Urethral edges were approximated using 5/0 vicryl suture. 10F foley's catheter was kept in place as a urethral stent. EBL was around 15 cc. The patient was shifted to the floor on IV acetaminophen and cefuroxime and was discharged home on oral acetaminophen, trimethoprim/sulfamethoxazole prophylaxis and foley catheter which was removed after 3 weeks. Follow-up VCUG at 3 months showed good caliber urethra with no strictures [Figure 7] and was able to empty his bladder completely since then.
Figure 5.
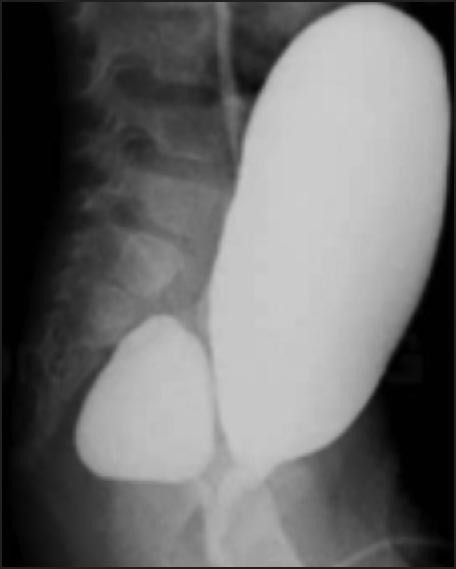
Voiding cystourethrogram showing huge diverticulum opening in the prostatic urethra
Figure 6.
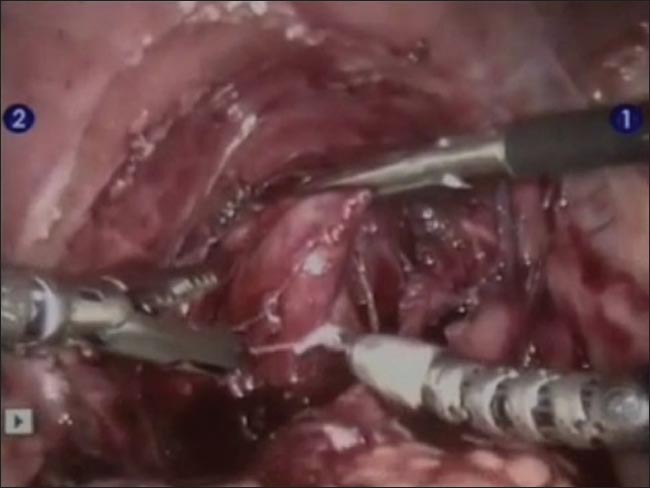
Robotic assisted laparoscopic posterior urethral diverticulectomy
Figure 7.
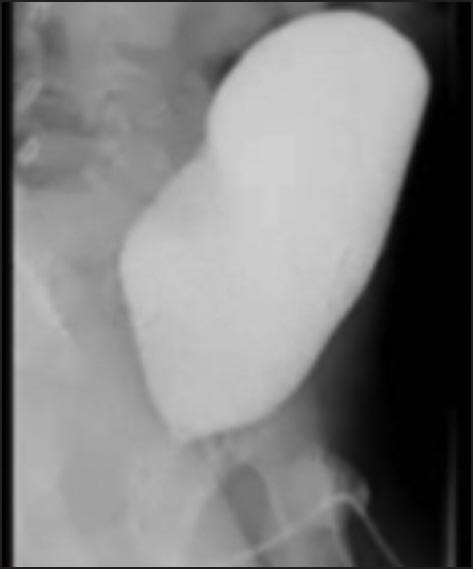
Post-operative voiding cystourethrogram showing normal prostatic urethra
DISCUSSION
Urethral diverticulum is defined as localized, epithelial-lined, saccular or fusiform out pouching of the urethra.[1,2] It's more common in females than males.[2] Male urethral diverticula are rare.[1,2] While most PUD cases reported in the literature are of acquired origin, congenital cases do exist.[1,2]
Congenital PUD usually form because of incomplete urethral duplication or more commonly where Mullerian remnant open in the urethra with a narrow neck.[1]
These may be prostatic utricles (PU) or Mullerian duct cysts (MDC).[1]
PU are usually small and have communication with the prostatic urethra.[3] Most don’t require any treatment unless very large causing recurrent UTIs, lower urinary tract symptoms or retention episodes.[1] MDC are cystic dilatations in the remnants of distal ends of the fused Mullerian ducts.[1] They rarely communicate with the urethra and if they do, they usually enter the midline of the verumontanum.[1]
Acquired diverticula mostly occur following pull through surgery of imperforate anus, trauma, infection or instrumentation.[1,4,5,6,7,8]
Clinically these patients may be completely asymptomatic or may present with difficulty in urination, urgency, lower abdominal swelling, perineal discomfort, recurrent UTI and urinary retention.[1,2]
Pelvic ultrasound and VCUG are usually sufficient to diagnose such cases.[2] However, giant PUD distorting the pelvic anatomy may be challenging to diagnose and additional imaging modality (e.g., magnetic resonance imaging) may be helpful in reaching the diagnosis and planning future surgery if needed.[2]
Not all PUD cases need to be treated. Asymptomatic small diverticula may be observed.[1] Different surgical approaches have been described to treat symptomatic PUD. Transurethral marsupialization of PUD are usually associated with high failure and recurrence rate specially if the diverticulum is large.[3] Open diverticulectomy through various approaches including the suprapubic, retrovesical, transvesical, posterior and perineal approaches have been described.[1,3] Because of the rarity of this disorder, the anatomical inaccessibility and proximity to pelvic nerves, rectum, vas deferens, ejaculatory ducts and ureters, open approach have been associated with significant morbidity including bladder, urethral, rectal and vasal injuries.[3] Treating such cases with minimally invasive techniques provided feasible alternative to open surgical excision with decreased morbidity, decreased post-operative pain, shorter hospital stay and reduced convalescence.[3] The current literature contains sporadic case reports on the use of minimally invasive surgery (MIS) for PUD.[3] To our experience MIS provided magnification, better visualization, meticulous dissection, precise approximation of the reconstructed urethra with minimal blood loss, minimal requirement of analgesia and short hospital stay. In addition, robotic assisted laparoscopic posterior urethral diverticulectomy had the advantage of providing 3D imaging and greater dexterity compared with laparoscopy which might be of advantage in treating such pathologies in a narrow space with so many vital structures around.
In conclusion, MIS can be an effective alternative to conventional open surgical approach in patients with congenital or acquired PUD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mousavi S, Mehrsai A, Nikoobakht M, Abedi AR, Salem S, Pourmand G. A giant congenital posterior urethral diverticulum associated with renal dysplasia. Urol J. 2006;3:247–9. [PubMed] [Google Scholar]
- 2.Kundum PR, Gupta AK, Thottom PV, Jana M. Technical note: Dynamic MRI in a complicated giant posterior urethral diverticulum. Indian J Radiol Imaging. 2010;20:300–3. doi: 10.4103/0971-3026.73536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong YK, Onal B, Diamond DA, Retik AB, Cendron M, Nguyen HT. Robot-assisted laparoscopic excision of symptomatic retrovesical cysts in boys and young adults. J Urol. 2011;186:2372–8. doi: 10.1016/j.juro.2011.07.113. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff A, Levitt MA, Peña A. Laparoscopy and its use in the repair of anorectal malformations. J Pediatr Surg. 2011;46:1609–17. doi: 10.1016/j.jpedsurg.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 5.Vinnicombe SJ, Good CD, Hall CM. Posterior urethral diverticula: A complication of surgery for high anorectal malformations. Pediatr Radiol. 1996;26:120–6. doi: 10.1007/BF01372089. [DOI] [PubMed] [Google Scholar]
- 6.Alam S, Lawal TA, Peña A, Sheldon C, Levitt MA. Acquired posterior urethral diverticulum following surgery for anorectal malformations. J Pediatr Surg. 2011;46:1231–5. doi: 10.1016/j.jpedsurg.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 7.Mickelson JJ, MacNeily AE, Blair GK. The posterior urethra in anorectal malformations. J Pediatr Surg. 2007;42:585–7. doi: 10.1016/j.jpedsurg.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.López PJ, Guelfand M, Angel L, Paulos A, Cadena Y, Escala JM, et al. Urethral diverticulum after laparoscopically-assisted anorectal pull-through (LAARP) for anorectal malformation: Is resection of the diverticulum always necessary? Arch Esp Urol. 2010;63:297–301. [PubMed] [Google Scholar]


