Abstract
Context:
The survival of atraumatic restorative treatment (ART) restorations would probably increase if near total elimination of cariogenic microorganisms could be done in the process of cavity cleaning before going ahead with the restoration. Thus, use of naturally occurring disinfecting agents for achieving this goal could herald a new beginning in the field of contemporary minimum intervention dentistry.
Aims:
To evaluate the efficacy of hand instruments in excavating dental caries and comparatively evaluate the roles of Aloe vera and propolis as potential cavity disinfecting agents after minimally invasive hand excavation of dental caries.
Settings and Designs:
Experimental, in vivo intergroup split mouth, randomized clinical trial.
Subjects and Methods:
The study included Group I (Control), Group II (A. vera) and Group III (propolis). Ten patients with three teeth each have occlusal/occlusoproximal lesions suitable for ART were selected. Dentinal samples were collected three times from each tooth viz., preexcavation, postexcavation and postdisinfection of the cavities. These dentinal samples were subjected to microbiological analyses for total viable count.
Statistical Analysis Used:
Repeated measures of analysis of variance (ANOVA) with Bonferroni post-hoc test and one-way ANOVA with Tukey post-hoc test.
Results:
In all the three groups, significant amount of bacteria were left behind after hand excavation. Group II and Group III, in which cavities were treated with A. vera and propolis extracts respectively, showed a significant reduction in the bacterial counts when compared to control the group.
Conclusions:
Hand excavation alone does not completely eliminate bacteria, which may predispose treated teeth to secondary caries. Both propolis and A. vera extracts can be used as potential natural disinfecting agents, thereby embracing the concept of phytotherapy in minimum intervention dentistry.
Keywords: Aloe vera, atraumatic restorative treatment, cavity disinfection, minimum intervention dentistry, phytotherapy, propolis, total viable count
Introduction
Minimum intervention, the key phrase in contemporary dentistry focuses on the least invasive treatment option available to minimize tissue loss and patient discomfort.[1] Atraumatic restorative treatment (ART) is one such minimally invasive modality, which uses hand instruments to remove soft, demineralized dental tissues followed by the placement of an adhesive restoration.[2]
Though this technique is most suited for the developing countries with sparse resources, it is gaining wide acceptance in the developed countries as well. ART is the currently the intervention of choice for (i) Treatment of early childhood caries,[2,3] (ii) very young children who are being introduced to oral care, (iii) patients who experience extreme fear or anxiety about dental procedures, (iv) mentally and/or physically handicapped patients, and (v) home-bound elderly patients and residents of nursing homes.[4]
The essence behind atraumatic restorative treatment
The goal of every stepwise intervention/excavation in both primary and permanent dentitions is to induce “self-repair”, to arrest the carious process and thus maintain pulp vitality.[5] Such an intervention bears fruit if we manage to perform the same in sterile conditions and then back it up with an adhesive restoration thereafter.
However, the available evidence pertaining to efficacy of hand instruments in elimination of cariogenic bacteria suggests that neither color nor dentin consistency is effective in differentiating the level of infection. Bacteria remain in the cavity even when excavation is carried to firm tissue and hence quite a few ART restorations fail due to secondary caries development subsequently.[2] Thus, the survival of ART restorations would probably increase if near total elimination of cariogenic microorganisms could be done in the process of cavity cleaning before going ahead with the restoration.[6] The use of disinfecting agents for achieving this goal could herald a new beginning for the field of contemporary, minimal intervention dentistry.[7]
Recently there has been a growing trend to seek natural remedies as part of medical and dental therapeutics, which has been termed as “phytotherapeutics” or “ethnopharmacology”.[8] Naturally occurring A. vera and propolis are emerging as very good antibacterial agents in various domains of dentistry.
Aloe barbadensis Mill (A. vera), is a short succulent herb resembling a cactus, with green dagger-shaped fleshy, spiny and marginated leaves, filled with a clear viscous gel which has potent antibacterial, antifungal, and antiviral properties.[9,10] Propolis is a resinous substance collected by honeybees (Apis mellifera), from different plant buds. Presence of flavonoids accounts for most of the biological activities of propolis, such as its’ antimicrobial, antioxidant, and antitumor activities.[11]
Though A. vera and propolis extracts have been used in dentistry for various purposes, no study till date has been conducted using them as cavity disinfectants. Thus, the present study was carried out to evaluate and compare the roles of A. vera and propolis as potential cavity disinfecting agents after minimally invasive hand excavation of dental caries.
Subjects and Methods
Children aged 5–12 years suitable for ART with at least three cavitated dentinal lesions in primary molars clinically involving occlusal and occlusoproximal surfaces, were selected irrespective of sex, race and socioeconomic status.
Study setting
Outpatient clinics of Department of Pedodontics and Preventive Dentistry at Bapuji Dental College and Hospital, Davanagere.
Study design
Experimental, in vivo, split mouth, randomized clinical trial.
Study groups
The study consisted of following groups [Table 1].
Table 1.
Study consisted of following groups

Inclusion/exclusion criteria and ethical clearance
Teeth with deep dentinal lesions with pulpal involvement, abscess, pain or swelling, developmental disorders and adjacent soft tissue lesions and children with systemic illness were excluded. Informed consent explaining the rationale of the study was read and signed by the parents of the children selected for the study. The study was conducted in strict adherence to the guidelines by Helsinki Declaration of 1975, (revised in 2000). Ethical clearance was obtained by institutional review board.
Study groups Preparation of cavity disinfectants
Propolis extract
Hand collected propolis samples produced by honeybees (A. mellifera) were kept desiccated in the dark before processing. The samples were ground mechanically and bottled in 10 g portions. The portions of 10 g were put into flasks, and 100 ml of 70% ethanol was added. Propolis was subjected to 14 days of extraction in order to obtain ethanolic extract of propolis. The flask was placed in laboratory shaker for 2 weeks at room temperature. After that the extract was cooled at 4°C for 24 h in order to precipitate all insoluble substances. Rough particles were removed from the propolis extract by filtering through filter paper (Whatman no. 1). The filtrate obtained was evaporated, using rotary vacuum evaporator at 40°C. This way, a viscous substance having brown color was obtained.[12]
Aloe vera extract
The leaves of the plant were washed with distilled water, cut opened, and fresh pulp was collected. The gel was dried in an oven at 800°C for 48 h and then powdered. An ethanolic extract was obtained by dissolving 20 g of the powder in 200 ml of ethanol. The contents were then filtered using Whatman filter paper no. 1, and the filtrate was evaporated for dryness.[13]
Procedure
Dentin sampling procedure
In each patient, three cavitated lesions were selected. A baseline sample of the carious lesion was obtained using a sterile spoon excavator from the center of each lesion, after isolation with a rubber dam prior to excavation of caries.
After the removal of caries, a second dentin sample for microbial evaluation was collected using another sterile spoon excavator from the hard dentin. Cavities of Groups I, II and III was disinfected with distilled water, propolis extract and A. vera extract respectively for 60 s. Then these cavities were air dried. New dentin samples were collected for microbial analysis using a spoon excavator from the same place. After collection of the third sample, the teeth were restored with glass ionomer cement (Fuji 9).[7]
Thus, dentinal samples were collected three times from each carious tooth, viz., baseline-before excavation of caries, after hand excavation of caries and after disinfection of the cavity. These samples were subjected to microbiological evaluation for total viable count (TVC).
Microbiological procedures
The samples collected were immediately transferred to brain heart infusion broth, then subjected to microbiological processing and were cultivated so that the total number of viable bacteria could be detected. The samples were homogenized in a tube shaker for 3 min, and 25 μl aliquots of this solution would be placed onto the plate surfaces containing blood agar, with a micropipette. Subsequently, the cultures were incubated after which it was possible to make a visual assessment of the total number of viable bacteria unit-forming colonies.[5]
Results
Table 2 shows a comparison of the viable bacterial colony count in each study group using repeated measures analysis of variance (ANOVA). A statistically significant reduction in the bacterial count was observed in all the three groups postexcavation (compared to preexcavation) and postcavity disinfection (compared to postexcavation) (P < 0.001).
Table 2.
Comparison of the bacterial colony count in each of the study groups using repeated measures ANOVA
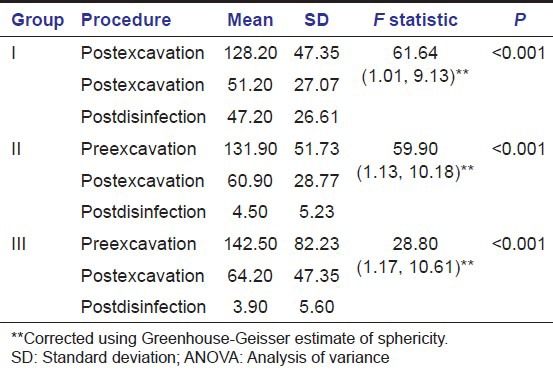
Pairwise comparison of viable bacterial colony counts at different phases viz preexcavation, postexcavation and postcavity disinfection was done in each of the study groups using Bonferroni post-hoc test. It showed statistically significant difference in the number of bacterial colonies between each phase in all the three study groups (P < 0.001) [Table 3].
Table 3.
Pairwise comparison of different phases in each of the study groups using Bonferroni post-hoc test

When inter group comparisons were made using one-way ANOVA [Table 4 and Illustration 1], and pair wise comparisons were done using post-hoc Tukey's test [Table 5], there was no statistically significant difference among the three study groups in the bacterial colony count at two phases of experiment viz preexcavation and also postexcavation. But when bacterial counts were compared postcavity disinfection there were statistically significant differences among the groups (P < 0.01). The reduction in the number of bacterial colonies after cavity disinfection was statistically significant in both Group II and Group III, when compared to Group I (P < 0.001). However, the difference observed between Group II and Group III in terms of TVC after cavity disinfection was not statistically significant (P = 0.99) [Figures 1–9].
Table 4.
Comparison of the bacterial colony counts between the study groups before excavation, after excavation and after disinfection using one-way ANOVA
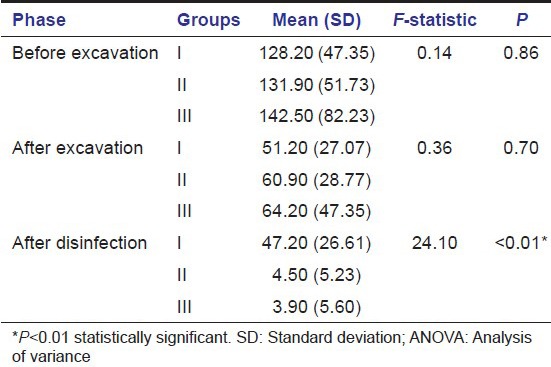
Illustration 1.
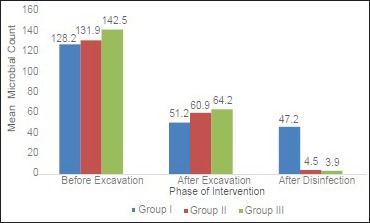
Comparison of the mean bacterial count between the study groups before excavation, after excavation and after cavity disinfection
Table 5.
Pairwise comparison of the number of bacterial colonies between the study groups before excavation, after excavation and after disinfection using Tukey post-hoc test
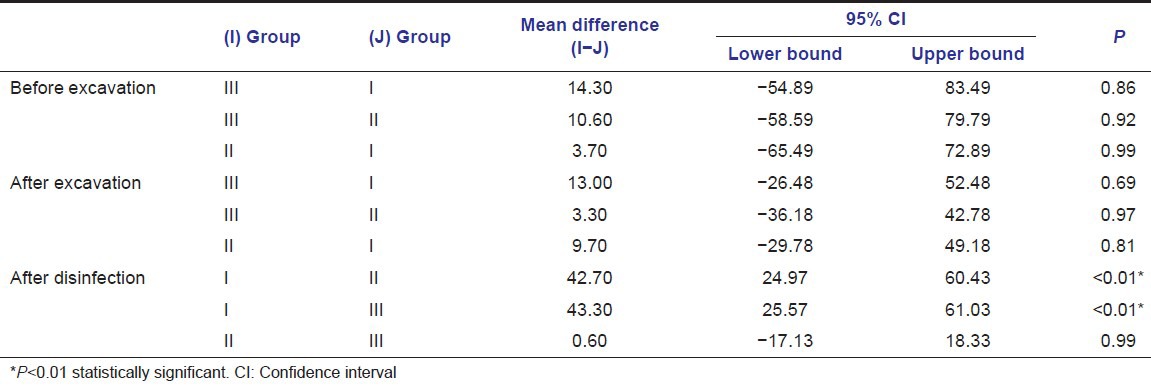
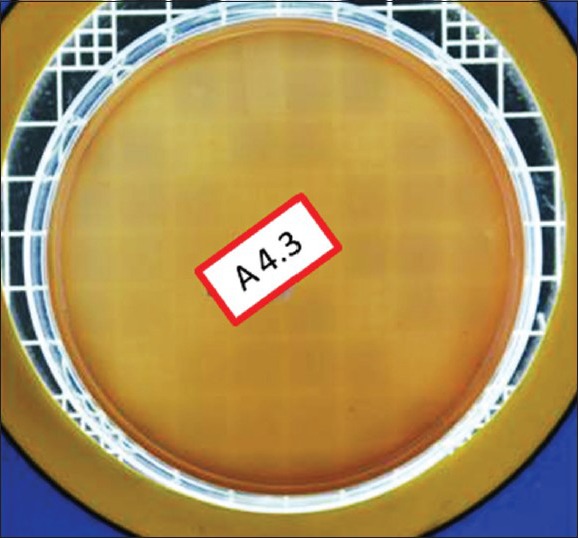
Figure 1.

Total viable count preexcavation in Group I
Figure 9.
Total viable count postdisinfection in Group III
Figure 2.
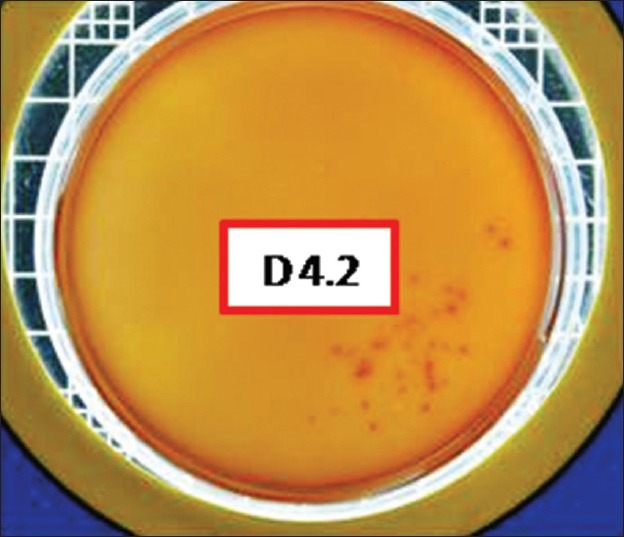
Total viable count postexcavation in Group I
Figure 3.
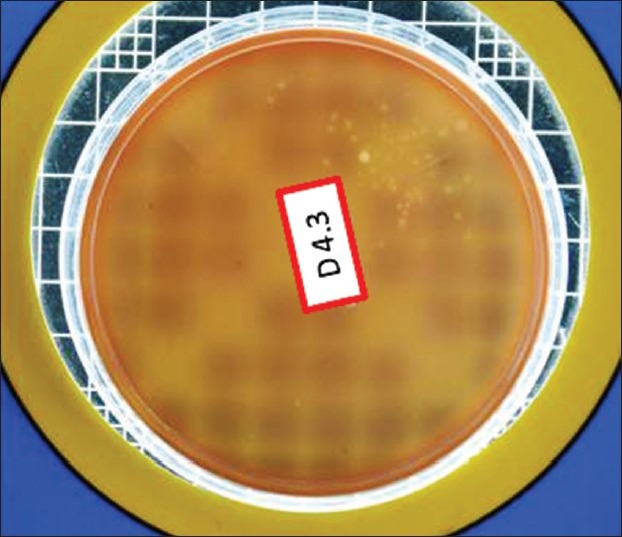
Total viable count postdisinfection in Group I
Figure 4.
Total viable count preexcavation in Group II
Figure 5.
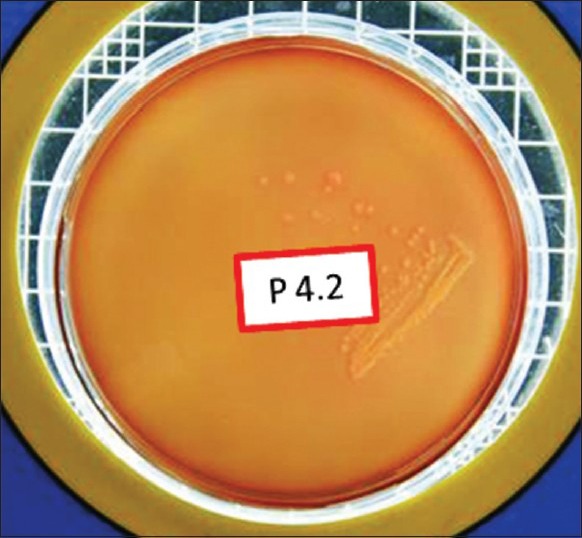
Total viable count postexcavation in Group II
Figure 6.
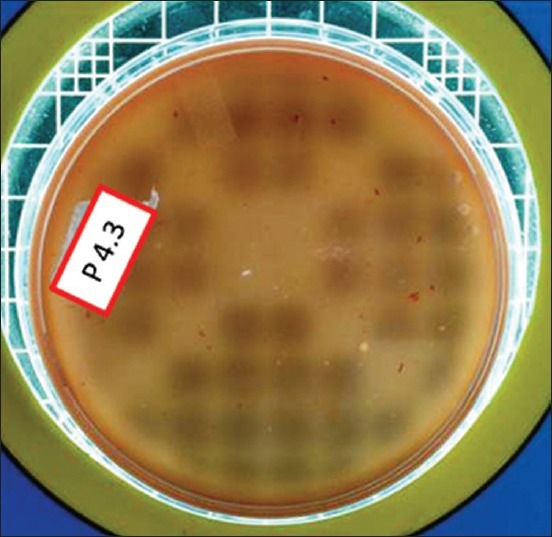
Total viable count postdisinfection in Group II
Figure 7.
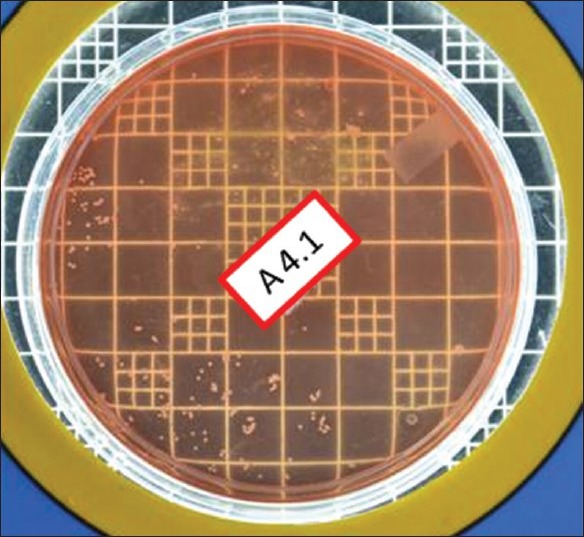
Total viable count preexcavation in Group III
Figure 8.
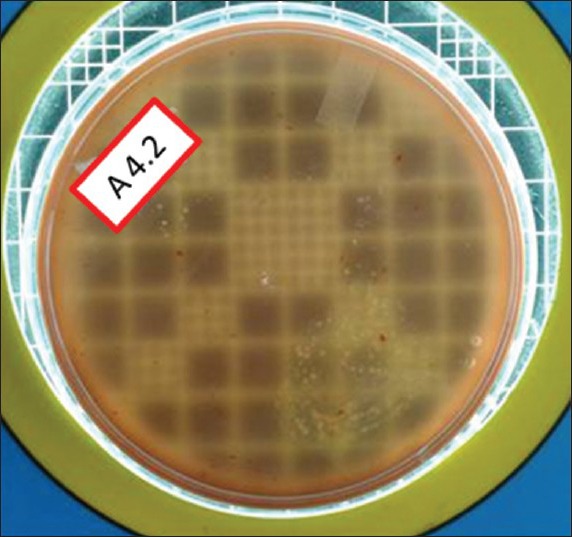
Total viable count postexcavation in Group III
Discussion
Restorative dentistry was traditionally based on the premise that bacterial infection of demineralized dentine should and must prompt operative intervention. However, the more recent concepts encompassing the MID philosophy emphasize the need to create a favorable environment for arresting the progress of caries with bare minimum operative intervention.[14] While using minimally invasive techniques such as ART, the limitations in terms of accessibility and fatigue of hand and wrists, increase the risk of incomplete excavation,[14,15] thereby allowing cariogenic bacteria to survive incarceration under adhesive restorations.[16] Even in conventional restorative techniques, residual or secondary caries is one of the most common reasons for the ultimate failure of the restoration.[14,17,18]
Several concepts using antimicrobial agents have been tried to eliminate bacteria underneath such restorations, the predominant one being the use of cavity disinfectants. In this regard, chlorhexidine has been studied extensively[7,19] and it was found to reduce residual bacteria after cavity excavation, but it has shown adverse effects on the microtensile[20] and shear bond strengths[21] as well as on microleakage.[20,22]
Thus, there is a global need for an alternate cavity disinfectant, which is safe, effective and economical. With the growing popularity of phytotherapy, natural products are increasingly being considered as better alternatives to synthetic chemicals.[23] Thus, in the present study, we used ethanolic extracts of naturally available A. vera and propolis for disinfecting the cavities before restorative procedure.
The results of our study showed that there were no statistically significant difference between the number of bacterial colonies among the three different study groups at various phases like preexcavation and postexcavation, thereby reiterating the importance of split mouth technique as well the use of a standardized method of caries excavation adapted in this study. When the TVC between pre and postexcavation of caries were compared, there was a significant reduction in the counts. However, hand excavation alone was not efficient enough to completely eliminate cariogenic bacteria. This is in agreement with previous studies, which have been critical about the ART technique based on the argument that hand excavation can never come close to its rotary counterpart as far as the efficiency of eliminating the carious tissues is concerned.[14,24]
An important and clinically relevant finding of the present study is that there was a significant reduction in the bacterial counts after cavity disinfection with propolis and A. vera when compared to the control group (Distilled water). These findings can be supported by the findings of previous investigators such as Mohammad Zadeh et al. (2007) who postulated that propolis had no significant clinical toxicity and was capable of preventing the growth of all the tested microorganisms including bacteria and fungi.[25] Similarly, Fani and Kohanteb (2012) had suggested that A. vera gel at optimum concentration could be used as an antiseptic for preventing dental caries and periodontal diseases.[26]
Mechanism of action of propolis
Flavonoids and Cinnamic acid derivatives have been widely cited as the main biologically active compounds in propolis.[27,28] Though, antimicrobial activities of propolis have usually been attributed to the aforementioned entities, other constituents of propolis too have been found to be contributing to the same cause. For instance, Ikeno et al. reported that cinnamic acid identified from Chinese and Japanese propolis demonstrated antimicrobial activity against Streptococcus mutan.[29] Similarly, Aga et al. stated that some hydroxycinnamic acid derivatives isolated in Brazilian propolis showed significant antimicrobial activity.[30] Further, Iio et al. demonstrated that Chrysin (one of the key components in propolis) inhibited glucosyltransferase (GTF) activity as well as glucan formation.[31] Results of several studies confirmed that other propolis constituents such as Pinocembrin and Naringenin possess antibacterial activity as well.[32,33] In addition, Duarte et al. found that fatty acids were the putative active compounds in Brazilian propolis and influenced some of the critical virulence factors associated with the pathogenesis of dental caries, including acid production, F-adenosinetriphosphatase Pase, and GTF activities.[34] Takaisi-Kikuni and Schilcher reported that propolis prevented bacterial cell division and also broke down bacterial walls and cytoplasm similar to the action of some antibiotics.[35] Thus, the antibacterial characteristic of propolis have been explained based on the synergy between these compounds along with unique properties of each constituent.[36,37,38]
Mechanism of action of Aloe vera
Aloe vera has been used therapeutically, since Roman times and perhaps long before.[39,40] The antimicrobial activity of A. vera is attributed to a number of pharmacologically active compounds including anthraquinones, aloin, aloe-emodin, aloetic acid, anthracene, aloe mannan, aloeride, antranol, chrysophanic acid, resistanol, and saponin.[41] Aloin, a bitter-tasting yellow compound, is the C-glycoside derivative of an anthraquinone.[42] Aloin and aloe-emodin possess strong antibacterial and antiviral activities as well as laxative, hepatoprotective, and antineoplastic characteristics.[43] Aloin and aloe emodin are the major anthrquinones in aloe plants, and these can inhibit protein synthesis from bacterial cells, thus explaining their antimicrobial activity.[44] It is noteworthy that some compounds like anthraquinones and saponin present in A. vera gel have direct antibacterial activities while some other components, such as acemannan, have been considered to exert indirect bactericidal activity through stimulation of phagocytosis.[45] The advantage of alcohol extract of A. vera is that the active ingredients of A. vera are highly soluble in this liquid extract media, thereby enhancing the antimicrobial activity.[46] Hence, we used an ethanolic extract of A. vera in the present study.
In the present study, though A. vera was found to be marginally more efficacious when compared to propolis, there was no statistically significant difference between their disinfecting abilities. Thus, it would be fair to suggest ethanolic extracts of both A. vera and propolis as potential cavity disinfecting agents although further long-term assessments are recommended.
Conclusion and Points of Clinical Relevance
Excavation alone cannot completely eliminate cariogenic bacteria
Ethanolic extracts of A. vera or propolis can be used as effective cavity disinfectants, which in turn may help to reduce secondary caries thus contributing to long-term restorative success.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rao A, Malhotra N. The role of remineralizing agents in dentistry: A review. Compend Contin Educ Dent. 2011;32:26–33. [PubMed] [Google Scholar]
- 2.Bönecker M, Toi C, Cleaton-Jones P. Mutans streptococci and lactobacilli in carious dentine before and after Atraumatic Restorative Treatment. J Dent. 2003;31:423–8. doi: 10.1016/s0300-5712(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 3.Smales RJ, Yip HK. The atraumatic restorative treatment (ART) approach for primary teeth: Review of literature. Pediatr Dent. 2000;22:294–8. [PubMed] [Google Scholar]
- 4.Honkala E, Behbehani J, Ibricevic H, Kerosuo E, Al-Jame G. The atraumatic restorative treatment (ART) approach to restoring primary teeth in a standard dental clinic. Int J Paediatr Dent. 2003;13:172–9. doi: 10.1046/j.1365-263x.2003.00455.x. [DOI] [PubMed] [Google Scholar]
- 5.Wicht MJ, Haak R, Schütt-Gerowitt H, Kneist S, Noack MJ. Suppression of caries-related microorganisms in dentine lesions after short-term chlorhexidine or antibiotic treatment. Caries Res. 2004;38:436–41. doi: 10.1159/000079624. [DOI] [PubMed] [Google Scholar]
- 6.Frencken JE, Imazato S, Toi C, Mulder J, Mickenautsch S, Takahashi Y, et al. Antibacterial effect of chlorhexidine- containing glass ionomer cement in vivo: A pilot study. Caries Res. 2007;41:102–7. doi: 10.1159/000098042. [DOI] [PubMed] [Google Scholar]
- 7.Ersin NK, Uzel A, Aykut A, Candan U, Eronat C. Inhibition of cultivable bacteria by chlorhexidine treatment of dentin lesions treated with the ART technique. Caries Res. 2006;40:172–7. doi: 10.1159/000091120. [DOI] [PubMed] [Google Scholar]
- 8.Neelakantan P, Jagannathan N, Nazar N. Ethnopharmacological approach in endodontic treatment: A Focused review. Int J Drug Res. 2011;3:68–77. [Google Scholar]
- 9.Ramasubramanian TS, Sivakumar VT, Thirumalai AV. Antimicrobial activity of Aloe vera (L.) Burm. f. against pathogenic microorganisms. J Biol Sci Res. 2010;4:251–8. [Google Scholar]
- 10.Arunkumar S, Muthuselvam M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci. 2009;5:572–6. [Google Scholar]
- 11.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–63. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 12.Dziedzic A, Kubina R, Wojtyczka RD, Kabala-Dzik A, Tanasiewicz M, Morawiec T. The antibacterial effect of ethanol extract of polish propolis on mutans streptococci and lactobacilli isolated from saliva. Evid Based Complement Alternat Med 2013. 2013 doi: 10.1155/2013/681891. 681891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athiban PP, Borthakur BJ, Ganesan S, Swathika B. Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones. J Conserv Dent. 2012;15:246–8. doi: 10.4103/0972-0707.97949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weerheijm KL, Groen HJ. The residual caries dilemma. Community Dent Oral Epidemiol. 1999;27:436–41. doi: 10.1111/j.1600-0528.1999.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 15.van Amerongen WE. Dental caries under glass ionomer restorations. J Public Health Dent. 1996;56:150–4. doi: 10.1111/j.1752-7325.1996.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 16.Foley J, Blackwell A. In vivo cariostatic effect of black copper cement on carious dentine. Caries Res. 2003;37:254–60. doi: 10.1159/000070867. [DOI] [PubMed] [Google Scholar]
- 17.Mjor IA. Frequency of secondary caries at various anatomical locations. Oper Dent. 1985;10:88–92. [PubMed] [Google Scholar]
- 18.Mjör IA. Glass-ionomer cement restorations and secondary caries: A preliminary report. Quintessence Int. 1996;27:171–4. [PubMed] [Google Scholar]
- 19.Borges FM, de Melo MA, Lima JP, Zanin IC, Rodrigues LK. Antimicrobial effect of chlorhexidine digluconate in dentin: In vitro and in situ study. J Conserv Dent. 2012;15:22–6. doi: 10.4103/0972-0707.92601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of 2% chlorhexidine on dentin microtensile bond strengths and nanoleakage of luting cements. J Dent. 2009;37:440–8. doi: 10.1016/j.jdent.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Vieira Rde S, da Silva IA., Jr Bond strength to primary tooth dentin following disinfection with a chlorhexidine solution: An in vitro study. Pediatr Dent. 2003;25:49–52. [PubMed] [Google Scholar]
- 22.Singla M, Aggarwal V, Kumar N. Effect of chlorhexidine cavity disinfection on microleakage in cavities restored with composite using a self-etching single bottle adhesive. J Conserv Dent. 2011;14:374–7. doi: 10.4103/0972-0707.87201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakrishna Y, Goda H, Baliga MS, Munshi AK. Decreasing cariogenic bacteria with a natural, alternative prevention therapy utilizing phytochemistry (plant extracts) J Clin Pediatr Dent. 2011;36:55–63. doi: 10.17796/jcpd.36.1.f485870h90174311. [DOI] [PubMed] [Google Scholar]
- 24.Bjørndal L, Larsen T, Thylstrup A. A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res. 1997;31:411–7. doi: 10.1159/000262431. [DOI] [PubMed] [Google Scholar]
- 25.Mohammad Zadeh SH, Shariatpanahi M, Hamedi M, Ahmadkhaniha R, Samadi N, Ostad S. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem. 2007;103:1097–103. [Google Scholar]
- 26.Fani M, Kohanteb J. Inhibitory activity of Aloe vera gel on some clinically isolated cariogenic and periodontopathic bacteria. J Oral Sci. 2012;54:15–21. doi: 10.2334/josnusd.54.15. [DOI] [PubMed] [Google Scholar]
- 27.Bonvehi JS, Coll FV, Jorda RE. The composition, active components and bacteriostatic activity of propolis in dietetics. J Am Oil Chem Soc. 1994;71:529–32. [Google Scholar]
- 28.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–71. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 29.Ikeno K, Ikeno T, Miyazawa C. Effects of propolis on dental caries in rats. Caries Res. 1991;25:347–51. doi: 10.1159/000261390. [DOI] [PubMed] [Google Scholar]
- 30.Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima S. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci Biotechnol Biochem. 1994;58:945–6. [Google Scholar]
- 31.Iio M, Uyeda M, Iwanami T, Nakagawa Y. Flavonoids as a possible preventive of dental caries. Agric Biol Chem. 1984;48:2143–5. [Google Scholar]
- 32.Houghton PJ, Woldemariam TZ, Davey W, Basar A, Lau C. Quantitation of the pinocembrin content of propolis by densitometry and high performance liquid chromatography. Phytochem Anal. 2008;6:207–10. [Google Scholar]
- 33.Denny BJ, West PW, Mathew TC. Antagonistic interactions between the flavonoids hesperetin and naringenin and beta-lactam antibiotics against Staphylococcus aureus. Br J Biomed Sci. 2008;65:145–7. doi: 10.1080/09674845.2008.11732819. [DOI] [PubMed] [Google Scholar]
- 34.Duarte S, Rosalen PL, Hayacibara MF, Cury JA, Bowen WH, Marquis RE, et al. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Arch Oral Biol. 2006;51:15–22. doi: 10.1016/j.archoralbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Takaisi-Kikuni NB, Schilcher H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med. 1994;60:222–7. doi: 10.1055/s-2006-959463. [DOI] [PubMed] [Google Scholar]
- 36.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117–24. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 37.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother. 2002;46:1302–9. doi: 10.1128/AAC.46.5.1302-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy S, Ramakrishna Y. Evaluation of antimicrobial efficacy of various root canal filling materials used in primary teeth: A microbiological study. J Clin Pediatr Dent. 2007;31:193–8. doi: 10.17796/jcpd.31.3.t73r4061424j2578. [DOI] [PubMed] [Google Scholar]
- 40.Möller AJ. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr. 1966;74(Suppl):1–380. [PubMed] [Google Scholar]
- 41.Kambizi L, Afolayan AJ. Extracts from Aloe ferox and Withania somnifera inhibit Candida albicans and Neisseria gonorrhea. Afr J Biotechnol. 2008;7:12–5. [Google Scholar]
- 42.Saccù D, Bogoni P, Procida G. Aloe exudate: Characterization by reversed phase HPLC and headspace GC-MS. J Agric Food Chem. 2001;49:4526–30. doi: 10.1021/jf010179c. [DOI] [PubMed] [Google Scholar]
- 43.Hatano T, Kusuda M, Inada K, Ogawa TO, Shiota S, Tsuchiya T, et al. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry. 2005;66:2047–55. doi: 10.1016/j.phytochem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Somboonwong J, Thanamittramanee S, Jariyapongskul A, Patumraj S. Therapeutic effects of Aloe vera on cutaneous microcirculation and wound healing in second degree burn model in rats. J Med Assoc Thai. 2000;83:417–25. [PubMed] [Google Scholar]
- 45.Ferro VA, Bradbury F, Cameron P, Shakir E, Rahman SR, Stimson WH. In vitro susceptibilities of Shigella flexneri and Streptococcus pyogenes to inner gel of Aloe barbadensis Miller. Antimicrob Agents Chemother. 2003;47:1137–9. doi: 10.1128/AAC.47.3.1137-1139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundarkar P, Govindwar R, Nyamati SB, Alladwar N, Thombre V, Soni A, et al. Use of Aloe vera in dentistry. J Indian Acad Oral Med Radiol. 2011;23:S389–91. [Google Scholar]


